Abstract
BACKGROUND
Blunted nocturnal blood pressure (NBP) dipping is a significant predictor of cardiovascular events. Lower socioeconomic position (SEP) may be an important predictor of NBP dipping, especially in African Americans (AA). However, the determinants of NBP dipping are not fully understood.
METHODS
The cross-sectional associations of individual and neighborhood SEP with NBP dipping, assessed by 24-h ambulatory BP monitoring, were examined among 837 AA adults (Mean age: 59.2 ± 10.7 years; 69.2% women), after adjustment for age, sex, hypertension status, body mass index (BMI), health behaviors, office, and 24-h systolic BP (SBP).
RESULTS
The mean hourly SBP was consistently lower among participants in the highest category of individual income compared to those in the lowest category, and these differences were most pronounced during sleeping hours. The odds of NBP dipping (defined as >10% decline in the mean asleep SBP compared to the mean awake SBP) increased by 31% (95% confidence interval: 13–53%) and 18% (95% confidence interval: 0–39%) for each s.d. increase in income and years of education, respectively, after multivariable adjustment.
CONCLUSIONS
NBP dipping is patterned by income and education in AA adults even after accounting for known risk factors. These results suggest that low SEP is a risk factor for insufficient NBP dipping in AA.
Keywords: ambulatory blood pressure monitoring, blood pressure, hypertension, Jackson Heart Study, nocturnal dipping, socioeconomic position, systole
A blunted blood pressure (BP) decline during sleep is a significant predictor of mortality1–3 and cardiovascular disease (CVD) related outcomes,4 including cardiac hypertrophy, dysfunction, and structure,5,6 congestive heart failure,7 and renal insufficiency.8 African Americans (AA) are more likely than whites to experience blunted BP dipping or absence of BP dipping during sleep.9 This insufficient BP decline may contribute to existing disparities in CVD and renal outcomes. Important questions remain regarding the main risk factors for nondipping BP, especially in AA.
The association of low socioeconomic position (SEP) with cardiovascular risk factors, including elevated BP is well recognized.10–14 Adverse neighborhood characteristics (e.g., socioeconomic disadvantage) have also been linked to poor cardiovascular outcomes and higher BP, even after adjustment for individual-level SEP (e.g., annual household income).12–19 Few studies have investigated the associations of individual and neighborhood SEP with BP dipping. Although three studies have reported associations of BP dipping with individual-level measures of SEP, sample sizes were small (the largest being 174 participants).20–22
We investigated associations of individual and neighborhood SEP measures with diurnal and nocturnal ambulatory BP measures in a large population-based sample of AA adults. We hypothesized that lower individual and neighborhood SEP would be associated with less BP dipping during sleep and that these associations would be independent of behavioral factors, body mass index (BMI), and other potential confounders.
METHODS
The Jackson Heart Study (JHS) is a population-based observational study of the epidemiology of CVD in AA. Details of the study design23 and recruitment24 are provided elsewhere. Institutional review board approval was obtained from the JHS institutions (Jackson State University, Tougaloo College, and the University of Mississippi Medical Center) and informed consent was received from all participants. All 5,301 JHS participants (mean age: 54.9 years (range: 21–94 years)) were invited to participate in the 24-h ambulatory BP monitoring (ABPM) during their scheduled baseline (2000–2004) clinic examination. In total, 1,146 (21.6%) participants underwent ABPM.
SEP
Education was categorized into three strata: greater than or equal to a high school diploma or graduate equivalency degree (36.0%), 1–3 years of college (25.2%), and ≥4 years of college (38.8%). Annual household income was self-reported in 11 categories ranging from under $5,000–$100,000 or more and collapsed into four categories: <$25,000 (36.0%), $25,000–$49,999 (26.4%), ≥$50,000 (27.7%), and unknown (9.9%). Education and income were also investigated as continuous predictors. Years of education was determined by assigning the corresponding numerical value for each year of school completed for grades 1–12, 12 years for a graduate equivalency degree, 13 years for a vocational or trade certificate and some college, but no degree, 14 years for an associate degree, 16 years for a bachelor degree, and 20 years for a graduate or professional degree. The median value of each income category was used to assign a continuous income measure to each participant. For participants classified with an “unknown” annual household income, we imputed the sex-specific median for the annual household income of people with the same education level as the respondent. For ease of comparison, continuous measures of income and education were transformed into s.d. units. Each participant’s residential mailing address was geocoded to the census tract and used as a proxy for neighborhood.25 Neighborhood SEP was defined as the median household income from 2000 US Census. Neighborhood SEP was divided into tertiles: ≤$25,900 (33.6%), $26,000–$35,000 (32.9%), and >$35,000 (33.6) and also examined continuously in s.d. units.
ABPM
Twenty-four hour ambulatory BP was obtained using a portable, noninvasive monitoring Spacelabs 90207 Oscillometric ABPM device (Medifacts, Rockville, MD). Participants were instructed in the proper use of the ABPM device by trained technicians. A monitoring device with the appropriate-sized cuff was placed on the nondominant arm and programmed to take readings at 20-min intervals. Each participant was instructed to proceed through a normal day and keep their arm still and extended at his/her side during each BP reading. With the participant in the seated position, 3–5 simultaneous monitor and office sphygmomanometer correlation BP readings measures were used to calibrate each ABPM device. According to the manufacturer’s protocol, the BP readings were considered within range if the mean office sphygmomanometer diastolic BP (DBP) did not differ from the mean office ambulatory DBP by ± 7 mm Hg. All BP readings were edited to eliminate out-of-range readings and errors due to motion artifacts or equipment problems. Predefined acceptable ranges included systolic BP (SBP): 60–261 mm Hg and DBP: 30–151 mm Hg. Participants were asked to maintain a diary of their sleep and wake times. The use of diary information compared to fixed interval sleep times to compute awake and sleep BP averages has been recently validated.26
Analyses were limited to participants with a minimum of 54 valid readings (75% of the 72 programmed readings), at least one valid reading per hour and complete diary information. For each participant, mean awake and sleep BPs were calculated using recorded awake and sleep times. We estimated three measures of awake and sleep SBP differences: (i) the absolute difference was calculated as the difference between the mean awake and asleep SBP; (ii) the percent difference was calculated as 1 minus the ratio of the mean sleep SBP to the mean awake SBP; and, (iii) a “dipper” was defined as a participant with >10% decline in the mean SBP during sleep compared to awake SBP.1–9,26 We used SBP as the key BP measure because it has been the most consistently used measure in studies linking BP dipping to health outcomes.1–8 In the present study, the SBP and DBP dipping measures were highly correlated (absolute difference: r = 0.79, P < 0.001; percent difference: r = 0.81, P value < 0.001).
Covariates
Risk factors for impaired BP dipping1,27–30 included self-reported cigarette smoking, physical inactivity, short sleep duration, binge alcohol consumption, increased dietary intake of sodium and potassium, and obesity. Other covariates included office BP, hypertension status, and use of antihypertensive medications. Cigarette smoking was classified as current, former, or never smoker. A summary score of physical activity (frequency and duration of watching television, walking and/or biking to work, school, or errands, and physical exercise during leisure time) was calculated from a 30-item validated questionnaire.31 Self-reported sleep duration was ascertained using the question: “During the past month, excluding naps, how many hours of actual sleep did you get at night (or day, if you work at night), on average?” Daily alcohol consumption and sodium and potassium intakes were assessed from a validated food frequency questionnaire administered face-to-face by trained interviewers.32 BMI was calculated from in-clinic measurements of standing weight and height using standardized procedures (kg/m2). Office BP was measured in the seated position after a 5-min rest using a random zero mercury sphygmomanometer with the appropriate size cuff; the mean of two measurements was reported. Hypertension was defined as a SBP ≥140 mm Hg, or DBP ≥90 mm Hg; self-reported use of antihypertensive medications during the 2 weeks prior to the baseline clinic examination; or, a self-report of a physician-diagnosis of hypertension and has been described previously.33 Antihypertensive medication use was defined as currently taking at least one of seven classes of BP lowering medications.33
Statistical analysis
All analyses were initially stratified by sex, hypertension status, and medication use, separately, to explore to explore heterogeneities in the associations of SEP with BP dipping by these factors. Statistical interactions between these factors and the SEP measures of interest with respect to the three BP dipping outcomes were tested in age-adjusted models. Since no consistent statistically significant interactions with SEP measures (in separate models) were observed in age-adjusted models (Ps > 0.10), subsequent analyses were pooled and adjusted for sex, hypertension status, and medication use. Associations of SEP with relative BP difference, with percent difference in BP, and with the odds of BP dipping were estimated using linear and logistic regression models. Hierarchical models with an identity or logit link (continuous or binary outcome, respectively) and a random intercept for each census tract were used to account for within tract correlations in selected analyses. Sequential multivariable models were fit to examine the mediating or confounding effect of different covariates on the associations of SEP with BP dipping. Model 1 (base model) adjusted for age and sex, Model 2 adjusted the base model for hypertension status and hypertension medication use, Model 3 added known risk factors (current cigarette smoking, physical activity, alcohol consumption, dietary intake of sodium and potassium, and BMI) and Models 4 and 5 added office SBP and mean 24-h SBP, respectively. In sensitivity analyses, (i) we further adjusted for prevalent diabetes34 and CVD35 defined using standard criteria, (ii) accounted for correlations among related participants using generalized estimating equations, and (iii) restricted analyses to persons not currently taking antihypertensive medications (n = 322). All tests were two-tailed and a probability value <0.05 was considered statistically significant.
RESULTS
Of the 1,146 participants who underwent ABPM, 837 (Mean age: 59.2 ± 10.7 years; 69.2% women) had the minimum number of valid BP readings and complete awake-sleep diary information as well as complete data on key socioeconomic indicators and covariates. The 837 participants included in these analyses did not differ substantially on socioeconomic indicators or office SBP from the other JHS participants but had lower office DBP and were more likely than the rest of the JHS cohort to be hypertensive and diabetic, taking antihypertensive medications, and to have CVD.
Similar to the prevalences reported in other samples of AA,9,27 the unadjusted prevalence of “dippers” (BP dipping >10% during sleep) was 31.5% in women and 33.2% in men. Compared to nondippers, dippers were younger, more likely to consume moderate amounts of alcohol and have less prevalent type 2 diabetes and CVD and lower BMI (Table 1).
Table 1.
Selected characteristics of the study population by blood pressure dipping status: Jackson Heart Study, 2000–2004
| Characteristic | Dipper (N = 268) |
Nondipper (N = 569) |
P value |
|---|---|---|---|
| Age, years | 57.4 (10.6) | 60.1 (10.7) | <0.001 |
| Female sex, % | 67.9 | 69.6 | 0.623 |
| Body mass index, kg/m2 | 30.1 (6.1) | 31.3 (6.6) | 0.007 |
| Current smoker, % | 12.7 | 8.8 | 0.08 |
| Alcohol consumption, grams/day | 6.1 (22.6) | 2.5 (10.4) | 0.015 |
| Physical activity score | 2.2 (0.8) | 2.1 (0.8) | 0.315 |
| Sleep duration, h | 6.4 (1.5) | 6.4 (1.5) | 0.669 |
| Sodium, grams/day | 3.9 (2.4) | 3.9 (2.0) | 0.974 |
| Potassium, grams/day | 2.7 (1.4) | 2.7 (1.2) | 0.670 |
| Office blood pressure, mm Hg | |||
| Systolic BP | 126.5 (17.0) | 127.8 (18.0) | 0.329 |
| Diastolic BP | 77.5 (10.5) | 77.0 (10.2) | 0.522 |
| Hypertension, % | 64.9 | 70.5 | 0.232 |
| Antihypertensive medication use, % | 54.9 | 61.3 | 0.121 |
| Use within 24-h of clinic visit, % | 81.2 | 80.6 | 0.336 |
| Diabetes, % | 15.7 | 22.3 | 0.013 |
| Cardiovascular disease, % | 6.4 | 11.0 | 0.034 |
A dipper is defined as a decline >10% in the mean systolic BP (SBP) during sleep compared to awake SBP.
BP, blood pressure.
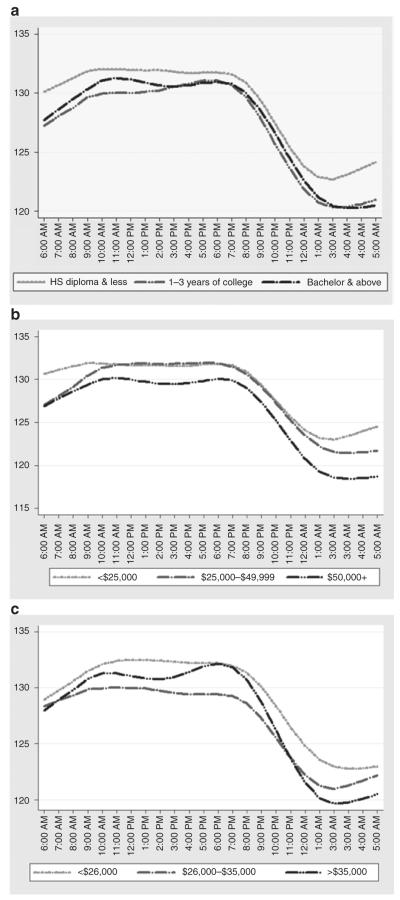
The mean hourly SBP was consistently lower among participants in the highest category of income compared to participants in the lowest category and differences by income appeared most pronounced during sleep hours (10:00 PM–6:00 AM) (Figure 1b). Similar differences in hourly SBP were observed across categories of education (Figure 1a) and neighborhood SEP (Figure 1c), although differences were not as pronounced.
Figure 1.
Twenty-four hour circadian rhythm of systolic blood pressure (SBP) among African-American men and women in the Jackson Heart Study, 2000–2004. Mean hourly unadjusted SBP across categories of (a) education, (b) income, and (c) median neighborhood income. HS, high school.
After adjustment for age, sex, hypertension status, and hypertension medication use (Model 2), higher income was associated with greater absolute and percent differences between awake and sleep mean SBP (Table 2). These differences were reduced but remained statistically significant after adjustment for standard risk factors (BMI, current cigarette smoking, physical activity, sleep duration, alcohol consumption, dietary intake of sodium and potassium, and BMI (Model 3)), indicating greater BP dipping in the higher income groups. These differences were not substantially attenuated after additional adjustment for office BP (Model 4) and mean 24-h SBP (Model 5). The largest attenuations in the associations of income with absolute and percent differences occurred after adjustment for standard risk factors. Associations of education with absolute and percent differences in SBP were in a similar direction to those observed for income but were not statistically significant.
Table 2.
Mean differences (in mm Hg) and odds ratio of BP dipping per 1-s.d. increase in SEP: Jackson Heart Study, 2000–2004
| Characteristics | Mean difference in absolute awake-sleep SBP difference |
Mean difference in percent awake-sleep SBP difference |
Odds ratio of BP dipping (defined as percent difference >10%) |
|---|---|---|---|
| Education | |||
| Model 1 | 0.62 (−0.03, 1.27)† | 0.45 (−0.04, 0.94)† | 1.18 (1.01, 1.37)* |
| Model 2 | 0.62 (−0.03, 1.27)† | 0.45 (−0.04, 0.94)† | 1.18 (1.01, 1.37)* |
| Model 3 | 0.49 (−0.18, 1.16) | 0.34 (−0.15, 0.83) | 1.17 (0.99, 1.37)† |
| Model 4 | 0.50 (−0.17, 1.17) | 0.34 (−0.15, 0.83) | 1.17 (0.99, 1.37)† |
| Model 5 | 0.54 (−0.13, 1.20) | 0.39 (−0.10, 0.88) | 1.18 (1.01, 1.39)* |
| Income | |||
| Model 1 | 1.22 (0.57, 1.87)*** | 0.91 (0.42, 1.40)*** | 1.30 (1.13, 1.51)*** |
| Model 2 | 1.21 (0.56, 1.86)*** | 0.90 (0.41, 1.39)*** | 1.31 (1.13, 1.51)*** |
| Model 3 | 1.06 (0.39, 1.73)** | 0.78 (0.39, 1.27)** | 1.32 (1.13, 1.54)*** |
| Model 4 | 1.06 (0.39, 1.73)** | 0.78 (0.39, 1.27)** | 1.32 (1.13, 1.54)*** |
| Model 5 | 1.05 (0.38, 1.72)** | 0.75 (0.26, 1.24)** | 1.32 (1.13, 1.54)*** |
| Neighborhood SEP | |||
| Model 1 | 0.46 (−0.19, 1.11) | 0.28 (−0.21, 0.77) | 1.00 (0.96, 1.03) |
| Model 2 | 0.47 (−0.18, 1.12) | 0.29 (−0.20, 0.78) | 1.00 (0.96, 1.03) |
| Model 3 | 0.37 (−0.30, 1.04) | 0.21 (−0.28, 0.70) | 0.99 (0.96, 1.03) |
| Model 4 | 0.36 (−0.31, 1.03) | 0.21 (−0.28, 0.70) | 0.99 (0.96, 1.03) |
| Model 5 | 0.44 (−0.23, 1.11) | 0.30 (−0.19, 0.79) | 1.00 (0.96, 1.03) |
BP, blood pressure; SBP, systolic BP; SEP, socioeconomic position.
Model 1 adjusts for age and sex; Model 2 adjusts for variables in Model 1 plus hypertension status and medication use; and, Model 3 adjusts for variables in Model 2 plus behavioral and anthropometric risk factors. Model 4 adjust for the variables in Model 3 plus office SBP. Model 5 adjusts for the variables in Model 4 plus mean 24-h SBP.
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.10.
Higher income and higher education were also associated with greater odds of BP dipping after adjustment for age, sex, hypertension status, and antihypertensive medication use (Model 2), although associations were stronger and more likely to be statistically significant for income than for education. Further adjustments for risk factors and office and 24-h SBP did not substantially alter these estimates. In the fully adjusted model, the odds of BP dipping increased by 32% (95% confidence interval: 13–54%) for each s.d. increase in income. The associations with education were in the same direction but only marginally statistically significant. Greater neighborhood SEP was associated with small differences in absolute and percent SBP differences but none of the associations were statistically significant after multivariable adjustment and no associations with the odds of BP dipping were observed.
Further adjustment for prevalent type 2 diabetes and CVD and accounting for clustering within related participants did not substantially modify results (data not shown). In models excluding participants undergoing antihypertensive therapy, similar associations between SEP and BP dipping were observed, although there were null associations between income and absolute and percent SBP differences (Table 3).
Table 3.
Mean differences (in mm Hg) and odds ratio of BP dipping per 1-s.d. increase in SEP among nonhypertensive individuals: Jackson Heart Study, 2000–2004
| Characteristics | Mean difference in absolute awake-sleep SBP difference |
Mean difference in percent awake-sleep SBP difference |
Odds ratio of BP dipping (defined as percent difference >10%) |
|---|---|---|---|
| Excluding hypertension medication use (n = 322) | |||
| Education | |||
| Model 1 | 0.54 (−0.32, 1.40) | 0.43 (−0.22, 1.08) | 1.26 (0.99, 1.61)** |
| Model 2 | 0.54 (−0.32, 1.40) | 0.43 (−0.22, 1.08) | 1.26 (0.99, 1.61)** |
| Model 3 | 0.55 (−0.31, 1.41) | 0.49 (−0.22, 1.20) | 1.38 (1.05, 1.80)* |
| Model 4 | 0.70 (−0.22, 1.62) | 0.55 (−0.14, 1.24) | 1.38 (1.05, 1.80)* |
| Model 5 | 0.73 (−0.19, 1.65) | 0.73 (−0.19, 1.65) | 1.40 (1.07, 1.84)* |
| Income | |||
| Model 1 | 0.92 (0.06, 1.78)* | 0.68 (0.03, 1.33)* | 1.13 (0.89, 1.42) |
| Model 2 | 0.92 (0.06, 1.78)* | 0.68 (0.03, 1.33)* | 1.13 (0.89, 1.42) |
| Model 3 | 1.09 (0.19, 1.99)* | 0.75 (0.06, 1.44)* | 1.23 (0.96, 1.58) |
| Model 4 | 1.09 (0.19, 1.99)* | 0.75 (0.06, 1.44)* | 1.23 (0.96, 1.58) |
| Model 5 | 1.12 (0.22, 2.02)* | 0.80 (0.13, 1.47)* | 1.25 (0.97, 1.60)** |
| Neighborhood SEP | |||
| Model 1 | 0.19 (−0.69, 1.07) | 0.04 (−0.63, 0.71) | 0.98 (0.93, 1.03) |
| Model 2 | 0.18 (−0.70, 1.06) | 0.05 (−0.62, 0.72) | 0.98 (0.93, 1.03) |
| Model 3 | 0.30 (−0.62, 1.22) | 0.12 (−0.57, 0.81) | 0.98 (0.93, 1.04) |
| Model 4 | 0.31 (−0.61, 1.23) | 0.12 (−0.57, 0.81) | 0.98 (0.93, 1.04) |
| Model 5 | 0.39 (−0.53, 1.31) | 0.25 (−0.44, 0.94) | 0.99 (0.94, 1.04) |
BP, blood pressure; SBP, systolic BP; SEP, socioeconomic position.
Model 1 adjusts for age and sex; Model 2 adjusts for variables in Model 1 plus hypertension status; and, Model 3 adjusts for variables in Model 2 plus behavioral and anthropometric risk factors. Model 4 adjust for the variables in Model 3 plus office SBP. Model 5 adjusts for the variables in Model 4 plus mean 24-h SBP.
P < 0.05,
P < 0.10.
DISCUSSION
In this population-based sample of AA adults, greater SEP was associated with greater BP dipping. The mean hourly SBP was consistently lower among participants in the highest income category compared to those in the lowest income category, and these differences were most pronounced during sleeping hours. A higher level of education was also associated with greater BP dipping, but this association was less pronounced. The odds of BP dipping increased by 32% and 18%, respectively for each s.d. in income and education, even after adjustment for known risk factors. Similar patterns were observed for absolute and percent differences between awake and asleep SBP.
Our findings are consistent with limited prior evidence suggesting that dippers are significantly healthier than nondippers and BP dipping is socioeconomically patterned; prior work has been based on small samples of mostly mildly hypertensive volunteer adults20,21 and undergraduates,22 the largest of which included only 42 AA participants.20 In 78 employed adults, Stepnowsky and colleagues20 reported that SEP (as measured by occupation and education) was positively associated with dipping. Spruill et al.21 observed that education was positively associated with greater BP dipping, even after controlling for race. Campbell and colleagues22 found that higher childhood socioeconomic status was associated with more BP dipping among 174 undergraduate students.
Our study is the first to examine the association between SEP and BP dipping in a large population-based sample of middle-aged and elderly AA. BP dipping was strongly patterned by SEP, especially income, even after adjustment for BP dipping risk factors. Although AA, as a group, have lower rates of BP dipping than whites,9 there is substantial socioeconomic heterogeneity in BP dipping within AA. Additional adjustment for antihypertensive medication use within 24-h of the ABPM did not materially change any of the observed associations, although we cannot rule out differential compliance with medication or use of different types of antihypertensive drugs by SEP as a possible explanation. Further investigation into the mechanisms through which SEP may affect BP dipping and contribute to racial differences in BP dipping is warranted.
Others have noted that education may be a weaker predictor of health in AA than in whites36 because of differential income returns to education in AA and whites. We found that income was the most consistent predictor of BP dipping in a large sample of AA: for nearly each $30,000 increase in annual household income, the odds of nocturnal BP (NBP) increased by 32%. When income and education were entered into the model simultaneously, education did not remain significant in any of the models (data not shown). Occupation was not associated with BP dipping in our analyses (data not shown) although challenges in the measurement of occupation in large population studies may have affected our ability to detect associations.
To our knowledge, no studies have examined associations of neighborhood characteristics with BP dipping in adults; one study of 212 adolescents reported that higher neighborhood income was associated with lower daytime SBP after adjustment for individual-level characteristics.37 We found no statistically significant associations of neighborhood SEP with BP dipping measures after multivariable adjustment. However, median household income may be a poor proxy for the neighborhood factors that may affect dipping. Additional research needs to investigate whether more specific environmental features of neighborhoods (e.g., noise or stressors) are related to BP dipping.
Associations of income and education with BP dipping persisted after adjustment for established risks factors. These findings are generally similar to those of other studies,20–22 although the risk factors investigated in our study were more comprehensive. While we cannot rule out measurement error, the fact that associations of SEP with BP dipping were not fully explained by established risk factors suggests that other mediators may be involved. Chronic stressors linked to SEP could play a role. Previous research22,38–40 has shown that various psychological stressors, including lack of social support, anger, and racism and discrimination, are associated with less BP dipping among AA and whites.
Strengths of our study include the availability of multiple SEP measures, the comprehensive covariate data to adjust for confounders and the statistical power provided by the use of a large population-based sample. The cross-sectional design of this study limits our ability to explore temporality. Another limitation of our study is the use of one ABPM to classify BP dipping. Nonetheless, we observed SEP differences in awake-sleep BP similar to studies with multiple ABPM.21 The lack of objective measures of obstructive sleep apnea data limited our ability to fully account for sleep disorders. Sample size and the high percentage of participants on antihypertensive medications limited our ability to fully investigate effect modification by hypertension status and medication use. The JHS cohort was not designed to be representative of AA in the entire United States and participants included in the present study were older, had lower office DBP and were more likely to be hypertensive, to undergo hypertension therapy and to have prevalent type 2 diabetes and CVD than the rest of the JHS cohort. It is not clear, however, that these selection factors would have affected the associations between income and BP dipping in this sample. Our sample remains one of the largest population-based samples of AA with ABPM data. We find that low SEP is a strong risk factor for less BP dipping in AA, even after adjustment for established risk factors for nondipping. Given that nondippers have an increased risk of CVD mortality of 51% compared to dippers,1 the magnitude of the associations we observed (e.g., a 31% increase in the odds of dipping) could have considerable population health impact. Taken together with known differences in SEP between AA and whites in the United States,23 our results imply that SEP may contribute to the large differences in BP dipping observed between AA and whites. Further investigation of the reasons for this strong income patterning of NBP dipping could provide important clues regarding the physiopathology of hypertension and hypertension-related mortality.
Acknowledgments
The authors thank the participants, staff, and interns of the Jackson Heart Study for their long-term commitment and important contributions to understanding the epidemiology of cardiovascular and other chronic diseases in African Americans. This work was supported by National Institutes of Health contracts (N01-HC-95170, N01-HC-95171, and N01-HC-95172) provided by the National Heart, Lung, and Blood Institute and the National Center for Minority Health and Health Disparities (NCMHD). This work was also supported in part by the University of Michigan Center for Integrative Approaches to Health Disparities (CIAHD), which is funded by the NCMHD (P60MD002249), and a pilot grant to the first author from CIAHD.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: a population-based study. Hypertension. 2005;45:499–504. doi: 10.1161/01.HYP.0000160402.39597.3b. [DOI] [PubMed] [Google Scholar]
- 2.Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens. 2008;21:92–97. doi: 10.1038/ajh.2007.7. [DOI] [PubMed] [Google Scholar]
- 3.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Satoh H, Hisamichi S. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 4.Routledge FS, McFetridge-Durdle JA, Dean CR, Canadian Hypertension Society Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol. 2007;23:132–138. doi: 10.1016/s0828-282x(07)70733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soylu A, Duzenli MA, Yazici M, Ozdemir K, Tokac M, Gok H. The effect of nondipping blood pressure patterns on cardiac structural changes and left ventricular diastolic functions in normotensives. Echocardiography. 2009;26:378–387. doi: 10.1111/j.1540-8175.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T, Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–438. doi: 10.1016/s0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 7.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 9.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33:1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 11.Diez Roux AV. Persistent social patterning of cardiovascular risk: rethinking the familiar. Circulation. 2005;111:3020–3021. doi: 10.1161/CIRCULATIONAHA.105.542845. [DOI] [PubMed] [Google Scholar]
- 12.Davey Smith G, Hart C, Hole D, MacKinnon P, Gillis C, Watt G, Blane D, Hawthorne V. Education and occupational social class: which is the more important indicator of mortality risk? J Epidemiol Community Health. 1998;52:153–160. doi: 10.1136/jech.52.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaix B, Bean K, Leal C, Thomas F, Havard S, Evans D, Jégo B, Pannier B. Individual/neighborhood social factors and blood pressure in the RECORD Cohort Study: which risk factors explain the associations? Hypertension. 2010;55:769–775. doi: 10.1161/HYPERTENSIONAHA.109.143206. [DOI] [PubMed] [Google Scholar]
- 14.Kawachi I, Berkman LF. Neighborhoods and Health. Oxford University Press; Oxford, New York: 2003. [Google Scholar]
- 15.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 16.Diez Roux AV, Chambless L, Merkin SS, Arnett D, Eigenbrodt M, Nieto FJ, Szklo M, Sorlie P. Socioeconomic disadvantage and change in blood pressure associated with aging. Circulation. 2002;106:703–710. doi: 10.1161/01.cir.0000025402.84600.cd. [DOI] [PubMed] [Google Scholar]
- 17.Chaix B, Ducimetière P, Lang T, Haas B, Montaye M, Ruidavets JB, Arveiler D, Amouyel P, Ferrières J, Bingham A, Chauvin P. Residential environment and blood pressure in the PRIME Study: is the association mediated by body mass index and waist circumference? J Hypertens. 2008;26:1078–1084. doi: 10.1097/HJH.0b013e3282fd991f. [DOI] [PubMed] [Google Scholar]
- 18.Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007;65:1853–1866. doi: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan TE, Cooper RS, Ni H, Shea S. Neighborhood characteristics and hypertension. Epidemiology. 2008;19:590–598. doi: 10.1097/EDE.0b013e3181772cb2. [DOI] [PubMed] [Google Scholar]
- 20.Stepnowsky CJ, Jr, Nelesen RA, DeJardin D, Dimsdale JE. Socioeconomic status is associated with nocturnal blood pressure dipping. Psychosom Med. 2004;66:651–655. doi: 10.1097/01.psy.0000138124.58216.6c. [DOI] [PubMed] [Google Scholar]
- 21.Spruill TM, Gerin W, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure dipping. Am J Hypertens. 2009;22:637–642. doi: 10.1038/ajh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell TS, Key BL, Ireland AD, Bacon SL, Ditto B. Early socioeconomic status is associated with adult nighttime blood pressure dipping. Psychosom Med. 2008;70:276–281. doi: 10.1097/PSY.0b013e3181647e30. [DOI] [PubMed] [Google Scholar]
- 23.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6–S4. [PubMed] [Google Scholar]
- 24.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6–18. [PubMed] [Google Scholar]
- 25.Robinson JC, Wyatt SB, Hickson D, Gwinn D, Faruque F, Sims M, Sarpong D, Taylor HA. Methods for retrospective geocoding in population studies: the Jackson Heart Study. J Urban Health. 2010;87:136–150. doi: 10.1007/s11524-009-9403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdecchia P, Angeli F, Sardone M, Borgioni C, Garofoli M, Reboldi G. Is the definition of daytime and nighttime blood pressure prognostically relevant? Blood Press Monit. 2008;13:153–155. doi: 10.1097/MBP.0b013e3282fd1709. [DOI] [PubMed] [Google Scholar]
- 27.Jehn ML, Brotman DJ, Appel LJ. Racial differences in diurnal blood pressure and heart rate patterns: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Arch Intern Med. 2008;168:996–1002. doi: 10.1001/archinte.168.9.996. [DOI] [PubMed] [Google Scholar]
- 28.Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–691. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- 29.Friedman O, Shukla Y, Logan AG. Relationship between self-reported sleep duration and changes in circadian blood pressure. Am J Hypertens. 2009;22:1205–1211. doi: 10.1038/ajh.2009.165. [DOI] [PubMed] [Google Scholar]
- 30.Sica DA. What are the influences of salt, potassium, the sympathetic nervous system, and the renin-angiotensin system on the circadian variation in blood pressure? Blood Press Monit. 1999;4(Suppl 2):S9–S16. [PubMed] [Google Scholar]
- 31.Smitherman TA, Dubbert PM, Grothe KB, Sung JH, Kendzor DE, Reis JP, Ainsworth BE, Newton RL, Jr, Lesniak KT, Taylor HA., Jr Validation of the Jackson Heart Study Physical Activity Survey in African Americans. J Phys Act Health. 2009;6(Suppl 1):S124–S132. doi: 10.1123/jpah.6.s1.s124. [DOI] [PubMed] [Google Scholar]
- 32.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA, Jr, Tucker KL. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109:1184–1193. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt SB, Akylbekova EL, Wofford MR, Coady SA, Walker ER, Andrew ME, Keahey WJ, Taylor HA, Jones DW. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51:650–656. doi: 10.1161/HYPERTENSIONAHA.107.100081. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 35.Keku E, Rosamond W, Taylor HA, Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6–62. [PubMed] [Google Scholar]
- 36.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 37.McGrath JJ, Matthews KA, Brady SS. Individual versus neighborhood socioeconomic status and race as predictors of adolescent ambulatory blood pressure and heart rate. Soc Sci Med. 2006;63:1442–1453. doi: 10.1016/j.socscimed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Beatty DL, Matthews KA. Unfair treatment and trait anger in relation to nighttime ambulatory blood pressure in African American and white adolescents. Psychosom Med. 2009;71:813–820. doi: 10.1097/PSY.0b013e3181b3b6f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas KS, Nelesen RA, Dimsdale JE. Relationships between hostility, anger expression, and blood pressure dipping in an ethnically diverse sample. Psychosom Med. 2004;66:298–304. doi: 10.1097/01.psy.0000126196.82317.9d. [DOI] [PubMed] [Google Scholar]
- 40.Brondolo E, Libby DJ, Denton EG, Thompson S, Beatty DL, Schwartz J, Sweeney M, Tobin JN, Cassells A, Pickering TG, Gerin W. Racism and ambulatory blood pressure in a community sample. Psychosom Med. 2008;70:49–56. doi: 10.1097/PSY.0b013e31815ff3bd. [DOI] [PubMed] [Google Scholar]