Abstract
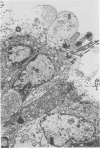
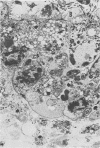
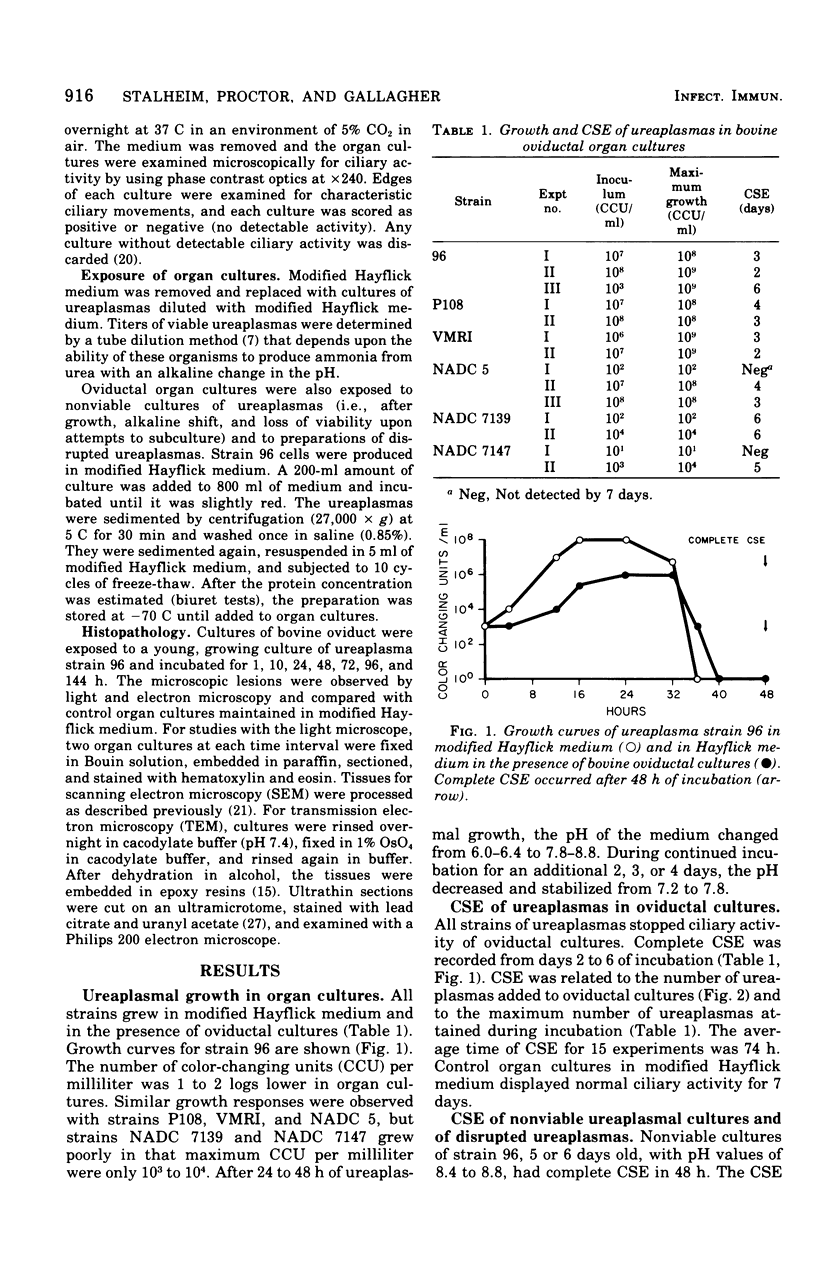
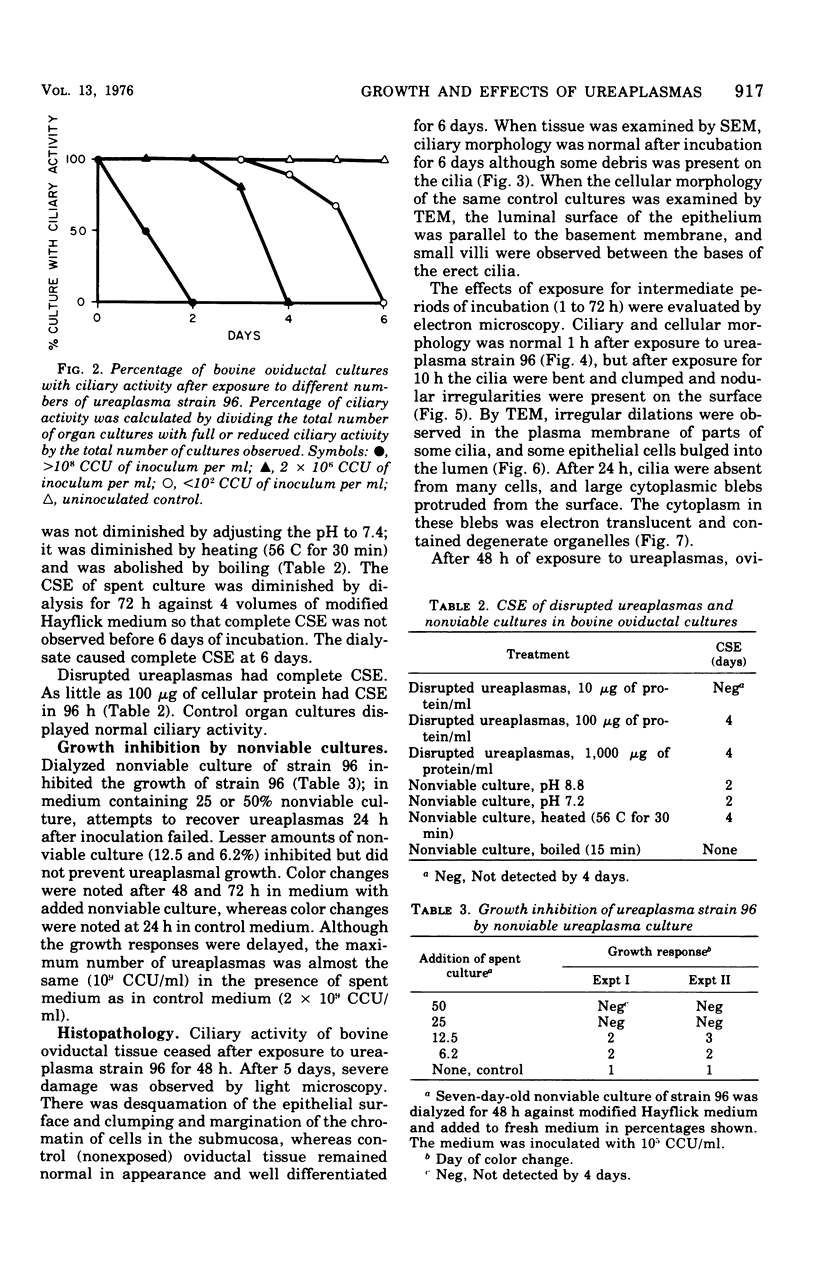
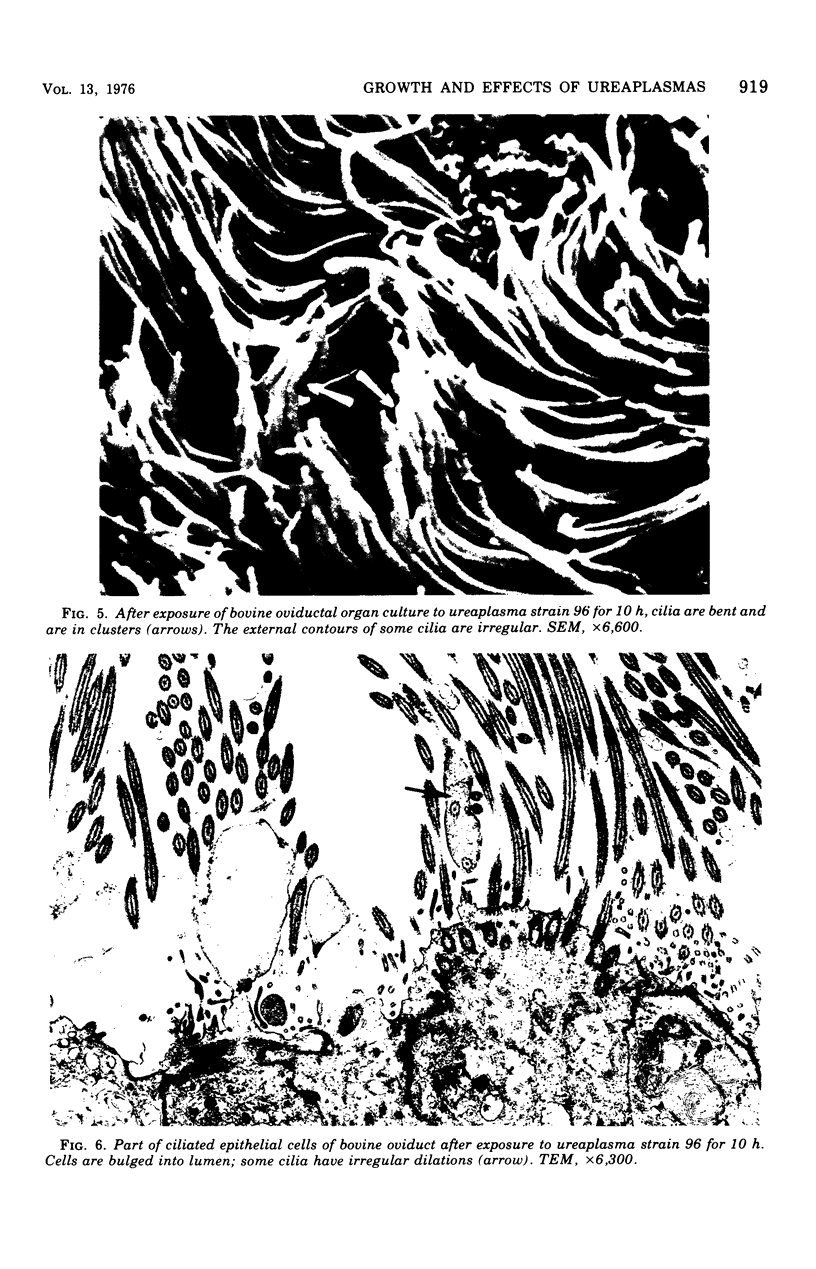
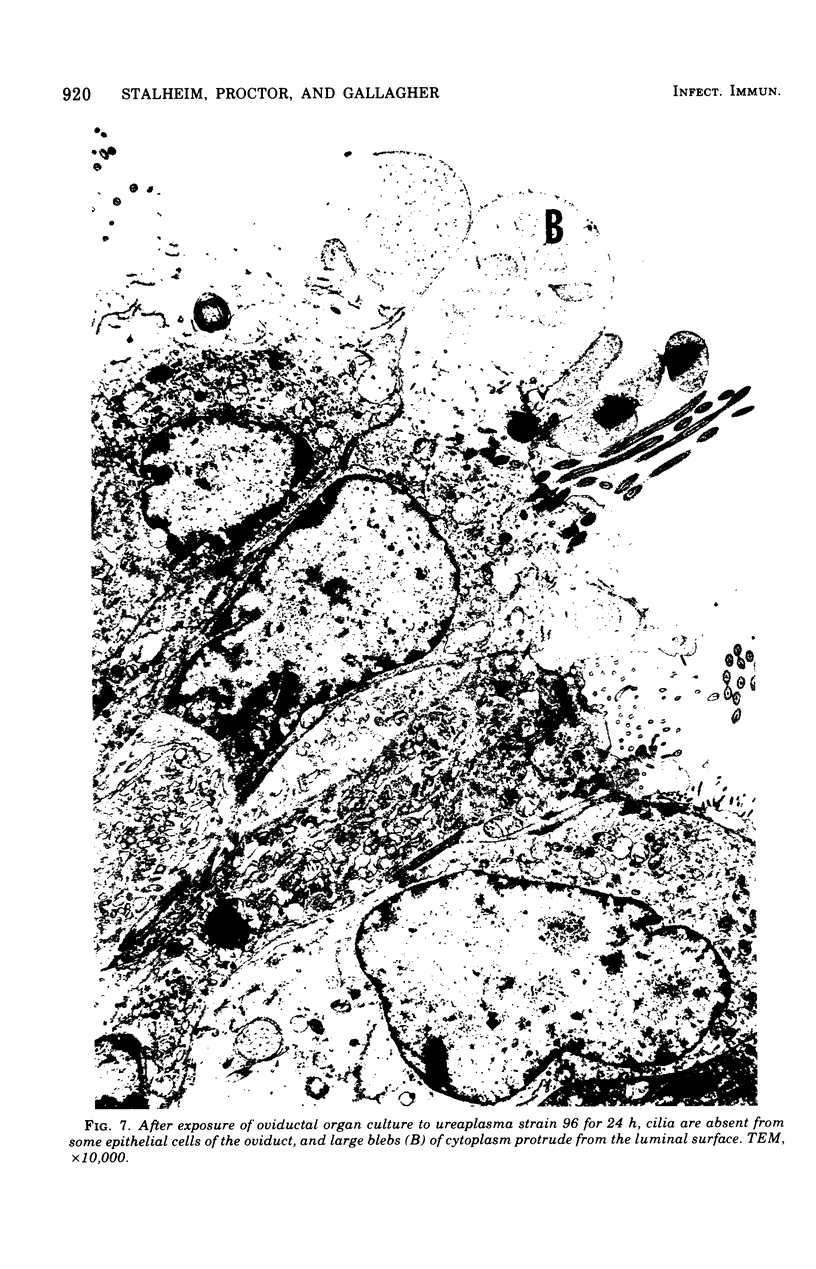
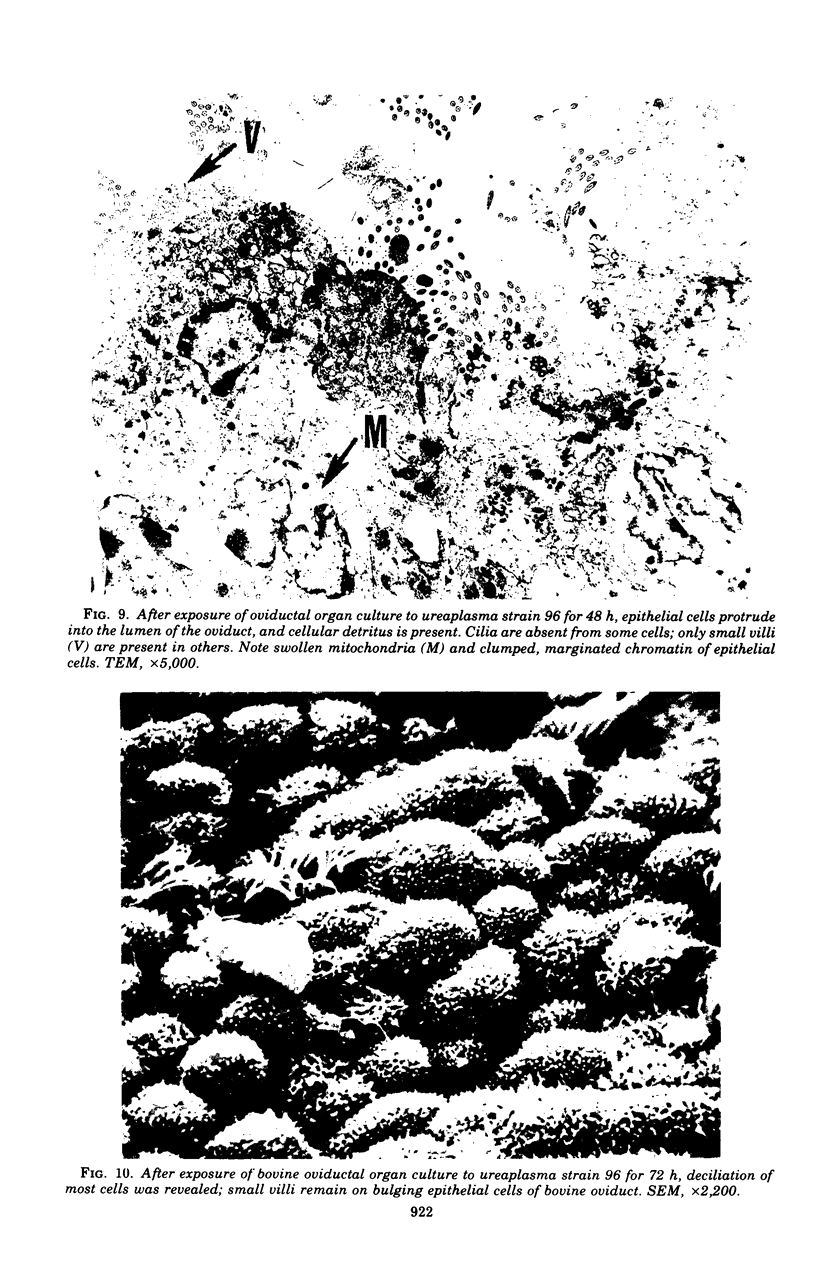
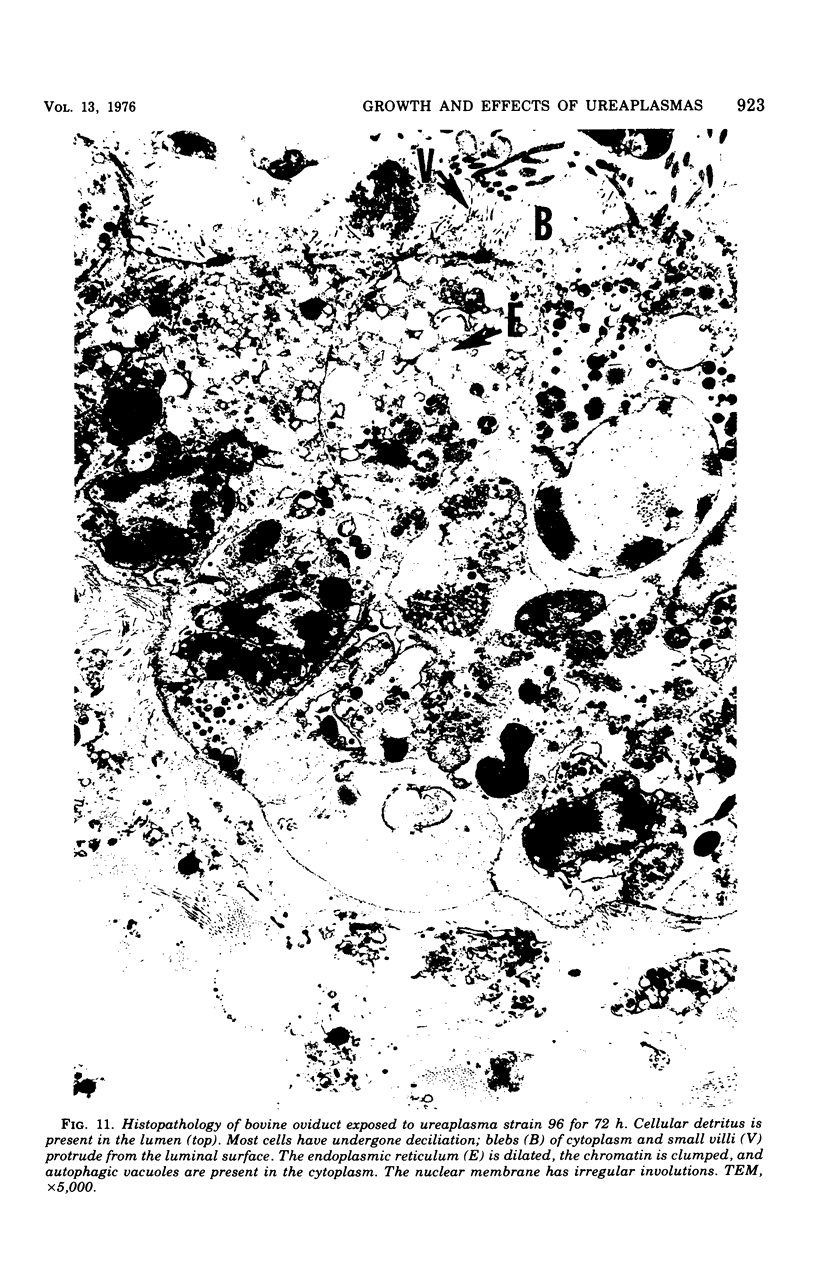
Ureaplasmas isolated from the human genital tract and from the genital and respiratory tracts of cattle were grown in association with organ cultures of bovine oviduct (uterine tube). All strains of unreaplasmas multiplied in organ cultures, stopped ciliary activity, and caused histological lesions. Most strains grew well, and 10(8) to 10(9) color-changing units were determined 18 to 144 h after inoculation. Twenty-four to 144 h after inoculation with unreaplasmas, ciliostasis was complete. Ciliostasis was also caused by additions of nonviable cultures at pH 8.8 (or adjusted to 7.4) or washed disrupted cells (100 mug of protein/ml); it occurred in 48 to 96 h. The cilia-stopping effect of nonviable cultures was diminished by heating (56 C for 30 min) and was abolished by boiling. When added to fresh medium in amounts exceeding 25%, nonviable unreaplasmal cultures completely inhibited ureaplasmal growth. By light, scanning, and transmission electron microscopy, cilia-stopping effect was correlated with collapse and sloughing of the cilia (the initial lesion was "bent" cilia), with bulging and vacuolization of secretory and ciliated cells, and finally with disorganization of the epithelium, necrosis, and desquamation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P., Besdine R. Tuboovarian abscess with recovery of T-mycoplasma. Am J Obstet Gynecol. 1973 Nov 15;117(6):861–862. doi: 10.1016/0002-9378(73)90508-5. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Growth and Pathogenesis of Mycoplasma mycoides var. capri in Chicken Embryo Tracheal Organ Cultures. Infect Immun. 1970 Oct;2(4):431–438. doi: 10.1128/iai.2.4.431-438.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness G. T-mycoplasmas: their growth and production of a toxic substance in broth. J Infect Dis. 1973 Jan;127(1):9–16. doi: 10.1093/infdis/127.1.9. [DOI] [PubMed] [Google Scholar]
- Gnarpe H., Friberg J. Mycoplasma and human reproductive failure. I. The occurrence of different Mycoplasmas in couples with reproductive failure. Am J Obstet Gynecol. 1972 Nov 15;114(6):727–731. [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Brownlie J. The production of mastitis in cows by the intramammary inoculation of T-mycoplasmas. J Hyg (Lond) 1972 Sep;70(3):511–521. doi: 10.1017/s0022172400063099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N. The isolation of T-strains of mycoplasma from pneumonic calf lungs. Res Vet Sci. 1968 Jul;9(4):376–377. [PubMed] [Google Scholar]
- Gourlay R. N., Thomas L. H. The experimental production of pneumonia in calves by the endobronchial inoculation of T-mycoplasmas. J Comp Pathol. 1970 Oct;80(4):585–594. doi: 10.1016/0021-9975(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Horne H. W., Hertig A. T., Kundsin R. B., Kosasa T. S. Sub-clinical endometrial inflammation and T-mycoplasma: a possible cause of human reproductive failure. Int J Fertil. 1973;18(4):226–231. [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N., Brownlie J. The virulence of T-mycoplasmas, isolated from various animal species, assayed by intramammary inoculation in cattle. J Hyg (Lond) 1973 Mar;71(1):163–170. doi: 10.1017/s0022172400046337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Thomas L. H. Inhibition by Mycoplasma dispar of ciliary activity in tracheal organ cultures. Infect Immun. 1974 Aug;10(2):405–408. doi: 10.1128/iai.10.2.405-408.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton W. H., Van Roekel H. Serological studies of mycoplasma (PPLO) of avian origin. Avian Dis. 1963 Aug;7(3):272–286. [PubMed] [Google Scholar]
- LOMBARD L., MORGAN B. B., McNUTT S. H. Some pathologic alterations of the bovine oviduct. Am J Vet Res. 1951 Apr;12(43):69–74. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston C. W., Jr Isolation of T-strain of mycoplasma from Texas feedlot cattle. Am J Vet Res. 1972 Oct;33(10):1925–1929. [PubMed] [Google Scholar]
- Reed S. E., Boyde A. Organ cultures of respiratory epithelium infected with rhinovirus or parainfluenza virus studied in a scanning electron microscope. Infect Immun. 1972 Jul;6(1):68–76. doi: 10.1128/iai.6.1.68-76.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N., Smith P. F., Mayberry W. R. Lipids of a T strain of Mycoplasma. J Bacteriol. 1972 Feb;109(2):565–569. doi: 10.1128/jb.109.2.565-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnke H. L., Von Dreumel A. A. The isolation of T-mycoplasma from pneumonic lungs of a calf. Can J Comp Med. 1972 Jul;36(3):317–318. [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H., Gallagher J. E., Deyoe B. L. Scanning electron microscopy of the bovine, equine, porcine, and caprine uterine tube (oviduct). Am J Vet Res. 1975 Aug;36(08):1069–1075. [PubMed] [Google Scholar]
- Stalheim O. H., Gallagher J. E. Effects of Mycoplasma spp, Trichomonas fetus, and Campylobacter fetus on ciliary activity of bovine uterine tube organ cultures. Am J Vet Res. 1975 Aug;36(08):1077–1080. [PubMed] [Google Scholar]
- Tan R. J., Miles J. A. Possible role of feline T-strain mycoplasmas in cat abortion. Aust Vet J. 1974 Apr;50(4):142–145. doi: 10.1111/j.1751-0813.1974.tb06877.x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Carney F. E., Jr Growth and effect of mycoplasmas in Fallopian tube organ cultures. Br J Vener Dis. 1974 Jun;50(3):212–216. doi: 10.1136/sti.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Carney F. E., Jr Growth and effect of mycoplasmas in Fallopian tube organ cultures. Br J Vener Dis. 1974 Jun;50(3):212–216. doi: 10.1136/sti.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Howard C. J. Effect of Mycoplasma dispar, Mycoplasma bovirhinis, Acholeplasma laidlawii and T-mycoplasmas on explant cultures of bovine trachea. J Comp Pathol. 1974 Apr;84(2):193–201. doi: 10.1016/0021-9975(74)90060-7. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff K. H., Schneider E., Unger L., Coalson J. J. Ultrastructural changes in hamster tracheal ring cultures exposed to Mycoplasma pneumoniae. Am J Pathol. 1973 Jul;72(1):91–102. [PMC free article] [PubMed] [Google Scholar]