Abstract
We have developed a synthesis of 4-substituted isoquinolones from the Rh(III)-catalyzed, C–H activation mediated, coupling of O-pivaloyl benzhydroxamic acids and 3,3-disubstituted cyclopropenes. Experiments suggest the formation of a [4.1.0] bicyclic-system, which can open under acidic conditions to generate the desired isoquinolone.
Keywords: C–H activation, heterocycles, isoquinolone, cyclopropene, donor-acceptor cyclopropane
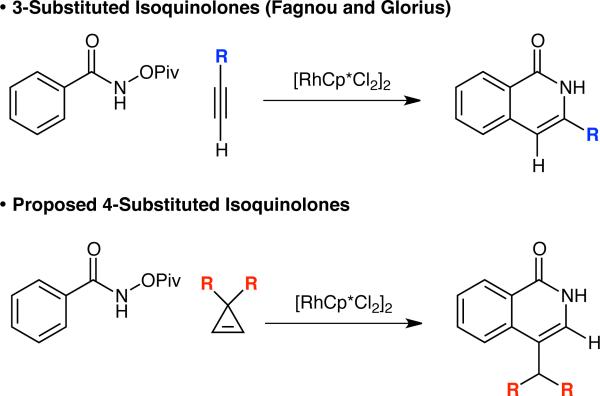
Isoquinolones are a ubiquitous motif found in numerous medicinally interesting targets.1 An approach to access this core is the Rh(III)-catalyzed coupling of amides with alkynes to provide 3,4-disubstituted isoquinolones.2 Early reports by our group2c and others2b,d found that terminal alkynes were incompatible with the oxidative reaction conditions because of unwanted Glaser-type couplings. In contrast, when an internal oxidant is utilized, terminal alkynes can be used to provide 3-substituted isoquinolones in excellent yield.2e Glorius expanded on this work by using MIDA-boronate terminal alkynes to access 3-MIDA boronate-substituted isoquinolones.2f Absent from these reports is the ability to access 4-substituted isoquinolones (Figure 1).
Figure 1.
Proposed approach to access mono-substituted isoquinolones
We imagined this substitution pattern could be accessed through formation of an intermediate donor–acceptor cyclopropane.3 We were intrigued by some recent reports on the use of nitrogen donors on activated cyclopropanes.4 We speculated that 3,3-disubstituted-cyclopropenes could be coupled to benzhydroxamic acids to generate a [4.1.0] bi-cyclic heterocycle. In the presence of either a carboxylic acid or the Lewis acidic Rh(III) catalyst, the resultant cyclopropane could be opened to generate a 4-substituted isoquinolone (Figure 1). In contrast to previous examples which used alkynylation to achieve isoquinolones, our approach requires an alkenylation. This reactivity has previously been reported independently by Fagnou2e and Glorius,5 and further developed by Rovis and Ward,6 Cramer,7 and Molander.8
 |
Equation 1 |
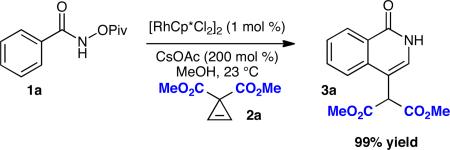
We were pleased to find that O-pivaloyl benzhydroxamic acid 1a could be coupled to activated cyclopropene 2a in MeOH with CsOAc (200 mol %) and [RhCp*Cl2]2 (1 mol %) to provide the desired 3-substituted isoquinolone 3a in excellent yield (99%) (Equation 1).
The reaction scope is illustrated in Table 1. A variety of para-substituents are tolerated providing product in excellent yield (Table 1, 3b-3e, 3h). Substitution at the meta-position provides variable levels of selectivity. Methyl substituted starting materials offer product in >10:1 regioselectivity in the C–H activation, favoring activation distal to the substituent (Table 1, 3f). In contrast, methoxy-substituents provide product, but at a 1.1:1 mixture of regioisomers (Table 1, 3g). Highly electron rich gallic acid derivatives provide product in excellent yield (Table 1, 3i). Finally, when di-ethyl malonate based cyclopropene is used, the desired product is isolated in exquisite yield without transesterification.
Table 1.
Isoquinolone Scopea
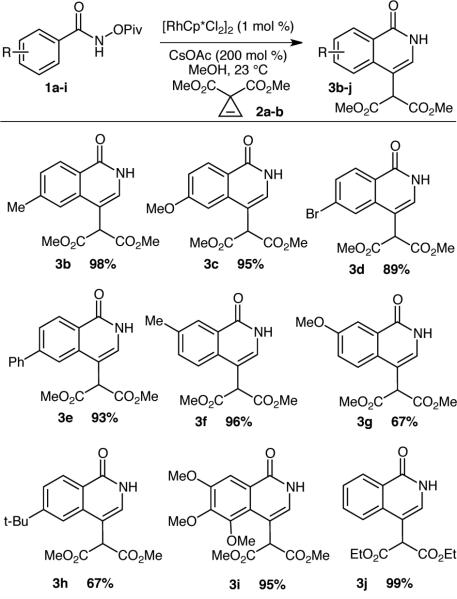
|
Reaction conditions: Amides (1 equiv), cyclopropene (1.1 equiv), [RhCp*Cl2]2 (1 mol %), CsOAc (200 mol %), MeOH (0.2 M), 23 °C, 8 hours.
Product provided as a 1.1:1 ratio of C-H activation regioisomers.
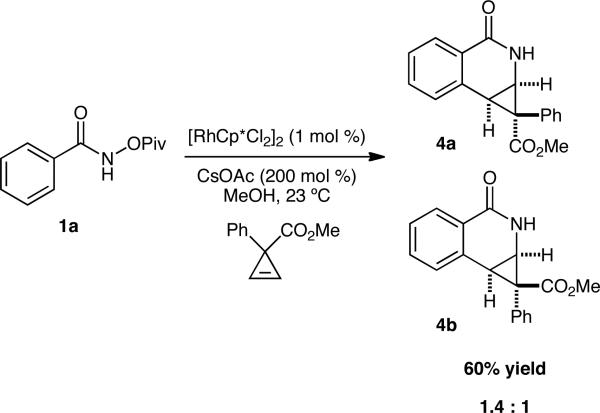
Unfortunately, our attempts to increase the scope of the cyclopropene component to include simple mono-activated cyclopropenes failed to provide the desired 4-substituted isoquinolone. We were, however, able to isolate the [4.1.0] bicyclic intermediate in 60% yield as a 1.4:1 ratio of inseparable diastereomers (Scheme 1).
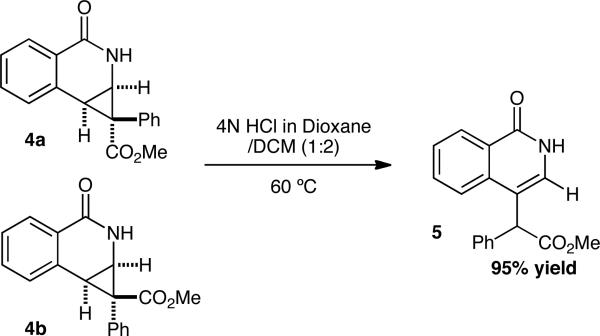
Disappointed that the cyclopropane did not open under the reaction conditions, we sought an alternative method to open the donor-acceptor cyclopropane. When cyclopropanes 4a and 4b were submitted to a 2:1 mixture of 4M HCl in 1,4 dioxane/DCM and heated to 60 °C for 2 hours, the desired isoquinolone 5 was furnished in 95% yield (Scheme 2).
Based on these experiments, we propose that a Rh(III) catalyzed olefination occurs as described by Fagnou and Glorius to afford the [4.1.0] bi-cycle. We suggest that pivalic acid or [RhCp*Cl2]2, acting as a Lewis acid, catalyzes the opening of the donor acceptor cyclopropane to generate an acyl-imine, which tautomerizes to form the desired isoquinolone. In the absence of two activating groups, a stronger acid is required to achieve opening.
In conclusion, we have discovered a method to access 4-substituted isoquinolones from cyclopropenes and amides under Rh(III) catalysis. Isolation of a [4.1.0] bicycle supports the mechanism occurs in two discrete steps.
Supplementary Material
Scheme 1.
mono-activated cyclopropenes
Scheme 2.
Cyclopropane Opening
References
- 1.For a review of isoquinolone synthesis; see: Glushkov VA, Shklyaev YV. Chem. Heterocycl. Compd. 2001;37:663.
- 2.a Guimond N, Gouliaras C, Fagnou K. J. Am. Chem. Soc. 2010;132:6908. doi: 10.1021/ja102571b. [DOI] [PubMed] [Google Scholar]; b Mochida S, Umeda N, Hirano K, Satoh T, Miura M. Chem. Lett. 2010;39:744. [Google Scholar]; c Hyster TK, Rovis T. J. Am. Chem. Soc. 2010;132:10565. doi: 10.1021/ja103776u. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Song G, Chen D, Pan C-L, Crabtree RH, Li X. J. Org. Chem. 2010;75:7487. doi: 10.1021/jo101596d. [DOI] [PubMed] [Google Scholar]; e Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]; f Wang H, Grohmann C, Nimphius C, Glorius F. J. Am. Chem. Soc. 2012;134:19592. doi: 10.1021/ja310153v. [DOI] [PubMed] [Google Scholar]
- 3.a Reissig H-U, Zimmer R. Chem. Rev. 2003;103:1151. doi: 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]; b Yu M, Pagenkopf BL. Tetrahedron. 2005;61:321. [Google Scholar]; c Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]; d Carson CA, Kerr MA. Chem. Soc. Rev. 2009;38:3051. doi: 10.1039/b901245c. [DOI] [PubMed] [Google Scholar]; e De Simone F, Waser J. Synthesis. 2009:3353. [Google Scholar]; f Campbell MJ, Johnson JS, Parsons AT, Pohlhaus PD, Sanders SD. J. Org. Chem. 2010;75:6317. doi: 10.1021/jo1010735. [DOI] [PubMed] [Google Scholar]
- 4.a De Simone F, Waser J. Synlett. 2009:593. [Google Scholar]; b De Simone F, Gertsch J, Waser J. Angew. Chem. Int. Ed. 2010;49:5767. doi: 10.1002/anie.201001853. [DOI] [PubMed] [Google Scholar]; c de Nanteuil F, Waser J. Angew. Chem. Int. Ed. 2011;50:12075. doi: 10.1002/anie.201106255. [DOI] [PubMed] [Google Scholar]; d Benfatti F, de Nanteuil F, Waser J. Org. Lett. 2012;14:386. doi: 10.1021/ol203144v. [DOI] [PubMed] [Google Scholar]; e Benfatti F, de Nanteuil F, Waser J. Chem. Eur. J. 2012;18:4844. doi: 10.1002/chem.201103971. [DOI] [PubMed] [Google Scholar]
- 5.a Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350. doi: 10.1021/ja109676d. [DOI] [PubMed] [Google Scholar]; b Wang H, Glorius F. Angew. Chem. Int. Ed. 2012;51:7318. doi: 10.1002/anie.201201273. [DOI] [PubMed] [Google Scholar]
- 6.Hyster TK, Knörr L, Ward TR, Rovis T. Science. 2012;338:500. doi: 10.1126/science.1226132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye B, Cramer N. Science. 2012;338:504. doi: 10.1126/science.1226938. [DOI] [PubMed] [Google Scholar]
- 8.Presset M, Oehlrich D, Rombouts F, Molander GA. Org. Lett. 2013;15:1528. doi: 10.1021/ol400307d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.General Proceedure for the synthesis of 3a A 1.5 dram vial was charged with a stir bar, 1a (0.2 mmol), [RhCp*Cl2]2 (1 mol %), CsOAc (0.4 mmol), and MeOH (1 mL). After stirring for 30 seconds, cyclopropene 2a (0.22 mmol) was added and the reaction mixture was allowed to stir for 8 hours at 23 °C. Upon completion, as determined by TLC, the reaction mixture was concentrated. The crude residue was purified via column chromatography (2:1 Hexanes/EtOAc with 1% Et3N) to provide the isoquinolone 3a as a white solid (99% yield).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.