Abstract
Background
Pain is one of the most commonly reported impairments after breast cancer treatment affecting anywhere from 16-73% of breast cancer survivors Despite the high reported incidence of pain from cancer and its treatments, the ability to evaluate cancer pain continues to be difficult due to the complexity of the disease and the subjective experience of pain. The Oncology Section Breast Cancer EDGE Task Force was created to evaluate the evidence behind clinical outcome measures of pain in women diagnosed with breast cancer.
Methods
The authors systematically reviewed the literature for pain outcome measures published in the research involving women diagnosed with breast cancer. The goal was to examine the reported psychometric properties that are reported in the literature in order to determine clinical utility.
Results
Visual Analog Scale, Numeric Rating Scale, Pressure Pain Threshold, McGill Pain Questionnaire, McGill Pain Questionnaire – Short Form, Brief Pain Inventory and Brief Pain Inventory – Short Form were highly recommended by the Task Force. The Task Force was unable to recommend two measures for use in the breast cancer population at the present time.
Conclusions
A variety of outcome measures were used to measure pain in women diagnosed with breast cancer. When assessing pain in women with breast cancer, researchers and clinicians need to determine whether a unidimensional or multidimensional tool is most appropriate as well as whether the tool has strong psychometric properties.
Keywords: Pain, Outcome Measure, Breast Cancer
INTRODUCTION
Breast cancer is the most common cancer affecting women in the United States, with an estimated 232,340 new cases in the United States for 2013.1 Currently there are approximately 2.9 million American women who are surviving breast cancer, commonly referred to as breast cancer survivors (BCS).2 Although improvements have been signifcant in breast cancer treatment producing more long-term survivors, breast cancer and its treatments continue to be associated with many undesirable symptoms and side effects. 3
Pain is one of the most commonly reported impairments after breast cancer treatment affecting anywhere from 16-73% of BCS4-10, and has a strong relationship to decreased quality of life and greater self-perceived disability.7 A cumulative prevalence of chronic pain has been reported in 43% of women 3 years after receiving a mastectomy for breast cancer.11 The presence of pain soon after breast cancer surgery is a predictive factor for chronic pain.12 Despite the high reported incidence of pain from cancer and its treatments, the ability to evaluate cancer pain continues to be difficult due to the complexity of the disease and the subjective experience of pain.13,14 The etiology of cancer pain may be from many causes such as the cancer itself, treatments (radiation, surgery or chemotherapy), musculoskeletal impairments secondary to treatment, or from unknown causes.15 Cancer pain can be acute, such as postoperative pain, or chronic lasting three months or more after breast surgery for cancer.16 The variability in the causes of cancer pain as well as the timing contributes to difficulty in its assessment as well as its control.17
The American Physical Therapy Association's (APTA) Evaluation Database to Guide Effectiveness (EDGE) Task Force was formed within the Section on Research in 2006. The Task Force's goal was to provide physical therapy professionals with a comprehensive list of outcome measures that can be administered to a specific patient population. The psychometric properties and clinical utility within a particular patient population were detailed with the ultimate goal of creating a central location for physical therapy professionals to have access to this valuable information for implementing evidence-based practice.18 The Task Force was expanded to include members from several other Sections of the APTA. After the success of the Neurology Section's StrokEDGE Task Force, where 57 outcome measures were assessed in patients with stroke, the Oncology Section created a Task Force with a focus on Breast Cancer Outcomes. The first assessment of breast cancer outcome tools from the Oncology section Task Force targeted scapula, shoulder and glenohumeral impairments and shoulder function and resulted in successful dissemination of the results at the APTA's Combined Sections Meeting in Chicago 2012, as well as four publications in the 2013 Rehabilitation Oncology Journal Volume 31, Number 1.18-21 Over the past 2 years, the purpose of the Breast Cancer EDGE Task Force has been to continue to assess breast cancer outcome measures with a focus on pain, lymphedema and fatigue. The purpose of this review is to identify evidence-based pain assessment tools in breast cancer survivors using the methodology of the EDGE Taskforce.
METHODS
A primary systematic search using PubMed was performed from April 6, 2012 up to June 1, 2013 and resulted in the retrieval of 872 publications. The search strategy began with the filters (“Breast Neoplasms”[MeSH Terms] AND ((((((“Radiotherapy”[MesSH Terms] OR “Mastectomy”[MeSH Terms]) OR (“Carcinoma/surgery”[MeSH Terms] OR “Carcinoma/therapy”[MeSH Terms])) OR “Lymph Node Excision”[MeSH Terms]) OR “Combined Modality Therapy”[MeSH Terms]) OR “Sentinel Lymph Node Biopsy”[MeSH Terms] OR (“Breast Neoplasms/drug therapy”[MeSH Terms] OR “Breast Neoplasms/radiotherapy”[MeSH Terms] OR “Breast Neoplasms/surgery”[MeSH Terms] OR “Breast Neoplasms/therapy”[MeSH Terms])) OR “Mammaplasty”[MeSH Terms])) AND (((“Pain”[MeSH Terms] OR “Pain Measurement”[MeSh Terms]) OR “Disability Evaluation”[MeSH Terms]) OR “Somatosensory Disorders”[MeSH Terms] OR pain[title]) AND English[lang]. There was no restriction on year of publication. A second systematic search strategy using CINAHL was performed from April 25, 2012 up to June 1, 2013 and yielded 205 publications using the following search terms: MH “Breast Neoplasms/RT /RH/SU”) (MH “Breast Reconstruction”) (MH “Sentinel Lymph Node Biopsy”) (MH “Lymph Node Excision+”) (MH “Mastectomy+”) (MH “Radiotherapy+”) (MH “Breast Neoplasms+”) (MH “Somatosensory Disorders+”) MH “Disability Evaluation”) (MH “Pain Measurement”) (MH “Pain+”)
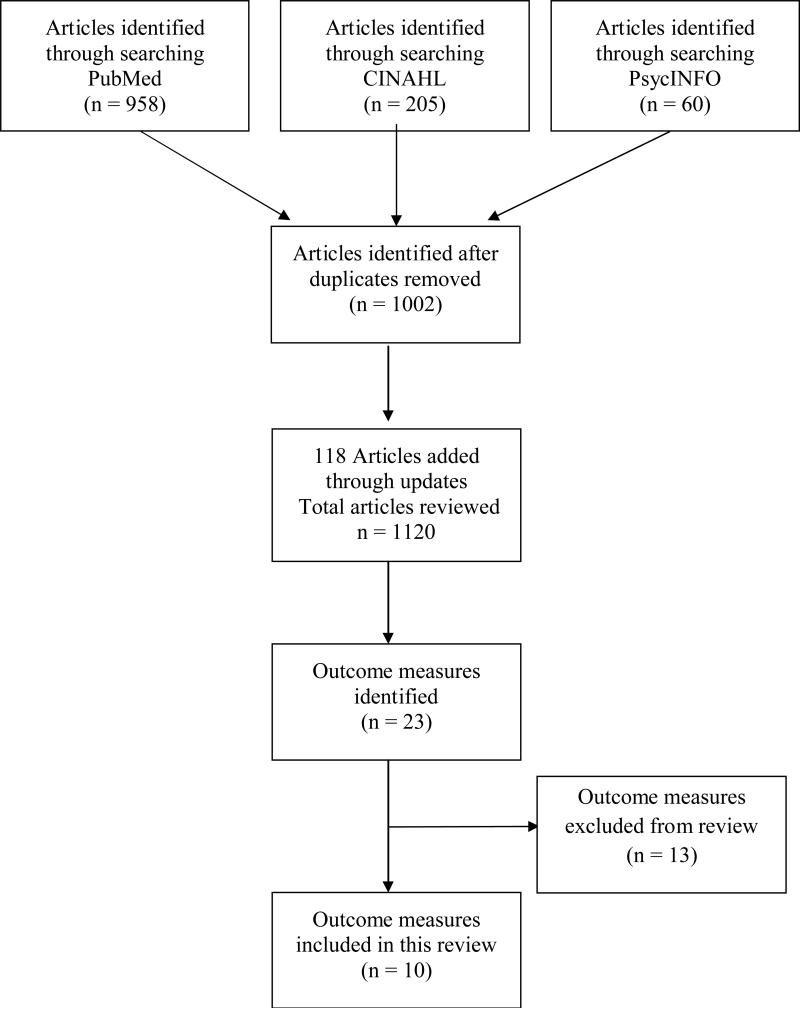
A third systematic search strategy using PsycINFO® from April, 2012 up to June 1, 2013 and yielded 28 publications using the following search terms (DE “Breast Neoplasms”) AND (DE “Radiation Therapy” OR DE “Mastectomy” OR DE “Plastic Surgery” OR DE “Surgery”) AND (DE “Pain” OR DE “Aphagia” OR DE “Chronic Pain” OR DE “Neuralgia” OR DE “Neuropathic Pain” OR DE “Somatoform Pain Disorder” OR DE “Pain Measurement” OR DE “Pain Perception” OR DE “Disability Evaluation” OR DE “Somatosensory Disorders”). These 3 searches were combined and duplicate publications removed, leaving a total of 1002 articles that included a pain measure in a breast cancer population for our review. The databases were monitored for updates throughout the months of data collection. Through this process an additional 86 articles were retrieved through PubMed and 32 articles through PsychInfo, without duplicates, for a final total of 1120 articles for review by the researchers. (Figure 1)
Figure 1.
PRISMA flow diagram
Titles and abstracts of the articles found in the search were divided among the researchers and reviewed for use of a measure of pain in the breast cancer population. Studies of assessment methodology, pain prevalence and epidemiology, and interventions for pain were all included. Articles were excluded that only measured acute surgical procedure pain and also references where pain was not a primary end point. In addition, the three authors examined reference lists from all selected publications to verify that no pertinent publications were missed during the above-described electronic searches. When warranted, full articles were obtained for review. The authors reviewed the included papers for the use of specific measures of pain, and then constructed a comprehensive list of measures that have been used in studying pain in the breast cancer population.
Once the list of pain assessment tools was compiled, the researchers held multiple conference calls to determine which tools were appropriate for full review as described below. Duplicate measures were excluded. Assessments that primarily measured other constructs such as function or quality of life, and those that consisted of a dichotomous question about the presence of pain (yes or no) were excluded. Included measures of pain were subdivided into the following categories: 1) pain intensity/severity, 2) pain quality, 3) pain-related disability, and 4) measures that combined multiple aspects of the pain experience such as both measuring pain intensity and quality. Based on the above criteria, the researchers came to consensus on a list of 10 pain outcome measures for review. (Figure 1) These measures were divided between the 3 researchers for an independent primary review of the psychometric properties and clinical utility. Reviewers conducted additional literature searches for papers on the psychometric properties of the assessment tools as well as researched cost and availability of the measures. The primary reviews were completed using the Cancer EDGE Task Force Outcome Measure Rating Form (Appendix A) for each of the selected pain outcome measures. In short, one of the authors rated each measure on the qualities of reliability, validity, availability of normal values, minimal clinical important difference (MCID) or minimal detectable change (MDC), and clinical utility. Once completed, a secondary review was conducted by a second author to ensure accuracy. Each pain outcome measure was then rated using a 0-4 scale by consensus of the three authors as a method to determine if a measure could be recommended for widespread clinical use. (Table 1)
Table 1.
Breast Cancer EDGE Rating Scale
| 4 | Highly Recommend | Highly recommended; the outcome has excellent psychometric properties and clinical utility; the measure has been used in research on individuals with or post breast cancer. |
| 3 | Recommend | Recommended; the outcome measure has good psychometric properties and good clinical utility; no published evidence that the measure has been applied to research on individuals with or post breast cancer. |
| 2A | Unable to Recommend at this time | Unable to recommend at this time; there is insufficient information to support a recommendation of this outcome measure; the measure has been used in research on individuals with or post breast cancer. |
| 2B | Unable to Recommend at this time | Unable to recommend at this time; there is insufficient information to support a recommendation of this outcome measure; no published evidence that the measure has been applied to research on individuals with or post breast cancer. |
| 1 | Do not Recommend | Poor psychometrics &/or poor clinical utility (time, equipment, cost, etc.) |
RESULTS
After a comprehensive review of the breast cancer literature, 23 different measures of pain were identified for potential inclusion in this review. After applying the inclusion and exclusion criteria, 10 measures were selected for full review using the Cancer EDGE Task Force Outcome Measure Rating. Of the ten measures reviewed (Table 2), a total of eight measures were given the highest rating of 4 (highly recommend) and are thus recommended for clinical use by the researchers of this Task Force. Of the measures of pain intensity/sensitivity, three of the recommended measures, the Visual Analog scale (VAS), Numeric Rating Scale (NRS) and Pressure Pain Threshold are highly recommended for use. In this same category, we are unable to recommend the Gaston – Johansson Painometer for clinical use at this time due to its limited availability and lack of full psychometric testing. Of the measures of pain quality, two of the recommended measures, the McGill Pain Questionnaire (MPQ) and McGill Pain Questionnaire – Short Form (MPQ – SF), are highly recommended for clinical use. Due to limited data on psychomentric properties, we are unable to recommend the Neuropathic Pain Scale – CIN at this time. For measurement of pain-related disability, one of the recommended measures, the Pain Disability Index (PDI), is highly recommended. For combined measures of pain intensity and interference, 2 measures, the Brief Pain Inventory (BPI) and the Brief Pain Inventory – Short Form (BPI – SF) are highly recommended.
Table 2.
Outcome Measures Sorted by Task Force Rating
| Measure | Rating |
|---|---|
| Pain Intensity/Sensitivity | |
| Visual Analog Scale | 4 – Highly Recommend |
| Numeric Pain Rating Scale | 4 – Highly Recommend |
| Pressure Pain Threshold | 4 – Highly Recommend |
| Gaston – Johansson Painometer | 2A – Unable to Recommend at this time |
| Pain Quality | |
| McGill Pain Questionnaire | 4 – Highly Recommend |
| McGill Pain Questionnaire – Short Form | 4 – Highly Recommend |
| Neuropathic Pain Scale – CIN | 2A – Unable to Recommend at this time |
| Pain-related Disability | |
| Pain Disability Index | 4 – Highly Recommend |
| Combined Pain Measures | |
| Brief Pain Inventory | 4 – Highly Recommend |
| Brief Pain Inventory – Short Form | 4 – Highly Recommend |
DISCUSSION
A number of different clinical measures of pain are available for use in the cancer population. This group has identified that the VAS, NRS, Pressure Pain Threshold, MPQ, MPQ – SF, PDI, BPI and BPI – SF are highly recommended for use in the breast cancer population. All of these measures have been used extensively in the breast cancer population and demonstrate excellent measurement properties within the populations for which they were developed. When determining what type of pain outcome scale to administer, researchers and clinicians need to define how they want to assess pain and whether to use a unidimensional or multidimensional tool. In the following sections we will review the properties of the recommended measures in order to allow readers to determine which of the measures may fit their specific needs.
Unidimensional Pain Intensity Measures
Unidimensional tools measure the intensity of pain, without examining the quality or impact of this pain. They are often administered when a single, clearly defined question is to be answered.22 There is both clinical and experimental evidence that shows pain has at least two dimensions, affective and sensory.23,24 Some believe that a unidimensional pain scale might not be adequate since there is no way to know which dimension of pain the individual is rating when using these types of scales.25 Nevertheless, these tools are often administered as they are easy to understand and place minimal burden on the patient and clinician.22 The unidimensional pain outcome measures we recommend as a result of this review include the VAS, NRS and Pressure Pain Threshold.
The VAS is a 10 cm-long horizontal line with the words “no pain” anchoring at one end and “pain as bad as it can be” at the other. The VAS has been validated in the acute,26 chronic,27 and cancer populations28 and has been used in over 90 breast cancer studies. This measure has shown acceptable test-retest reliability of 0.80,29 concurrent validity with other pain scales in a cancer population of 0.70,29,30 and has an established MCID of 9-11 mm in the breast cancer population.31
The NRS has several iterations, but the most commonly used one is the 11-item version where individuals are asked what number would they rate their pain from 0 – 10 where 0 is no pain and 10 is the most severe pain.32 This measure has been validated in a variety of pain populations including chronic33,34 low back35, musculoskeletal36, cancer29,37 and specifically breast cancer.38 It has been used in approximately 20 studies involving breast cancer survivors. The NRS has established reliability of 0.8733,35-37 and convergent validity of 0.8529,39 across many populations with pain including breast cancer-related pain.40 A reported 2-point change represents a clinically meaningful difference34,35 and it has been reported that when an individual with breast cancer rates their pain ≥ 5, health related quality of life is impacted.41
The third unidimensional tool the Task Force recommends is Pressure Pain Threshold. Pressure pain threshold is commonly used to assess the hyperexcitability of the central nervous system.42 Pressure pain threshold is defined as “the minimal amount of pressure where a sensation of pressure first changes to pain”.43 Pressure pain threshold is assessed using a device called an algometer in which a circular probe is attached to a pressure gauge. Pressure is applied at a constant rate to the tissue being tested and is stopped when individuals identify when the sensation first changes from pressure to pain.31 This measure has been validated in a variety of pain populations including temporomandibular disorders,44,45 patellar tendinopathy,46 low back pain,47 knee osteoarthritis,48 myofascial pain49 and has been used in at least five breast cancer studies.31,50-53 Pressure pain threshold has established reliability (0.60 – 0.94 with electric algometers being more reliable than force-gauge models)31,46,53-57 and good construct and concurrent validity.58,59 Prushansky et al 200460 reported a 20% change in pressure is needed to indicate significant change, and results can be compared to published normal values.31,52,53,55 According to the oncology section EDGE criteria, a measure can be given a ‘highly recommend’ if it has good psychometric properties and has been used in research with BCS.19 The acceptable psychometric properties found in multiple populations, and the reference values give the pressure pain threshold a “highly recommended” rating, though the authors acknowledge that the lack of a MDC or MCID in BCS could make it more challenging for clinicians to make decisions based on the results of the measure.
The Gaston-Johansson Painometer was originally developed for the assessment of acute and chronic pain in rheumatoid arthritis, women in labor, and post-operative pain.61 It includes a visual analog scale for measurement of pain intensity and a list of pain descriptors, although it must be noted that in some investigations the provided descriptive terms were found to be inadequate.62 Given the questioning of descriptors used and a lack of sensitivity data and reference values, this measure is not recommended for use at this time.
Multidimensional Pain Measures
While the unidimensional tools primarily measure the intensity of pain, multidimensional tools take into consideration other factors that influence pain perception.22 These factors include the affective contributions, quality and the temporal sequence of pain, and an individual's belief system.22 While multidimensional tools take a more comprehensive approach, the interpretation and use of these tools can be difficult because of their complexity.22 Additionally, multidimensional tools generally take a longer time to complete and can be difficult to understand by the individual. The multidimensional pain outcome measures we recommend as a result of this review include the MPQ, MPQ – SF, PDI, BPI and BPI – SF. Some of the multidimentional pain measures intend to assess the differing qualities of pain, such as the MPQ and the NPS-CIN, while others, such as the PDI, intend to assess the impact of pain on the individual.
Pain Quality Measures
The MPQ is a unique measure because it assesses pain using a multidimensional approach based on the gate control theoretical framework.15 The MPQ contains three major classes of word descriptors: sensory, affective and evaluative.63 There are three parts to the MPQ including the pain rating index, the number of words chosen and the present pain intensity.63 This measure was developed in an adult population with a wide variety of conditions including cancer.63 The MPQ has been validated in several diagnoses including breast cancer.15 The MPQ has been used in approximately 10 research studies involving the breast cancer population. The MPQ has demonstrated a good test-retest reliability of 0.70,63 construct validity,14,63-65 concurrent validity (r=0.31-0.40)64,66-69 and predictive validity.70-74 Reported MDC or MCID for this measure were unavailable, which could make it challenging to make clinical decisions based on the results.
The MPQ – SF was developed from the MPQ to make the multidimensional approach to pain assessment easier and more efficient to administer.63,75 The MPQ-SF is comprised of 3 parts: 1) 15 word descriptors that describe two dimensions of pain: sensory and affective, 2) Present Pain Intensity scale and 3) VAS.75 The MPQ-SF has been validated and used in pain assessment in a variety of pain conditions including metastatic cancer pain.75 The MPQ-SF has been used in over 10 studies examining women with breast cancer. Reliability for the MPQ-SF in individuals with cancer has been shown to be 0.94 (Cronbach's alpha).76 Concurrent validity with the long form MPQ was found to be r = .77 to .88 in patients with cancer pain.75 MDC or MCID for this measure was unavailable, which could make it challenging to make clinical decisions based on these results.
The NPS-CIN was developed by combining items from the original Neuropathic Pain Scale and the Pain Quality Assessment Scale in order to measure neuropathic pain from cancer treatment.77,78 At the current time, the psychometric properties are incomplete. Initial validity studies have been completed but information on reliability and sensitivity to change is lacking.77,78 Because this measure assesses neuropathic pain specific to cancer treatment and has good clinical utility, it may be a useful measure for the BCS population if all of the psychometric properties are found to be favorable.
Pain Disability Measures
The PDI is a multidimensional tool designed to measure the degree in which chronic pain affects an individual's ability to perform a variety of activities.79 The PDI contains seven categories: 1) family/home responsibility, 2) recreation, 3) social activity, 4) occupation, 5) sexual behavior, 6) self-care, and 7) life support activity.80 Individuals are asked to rate their level of disability on a rating scale (0 = no disability to 10 = total disability).80 An overall score is calculated by summing the ratings of the seven categories (0 – 70).80 The PDI was developed in individuals with chronic pain from multiple causes as well as low back pain80,81 and has been validated in individuals post-surgery,80 as well as outpatients and inpatients.81 The PDI has been used in 3 cross-sectional studies in women diagnosed with breast cancer.10,82,83 Reliability of the PDI when administered to the general chronic pain population was 0.87 (Cronbach's alpha).81 The PDI has been shown to have acceptable concurrent and construct validity in individuals with chronic pain.81 The PDI has a reported MCID of 6 points for individuals with low back pain.84 While the PDI was developed for patients with chronic pain from multiple causes, including cancer-related pain, published psychometrics for the PDI when administered to only a cancer population could not be found. Since the measure has good psychometric properties in mixed chronic pain populations, it is therefore still a recommended measure.
Combined Pain Intensity and Interference Measure
The BPI is a multimodal scale comprised of questions on pain intensity and pain-related interference with function.85-87 There is a total of 32 items on the BPI. Individuals rate their worst, least, average and current pain intensity (including the last 24 hours) as well as the degree to which pain interferes with 7 domains of function: 1) general activity, 2) mood, 3) walking ability, 4) normal work, 5) relations with other persons, 6) sleep, and 7) enjoyment of life using a scale from 0 (no pain) to 10 (pain as bad as you can imagine).88 The BPI was developed specifically for use in individuals with cancer85 and has been validated in individuals with bone metastases, breast cancer and postoperative cancer patients.89 The BPI has been used in over 25 studies involving women with breast cancer. Test-retest reliability in a mixed cancer population ranged from 0.59-0.93.90 Reliability of the BPI when administered to a mixed cancer population with metastatic pain ranged from 0.81 – 0.89 (Cronbach's alpha).91 Construct validity found three factors, pain intensity, activity interference and affective interference that were invariant across age, disease and ethnicity.91,92 Although there is no reported MCID or MDC in the literature, increased pain management strategies are recommended when the average of the severity and interaction scores reaches five.93
The BPI-SF is a tool developed specifically for use in individuals with cancer that was modified from the BPI. Due to the amount of time it takes for an individual to complete the BPI (10-15 minutes) as well as the time needed to score the tool,85 the BPI – SF was developed. The BPI-SF asks individuals to use a 1-week recall of their pain experience as opposed to a 24-hour recall and has 9 total items as compared to 32 on the BPI.94,95 The BPI-SF evaluates the severity of pain as well as the impact pain has on daily function.85 The BPI-SF has been used in approximately six studies involving women with breast cancer. Cronbach's alpha was found to be 0.89 in 36 women diagnosed with stage I-IIIA breast cancer.96 Construct validity has been reported high for the pain interference (0.71-0.94) and pain severity (0.70-0.91) constructs of the BPI-SF.97 The Minimal Important Difference has been reported as 1.2 points for pain severity, 1.6 points for activity-related pain interference, and 1.5 points for mood-related pain interference.94
Limitations and Conclusions
There are several factors that should be considered when interpreting the Task Force recommendations. An outcome measure may have been excluded in this review due to a lack of published data; the authors are aware that new studies may have been published after June 1, 2013. For measures that could not be recommended at this time, additional information may become available that might elevate the task force recommendation in the future. The literature search was limited to English-language journals therefore journals in other languages were not reviewed and could limit the number of measures reviewed. Researchers and clinicians are encouraged to review the Task Force recommendations as well as each specific outcome measure for more extensive information. While this article can serve as a guide, ultimately, it is up to the clinician and researcher to identify the best available evidence in addition to patient values and expectations in order to appropriately administer the correct pain outcome measure in the breast cancer population.98
ACKNOWLEDGEMENTS
Thank you to Taylor Lundy BS, Andrew Scheimann SPT and Sarah Darst Weaving, PT, DPT for their assistance with the literature search and EDGE forms.
Appendix
Appendix 1. Cancer EDGE Taskforce Outcome Measure Rating Form
References
- 1.American Cancer Society [July 1, 2013];How Many Women Get Breast Cancer? 2013 http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-overview-key-statistics. 2013.
- 2.Society AC. What are the Key Statistics about Breast Cancer? 2012 http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics.
- 3.Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. Journal of behavioral medicine. 2006 Feb;29(1):17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 4.Ververs JM, Roumen RM, Vingerhoets AJ, et al. Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancer. European journal of cancer. 2001 May;37(8):991–999. doi: 10.1016/s0959-8049(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 5.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1995 May;6(5):453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 6.Ryttov N, Blichert-Toft M, Madsen EL, Weber J. Influence of adjuvant irradiation on shoulder joint function after mastectomy for breast carcinoma. Acta radiologica. Oncology. 1983;22(1):29–33. doi: 10.3109/02841868309134336. [DOI] [PubMed] [Google Scholar]
- 7.Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disability and rehabilitation. 2004 Jan 21;26(2):78–84. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 8.Maunsell E, Brisson J, Deschenes L. Arm problems and psychological distress after surgery for breast cancer. Canadian journal of surgery. Journal canadien de chirurgie. 1993 Aug;36(4):315–320. [PubMed] [Google Scholar]
- 9.Hladiuk M, Huchcroft S, Temple W, Schnurr BE. Arm function after axillary dissection for breast cancer: a pilot study to provide parameter estimates. Journal of surgical oncology. 1992 May;50(1):47–52. doi: 10.1002/jso.2930500114. [DOI] [PubMed] [Google Scholar]
- 10.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 Jan;17(1):143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. British journal of cancer. 2005 Jan 31;92(2):225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000 Oct;93(4):1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 13.Wilkie DJ, Monreal DW. Principles of Practice for the Acute Care Nurse Practitioner. Appleton and Lange; Stamford, CT: 1999. [Google Scholar]
- 14.McGuire DB. Assessment of pain in cancer inpatients using the McGill Pain Questionnaire. Oncology nursing forum. 1984 Nov-Dec;11(6):32–37. [PubMed] [Google Scholar]
- 15.Ngamkham S, Vincent C, Finnegan L, Holden JE, Wang ZJ, Wilkie DJ. The McGill Pain Questionnaire as a multidimensional measure in people with cancer: an integrative review. Pain management nursing : official journal of the American Society of Pain Management Nurses. 2012 Mar;13(1):27–51. doi: 10.1016/j.pmn.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesthesia and analgesia. 2005 Nov;101(5):1427–1432. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 17.Ngamkham S, Holden JE, Wilkie DJ. Differences in pain location, intensity, and quality by pain pattern in outpatients with cancer. Cancer nursing. 2011 May-Jun;34(3):228–237. doi: 10.1097/NCC.0b013e3181faab63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miale S, Harrington S, Kendig T. Oncology Section Taskforce on Breast Cancer Outcomes: Clinical Measures of Upper Extremity Function. Rehabilitation Oncology. 2013;31(1):27–34. [Google Scholar]
- 19.Levangie PKFM. Oncology Section Task Force on Breast Cancer Outcomes: An Introduction to the EDGE Task Force and Clinical Measures of Upper Extremity Function. Rehabilitation Oncology. 2013;31(1):6–10. [Google Scholar]
- 20.Fisher MILP. Oncology Section Task Force on Breast Cancer Outcomes: Scapular Assessment. Rehabilitation Oncology. 2013;31(1):11–18. [Google Scholar]
- 21.Perdomo MSC, Davies C. Oncology Section Task Force on Breast Cancer Outcomes: Shoulder and Glenohumeral Outcome Measures. Rehabilitation Oncology. 2013;31(1):19–26. [Google Scholar]
- 22.Ho K, Spence J, Murphy MF. Review of pain-measurement tools. Annals of emergency medicine. 1996 Apr;27(4):427–432. doi: 10.1016/s0196-0644(96)70223-8. [DOI] [PubMed] [Google Scholar]
- 23.Lowe NK, Walker SN, MacCallum RC. Confirming the theoretical structure of the McGill Pain Questionnaire in acute clinical pain. Pain. 1991 Jul;46(1):53–60. doi: 10.1016/0304-3959(91)90033-T. [DOI] [PubMed] [Google Scholar]
- 24.De Gagne TA, Mikail SF, D'Eon JL. Confirmatory factor analysis of a 4-factor model of chronic pain evaluation. Pain. 1995 Feb;60(2):195–202. doi: 10.1016/0304-3959(94)00114-T. [DOI] [PubMed] [Google Scholar]
- 25.Clark WC, Ferrer-Brechner T, Janal MN, Carroll JD, Yang JC. The dimensions of pain: a multidimensional scaling comparison of cancer patients and healthy volunteers. Pain. 1989 Apr;37(1):23–32. doi: 10.1016/0304-3959(89)90149-8. [DOI] [PubMed] [Google Scholar]
- 26.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976 Jun;2(2):175–184. [PubMed] [Google Scholar]
- 27.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Annals of emergency medicine. 2001 Dec;38(6):633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986 Oct;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MP. The Validity and Reliability of Pain Measures in Adults with Cancer. The Journal of Pain. 2003;4(1):2–12. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 30.Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain. 1981 Apr;10(2):241–248. doi: 10.1016/0304-3959(81)90199-8. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Lao C, Cantarero-Villanueva I, Fernandez-de-las-Penas C, Del-Moral-Avila R, Menjon-Beltran S, Arroyo-Morales M. Widespread mechanical pain hypersensitivity as a sign of central sensitization after breast cancer surgery: comparison between mastectomy and lumpectomy. Pain medicine. 2011 Jan;12(1):72–78. doi: 10.1111/j.1526-4637.2010.01027.x. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP. What is the Maximum Number of Levels Needed in Pain Intensity Measurement? Pain. 1994;58(3):387–392. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999 Nov;83(2):157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 34.Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001 Nov;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 35.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005 Jun 1;30(11):1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 36.Stratford PW SG. The Reliability, Consistency, and Clinical Applicatoin of a Numeric Pain Rating Scale. Physiotherpy Canada. 2001;53(2):88–91. [Google Scholar]
- 37.Brunelli C, Zecca E, Martini C, et al. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health and quality of life outcomes. 2010;8:42. doi: 10.1186/1477-7525-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen MP, Chang HY, Lai YH, Syrjala KL, Fann JR, Gralow JR. Pain in long-term breast cancer survivors: frequency, severity, and impact. Pain medicine. 2010 Jul;11(7):1099–1106. doi: 10.1111/j.1526-4637.2010.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paice JA CF. Validity of a Verbally Administered Numerci Rating Scale to Measure Cancer Pain Intensity. Cancer nursing. 1997;20(2):88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005 Jan;113(1-2):37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005 Dec 15;119(1-3):16–25. doi: 10.1016/j.pain.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003 Mar 25;60(6):898–904. doi: 10.1212/01.wnl.0000058546.16985.11. [DOI] [PubMed] [Google Scholar]
- 43.Vanderweeen L, Oostendorp RA, Vaes P, Duquet W. Pressure algometry in manual therapy. Manual therapy. 1996 Dec;1(5):258–265. doi: 10.1054/math.1996.0276. [DOI] [PubMed] [Google Scholar]
- 44.Ohrbach R, Gale EN. Pressure pain thresholds in normal muscles: reliability, measurement effects, and topographic differences. Pain. 1989 Jun;37(3):257–263. doi: 10.1016/0304-3959(89)90189-9. [DOI] [PubMed] [Google Scholar]
- 45.Gomes MB, Guimaraes JP, Guimaraes FC, Neves AC. Palpation and pressure pain threshold: reliability and validity in patients with temporomandibular disorders. Cranio : the journal of craniomandibular practice. Jul. 2008;26(3):202–210. doi: 10.1179/crn.2008.027. [DOI] [PubMed] [Google Scholar]
- 46.van Wilgen P, van der Noord R, Zwerver J. Feasibility and reliability of pain pressure threshold measurements in patellar tendinopathy. Journal of science and medicine in sport / Sports Medicine Australia. 2011 Nov;14(6):477–481. doi: 10.1016/j.jsams.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Schenk P, Laeubli T, Klipstein A. Validity of pressure pain thresholds in female workers with and without recurrent low back pain. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2007 Feb;16(2):267–275. doi: 10.1007/s00586-006-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessel J. The reliability and validity of pain threshold measurements in osteoarthritis of the knee. Scandinavian journal of rheumatology. 1995;24(4):238–242. doi: 10.3109/03009749509100881. [DOI] [PubMed] [Google Scholar]
- 49.Park G, Kim CW, Park SB, Kim MJ, Jang SH. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Annals of rehabilitation medicine. 2011 Jun;35(3):412–417. doi: 10.5535/arm.2011.35.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantarero-Villanueva I, Fernandez-Lao C, Caro-Moran E, et al. Aquatic exercise in a chest-high pool for hormone therapy-induced arthralgia in breast cancer survivors: a pragmatic controlled trial. Clinical rehabilitation. 2013 Feb;27(2):123–132. doi: 10.1177/0269215512448256. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Lao C, Cantarero-Villanueva I, Diaz-Rodriguez L, Fernandez-de-las-Penas C, Sanchez-Salado C, Arroyo-Morales M. The influence of patient attitude toward massage on pressure pain sensitivity and immune system after application of myofascial release in breast cancer survivors: a randomized, controlled crossover study. Journal of manipulative and physiological therapeutics. 2012 Feb;35(2):94–100. doi: 10.1016/j.jmpt.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Lao C, Cantarero-Villanueva I, Fernandez-de-Las-Penas C, del Moral-Avila R, Castro-Sanchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. The Clinical journal of pain. 2012 Feb;28(2):113–121. doi: 10.1097/AJP.0b013e318225dc02. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Lao C, Cantarero-Villanueva I, Fernandez-de-Las-Penas C, Del-Moral-Avila R, Arendt-Nielsen L, Arroyo-Morales M. Myofascial trigger points in neck and shoulder muscles and widespread pressure pain hypersensitivtiy in patients with postmastectomy pain: evidence of peripheral and central sensitization. The Clinical journal of pain. 2010 Nov-Dec;26(9):798–806. doi: 10.1097/AJP.0b013e3181f18c36. [DOI] [PubMed] [Google Scholar]
- 54.Persson A BC, Sjolund BH. Tender or Not Tender: Test-Restest Repeatability of Pressure Pain Thresholds in the Trapezius and Deltoid Muscles of Healthy Women. Journal of Rehabilitation Medicine. 2004;36(1):17–27. doi: 10.1080/16501970310015218. [DOI] [PubMed] [Google Scholar]
- 55.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987 Jul;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 56.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. The Clinical journal of pain. 2007 Nov-Dec;23(9):760–766. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 57.Binderup AT, Arendt-Nielsen L, Madeleine P. Pressure pain sensitivity maps of the neck-shoulder and the low back regions in men and women. BMC musculoskeletal disorders. 2010;11:234. doi: 10.1186/1471-2474-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinser AM, Sands WA, Stone MH. Reliability and validity of a pressure algometer. Journal of strength and conditioning research / National Strength & Conditioning Association. 2009 Jan;23(1):312–314. doi: 10.1519/jsc.0b013e31818f051c. [DOI] [PubMed] [Google Scholar]
- 59.Goolkasian P, Wheeler AH, Gretz SS. The neck pain and disability scale: test-retest reliability and construct validity. The Clinical journal of pain. 2002 Jul-Aug;18(4):245–250. doi: 10.1097/00002508-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Prushansky T, Dvir Z, Defrin-Assa R. Reproducibility indices applied to cervical pressure pain threshold measurements in healthy subjects. The Clinical journal of pain. 2004 Sep-Oct;20(5):341–347. doi: 10.1097/00002508-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Gaston-Johansson F. Measurement of pain: the psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. Journal of pain and symptom management. 1996 Sep;12(3):172–181. doi: 10.1016/0885-3924(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 62.Bergh I, Gunnarsson M, Allwood J, Oden A, Sjostrom B, Steen B. Descriptions of pain in elderly patients following orthopaedic surgery. Scandinavian journal of caring sciences. 2005 Jun;19(2):110–118. doi: 10.1111/j.1471-6712.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 63.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975 Sep;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 64.Sist TC FG, Miner MF, Lema MJ, Zevon MA. The Relationship Between Depressoin and Pain Language in Cancer and Chronic Non-Cancer Pain Patients. Journal of pain and symptom management. 1998;15(6):350–358. doi: 10.1016/s0885-3924(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 65.Kremer EF, Atkinson JH, Jr., Ignelzi RJ. Pain measurement: the affective dimensional measure of the McGill pain questionnaire with a cancer pain population. Pain. 1982 Feb;12(2):153–163. doi: 10.1016/0304-3959(82)90191-9. [DOI] [PubMed] [Google Scholar]
- 66.Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. Journal of pain and symptom management. 2001 Nov;22(5):899–910. doi: 10.1016/s0885-3924(01)00351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer DJ, Villines D, Kim YO, Epstein JB, Wilkie DJ. Anxiety, depression, and pain: differences by primary cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010 Jul;18(7):801–810. doi: 10.1007/s00520-009-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beck SL. The Therapeutic Use of Musci for Cancer-Related Pain. Oncology nursing forum. 1990;18(8) [PubMed] [Google Scholar]
- 69.Ahles TA, Martin JB. Cancer pain: a multidimensional perspective. The Hospice journal. 1992;8(1-2):25–48. doi: 10.1080/0742-969x.1992.11882718. [DOI] [PubMed] [Google Scholar]
- 70.Wilkie DJ, Keefe FJ. Coping strategies of patients with lung cancer-related pain. The Clinical journal of pain. 1991 Dec;7(4):292–299. doi: 10.1097/00002508-199112000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Wagstaff S, Smith OV, Wood PH. Verbal pain descriptors used by patients with arthritis. Annals of the rheumatic diseases. 1985 Apr;44(4):262–265. doi: 10.1136/ard.44.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubuisson D, Melzack R. Classification of clinical pain descriptions by multiple group discriminant analysis. Experimental neurology. 1976 May;51(2):480–487. doi: 10.1016/0014-4886(76)90271-5. [DOI] [PubMed] [Google Scholar]
- 73.Boureau F, Doubrere JF, Luu M. Study of verbal description in neuropathic pain. Pain. 1990 Aug;42(2):145–152. doi: 10.1016/0304-3959(90)91158-F. [DOI] [PubMed] [Google Scholar]
- 74.Berry DL, Wilkie DJ, Huang HY, Blumenstein BA. Cancer pain and common pain: a comparison of patient-reported intensities. Oncology nursing forum. 1999 May;26(4):721–726. [PubMed] [Google Scholar]
- 75.Dudgeon D, Raubertas RF, Rosenthal SN. The short-form McGill Pain Questionnaire in chronic cancer pain. Journal of pain and symptom management. 1993 May;8(4):191–195. doi: 10.1016/0885-3924(93)90126-g. [DOI] [PubMed] [Google Scholar]
- 76.Shin H, Kim K, Young Hee K, Chee W, Im EO. A comparison of two pain measures for Asian American cancer patients. Western journal of nursing research. 2007 Aug;29(5):545–560. doi: 10.1177/0193945906298696. [DOI] [PubMed] [Google Scholar]
- 77.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL. The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncology nursing forum. 2011 Mar;38(2):133–142. doi: 10.1188/11.ONF.133-142. [DOI] [PubMed] [Google Scholar]
- 78.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer nursing. 2010 May-Jun;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 79.Pollard CA. The Relationship of Family Environment to Chronic Pain Disability [Doctoral Dissertation]. Dissertation Abstracts International, California School of Professional Psychology -San Diego. 1981 [Google Scholar]
- 80.Pollard CA. Preliminary validity study of the pain disability index. Perceptual and motor skills. 1984 Dec;59(3):974. doi: 10.2466/pms.1984.59.3.974. [DOI] [PubMed] [Google Scholar]
- 81.Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Archives of physical medicine and rehabilitation. 1987 Jul;68(7):438–441. [PubMed] [Google Scholar]
- 82.Kudel I, Edwards RR, Kozachik S, et al. Predictors and consequences of multiple persistent postmastectomy pains. Journal of pain and symptom management. 2007 Dec;34(6):619–627. doi: 10.1016/j.jpainsymman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Bishop SR, Warr D. Coping, catastrophizing and chronic pain in breast cancer. Journal of behavioral medicine. 2003 Jun;26(3):265–281. doi: 10.1023/a:1023464621554. [DOI] [PubMed] [Google Scholar]
- 84.Hawk C, Rupert RL, Colonvega M, Boyd J, Hall S. Comparison of bioenergetic synchronization technique and customary chiropractic care for older adults with chronic musculoskeletal pain. Journal of manipulative and physiological therapeutics. 2006 Sep;29(7):540–549. doi: 10.1016/j.jmpt.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994 Mar;23(2):129–138. [PubMed] [Google Scholar]
- 86.Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996 Oct;67(2-3):267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 87.Caraceni A, Mendoza TR, Mencaglia E, et al. A validation study of an Italian version of the Brief Pain Inventory (Breve Questionario per la Valutazione del Dolore). Pain. 1996 Apr;65(1):87–92. doi: 10.1016/0304-3959(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 88.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. The journal of pain : official journal of the American Pain Society. 2004 Mar;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Kumar SP. Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian journal of palliative care. 2011 May;17(2):108–115. doi: 10.4103/0973-1075.84531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983 Oct;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 91.Wu JS, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. Journal of pain and symptom management. 2010 Feb;39(2):230–240. doi: 10.1016/j.jpainsymman.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Atkinson TM, Rosenfeld BD, Sit L, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). Journal of pain and symptom management. 2011 Mar;41(3):558–565. doi: 10.1016/j.jpainsymman.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castel LD, Abernethy AP, Li Y, Depuy V, Saville BR, Hartmann KE. Hazards for pain severity and pain interference with daily living, with exploration of brief pain inventory cutpoints, among women with metastatic breast cancer. Journal of pain and symptom management. 2007 Oct;34(4):380–392. doi: 10.1016/j.jpainsymman.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Kaufman B, Wu Y, Amonkar MM, et al. Impact of lapatinib monotherapy on QOL and pain symptoms in patients with HER2+ relapsed or refractory inflammatory breast cancer. Current medical research and opinion. 2010 May;26(5):1065–1073. doi: 10.1185/03007991003680323. [DOI] [PubMed] [Google Scholar]
- 95.Cleeland CS. The Brief Pain Inventory User Guide. 2009 [Google Scholar]
- 96.Lyon DE, Schubert C, Taylor AG. Pilot study of cranial stimulation for symptom management in breast cancer. Oncology nursing forum. 2010 Jul;37(4):476–483. doi: 10.1188/10.ONF.476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leppert W, Majkowicz M. Polish brief pain inventory for pain assessment and monitoring of pain treatment in patients with cancer. Journal of palliative medicine. 2010 Jun;13(6):663–668. doi: 10.1089/jpm.2009.0326. [DOI] [PubMed] [Google Scholar]
- 98.Sackett DL SS, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Churchill Livingstone; London: 2000. [Google Scholar]