Abstract
Blood transfusions are routinely done in every medical regimen and a worldwide established collection, processing/storage centers provide their services for the same. There have been extreme global demands for both raising the current collections and supply of safe/adequate blood due to increasingly demanding population. With, various risks remain associated with the donor derived blood, and a number of post collection blood screening and processing methods put extreme constraints on supply system especially in the underdeveloped countries. A logistic approach to manufacture erythrocytes ex-vivo by using modern tissue culture techniques have surfaced in the past few years. There are several reports showing the possibilities of RBCs (and even platelets/neutrophils) expansion under tightly regulated conditions. In fact, ex vivo synthesis of the few units of clinical grade RBCs from a single dose of starting material such as umbilical cord blood (CB) has been well established. Similarly, many different sources are also being explored for the same purpose, such as embryonic stem cells, induced pluripotent stem cells. However, the major concerns remain elusive before the manufacture and clinical use of different blood components may be used to successfully replace the present system of donor derived blood transfusion. The most important factor shall include the large scale of RBCs production from each donated unit within a limited time period and cost of their production, both of these issues need to be handled carefully since many of the recipients among developing countries are unable to pay even for the freely available donor derived blood. Anyways, keeping these issues in mind, present article shall be focused on the possibilities of blood production and their use in the near future.
Keywords: RBCs, ex-vivo erythrocytes, manufacturing blood, hematopoietic stem cells, induced pluripotent stem cells
Introduction
Initially, started by Harvey's studies of blood circulation system, blood transfusion began in the 17th century with animal blood transfusion experiments. The first fully documented report on blood transfusion in humans was from Dr. Jean-Baptiste Denys, who in 1665 successfully transfused blood from a sheep in a 15 years old boy. Though, he could not succeed in later transfusions as recipients died after transfusions were made. In similar studies, Dr. Richard Lower demonstrated the effects of changes in blood volume in circulatory function and developed methods for cross-circulatory study of animals. The first successful human blood transfusion was reported by Dr. James Blundel (1818) between a married couple for a postpartum hemorrhage. However, in 1901, the breakthrough was achieved in human transfusion with the discovery of blood group antigen by Austrian researcher Karl Landsteiner, who discovered that red blood cells got clumped when incompatible blood types were mixed and immunological reaction occurred if the recipient of a blood transfusion had antibodies against the donor blood cells. This “Nobel Prize” (1930) winning discovery made it possible to determine blood type and paved the way for safe blood transfusions. Since then many other blood groups have been discovered. Following to these discoveries a number of blood banks were established during 1940–1950s and it is an inevitable part of all the modern clinical modalities (Alter and Klein, 2008).
The global blood collection was reported to be about 103 million units (www.who.int/worldblooddonorday/en/) (Department of Health and Human Services, 2010, 2013; World Health Organization, 2011). The quality and quantity of donor derived blood collection remain unevenly scattered in economically developed and developing countries. Almost 50% of these blood collections is made in developed countries, which accommodate only a mere 15% fraction of the world's population. Presently, the blood collection seems to be sufficient in economically developed countries. It is supported by reports showing 30,000 annual blood donations on an average per blood center through ~8000 blood centers scattered in 159 high-income countries (Department of Health and Human Services, 2010, 2013). For example, in U.S. the total no. of blood unit collected were 5% more than the actual transfusion made during year 2011 (Department of Health and Human Services, 2013). On the contrary, this number of collections/per center is very less (3700) in developing countries. As per WHO report 82 low income and middle income countries have only 10 donations per 1000 people in the population that would remain highly insufficient to supply a comparable large population residing in these countries (World Health Organization, 2011). Further, the screening facilities are very much inefficient in most of the developing countries. As per WHO record, 39 countries are not able to screen all blood donations for one or more of the following transfusion-transmissible infections (TTIs): HIV, hepatitis B, hepatitis C, and syphilis (Department of Health and Human Services, 2013). Again, there are only 106 countries which have national guidelines on the appropriate clinical use of blood. It would be worth noticing that only 13% of low-income countries have a national haemovigilance system to monitor and improve the safety of the transfusion process. Moreover, the blood supply may look sufficient for the time being in developed countries, it likely becomes inefficient to keep supporting a rapidly growing proportion of elderly population (>60 years age) and burgeoning demand for blood transfusions for surgical treatments by the year of 2050 (U.S. Census Bureau, 2004; Ali et al., 2010).
One of the major challenges in clinical settings is to find blood group compatibility for more than 30 blood group system (308 recognized antigens) including ABO & Rh antigens (Daniels et al., 2009).
There are a number of situations such as rare phenotypes, hemoglobinopathies, polytransfusion patients and polyimmunization that may result in significant complications (World Health Organization, 2011) adding more complexity to the supply constraints. Recent technologies such as antigen masking and enzymatic cleavage to generate universal donor blood groups (O and RhD antigens) have been proposed. But these methods are yet to be developed (Bagnis et al., 2011). While keeping these issues in mind, it is worth noticing that the donated blood is tested for various kinds of infections before any transfusion could be made [e.g., HIV-1, HIV-2, HTLV-1, HTLV-2, Hepatitis B, Hepatitis C, Syphilis (T pallidum), Chagas disease (T cruzi), and West Nile Virus, Cytomegalovirus (CMV)], and a number of precautionary measures are followed during transfusion procedures. Even then blood transfusions are often associated with several complications (Alter and Klein, 2008; World Health Organization, 2011). These complications not only compromise the quality of treatment, but may add to the overall cost of the regime also which remain associated with it in any clinical setting (Department of Health and Human Services, 2010). This is true even in the developed countries where 0.24 % transfusion has been reported to be associated with adverse reactions (Department of Health and Human Services, 2013). For example, adverse events from transfusions in the US only may account for approximately $17 Billion every year. These complications depend upon the patient status or specific transfusion quantity involved. These situations have evoked researchers to develop alternate methods to get blood from various non-donor derived sources. In the search of blood alternates researchers have developed various artificial molecules mimicking functionalities of blood RBCs. The majority of these molecules is either hemoglobin based oxygen carriers or perfluorocarbon solutions. Hemoglobin based carriers are reported to be inefficient as compared to the oxygen carrying capacity of native RBCs. Whereas, perflorocarbons could not become popular due to their limited functional applications (Winslow, 2006; Henkel-Honke and Oleck, 2007; Natanson et al., 2008; Silverman and Weiskopf, 2009; Castro and Briceno, 2010).
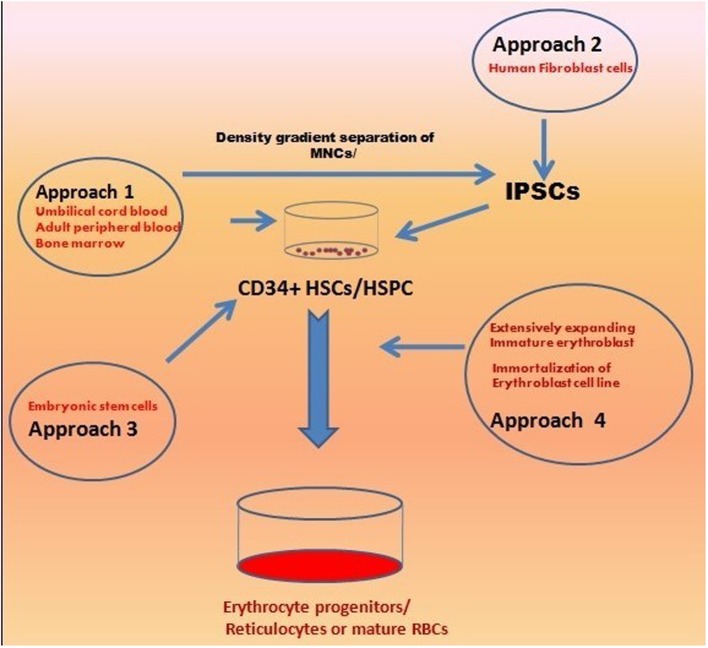
Ex vivo expansion of erythrocytes or manufacturing blood from stem cell is the most attracting approach among the global research community for the last few years. The basis for the principle that ex vivo cultivated RBCs (or other blood cells) may be of clinical use was provided by Nakamura and his colleagues who have demonstrated that RBCs derived from the immobilized ESCs cell line can protect mice from lethal anemia (Hiroyama et al., 2008). Recently, the proof-of-concept was described by first in-human blood transfusion using ex-vivo generated RBCs (Giarratana et al., 2011). In a major landmark study, Giarratana et al. shown the survival capacity of ex vivo cultivated RBCs in humans. As described by them, the CD34+ cells obtained from volunteer donors as an aphaeresis product were cultured in a serum free media (with the addition of Stem cell factor (SCF), Interleukin-3 (IL-3), and Erythropoietin (EPO) resulting into 2 ml of blood RBCs equivalent (1010 cells) under GMP conditions. The ex vivo expanded RBCs were labeled with 51Cs to trace their fate in patients as approved by FDA U.S. (Giarratana et al., 2011) and demonstrated to be comparable with any native RBCs transfusion. These findings established the proof-of-concept for clinical utilization of ex vivo generated RBCs globally. As discussed in the next section, a number of methods have been demonstrated so far (Figure 2), which ensure the feasibilities of ex vivo RBCs expansion from various source materials (e.g., CD34+ HSCs, Embryonic stem cells, and IPSCs) (Figure 3). There are various factors which essentially regulate the yield and the level of RBCs maturation through these protocols. Reports are there, showing numerical expansion of erythrocyte precursors up to ~0.5 transfusion unit/donation from UCB CD34+ cells, and approximately 2 million fold expansions of enucleated erythrocytes functionally comparable to native RBCs through slight modification of culturing techniques (Neildez-Nguyen et al., 2002). Further, large scale production (up to 10 transfusion unit) from two units of UCB have been demonstrated by technically complex protocols (described in later sections) which presently may appear ill suited from the commercial scale production point of view but strengthen the concept that with the advancement of techniques sufficient transfusable units may be produced (Fujimi et al., 2008). Besides that, RBCs precursors, cellular intermediates/nucleated erythroid precursors are generated through these protocols, which may also be of use if transfused. It is expected that these cells are likely to become functionally matured in vivo while interacting with internal factors governing their maturation. The same has been reported both in human and animal models (Ende and Ende, 1972; Neildez-Nguyen et al., 2002). Conceptually, the maturing cells along with functionally matured cells may aid to maintain the cellular content at the time of transfusion. These maturing cells may proliferate and each cell may give rise to 4–64 cells before they get enucleated and functionally matured in vivo. This hypothesis is supported by the data available from clinical observations in developing countries where 40–80 ml of compatible CB blood (containing 4–7 × 1010 RBCs and 2–8 × 109 erythroblast cells) is occasionally used for transfusion in emergent conditions (Ende and Ende, 1972). Together, these reports strengthen the concept that ex vivo RBCs expansion likely support the different clinical short-comes to exist due to limited supply and various rare blood based disorder.
Figure 2.
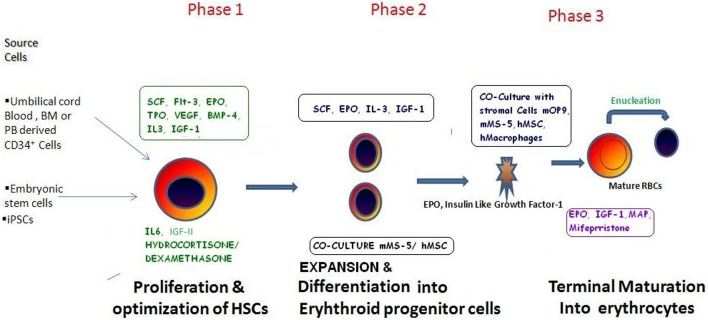
The various methods/protocols described so far for the ex vivo RBCs expansion. As discussed in the main text, manufacturing blood may involve various step which are to be categorized in three main phases as depicted in the figure. In phase 1: initial source material is to be collected from a variety of source material(s) on the basis of their availability, suitability, and expansion potential and grown in medium generally supplemented with growth factors to enhance HSCs proliferation: subsequently these cells are cultured in the presence of Erythropoietin to induce their differentiation and maintenance into erythropoietic progenitor stage. Finally, in Phase 3 cultures these Erythropoietic progenitors may be co cultured with murine/human stromal cell line support to induce their maturation and enucleation resulting into mature RBCs. These RBCs are evaluated for their biochemical properties and various antigenic profiles to ensure their nativeness.
Figure 3.
An overview of the various approaches used for ex vivo Erythropoiesis. The different approaches are in use for develoing large amount of transfusable clinical grade RBCs include CD34+ HSPCs (from CB, PB, BM), ESCs/IPSCs derived erythroid progenitors, and highly expanding erythroid progenitors due to stress erythropoiesis. All these approaches has been discussed in detail in text.
However, a number of factors such as technological barriers associated with large scale production and per unit production cost remain to be considered in order to support a large population. The present scale of RBCs production would require to be enhanced several times in a more cost-effective and timely manner. Commercial production of blood may lack essential background financial support in developing countries which are suffering from even more sensitive issues. Moreover, in the absence of more realistic, achievable goals it may become hard even in the capitalistic developed countries. Present article focuses on various issues related to current methods and their feasibilities to realize limited “blood pharming” in near future.
Erythropoiesis in vivo
Hematopoiesis, a dynamic process, is regulated both temporally and spatially. It may be classified as “primitive hematopoiesis” in featus where specified cells termed as “blood islands” are generated by yolk sac (YS). In YS, nucleated RBCs exclusively expresses embryonic Hbs (Gower-1, Gower-2, and Hb Portland) are generated for a small period of time. Following to that, a second wave of hematopoiesis or “definitive hematopoiesis” produce hematopoietic stem cells, which have the potential of transfusion and includes enucleated RBCs along with various other hematopoietic cells. This event takes place largely in the fetal liver and up to a little extent in YS. In later stages of featus development Definitive hematopoiesis occurs in bone marrow (BM) and that serves as the only site of hematopoiesis in adults through their life. The hematopoietic cells from fetal and adult definitive hematopoietic stages may be differentiated on the basis of their HB contents. In the fetus hematopoiesis α and γ globins, (the components of fetal Hb) (Hb F), are expressed, and in the later β globins (the components of adult-type) (HbA), are expressed (Palis, 2008; Baron et al., 2012).
Erythropoiesis or generation of RBCs includes hematopoietic progenitor cells, which are first differentiated into committed erythroid progenitors and thereafter proliferate/converted to functional red blood cells in a highly regulated hierarchal manner. This dynamic process is tightly regulated through a number of growth factors which ensure the maintenance of regular homeostasis of the RBCs mass.
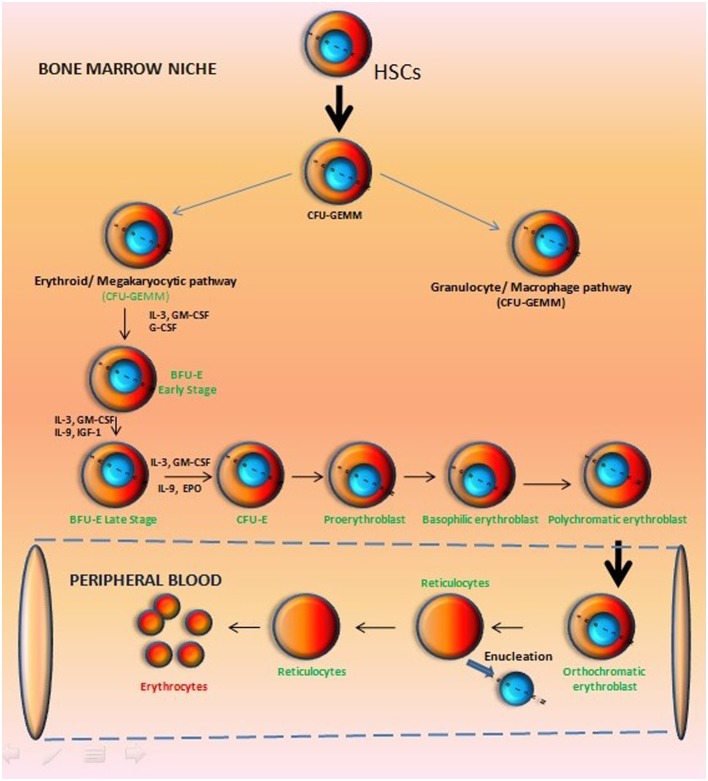
The entire process of erythropoiesis may be defined to occur in three stages: (1) Erythropoietic commitment of HSCs: this includes development of erythroid committed blast cells from multipotent hematopoietic progenitors; (2) Division and differentiation of these morphologically identifiable erythroid progenitor cells; and (3) Terminal differentiation including cellular morphological changes such as enucleation, to produce reticulocytes and ultimately maturation: of RBCs (Figure 1).
Figure 1.
Hierarchy of erythropoietic development in vertebrates. In adult Bone Marrow, committed progenitors arising from hematopoietic stem cells give rise to erythroblasts, and as the progeny of a stem cell progress through development, there is a loss of their multipotency while increasing lineage restriction. The various cellular stages in erythrocyte development are identified by their ability to form colonies in semisolid medium supplemented with specific cytokines and by cell surface markers.
The Hematopoietic stem cells are generated in YS and may be differentiated into a common myeloid progenitor that, in turn, gives rise to bipotent progenitors restricted to either the granulocyte/macrophage or the erythroid/megakaryocytic pathways (Suda et al., 1983; Debili et al., 1996; McLeod et al., 1996; Akashi et al., 2000). A comparable stage of development defined in vitro using cytokine containing semisolid medium (Figure 1) is the colony-forming unit granulocyte, erythroid, macrophage, megakaryocytic (CFU-GEMM) precursor that gives rise to bipotent progenitors restricted to either granulocyte/macrophage or erythroid/megakaryocytic pathways (Suda et al., 1983; Debili et al., 1996; McLeod et al., 1996). Those progenitors which express erythropoietin receptor (EPOR) remain responsive to erythropoietin (EPO) and are committed to the erythroid/megakaryocytic pathway.
On the other hand, EPOR deficient progenitor cells are committed to the myeloid pathway. The burst-forming unit–erythroid (BFU–E), which gives rise to colony-forming unit–erythroid (CFU–E) is termed as the most immature erythroid-restricted progenitor. Among them, early BFU–E (blast-like cells) are highly proliferative, and give rise to clustered burst colonies of up to 20,000 cells in semisolid culture assays.
However, BFU–E bear comparatively small number of EPOR but later progenies (daughter cells) derived from them acquire a high expression level of EPOR and become EPO responsive, transferrin receptor positive, and begin to express hemoglobin (Heath et al., 1976; Lichtman et al., 2007). Later on, CFU–E are derived from these cells, which are highly responsive to EPO, but generate smaller colonies, and express many of the gene products required for definitive erythroid development (Figure 1). There are reports describing a cell division stimulating anti-apoptotic role of EPO in late-stage erythroid cells derived from CFU–E. At this stage of development cells may start the synthesis of hemoglobin and acquire cytoskeletal proteins in tissue culture systems. Moreover, cellular adhesion molecules are expressed in these cells that help define them as nucleated erythroblasts. Thereafter morphologically identifiable nucleated erythroid precursor become visible, which progress from the pro-erythroblast to basophilic, polychromatophilic, and orthochromatic cell forms leading to the reticulocyte (Figure 1) (Stephenson et al., 1971; Lichtman et al., 2007; Jing et al., 2013).
Erythroid differentiation encompasses four distinct major cellular processes such as, (1) the accumulation of hemoglobin that participates in driving the basophilic to acidophilic cytoplasmic changes seen during maturation, (2) limited erythroblast expansion, (3) a continued decrease in cell size, (4) and nuclear condensation leading to their final enucleation. These highly regulated developmental changes take place in the microenvironment of BM niche where “erythroblast islands” are closely associated with macrophages that serve as stromal or nurse cells (Bessis et al., 1978; Manwani and Bieker, 2008; Rhodes et al., 2008). These erythroid development stages end up with the exit of reticulocytes from the marrow, which now enter the circulation and get matured into erythrocytes. The characteristic features of this stage include disassembly of ribosomes, Golgi bodies, and other cellular machinery, removal of organelles, enucleation, changes in the cytoskeleton leading to the classic biconcave discoid shape, and then release into the circulation (Department of Health and Human Services, 2013). Terminal maturation takes place in the erythroblast islands, which involves participation macrophages of with maturing erythrocytes (Bessis et al., 1978; Manwani and Bieker, 2008; Rhodes et al., 2008).
The mature erythrocyte have a life span of 120 days in blood circulation, and afterwards “senescence” occurs reflected through changes in surface antigen expression or physical characteristics of the cell triggering their removal from the blood by macrophages/reticuloendothelial system (Gifford et al., 2006). Thus, hematopoiesis is regulated through changes in body compartments during mammalian development, from embryo to adult, and depending on the age of development, specialized microenvirnmental niches precisely regulate hematopoietic development (Migliaccio et al., 1986; Palis and Yoder, 2001; Baron and Fraser, 2005; Mikkola et al., 2005; McGrath and Palis, 2008).
Ex vivo erythropoiesis: an overview
There has been considerable progress in the field of developing biological control over the expansion of erythrocytes to generate terminally differentiated, fully functional RBCs (Migliaccio et al., 2012). The in vivo hematopoietic development process have been studied (Figure 1) and an in depth knowledge of the generation of erythroblast regulated through various growth factors have paved the way to mimic the process in vitro (Lodish et al., 2010). Altogether, these reports demonstrated the enormous potential inherited by three major cell types including (i) CD34+ HSPCs, (II) Embryonic stem cells/induced pluripotent stem cells, and (III) immortalized erythroid precursors which can be differentiated into erythroid lineages by using almost similar/overlapping protocols (Figure 2) (Table 1). Regardless of their types, stem cells are promoted to become erythroid progenitor in three basic steps viz. commitment, expansion, and maturation. There are a number of reports showing use of various cytokines/growth factors (SCF and EPO are most common) to generate variable yields of immature progenitors or mature RBCs with or without using animal/human derived feeder coculture system (Figure 2). Recently, a new concept of transdifferentiation (directly differentiating human fibroblast cells into erythroblasts) has been shown to bypass the HSPC state (Figure 3) (Szabo et al., 2010). Although promising, but the technique remains to be evaluated for their potential to generate large scale RBCs. Most of these protocols suffer from similar problems such as degree of enucleation or erythrocyte formation, predominate form of Hb expressed by them, use of animal derived products such as serum and feeder cell coculture, and clinical transfusion related problems such as blood type matching and unavailability of sufficient number of RBCs for transfusion. All these issues can be summarized in the following sections.
Table 1.
Progression in the field of ex vivo RBCs expansion during last two decades.
| S. no. | Cell source and culture time | Growth factors | Co-culture | Expansion | Units of blood | Enucleation rate | Animal or human serum/plasma | Key points | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CB CD34+ 21 days | FLT-3, SCF, TPO, EPO, IGF-1 | NO | NA | NA | 4% in vitro | No | Basis for transfusion potential of ex vivo cultured RBCs in animal model | Neildez-Nguyen et al., 2002 |
| 99% in vivo | |||||||||
| 2 | Cord blood 21 days | SCF, IL-3, EPO hydrocortisone | Murine stromal mMS-5 | 1.95 × 106 | 4.6/CB | 95% | No | First in vitro production of RBCs | Giarratana et al., 2005 |
| 3 | Cord blood 60 days | EPO, SCF, IGF-1 dex and lipid mix | No | 109 | -NA | −100% | Yes | Prolonged expansion protocol up to 60 days for adult globin switching | Leberbauer et al., 2005 |
| 4 | Cord blood 20 days | SCF, IL-3, EPO VEGF, IGF-2 | No | 7.2 × 105 | 104/CB | 77.5% | Yes | Low expansion and enucleation rates | Miharada et al., 2006 |
| 5 | Cord blood 21 days | SCF, IL-3, EPO, TPO, Flt-3 | Human MSCs | 8 × 103 | 0.02/CB | 64% | No | Less allogenicity, lower expansion, and enucleation rates, replaced BM derived feeder cells with UCB derived cells | Baek et al., 2008 |
| 6 | Cord blood 38 days | SCF+Flt-3+TPO IL-3+EPO | hTERT+ Macrophages | 3.5 × 106 | 8.8/CB | 100% | Yes | Impossible to scale up | Fujimi et al., 2008 |
| 7 | hESCs 59 days | Hydrocortisone, IL3, BMP-4, SCF, EPO, IGF-1 | hMSCs, mMS-5 | 4 × 107 cells | NA | (6.5%, starting from CD34+ cells) | Yes | Globin switching | Qiu et al., 2008 |
| FH-B-hTERT | |||||||||
| 8 | hESCs 42 days | SCF, Epo, BMP-4, VEGF, bFGF, TPO, FLT3 L | MEFs, OP9 | 1010–1011 cells/6-well plate of hESCs | NA | 10–65% | No | Functional oxygen carrying capacity of ESCs derived RBCs | Lu et al., 2008 |
| 10 | hiPSCs,(MR90, FD-136) | SCF,TPO, FLT3-L, TPO, BMP-4; VEGF- IL-3, IL-6 | hIPSCs | NA | 4–10% | No | First time complete differentiation of hiPSCs cells into definitive erythrocytes capable of maturation up to enucleated RBCs (fetal hemoglobin in a functional tetrameric form) | Lapillonne et al., 2010 | |
| hESCs(H1I) 46 days | 4.4 × 108 | 52–66% | |||||||
| hESCs 35 × 108 | |||||||||
| 11 | Cord blood 33 days | SCF, IL-3, EPO hydrocortisone | No | 2.25 × 108 | −500 units/UCB | >90% | No | First serum-free culture, first demonstration of RBC culture in a large-scale bioreactor | Timmins et al., 2011 |
| 12 | Cord blood 18 days | SCF, EPO hydrocortisone | No | 4.3 × 107 | 75/CB | 70% | Yes | Best yield to date | Giarratana et al., 2011 |
| 13 | hiPSCs 59 days | Hydrocortisone, IL-3 BMP-4, Flt3L, SCF, EPO, IGF-1 | hMSCs/Matrigel. | 0.5–8 × 106 | NA | NA | Yes | Production of large number of erythroid cells with embryonic and fetal-like characteristics regardless of the age of donor tissue | Chang et al., 2011 |
| hTERT immortalized fetal liver cells | |||||||||
| 14 | hESCs hiPSCs 60–125 days | Iron saturated transferrin, dexamethasone, insulin, SCF, EPO, TPO, IL-3, IL-6 | MEFs, mOP9 mMS-5 | 2 × 105 ESCs | NA | 2–10% | Yes | RBCs production from transgenic and transgene-free iPSCs using the OP9 coculture method with efficiency comparable to hESCs | Dias et al., 2011 |
| 15 | hiPSCs 52 days | holo-human transferrin recombinant human insulin heparin, and 5% human plasma SCF, TPO, FLT3 ligand, BMP4, VEGF- n-3, IL-Epo | MEFs | 15–28.3 × 108 | NA | 20–26% RBC and 74–80% orthochromatic erythroblasts | 5–10% Hu plasma | First time in a normal and a pathological erythropoietic differentiation models that hiPSC are intrinsically able to mature into adult hemoglobin synthesizing cells | Kobari et al., 2012 |
CD34+ hematopoietic cells from various sources and their use for ex vivo RBCs culture
CD34+ cells from peripheral blood (PB), cord blood (CB), and BM have been in use for clinical transplantations for more than two decades (Carmelo et al., 1995). Initialed by Fibach et al. the methods to generate RBCs from CD34+ HSPCs have been existing for a long time (Fibach et al., 1989, 1991; Wada et al., 1990). Douay et al. were quick to demonstrate functional characteristics of the erythrocytes generated by CB derived CD34+ HSPCs (Douay, 2001; Neildez-Nguyen et al., 2002). Their studies confirmed a higher erythrocyte generating potential in comparison to myeloid precursors with low (4%) enucleation efficiency (Neildez-Nguyen et al., 2002). Giarratana et al. further modified the protocol and demonstrated 90% enucleation efficiency in a similar 3 step method for adult blood/BM derived CD34+ cells (Giarratana et al., 2005) by co-culturing them with murine stromal cell line (MS5) or Human mesenchymal stem cells (HuMSC). The procedure resulted in, 20,000 fold, 30000 fold and 2 × 105 fold increment in the erythrocytic cell generation when BM, GM-CSF mobilized PB, and CB derived CD34+ cells were used respectively (Douay and Andreu, 2007; Douay and Giarratana, 2009; Douay et al., 2009). This group further reported 5–10 units of blood cells on prolonged culture with feeder cell coculture system (Douay and Andreu, 2007; Douay and Giarratana, 2009; Douay et al., 2009). The cells were reported to have native RBCs like functional characteristics as described by normal glucose-6-phosphate dehydrogenase (G6PD) and Pyruvate Kinase (PK) enzyme levels, membrane deformability and oxygen dissociation characteristics. This was also evident by their similar survival rate in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice following to intraperitoneal infusion of carboxyfluorescein diacetate succinidyl acetate (CFSE) labeled cells.
These methods suffer from two major drawbacks such as (i) use of animal serum and co-culture system and (ii) low yield of functionally matured RBCs per unit of blood cells, which tempted researchers to identify more defined cocktail of growth factors to achieve a better control over erythrocytes proliferation and differentiation. Malik et al. demonstrated generation of ~10–40% enucleated erythrocytes by enriched CD34+ cells from CB, PB, and BM (Malik et al., 1998) in a serum free liquid culture system (with Flt-3, GM-CSF, EPO). This report indicated the possibilities of generating erythrocytic progenitors in the absence of animal derived serum that is strongly recommended by most clinicians. Following to these reports 105 − 1.5 × 108 folds erythroid cell expansion have been reported in similar serum free environment. (Panzenböck et al., 1998; Freyssinier et al., 1999).
Fujimi et al. demonstrated massive production of terminally matured erythrocytes from CD34+ CB cells in the serum free environment while avoiding xenogenic feeder by using hTERT transduced human stromal cell line (Fujimi et al., 2008). These methods could result in massive growth of 6 × 1012 RBCs from one unit of CB and also the highest RBC number per starting cell (3.52 × 106) in any static culture. However, use of the AB blood group, serum to derive macrophages in a 14 days culture and an additional step of magnetic separation leaves this method ill-suited for the scaling up purposes. Beak et al. showed up to 64% enucleation efficiency by using CB or BM derived mesenchymal stem cells as feeder layer which would require an additional MSCs donor for the feeder cells and thus may not be suitable for large scale production procedures (Baek et al., 2008). Xi et al. has demonstrated an in vitro model of human erythropoiesis to study the erythroid differentiation in normal and pathological conditions (Xi et al., 2013). CD34+ cells were shown to undergo up to 35 doublings in a prolonged 50 days culture in serum free media supplemented with EPO, synthetic glucocorticoid Dex, IGF-1, SCF, and iron saturated human transferrin. In a subsequent step, matured RBCs could be generated by coculturing erythroid progenitors with human fetal liver stromal cells. The procedure resulted in 109 fold expansion of erythrocytes (Xi et al., 2013).
Recent findings have indicated a significant erythropoietic potential in the CD34neg cell fraction which should also be exploited to enhance the production (Leberbauer et al., 2005; Van den Akker et al., 2010; Tirelli et al., 2011a). Leberbauer et al. described a culture method which exploited the signal transduction cascades of stress erythropoiesis, achieving a 109-fold expansion of erythroblasts of CB cells without prior CD34+ isolation (Leberbauer et al., 2005). Akker et al. describe a culture method modified from Leberbauer et al, and obtained a homogenous population of erythroblasts from peripheral blood mononuclear cells (PBMC) without prior purification of CD34+ cells. This pure population of immature erythroblasts can be expanded to obtain 4 × 108 erythroblasts from 1 × 108 PBMC after 13–14 days in culture. Upon synchronizing differentiation, high levels of enucleation (80–90%) and low levels of cell death (<10%) are achieved. These studies indicated CD34Neg cells as most significant early erythroid progenitor population (Van den Akker et al., 2010). Tirelli et al. also demonstrated significant enhancement (log scale) in the erythroid cell generation by using total MNC in Human Erythroid Massive Culture (HEMA) (Tirelli et al., 2011a). The careful phenotypes examination showed a significantly more erythropoietic potential of CD34neg population as compared to CD34+ cells (Tirelli et al., 2011a).
One of the major criteria for calculating efficiency of various ex vivo expansion protocols remains to describe the degree of enucleation of these maturing erythrocytic cells. The interactions with macrophages/stromal cells play a crucial role in developing high degree of enucleated erythrocytes as described through various researchers (Kawano et al., 2003). However, most of the stromal cells support used by various researchers are of animal origin and may conflict with the interests of the few clinicians to describe these cells as clinical grade blood products. These issues raised the interest of the research community to define methods without a need for animal based products. Miharada et al. who showed generation of 4.5 × 1012 erythroid cells from 5 × 106 CD34+ cells in the absence of any feeder cell layer or macrophage cells by using VEGF-1, TGF-1 and a glucocorticoid antagonist Mifepriostone to avoid dependence of these methods on feeder cells (Miharada et al., 2006). Maggakis-Kelemen et al. also reported similar results to improve the yield by using DMSO, Ferrous Citrate and Transferrin in the culture medium (Maggakis-Kelemen et al., 2003). Though these factors significantly increased the yield, but precursor cells shown higher degree of deformability and reticulocytes/erythrocytes were demonstrated to have reduced shear modulus. Beak et al. demonstrated the use of a polymer Poloxamer 188 to yield >95% enucleated RBCs in similar protocols without using any feeder cell support (Baek et al., 2009). Poloxamer 188 enhances the production by protecting cellular lysis during nucleation due to hydrodynamic stress. It provides support to the membranes and protects fragile cells from lysis at the time of enucleation. Thus, it seems that a large number of terminally matured clinical grade RBCs may be obtained regularly with the advent of similar protocol, while introducing newer alternatives at different levels of their expansion. Earlier, similar results were reported by Timmins et al. who demonstrated feeder free culture system by using a bioreactor and resulted in a massive 500 units (108 fold) per UCB donations (Timmins et al., 2011).
While considering these findings, it seems that both CB and PB derived CD34+ cells possess enormous expansion potential with some marginal differences such as the predominant form of Hb expressed and % yield of enucleated RBCs. For example, RBCs from CB derived cells are predominantly rich in fetal Hb (Neildez-Nguyen et al., 2002; Giarratana et al., 2005, 2011), whereas, RBCs from PB derived cells majorly express adult Hb (Giarratana et al., 2005, 2011). Apart from that, there have been a significant difference in the expansion potential of CB/PB derived HSPCs. With the current technologies available for their expansion PB derived cells have shown comparatively low (120,000 fold) yields (Giarratana et al., 2005). In contrast, CB derived cells are reported to expand up to 2 million folds. Even more, a single unit of CB derived cells can result into 500 units of transfusable RBCs (Timmins et al., 2011). However, the later method has not been applied for the PB derived cells so far. Thus, it seems that CB shall provide a better option for initial source to be selected under present circumstances, and, if presence of fetal Hb is acceptable (Conley et al., 1963; Weatherall and Clegg, 1975; Thomson et al., 1998; Foeken et al., 2010), it shall remain the most attractive alternate for RBCs expansion protocols. The infrastructure for CB collection is well developed. According to a report in 2010, about 4,50,000 CB units were collected through 131 CB Banks worldwide (Foeken et al., 2010). There is enough information available through these blood bank registries regarding the blood types and combinations of the antigen present on these blood groups for each CB donation. Though, the exact percentage for each rare blood type may not be available and even if the specialized donor could be found for each rare antigen combination, it may be difficult to fulfill a regular demand of transfusion in every 3–4 weeks due to the limited life span of these cells. However, even then a large fraction of the population may be benefitted from the existing technologies without any risk for rare antigen combinations.
Embryonic stem cells and induced pluripotent stem cells
Embryonic stem cells (Thomson et al., 1998) and induced pluripotent stem cells (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) are defined as immortal cells with the self-renewing capacity and if the requisite growth environment is made available have potential to differentiate into all kinds of cells of the organism. Embryonic stem cells were first isolated from 3–4 days old Blastocysts (Takahashi et al., 2007), and subsequent studies by various research groups described their capabilities to be maintained indefinitely in cultures due to high telomerase activities. Takahashi and Yamanaka's group firstly demonstrated iPSCs or ESC-like cells that may be generated by reprogramming somatic cells. They showed the transformation of murine fibroblast cells in to ESC by transfection of transcription factors such as POUSF-1, KLF-4, SOX-2,and Myc (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). These cells were further demonstrated to form teratoma on infusion in NOD-SCID mice and differentiation into cells from all the three germ cell lines respectively (Okita et al., 2007; Wernig et al., 2007). Generation of Human IPSCs (hips) was also defined by transformation of human neonatal or adult fibroblast by the same set of genes (Takahashi et al., 2007). Yu et al. also generated of hIPSCs from fibroblast cell by using lentivirus mediated transfection of Nanog and LIN28 to reduce the undesired effects of c-Myc (Yu et al., 2007). Subsequently, other methods of generating IPSCs have been reported such as ectopic expression of the same set of transcription factors (Stadtfeld et al., 2008) and use of excisable transposons (Lacoste et al., 2009). Alternatively, these factors could also be provided from outside attached to a cell penetrating peptide sequence (Zhou et al., 2009). Together these cells provide an alternate to generate HSPC. The mononuclear cells (CB/PB) may be reprogrammed in IPSCs and in subsequent procedure human IPSCs could be differentiated into CD34+CD45+ HSPC by using similar protocols used for ESCs (Ng et al., 2005; Choi et al., 2009).
There are a number of groups who have demonstrated hematopoietic differentiation of hESCs/IPSCs (Lu et al., 2008; Chang et al., 2011; Kobari et al., 2012) (Table 1). These methods can largely be grouped into two categories of (1) Embryoid body formation through suspension culture of hESCs without feeder cells, and (2) co culture of hESCs with murine stromal cell lines such as S17 or OP9 (Chadwick et al., 2003; Vodyanik et al., 2005, 2006; Chang et al., 2006). In the first approach, cells grow in suspension culture resulting in 3-dimensional structured aggregates of differentiating cells called Erythroid Bodies (EBs) (Vodyanik et al., 2005). Chang et al. described a method of generating erythroid cells from embryoid bodies (Vodyanik et al., 2006). Nakamura's group described a method for the generation of enucleated erythrocytes from mouse ESCs (Hiroyama et al., 2008) which was followed by several researchers who demonstrated procedures for the generation of enucleated erythrocytes by using human ESCs (Lu et al., 2008; Qiu et al., 2008; Lapillonne et al., 2010; Dias et al., 2011)
During last 10–11 years significant development has occurred in the procedure to develop hESCs/iPSCs and high yields of approximately up to 1011–1012 erythrocytic cells have been achieved from a single plate of hESCs (Lu et al., 2008). While comparing these reports, most procedures have indicated an important role of erythropoietin (along with other growth factors BMP-4, VEGF, bFGF etc.) and coculturing them with a feeder cell to derive sufficient yield of enucleated RBCs (Kaufman et al., 2001; Keller, 2005; Ng et al., 2005; Ledran et al., 2008; Zambidis et al., 2008). His can be a Co cultured with a number of feeder cells available, including mesenchymal cell Murine stromal cell line S17, OP9 (Vodyanik et al., 2005, 2006). YS endothelial cell lines (Kaufman et al., 2001), cell lines derived from murine AGM stroma, liver or other developmental niche (Ledran et al., 2008) (Murine AGM derived AM20-1B4 cells) are described as best supporting cells providing higher yields.
In order to achieve animal cell free clinical grade blood products efforts have been made by various researchers. It has been described that the use of feeder cell layer derived from human tissue itself e.g., human foreskin derived fibroblast cells support to generate similar results (Amit et al., 2003; Hovatta et al., 2003; Koivisto et al., 2004). Genbacey and his colleagues, has reported expansion of hESCs in serum free conditions by growing them on cells derived from human early gestation placental fibroblast cells or human placental laminin substrates (Genbacev et al., 2005). hESCs can also be grown in serum free and feeder free conditions by using fibronectin, TGF beta 1, bFGF, and leukemia inhibitory factor (Amit et al., 2003). Bouhasara's group demonstrated hESCs culture on hTERT cell line and their subsequent differentiation into erythrocytic cells in suspension culture method (Qiu et al., 2005; Olivier et al., 2006).
However, there have been ambiguities about the role of EPO/feeder layer played in erythrocytic enucleation; their presence in the final step of most protocols seems to remain inevitable. This is evident from the results obtained by growing these cells in the absence of both the above mentioned factors that result to meager 10% enucleating efficiency (Qiu et al., 2008; Dias et al., 2011). Whereas, inclusion of both EPO/feeder cells has been shown to yield remarkable up to 65% enucleation efficiency of these methods (Lu et al., 2008; Lapillonne et al., 2010). Similarly, IPSCS derived erythrocytes have been shown to depend on a feeder layer for their enucleation in final steps of maturation (Lapillonne et al., 2010; Chang et al., 2011; Dias et al., 2011; Kobari et al., 2012). Recently, heparin and insulin have been reported to enhance enucleation efficiency of IPSCs derived erythrocytes in a feeder free system, but that also remains only 26% (Kobari et al., 2012)
Another important factor is the expression of fetal Hb as a predominant form of protein by the erythrocytes generated in almost all these protocols (Lu et al., 2008; Lapillonne et al., 2010; Chang et al., 2011; Dias et al., 2011; Kobari et al., 2012). This is quite obvious because of the embryonic state of development of both ESCs/IPSc and a more primary route of hematopoietic development (indefinitive) likely to yield predominant expression of fetal/embryonic Hb through these protocols. However, researchers have demonstrated the possibilities of selective expression of adult Hb in erythrocytes by either increasing the time length for coculture (Qiu et al., 2008), or enforced transgene expression of RUNX1a to enhance hematopoiesis from human ESCs and iPSCs (Tirelli et al., 2011a) reporting a higher level of β - globin expression in differentiated erythrocytes (Ran et al., 2013).
So far, there has been no significant difference in the erythroid expansion potential of ESCs and IPSC lines (Dias et al., 2011). The findings reported by Douay's group about 7–8 fold higher erythrocytic expansion potential of human H1 ESC line in comparison to the human IPSCs line (IMR90-16) may be attributed to the viral vectors used for deriving IPSC that has been reported to decrease hematopoietic and erythrocytic expansion potential (Feng et al., 2010). Further, epigenetic variation (integration of viral genomes at different loci) associated with IPSCs generating methods could also contribute to these variations. Similarly, altered hematopoietic potential have been reported for various hESCs lines (Chang et al., 2008).
IPSCs may be advantageous in the conditions where no other alternate in present of generating rare blood group such as O−Rh− type of blood group. It has been postulated that only three selected human IPSC lines may be sufficient to generate required blood groups for almost 95.5% of patients in France (Peyrard et al., 2011). While, no such ESC line lines with universal blood donor group has been identified so far in the current list of NIH-approved-for-research (Lu et al., 2008). In addition, IPSCs can be helpful to support autologous transfusion which is highly recommended for alloimmune patients. On the other hand, it has promising reports to generate functional RBCs to cure rare hemoglobinopathies such as sickle cell anemia and β-Thalassemia (Hanna et al., 2007; Zou et al., 2011).
Erythroid precursor from stress erythropoiesis
In addition to the above mentioned stem cells, researchers have shown a significant RBCs generation potential in the extensively proliferating erythroid precursors which are developed due to stress conditions such as erythrolysis or hypoxia (Figure 3). The process, generally termed as “stress erythropoiesis,” relies upon glucocorticoids and their counter receptor interactions. There are reports showing up to 1010-fold expansion of CB-derived erythroid precursors using Dexamethasone as a glucocorticoid agonist along with other growth factors, e.g., SCF, EPO, and IGF-1 (Bauer et al., 1999; Von Lindern et al., 1999; Leberbauer et al., 2005). Similar results could be obtained for the PB derived erythroid precursors with the use of IL-3 along with above mentioned cytokines (Migliaccio et al., 2002). However, these approaches were reported to be of limited use due to their minimal fold expansion (<20 fold) which remain insufficient as per clinical transfusion is concerned (Migliaccio et al., 2010).
In contrast, there have been advancements in generating immortalized or extensively expanding erythroblast cell lines from embryonic stem cells with the ability to produce enucleated erythrocytes in mouse (Carotta et al., 2004; Hiroyama et al., 2008). Similar extensive expansion potential has been demonstrated by mouse embryos (England et al., 2011), which indicated a higher proliferation potential of embryonic erythroid progenitors than postnatal cells. It is hypothesized that if postnatal erythroid cells could be reprogrammed by using existing techniques to the state of embryonic erythroid progenitor cells they could also proliferate up to a similar extent. In fact, it has been demonstrated by Cheng's group (Huang et al., 2013), who has demonstrated immortalization of human CB-derived erythroblast by using similar reprogramming factors defined by Yamasaki's group (Takahashi and Yamanaka, 2006). The Author has demonstrated a significant 1068-fold expansion of CB-derived erythroblasts (in~12 months) in a serum-free culture. The ectopic expression of three genetic factors Sox2, c-Myc, and a shRNA against TP53 gene enabled these cells to undergo an expanded expansion in similar culture condition. These cells showed normal erythroblast phenotypes and morphology, a normal diploid karyotype and dependence on a specific combination of growth factors for proliferation throughout the expansion period. The coculture of these cells with irradiated mouse OP9 cell line in the presence of serum yield to 30% enucleation efficiency with increased fetal Hb expression level. Similarly, Kurita et al, demonstrated immortalization of human IPSC or CB derived cells by inducible expression of TAL-1 and HPV-16-E6/E7 viral gene (Kurita et al., 2013). Thus, it is expected that with more advancements in the reprogramming technology such as the use of nonviral episomal plasmids, synthetic RNA, and small molecules, development of more donor cell line would facilitate generation of all blood types (Chou et al., 2011; Hou et al., 2013; Yoshioka et al., 2013).
Ex-vivo culturing of other blood components
Platelets
Platelets are small anuleated, discoid shaped, 1–2 μm cell fragments that play essential role in the maintenance of homeostasis, stopping bleeding, or wound healing (thrombosis), inflammation, and innate immunity. There is a burgeoning use of plasma transfusions to control massive bleeding in clinics. The combination of red blood cells, platelet concentrates, and plasma is used for the same Platelets are present in much higher densities in vertebrate blood (5.5 platelets × 1010/unit) that are generated from megakaryocytes and their precursor cells in BM (Machlus and Italiano, 2013).
Platelets are generated from megakaryocytic cells in the BM niche under the regulation of various growth factors. Unlike RBCs, committed HPCs can be easily recruited into megakaryocytic lineage differentiation pathways by their induction through a single growth factor TPO. This straight forward mechanism has tempted many researchers to mimic similar differentiation under research settings. Like RBCs, there have been efforts to generate platelets from all different sources, including CD34+ HSPC., ESCs/iPSCs and immature megakaryotic precursor cells. Christian et al. have demonstrated the generation of platelet precursor cells from human CD34+ cells by using SCF, TPO, IL-3/IL-11, and Fetal Liver Tyrosine kinase-3 ligand (Flt-3L) in the liquid culture system (Christian et al., 2010). However, these precursors were shown to undergo recirculation in hematopoietic tissues on transfusion in mice. Proulx et al. demonstrated ~300 fold expansion of MK/CD34+ by growing UCB derived CD34+ cells with SCF, Flt-3L, TPO, and IL-6 at high concentrations (Proulx et al., 2003, 2004). Growth of MK/CD34+ may further be improved by optimizing culture conditions such as temp (39°C), O2 tension and addition of additional factors such as SDF-1α and Nicotinamide (Guerriero et al., 2001; Mostafa et al., 2001; Cortin et al., 2005; Giamniona et al., 2006). Matsunaga et al. (2006), demonstrated generation of 3.4 unit platelets from UCB-CD34+ cells by using hTERT mesenchymal cell coculture method in 33 days (Matsunaga et al., 2006). Sullenberger, also reported a 3D cartridge based perfusion bioreactor with the capacity of continuously producing platelets over a period of 30 days (Sullenharger et al., 2009). Moreover, the efforts described so far for producing platelets from UCB derived cells remain largely insufficient to fulfill even a fraction of global demand.
Recent advancements have demonstrated enormous in vitro platelets generation potential in pluripotent stem cells (including iPSCs/ESCs), and a comparatively new type of cells called induced MKs (iMKs) (Masuda et al., 2013). Matsubara et al. showed a direct conversion of fibroblasts (mouse/human) into megakaryocytes (MKs) by using three factors p45NF-E2, Maf G, and Maf K, which subsequently can release platelets (Ono et al., 2012). The authors identified significance of these factors in generating abundant MKs and platelets from human subcutaneous adipose tissues (Matsubara et al., 2009, 2010; Ono et al., 2012).
In another approach, ESCs/IPSC mediated generation of platelets in humans has been reported (Takayama et al., 2008, 2010). Eto et al. showed that c-Myc expression (essential for reprogramming of IPSCs) must be reactivated transiently and then shut-off (Takayama et al., 2010) and to ensure efficient platelet production from human IPSCs. This transient expression might be essential because continuous excessive c-Myc expression in IPSCs derived MKs was shown to increase p14 (ARF) and p16 (INK4A) expression, and decreased GATA1 and NF-E2 expression, which eventually resulted MK senescence and apoptosis, as well as impaired production of functional platelets (Takayama et al., 2010).
More recently, Eto and colleagues have established an immortalized MK cell line (MKCL) derived from human IPSCs (Masuda et al., 2013). The immortalized MKCL could potentially provide a stable supply of high quality platelets for transfusion medicine. However, this method remains elusive and a detailed protocol suitable for commercial production is yet to come.
Thus, recent findings indicate strong possibilities of a regular non donor derived platelet supply in near future. However, there are several factors that shall be discussed to compare existing approaches. While comparing with IPSCs/ESCs based methods, the iMK cells (derived in these studies) are rapidly converted (2 weeks) into MKs cells, but yield remain poor as fewer platelets (5–10 platelets per iMK cell was generated. In contrast, single MKs may give rise to up to 2000 platelets under in vivo conditions. Hence, one of the important factors shall remain to be discussed is the total number of cells that can be obtained from each cultural setting. For example, Matsubara et al reported generation of 8–10 × 105 iMKs from 20 × 106 Human Derived Fibroblasts (HDFs) (Ono et al., 2012). HDFs are easily expanded following to their initiation of direct conversion with limited number of transfected cells, whereas, 200–300 IPS clones from 105 HDFs were reported by Eto and colleagues in their studies. Further, 1 × 105 cultured IPSCs gave rise to 17 × 105 MKs (Takayama et al., 2010). Thus, these results indicated low platelet production efficiency per MK in both strategies.
Another important issue is the time taken for the production of MKs and platelets through these protocols. iMKs and iPSC-mediated MKs are induced in approximately 2 weeks and 2 months, respectively. However, it is yet to be established, but MKCLs may be a much faster approach to generate platelet sources for platelet production due to their direct expandability into MKs.
Further IPSC-mediated MKs are not completely free from the possible risk of some residual undifferentiated cells However, a possible approach may be to avoid transfusion of MKs while preferring fully matured platelets derived from these methods. Platelets (anucleated cells) may be irradiated before transfusion and thus may become free of any contamination of residual undifferentiated cells.
IPSCs and MKCLs are immortal cell lines and thus are comparatively easy to freeze down. Similarly, HDFs are also suitable for cryopreservationm from where they can be taken out and used as starting materials for iMK induction. HDFs in comparison to platelets show better suitability for cryopreservation. The functionality of iMKs has been assessed through their infusion into irradiated immunodeficient NOD/Shi-scid/IL-2Rγnull (NOG) mice showing normal release of human platelets in vivo (5–10 platelets per infused IMK) cells and equal capabilities of forming a thrombus in ex vivo conditions formation under flow condition (Ono et al., 2012).
The iMKs are reported very recently and the early outcomes indicate them as a valuable option for transfusion medicine. However, there are some important issue which shall be considered in much detail such as the molecular mechanism by which iMKs are induced through the p45NF-E2 transcriptional complex, and identification of most suitable cells for direct conversion into iMKs (or for establishment of MKCLs).
One of the important issues to be discussed here is to explore the necessity of generating autologous iMKs through direct conversion for clinical transfusion. Under most clinical settings, platelet transfusion is done without any prior matching of human leukocyte antigen (HLA) between the donor and recipient. However, in rare conditions repeated platelet transfusions can elicit undesired production of anti-HLA antibodies against transfused platelets leading to an inevitable demand to identify HLA-matched platelet donor (Stroncek and Rebulla, 2007). A possible alternate may be provided by using autologous iMKs based strategies. In addition, establishment of iPSC banks shall be an alternative approach to deal with this problem. These banks shall store homozygous HLA-typed iPSCs which would be deposited for HLA-matched tissue transplantation (Taylor et al., 2012). It has been demonstrated that a tissue bank from 150 selected homozygous HLA-typed volunteers could match 93% of the UK population (Taylor et al., 2012). Yamanaka et al has also postulated that 140 unique HLA homozygous donors shall remain sufficient for 90% of the Japanese population (Okita et al., 2011).
Neutrophils
Neutrophils play important role in cellular immunity, but unlike RBCs and Platelets they are not routinely collected in clinics. Neutrophils are need to be transfused in relatively large amount, i.e., >1010 calls every day in neutropenic patients. Ex vivo expansion of neutrophils has been reported by some groups showing ~400 fold expansion from single units of UCB-CD34+ cells (5 × 106 CD34+ cells), which equivalents to a mere 20% of the required daily dose. Although, sufficient cells may be produced by using PB mobilized by cytokines as demonstrated by Dick et al. (2008), who showed ~534 × fold (equivalent to 10 neutrophil units) expansion on ex vivo culture of these cells. But this would mean one donor per recipient and supply constraints largely remain unsolved. Similar efforts to generate neutrophils from hESCs have also resulted in very low yields (Saeki et al., 2008). These reports indicated the need for developing more efficient and simple procedures to be developed first for the generation of neutrophils from either UCB-CD34+, BM derived cells and/or houses before their commercial manufacturing could be planned.
Factors regulating the fate of commercial blood manufacture and future prospects
Source material suitability and availability
Scientists have been trying to develop a consensus on the different variables controlling the fate of ex vivo RBCs expansion and clinical use. The most important of them is the source materials for various methods being explored to expand RBCs in large scale. Ideally, a source material should be a discarded material so that no extra cost would be aided in the commercial production process. At the same time, if a regular supply chain of ex vivo manufactured blood has to be maintained the source material would also be required on a regular basis i.e., unlimited availability of the source material should be ensured. The most important factor is the capability of the source cells to get developed into the finally matured RBCs cells with absolute efficiency without raising any immune response in the host i.e., it should be non-immunogenic. The criterion fits well with umbilical CB, which is available abundantly and is a waste product in all the maternity hospitals. In fact, a significant number of transfusion can be made for the patients who are suffering from rare hemoglobinopathies (~ 1%) by using ex vivo cultivated RBCs units that might be obtained from these sub threshold units of CB (>90 ml) discarded regularly. Each discarded unit of CB may generate 10–75 RBCs products which might be useful in transfusions for rare hemoglobinopathies (Peyrard et al., 2011). Similarly, Leukopheresis is routinely done in clinical centers where transfusions are made to treat various leukemia patients. In these protocols the leuko-reduction buffy coat is produced as a byproduct and this contains significant amounts of RBCs producing cells (equivalent to umbilical CB) (Zhou et al., 2009). The proof-of-the concept that these ex-vivo cultivated cells may be of clinical use was provided by demonstrating successful transfusion of CD34+ cells which were obtained from a G-CSF mobilized donor (Migliaccio et al., 2010). Apart from UCB derived CD34+ cells, ESCs, and hiPSCs as described in previous sections might serve as a potential source for regular manufacturing processes with the advancement in technologies.
Quantitative issues
The most frequently transfused blood component, RBCs, are present in 2 × 1012 cells/unit (200 ml of blood) with an expected cell density of 3 × 106 cells/ml. According to the WHO data >100 million units of blood is collected every year worldwide. In order to replace this huge volume with synthetic blood/blood components, an extremely large amount of ingredients along with skilled manpower would be an inevitable requirement. It is hypothesized that a cell density of 5 × 107 cells/ml would be essential to produce regularly to support this much demand. Further, according to an assumption, manufacturing of a single unit of blood by using present static culture methods, would require 660 liters of culturing medium and 9500 lab scale 175 cm2 culturing flasks (70 ml medium/flask) (Timmins and Nielsen, 2009; Zeuner et al., 2012).
Ensuring such a high concentration of RBCs would attract development of more automated methods like bioreactors that might be helpful in reducing the culturing assets and associated labor. Fortunately a wealth of information regarding the use of various types of bioreactors for HSCs expansion has been accumulating during the last two decades. This could help researchers achieve required cell density. It was reported by Timmins et al that similar density of RBCs as in static culture can be produced in a wave bioreactor (Timmins et al., 2011). A maximum cell density of 107 cells/ml was also reported in a stirred small scale bioreactor (Ratcliffe et al., 2012). Housler et al, reported a massive yield of 2 × 108 cells/ml in a hollow fiber bioreactor (Housler et al., 2012). These 3D bioreactors can be scaled up with current design to produce 1–2 units of RBCs in 3–4 weeks of time. These reports indicate possibilities of ensuring a regular production of large amounts of RBCs, however, a number of factors such as degree of cell maturation and various cultural parameters are yet to be defined in a more elaborated manner.
Further, an important achievement could be the development of methods to commit the source material directly into the erythropoietic lineages reducing both the time and cost of production. There are few preliminary reports about various efforts to define alternate sources such as (i) generation of erythropoietic cell lines from ESCs/IPSCc (i) reprogramming any somatic cells directly into erythroblasts by passing the pluripotent state through over expression of suitable genes.
This has been reported for megakaryocytic differentiation also where human fibroblast cells are directed to differentiate directly into megakaryocytic cells through over expression of p45NF and E2/MaF transcription factors (England et al., 2011; Wang et al., 2011). Cheng's group reported in humans the differentiated erythroid cells obtained from human embryo might be used as a potential initial source for RBCs production (Huang et al., 2013; Kurita et al., 2013). Thus, it is conceivable that with more advancements in the culture methods and initial source material a significant amount of RBCs could be produced for limited clinical uses.
Growth factors
Growth factors used in various phases of ex vivo culturing should also be explored to regulate the fate of commercial manufacturing. Both the “concentration” and “time” of their administration would play an important role in the optimization of the expansion process. It is also to be noticed that there are various growth factors which express gene polymorphism, for example, >260 isoforms of human glucocorticoids receptors are expressed which are likely to differ in their potency and effects. In order to secure a large scale production of RBCs, it is conceivable to determine an in depth knowledge about the functioning and efficiency of various isoforms through high throughput screening methods regularly. A good example might be the use of reverse phase proteomic analysis of CD34+ cells to define molecular mechanism of signaling pathways activated during erythroid differentiation. Recently, similar studies have been reported by various groups showing activation of different set of gene(s) in CD34+ cells and erythroblasts. Genes that are involved in the inhibition of apoptosis, transcription, and proliferation are more active in CD34+ cells, whereas erythroblasts predominantly express those genes which play important roles in regulation of cell cycle, transcription and translation (Ratcliffe et al., 2012). This information may be of use if specific pharmacological agents enhancing erythrocytic pathways and blocking apoptotic pathways may be introduced at various phases of ex vivo expansion.
Biophysical and biochemical parameters
Generation of clinical grade RBCs would require development of stringent quality checks for ex vivo expanded RBCs. The major parameters would include determination of antigenic profiles, hemoglobin contents and physical progenies derived from these culture processes. As described by various laboratories ex vivo generated RBCs are slightly macrocytic but normal in size. These RBCs also expresses a great level of α-hemoglobin stabilizing proteins (AHSP), BCL11A and globin gene with great donor to donor heterogeneity (Keel et al., 2008). It is also reported that ex vivo generated erythroblasts contain γ-globin in a slightly greater amount in comparison to RBCS which are generated in vivo (0.12–0.20 pg/cell with respect to total protein content of 18.723.7 per cell) (Lu et al., 2008).
Antigenic profile
Surface antigens are vital for the function of RBCs and a large number of surface antigen have been identified on the surface of mature RBCs. Ex vivo generated RBCs are reported to express normal level of antigens present on both the Ankyrin A (GPA, M/N, Ena FS, RhAO, and band 3/4.1 R) and Glycophorin B complex, the urea transporter, the complement receptor, and receptors that protect RBCs from complement mediated lysis Recent reports about the surface antigenic analysis of the ex vivo generated RBCs ensured that appropriate level of surface antigen such as band 3 and Rh antigen is assured at the beginning of the maturation process (Satchwell et al., 2011).
Ethnicity
Recently, there have been some reports demonstrating effects of ethnicity on the source material used for ex vivo expansion. African-American donor derived MNC were found to be found to be more useful as per as fold expansion is concerned since they could be expanded up to 14–40 fold in comparison to the Caucasian population derived MNCs which give rise to 1.7–30 fold expansion (Tirelli et al., 2011b; Carilli et al., 2012)
Enucleation of RBCs
Since, Ex vivo expanded RBCs shall essentially be evaluated for their degree of terminal maturation in all the production units. Hence, high level of enucleation of maturing erythroblast is an inevitable requirement. Erythroblasts derived from ESCs are demonstrated to yield >60% enucleated RBCs (Lu et al., 2008) while the nucleation efficiency of maturing erythroblast from ups could be demonstrated to a mere 4–10% of RBCs produced (Lapillonne et al., 2010). Recently, there have been efforts to define the factors regulating enucleation efficiency of RBCs indicating the significance of proteins such as Huston deacetylase (specifically HDAC-2 isoform) which are required for chromatin condensation and enucleation in mice fetal erythroblast (Chang et al., 2011). Since, glucocorticoids play important negative regulators for these proteins as mentioned above, application of molecular agents which specifically activates HDACs could be employed in phase-3 cultures to enhance the enucleation yields. As mentioned in a number of reports, co -culturing of maturating erythroblast along with stromal cells may enhance their terminal maturation, but on the other hand it might impose an additional risk of poorly defined potentially immunogenic and/or infectious agent which may compromise the production of GMP for RBCs in the developmental process. These issues indicate the need for the development of biochemical alternatives to avoid the use of stromal co-cultures in these protocols. There are preliminary reports on the use of drugs like Mifepristone and plasmanate which are defined to have similar effects on promoting enucleation efficiency during ex vivo expansion of RBCs (Miharada et al., 2006). In addition, factors promoting the functioning of proteins involved in vesicle trafficking such as “vaccuoline” may also be used to improve the enucleation efficiency if phase-3 cultures (Keerthivasan et al., 2010).
Production cost
The regular commercial production of RBCs would be heavily affected by their cost of production. According to a hypothetical estimation, producing RBCs in desired range would cost approximately $8000–15000 per unit (Timmins and Nielsen, 2009; Zeuner et al., 2012). Whereas, the hospital cost for leuco-reduced RBCs is only $225 that is significantly lower in comparison to synthetic product. There are situations such as matching of rare phenotypes of RBCs and the present cost of a phenotypically matched unit of RBCs is ~$700–1200. Producing blood on a commercial scale would require a huge monetary investment and it will be difficult to keep the momentum in the direction of R&D for developing more and more efficient protocols due to lack of money especially in low income grade countries. It would be important to find out immediate goals which can be achieved from the outcomes of the existing research findings to generate alternate sources of money. For example, immediate use of these small scale products may be useful in developing various immediately achievable goals. There are few promising examples, such as the use of ex vivo cultured cells for Reagent RBCs diagnostics in specifically alloimmunized patients and they can be used as drug delivery vehicles for personalized therapies. The requirement of relatively small no. of RBCs in these methods makes them most suitable for these kinds of assays, for example, ~2 × 108 RBCs will be sufficient to perform 100× s of these assays in clinical settings and with the present methodologies 109–1010 RBCs can be expanded regularly.
Summary
In brief, it seems feasible that commercial production of RBCs may take place in near future. There are enough technological evidences available supporting the concept and with the advent of newer source materials, optimal methods for ex vivo cultivation of RBCs and highly stringent, comprehensive quality control, analytical measures, the regular production of blood may take place to support a smaller fraction of the population at least. All the three major sources of like CD34+− HSCs (UCB, PB, and BM derived cells), hESCs and, iPSCs have enough potential of generating huge amounts of RBCs and if more sophisticated methods are generated such as development of erythropoietic hESC cell lines, it would be possible to generate an unlimited source of initial source material for the production measures.
Moreover, with the development of more in depth knowledge about the molecular mechanisms involved in various stages of ex vivo erythropoiesis. It would become more efficient to support even larger population's blood demands. The fundamental information shall also be helpful in developing pharmacological alternates for the various stages of erythropoietic development which presently depend upon their interactions with feeder cell support systems in most of the protocols. Development of pharmacological alternates is essential to simplify the production processes for clinical grade blood. Avoiding the need of feeder cells may also help in reducing the unexpected risks associated with their use due to undefined immunogenic and infectious exposures which are likely imposed to the recipients by animal derived feeder cell support methods. The omission of feeder cells from ex vivo RBCs expansion protocols would also simplify the commercial scale production methods and may also be helpful in reducing the cost of production. As per as cost is concerned, a small fraction of the population may be capable of bearing the cost of commercially produced blood in economically developed countries. However, more liberal government policies such as lowering both the sale and services taxes, provisions for subsidized input materials (like electricity, water, etc.), direct subsidy offered for the products may be helpful in supporting the low cost production of blood. Besides that, a small amount of remuneration may also be generated from the various smaller/specific products such as reagent RBCs, RBCs for the specific drug delivery targets to cure rare hemoglobinopathies and that might support the ongoing research and development in the same direction.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Honorable Chairman and the Honorable Vice-Chancellor of the Delhi Technological University, Shahbad Daulatput, Bawana road, Delhi-42, for support. Dr. Vimal K. Singh particularly thanks the Department of Science and Technology and Indian National Science Academy (INSA), INDIA, for the research grant.
References
- Akashi K., Traver D., Miyamoto T., Weissman I. L. (2000). A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197. 10.1038/35004599 [DOI] [PubMed] [Google Scholar]
- Ali A., Auvinen M. K., Rautonen J. (2010). The aging population poses a global challenge for blood services. Transfusion 50, 584–588. 10.1111/j.1537-2995.2009.02490.x [DOI] [PubMed] [Google Scholar]
- Alter H. J., Klein H. G. (2008). The hazards of blood transfusion in historical perspective. Blood 112, 2617–2626. 10.1182/blood-2008-07-077370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit M., Margulets V., Segev H., Shariki K., Laevsky I., Coleman R., et al. (2003). Human feeder layers for human embryonic stem cells. Biol. Reprod. 68, 2150–2156. 10.1095/biolreprod.102.012583 [DOI] [PubMed] [Google Scholar]
- Baek E. J., Kim H. S., Kim J. H., Kim N. J., Kim H. O. (2009). Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion 49, 2285–2295. 10.1111/j.1537-2995.2009.02303.x [DOI] [PubMed] [Google Scholar]
- Baek E. J., Kim H. S., Kim S., Jin H., Choi T. Y., Kim H. O. (2008). In vitro clinical grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion 48, 2235–2245. 10.1111/j.1537-2995.2008.01828.x [DOI] [PubMed] [Google Scholar]
- Bagnis C., Chiaroni J., Bailly P. (2011). Elimination of blood group antigens: Hope and reality. Br. J. Haematol. 152, 392–400. 10.1111/j.1365-2141.2010.08561.x [DOI] [PubMed] [Google Scholar]
- Baron M. H., Fraser S. T. (2005). The specification of early hematopoiesis in the mammal. Curr. Opin. Hematol. 12, 217–221. 10.1097/01.moh.0000163217.14462.58 [DOI] [PubMed] [Google Scholar]
- Baron M. H., Isern J., Fraser S. T. (2012). The embryonic origins of erythropoiesis in mammals. Blood 119, 4828–4837. 10.1182/blood-2012-01-153486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Tronche F., Wessely O., Kellendonk C., Reichardt H. M., Steinlein P., et al. (1999). The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 13, 2996–3002. 10.1101/gad.13.22.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis M., Mize C., Prenant M. (1978). Erythropoiesis: comparison of in vivo and in vitro amplification. Blood. Cells 4, 155–174. [PubMed] [Google Scholar]
- Carilli A. R., Sugrue M. W., Rosenau E. H., Chang M., Fisk D., Medei-Hill M., et al. (2012). African American adult apheresis donors respond to granulocyte-colony-stimulatiflg factor with neutrophil and progenitor cell yields comparable to those of Cauc asian and Hispanic donors. Transfusion 52, 166–172. 10.1111/j.1537-2995.2011.03253.x [DOI] [PubMed] [Google Scholar]
- Carmelo C. S., Mario C., Paolo D. F., Armando D. V., Lessandro M. G., Francesco L., et al. (1995). CD34-positive cells: biology and clinical relevance Haematologica 80, 367–387. [PubMed] [Google Scholar]
- Carotta S., Pilat S., Mairhofer A., Schmidt U., Dolznig H., Steinlein P., et al. (2004). Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood 104, 1873–1880. 10.1182/blood-2004-02-0570 [DOI] [PubMed] [Google Scholar]
- Castro C. I., Briceno J. C. (2010). Perfluorocarbonbased oxygen carriers: Review of products and trials. Artif. Organs 34, 622–634. 10.1111/j.1525-1594.2009.00944.x [DOI] [PubMed] [Google Scholar]
- Chadwick K., Wang L., Li L., Menendez P., Murdoch P., Rouleau A., et al. (2003). Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood 102, 906–915. 10.1182/blood-2003-03-0832 [DOI] [PubMed] [Google Scholar]
- Chang C. J., Mitra K., Koya M., Velho M., Desprat R., Lenz J., et al. (2011). Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS ONE 6:10. 10.1371/journal.pone.0025761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Nelson A. M., Cao H., Wang L., Nakamoto B., Ware C. B., et al. (2006). Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood 108, 1515–1523. 10.1182/blood-2005-11-011874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Nelson A. M., Fields P. A., Hesson J. L., Ulyanova T., Cao H., et al. (2008). Diverse hematopoietic potentials of five human embryonic stem cell lines. Exp. Cell. Res. 314, 2930–2940. 10.1016/j.yexcr.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. D., Yu J., Smuga-Otto K., Salvagiotto G., Rehrauer W., Vodyanik M., et al. (2009). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27, 559–567. 10.1634/stemcells.2008-0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou B. K., Mali P., Huang X., Ye Z., Dowey S. N., Resar L. M., et al. (2011). Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 21, 518–529. 10.1038/cr.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian H., Rüster B., Seifried E., Henschler R. (2010). Platelet precursor cells can be generated from cultured human CD34+ progenitor cells but display recirculation into hematopoietic tissue upon transfusion in mice. Transfus. Med. Hemother. 37, 185–190. 10.1159/000316975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley C. L., Weatherall D. J., Richardson S. N., Shepard M. K., Charache S. (1963). Hereditary persistence of fetal hemoglobin: a study of 79 affected persons in 15 Negro families in Baltimore. Blood 21, 261–281. [PubMed] [Google Scholar]
- Cortin V., Garnier A., Pineault N., Lemieux R., Boyer L., Proulx C. (2005). Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp. Hematol. 33, 1182–1191. 10.1016/j.exphem.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Daniels G., Castilho L., Flegel W. A., Fletcher A., Garratty G., Levene C., et al. (2009). International society of blood transfusion committee on terminology for red blood cell surface antigens: macao report. Vox. Sang. 96, 153–156. 10.1111/j.1423-0410.2008.01133.x [DOI] [PubMed] [Google Scholar]
- Debili N., Coulombel L., Croisille L., Katz A., Guichardv J., Breton-Gorius J., et al. (1996). Characterization of a bipotent erythro megakaryocyticprogenitor in human bone marrow. Blood 88, 1284–1296. [PubMed] [Google Scholar]
- Department of Health and Human Services. (2010). The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: DHHS. Available online at: http://www.aabb.org/programs/biovigilance/nbcus/Documents/09-nbcus-report.pdf (Accessed May 4, 2014). [Google Scholar]
- Department of Health and Human Services. (2013). The 2011 National Blood Collection and Utilization Survey Report. Washington, DC: DHHS. Available online at: http://www.aabb.org/programs/biovigilance/nbcus/Documents/11-nbcus-report.pdf (Accessed May 4, 2014). [Google Scholar]
- Dias J., Gumenyuk M., Kang H., Vodyanik M., Yu J., Thomson J. A., et al. (2011). Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 20, 1639–1647. 10.1089/scd.2011.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick E. P., Rebecca L., Sabroe P. I. (2008). Ex vivo-expanded bone marrow cd34+ derived neutrophils have limited bactericidal ability. Stem Cells 26, 2552–2563. 10.1634/stemcells.2008-0328 [DOI] [PubMed] [Google Scholar]
- Douay L. (2001). Experimental culture conditions are critical for ex vivo expansion of hematopoietic cells. J. Hematother. Stem. Cell. Res. 10, 341–346. 10.1089/152581601750288948 [DOI] [PubMed] [Google Scholar]
- Douay L., Andreu G. (2007). Ex vivo production of human red blood cells from hematopoietic stem cells: what is the future in transfusion? Transfus. Med. Rev. 21, 91–100. 10.1016/j.tmrv.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Douay L., Giarratana M. C. (2009). Ex vivo production of human red blood cells: a newadvance in stem cell engineering. Methods Mol. Biol. 482, 127–140. 10.1007/978-1-59745-060-7_8 [DOI] [PubMed] [Google Scholar]
- Douay L., Lapillonne H., Turhan A. G. (2009). Stem cells-A source of adult red blood cells for transfusion purposes: present and future. Crit. Care Clinics 25, 383–398. 10.1016/j.ccc.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Ende M., Ende N. (1972). Hematopoietic transplantation by means of fetal (cord) blood. A new method. Va. Med. Mon. 99, 276–280. [PubMed] [Google Scholar]
- England S. J., McGrath K. E., Frame J. M., Palis J. (2011). Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood 117, 2708–2717. 10.1182/blood-2010-07-299743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Lu S. J., Klimanskaya I., Gomes I., Kim D., Chung Y., et al. (2010). Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28, 704–712. 10.1002/stem.321 [DOI] [PubMed] [Google Scholar]
- Fibach E., Manor D., Oppenheim A., Rachmilewitz E. A. (1989). Proliferation and maturation of human erythroid progenitors in liquid culture. Blood 73, 100–103. [PubMed] [Google Scholar]
- Fibach E., Manor D., Oppenheim A., Rachmilewitz E. A. (1991). Growth of human normal erythroid progenitors in liquid culture: a comparison with colony growth in semisolid culture. Int. J. Cell. Cloning 9, 57–64 10.1002/stem.5530090108 [DOI] [PubMed] [Google Scholar]
- Foeken L. M., Green A., Hurley C. K., Marry E., Wiegand T., Oudshoorn M. (2010). Monitoring the international use of unrelated donors for transplantation: The WMDA annual reports. Bone Marrow Transplant. 45, 811–818. 10.1038/bmt.2010.9 [DOI] [PubMed] [Google Scholar]
- Freyssinier J. M., Lecoq-Lafon C., Amsellem S., Picard F., Ducrocq R., Mayeux P., et al. (1999). Purification, amplification and characterization of a population of human erythroid progenitors. J. Haematol. 106, 912–922. 10.1046/j.1365-2141.1999.01639.x [DOI] [PubMed] [Google Scholar]
- Fujimi A., Matsunaga T., Kobune M., Kawano Y., Nagaya T., Tanaka I., et al. (2008). Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int. J. Hematol. 87, 339–350. 10.1007/s12185-008-0062-y [DOI] [PubMed] [Google Scholar]
- Genbacev O., Krtolica A., Zdravkovic T., Brunette E., Powell S., Nath A., et al. (2005). Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 83, 1517–1529. 10.1016/j.fertnstert.2005.01.086 [DOI] [PubMed] [Google Scholar]
- Giamniona L. M., Fuhrken P. G., Papoustsakis E. T., Miller W. M. (2006). Nicotinamide (vitamin B3) increases the polyploidisation and propletelet formation of cultured primary human megakaryocytes. Br. J. Haemotol. 135, 554–566. 10.1111/j.1365-2141.2006.06341.x [DOI] [PubMed] [Google Scholar]
- Giarratana M. C., Kobari L., Lapillonne H., Chalmers D., Kiger L., Cynober T., et al. (2005). Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat. Biotechnol. 23, 69–74. 10.1038/nbt1047 [DOI] [PubMed] [Google Scholar]
- Giarratana M. C., Rouard H., Dumont A., Kiger L., Safeukui I., Le Pennec P. Y., et al. (2011). Proof of principle for transfusion of in vitro-generated red blood cells. Blood 118, 5071–5079. 10.1182/blood-2011-06-362038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford S. C., Derganc J., Shevkoplyas S. S., Yoshida T., Bitensky M. W. (2006). A detailed study of time-dependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br. J. Haematol. 135, 395–404. 10.1111/j.1365-2141.2006.06279.x [DOI] [PubMed] [Google Scholar]
- Guerriero R., Mattia G., Testa U., Chelucci C., Macioce G., Casella I., et al. (2001). Stromal cell-derived factor in increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood 97, 2587–2595. 10.1182/blood.V97.9.2587 [DOI] [PubMed] [Google Scholar]
- Hanna J., Wernig M., Markoulaki S., Sun C. W., Meissner A., Cassady J. P., et al. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1923. 10.1126/science.1152092 [DOI] [PubMed] [Google Scholar]
- Heath D. S., Axelrad A. A., McLeod D. L., Shreeve M. M. (1976). Separation of the erythropoietin-responsive progenitors BFU–E and CFU–E in mouse bone marrow by unit gravity sedimentation. Blood 47, 777–792. [PubMed] [Google Scholar]
- Henkel-Honke T., Oleck M. (2007). Artificial oxygen carriers: a current review. AANA. J. 75, 205–211. [PubMed] [Google Scholar]
- Hiroyama T., Miharada K., Sudo K., Danjo I., Aoki N., Nakamura Y. (2008). Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS ONE 6:3. 10.1371/journal.pone.0001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., et al. (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341, 651–654. 10.1126/science.1239278 [DOI] [PubMed] [Google Scholar]
- Housler G. J., Miki T., Schmelzer E., Pekor C., Zhang X., Kang L., et al. (2012). Compartmental hollow fiber capillary membrane-based bioreactor technology for in vitro studies on red blood cell lineage direction of hematopoietic stem cells. Tissue Eng. Part. C Methods 18, 133–142. 10.1089/ten.tec.2011.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O., Mikkola M., Gertow K., Stromberg A. M., Inzunza J., Hreinsson J., et al. (2003). A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 18, 1–6. 10.1093/humrep/deg290 [DOI] [PubMed] [Google Scholar]
- Huang X., Shah S., Wang J., Ye Z., Dowey S. N., Tsang K. M., et al. (2013). Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol. Ther. 22, 451–463. 10.1038/mt.2013.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Jianhua Z., Yelena G., Huihui L., Fumin X., Lucia D. F., et al. (2013). An: quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood 121, e43–e49. 10.1182/blood-2012-09-456079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. S., Hanson E. T., Lewis R. L., Auerbach R., Thomson J. A. (2001). Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 98, 10716–10721. 10.1073/pnas.191362598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Kobune M., Yamaguchi M., Nakamura K., Ito Y., Sasaki K., et al. (2003). Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood 101, 532–540. 10.1182/blood-2002-04-1268 [DOI] [PubMed] [Google Scholar]
- Keel S. B., Doty R. T., Yang Z., Quigley J. G., Chen J., Knoblaugh S., et al. (2008). A heme export protein is required for red blood cell differentiation and iron homeosiasis. Science 319, 825–828. 10.1126/science.1151133 [DOI] [PubMed] [Google Scholar]
- Keerthivasan G., Small S., Liu H., Wickrema A., Crispino J. D. (2010). Vesicle trafficking plays a novel role in erythroblast enucleation. Blood 116, 3331–3340. 10.1182/blood-2010-03-277426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. (2005). Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155. 10.1101/gad.1303605 [DOI] [PubMed] [Google Scholar]
- Kobari L., Yates F., Oudrhiri N., Francina A., Kiger L., Mazurier C., et al. (2012). Human induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 97, 1795–1803. 10.3324/haematol.2011.055566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto H., Hyvärinen M., Strömberg A. M., Inzunza J., Matilainen E., Mikkola M., et al. (2004). Cultures of human embryonic stem cells: serum replacement medium or serum-containing media and the effect of basic fibroblast growth factor. Reprod. Biomed. Online 9, 330–337. 10.1016/S1472-6483(10)62150-5 [DOI] [PubMed] [Google Scholar]
- Kurita R., Suda N., Sudo K., Miharada K., Hiroyama T., Miyoshi H., et al. (2013). Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE 8:3 10.1371/journal.pone.0059890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste A., Berenshteyn F., Brivanlou A. H. (2009). An efficient and reversible transposable system for gene delivery and lineage-specific differentiation in human embryonic stem cells. Cell Stem Cell 5, 332–342. 10.1016/j.stem.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Lapillonne H., Kobari L., Mazurier C., Tropel P., Giarratana M. C., Cleon I. Z., et al. (2010). Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica 95, 1651–1659. 10.3324/haematol.2010.023556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberbauer C., Boulme F., Unfried G., Huber J., Beug H., Mullner E. W. (2005). Different steroids coregulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105, 85–94. 10.1182/blood-2004-03-1002 [DOI] [PubMed] [Google Scholar]
- Ledran M. H., Krassowska A., Armstrong L., Dimmick I., Renstrom J., Lang R., et al. (2008). Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3, 85–98. 10.1016/j.stem.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Beutler E., Kipps T. J., Kaushansky K., Seligsohn U., Prchal J. (2007). Williams Hematology. New York, NY: McGraw-Hill [Google Scholar]
- Lodish H., Flygare J., Chou S. (2010). From stem cell to erythroblast: regulation of red cell production at multiple levels by multiple hormones. IUBMB Life 62, 492–496. 10.1002/iub.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. J., Feng Q., Park J. S., Vida L., Lee B. S., Strausbauch M., et al. (2008). Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood 112, 4475–4484. 10.1182/blood-2008-05-157198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlus K. R., Italiano J. E. (2013). The incredible journey: from megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796. 10.1083/jcb.201304054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggakis-Kelemen C., Bork M., Kayser P., Biselli M., Artmann G. M. (2003). Biological and mechanical quality of red blood cells cultured from human umbilical cord blood stem cells. Med. Biol. Eng. Compuz. 41, 350–356 10.1007/BF02348442 [DOI] [PubMed] [Google Scholar]
- Malik P., Fisher T. C., Barsky L. L. W., Zeng L., Izadi P., Hiti A. L., et al. (1998). An in vitro model of human red blood cell production from hematopoietic progenitor cells. Blood 91, 2664–2671. [PubMed] [Google Scholar]
- Manwani D., Bieker J. J. (2008). The erythroblastic island. Curr. Top. Dev. Biol. 82, 23–53. 10.1016/S0070-2153(07)00002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S., Li M., Izpisua Belmonte J. C. (2013). In vitro generation of platelets through direct conversion: first report in My Knowledge (iMK). Cell. Res. 23, 176–178. 10.1038/cr.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara Y., Saito E., Suzuki H., Watanabe N., Murata M., Ikeda Y. (2009). Generation of megakaryocytes and platelets from human subcutaneous adipose tissues. Biochem. Biophys. Res. Commun. 378, 716–720. 10.1016/j.bbrc.2008.11.117 [DOI] [PubMed] [Google Scholar]
- Matsubara Y., Suzuki H., Ikeda Y., Murata M. (2010). Generation of megakaryocytes and platelets from preadipocyte cell line 3T3-L1, but not the parent cell line 3T3, in vitro. Biochem. Biophys. Res. Commun. 402, 768–800. 10.1016/j.bbrc.2010.10.120 [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Tanaka I., Kobune M., Kawano Y., Tanaka M., Kuribayashi K., et al. (2006). Ex vivo large-scale generation of human platelets from cord blood CD34+. Cells Stem Cells 24, 2877–2887. 10.1634/stemcells.2006-0309 [DOI] [PubMed] [Google Scholar]
- McGrath K., Palis J. (2008). Ontogeny of erythropoiesis in the mammalian embryo. Curr. Top. Dev. Biol. 82, 1–22. 10.1016/S0070-2153(07)00001-4 [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreve M. M., Axelrad A. A. (1996). Induction of megakaryocyte colonies with platelet formation in vitro. Nature 88, 492–494 [DOI] [PubMed] [Google Scholar]
- Migliaccio A. R., Masselli E., Varricchio L., Whitsett C. (2012). Ex-vivo expansion of red blood cells: how real for transfusion in humans? Blood Rev. 26, 81–95. 10.1016/j.blre.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G., Di Pietro R., Di Giacomo V., Di Baldassarre A., Migliaccio A. R., Maccioni L., et al. (2002). In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol. Dis. 28, 169–180. 10.1006/bcmd.2002.0502 [DOI] [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Petti S., Mavilio F., Russo G., Lazzaro D., et al. (1986). Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac – liver transition. J. Clin. Invest. 78, 51–60. 10.1172/JCI112572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G., Sanchez M., Masiello F., Tirelli V., Varricchio L., Whitsett C., et al. (2010). Humanized culture medium for clinical expansion of human erythroblasts. Cell Transplant. 19, 453–469. 10.3727/096368909X485049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miharada K., Hiroyama T., Sudo K., Nagasawa T., Nakamura Y. (2006). Efficient enucleation of eryth' roblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat. Biotechnol. 24, 1255–1256. 10.1038/nbt1245 [DOI] [PubMed] [Google Scholar]
- Mikkola H. K., Gekas C., Orkin S. H., Dieterlen-Lievre F. (2005). Placenta as a site for hematopoietic stem cell development. Exp. Hematol. 33, 1048–1054. 10.1016/j.exphem.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Mostafa S. S., Papoutsakis E. T., Miller W. M. (2001). Oxygen tension modulates the expression of cytokine receptors, transcription factors and lineage-specific markers in cultured human megakaryocytes. Exp. Hematol. 29, 873–883. 10.1016/S0301-472X(01)00658-0 [DOI] [PubMed] [Google Scholar]
- Natanson C., Kern S. J., Lurie P., Banks S. M., Wolfe S. M. (2008). Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. J. Am. Med. Assoc. 299, 2304–2312. 10.1001/jama.299.19.jrv80007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neildez-Nguyen T. M., Wajcman H., Marden M. C., Bensidhoum M., Moncollin V., Giarratana M. C., et al. (2002). Human erythroid cells produced cx vivo at large scale differentiate into red blood cells in vivo. Nat. Biotechnol. 20, 467–472. 10.1038/nbt0502-467 [DOI] [PubMed] [Google Scholar]
- Ng E. S., Davis R. P., Azzola L., Stanley E. G., Elefanty A. G. (2005). Forced aggregation of defined numbers of human embryonicstem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood 106, 1601–1603. 10.1182/blood-2005-03-0987 [DOI] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. (2007). Generation of germlilne-competent induced pluripotent stem cells. Nature 448, 313–317. 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., et al. (2011). More efficient method to generate integration-free human iPS cells. Nat. Methods 8, 409–412. 10.1038/nmeth.1591 [DOI] [PubMed] [Google Scholar]
- Olivier E. N., Qiu C., Velho M., Hirsch R. E., Bouhassira E. E. (2006). Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp. Hematol. 34, 1635–1642. 10.1016/j.exphem.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Ono Y., Wang Y., Suzuki H., Okamoto S., Ikeda Y., Murata M., et al. (2012). Induction of functional platelets from mouse and human fibroblasts by p45NF-E2/Maf. Blood 120, 3812–3821. 10.1182/blood-2012-02-413617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J. (2008). Ontogeny of erythropoiesis. Curr. Opin. Hematol. 15, 155–161 10.1097/MOH.0b013e3282f97ae1 [DOI] [PubMed] [Google Scholar]
- Palis J., Yoder M. C. (2001). Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29, 927–936. 10.1016/S0301-472X(01)00669-5 [DOI] [PubMed] [Google Scholar]
- Panzenböck B., Bartunek P., Mapara M. Y., Zenke M. (1998). Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood 15, 3658–3668. [PubMed] [Google Scholar]
- Peyrard T., Bardiaux L., Krause C., Kobari L., Lapillonne H., Andreu G., et al. (2011). Banking of pluripotent adult stem cells as an unlimited source for red blood cell production; Potential applications for alloimmunized patients and rare blood challenges. Transfus. Med. Rev. 25, 206–216. 10.1016/j.tmrv.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Proulx C., Boyer L., Humanen D. R., Lemieux R. (2003). Preferential ex vivo expansion of megakaryocytes from human cord blood CD34+-enriched cells in the presence of thrombopoietin and limiting amounts of stem cell factor and Flt-3 ligand. J. Hematother. Stem. Cell. Res. 12, 179–188. 10.1089/152581603321628322 [DOI] [PubMed] [Google Scholar]
- Proulx C., Dupuis N., Amour I. S., Boyer L., Lemieux R. (2004). Increased megakaryopoiesis in cultures of CD34-enriched cord blood cells maintained at 39 degrees C. Biotechnol. Bioeng. 88, 675–680. 10.1002/bit.20288 [DOI] [PubMed] [Google Scholar]
- Qiu C., Hanson E., Olivier E., Inada M., Kaufman D. S., Gupta S., et al. (2005). Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp. Hematol. 33, 1450–1458. 10.1016/j.exphem.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Qiu C., Olivier E. N., Velho M., Bouhassira E. E. (2008). Globin switches in yolk sac-like primitive and fetal like definitive red blood cells produced from human embryonic stem cells. Blood 111, 2400–2408 10.1182/blood-2007-07-102087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran D., Shia W. J., Lo M. C., Fan J. B., Knorr D. A., Ferrell P. I., et al. (2013). RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood 121, 2882–2890. 10.1182/blood-2012-08-451641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe E1., Glen K. E., Workman V. L., Stacey A. J., Thomas R. J. (2012). A novel automated bioreactor for scalable process optimisation of haematopoietic stem cell culture. J. Biotechnol. 161, 387–390. 10.1016/j.jbiotec.2012.06.025 [DOI] [PubMed] [Google Scholar]
- Rhodes M. M., Kopsombut P., Bondurant M. C., Price J. O., Koury M. J. (2008). Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood 111, 1700–1708. 10.1182/blood-2007-06-098178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K., Nakahara M., Matsuyama S., Nakamura N., Yogiashi Y., Yoneda A., et al. (2008). A feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells 27, 59–67. 10.1634/stemcells.2007-0980 [DOI] [PubMed] [Google Scholar]
- Satchwell T. J., Bell A. J., Pellegrin S., Kuziq S., Ridgwell K., Daniels G., et al. (2011). Critical band 3 multiprotein complex interactions establish early during human erythropoiesis. Blood 118, 182–191. 10.1182/blood-2010-10-314187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman T. A., Weiskopf R. B. (2009). Hemoglobinbased oxygen carriers: Current status and future directions. Transfusion 49, 2495–2515. 10.1111/j.1537-2995.2009.02356.x [DOI] [PubMed] [Google Scholar]
- Stadtfeld M., Nagaya M., Utikal J., Weir G., Hochedlinger K. (2008). Induced pluripotent stem cells generated without viral integration. Science 322, 945–949. 10.1126/science.1162494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreevel M. M. (1971). Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc. Natl. Acad. Sci. U.S.A. 68, 1542–1546. 10.1073/pnas.68.7.1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroncek D. F., Rebulla P. (2007). Platelet transfusions. Lancet 370, 427–438. 10.1016/S0140-6736(07)61198-2 [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. (1983). Single-cell origin of mouse hemopoietic colonies expressing multiplelineages in variable combinations. Proc. Natl. Acad. Sci. U.S.A. 80, 6689–6693. 10.1073/pnas.80.21.6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenharger B., Bhang J. H., Gruner R., Kotov N., Lasky L. C. (2009). Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp. Hematol. 37, 101–110. 10.1016/j.exphem.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E., Rampalli S., Risueno R. M., Schnerch A., Mitchell R., Fiebig-Comyn A., et al. (2010). Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468, 521–526. 10.1038/nature09591 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takayama N., Nishikii H., Usui J., Tsukui H., Sawaguchi A., Hiroyama T., et al. (2008). Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood 111, 5298–5306. 10.1182/blood-2007-10-117622 [DOI] [PubMed] [Google Scholar]
- Takayama N., Nishimura S., Nakamura S., Shimizu T., Ohnishi R., Endo H., et al. (2010). Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 207, 2817–2830. 10.1084/jem.20100844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Peacock S., Chaudhry A. N., Bradley J. A., Bolton E. M. (2012). Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152. 10.1016/j.stem.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Timmins N. E., Athanasas S., Günther M., Buntine P., Nielsen L. K. (2011). Ultrahigh-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng. Part. C Methods 17, 1131–1137. 10.1089/ten.TEC.2011.0207 [DOI] [PubMed] [Google Scholar]
- Timmins N. E., Nielsen L. K. (2009). Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 27, 415–422. 10.1016/j.tibtech.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Tirelli V., Ghinassi B., Migliaccio A. R., Whitsett C., Masiello F., Sanchez M., et al. (2011a). Phenotypic definition of the progenitor cells with erythroid differentiation potential present in human adult blood. Stem Cells Int. 2011:602483. 10.4061/2011/602483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli V., Masiello F., Sanchez M., Ghinassi B., Whitsett C., Migliaccio G., et al. (2011b). Effects of ontogeny, ethnicity, gender and loss of companion cells on ex-vivo expansion of erythroid cells for transfusion. Blood 118, 563 [Google Scholar]
- U.S. Census Bureau. (2004). Global Population Composition. Available online at: http://www.census.gov/population/international/files/wp02/wp-02004.pdf (Accessed September 14, 2012).
- Van den Akker E., Satchwell T. J., Pellegrin S., Daniels G., Toye A. M. (2010). The majority of the in vitro erythroid expansion potential resides in CD34 (-) cells, outweighing the contribution of CD34 (+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 95, 1594–1598. 10.3324/haematol.2009.019828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M. A., Bork J. A., Thomson J. A., Slukvin I. I. (2005). Human embryonic stem cell-derived CD34+cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 105, 617–626. 10.1182/blood-2004-04-1649 [DOI] [PubMed] [Google Scholar]
- Vodyanik M. A., Thomson J. A., Slukvin I. I. (2006). Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108, 2095–2105. 10.1182/blood-2006-02-003327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lindern M., Zauner W., Mellitzer G., Steinlein P., Fritsch G., Huber K., et al. (1999). The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood 94, 550–559. [PubMed] [Google Scholar]
- Wada H., Suda T., Miura Y., Kajii E., Ikemoto S., Yawata Y. (1990). Expression of major blood group antigens on human erythroid cells in a two phase liquid culture system. Blood 75, 505–511. [PubMed] [Google Scholar]
- Wang Y., Ono Y., Ikeda Y. (2011). Induction of megakaryocytes from fibroblasts by p45NF-E2fMaf. Blood 11:908. 22855609 [Google Scholar]
- Weatherall D. J., Clegg J. B. (1975). Hereditary persistence of fetal haemoglobin. Br. J Haematol. 29, 191–198 10.1111/j.1365-2141.1975.tb01813.xx [DOI] [PubMed] [Google Scholar]
- Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., et al. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324. 10.1038/nature05944 [DOI] [PubMed] [Google Scholar]
- Winslow R. M. (2006). Current status of oxygen carriers (“blood substitutes”): 2006. Vox Sang. 91, 102–110. 10.1111/j.1423-0410.2006.00789.x [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). Global Database on Blood Safety: Summary Report 2011. Available online at: http://www.who.int/bloodsafety/global_database/GDBS_Summary_Report_2011.pdf(Accessed May 4, 2014).
- Xi J., Li Y., Wang R., Wang Y., Nan X., He L., et al. (2013). In vitro large scale production of human mature red blood cells from hematopoietic stem cells by coculturing with human fetal liver stromal cells. Biomed. Res. Int. 2013:807863. 10.1155/2013/807863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N., Gros E., Li H. R., Kumar S., Deacon D. C., Maron C., et al. (2013). Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell 13, 246–254. 10.1016/j.stem.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourrget J., Frane J. L., Tian S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Zambidis E. T., Park T. S., Yu W., Tam A., Levine M., Yuan X., et al. (2008). Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 112, 3601–3614. 10.1182/blood-2008-03-144766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner A., Martelli F., Vaglio S., Federici G., Whitsett C., Migliaccio A. R. (2012). Concise review: stem cell-derived erythrocytes as upcoming players in blood transfusion. Stem Cells 30, 1587–1596. 10.1002/stem.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wu S., Joo J. Y., Zhu S., Han D. W., Lin T., et al. (2009). Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384. 10.1016/j.stem.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Mali P., Huang X., Dowey S. N., Cheng L. (2011). Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood 118, 4599–4608. 10.1182/blood-2011-02-335554 [DOI] [PMC free article] [PubMed] [Google Scholar]