Abstract
BACKGROUND
Evidence from randomized trials in the pre-sentinel node biopsy era indicate that adjuvant treatment with high-dose interferon-α (IFN) increases relapse-free survival (RFS) in patients with high-risk melanoma. However, the role of this treatment in selected patients with early stage III disease has not been well studied.
METHODS
We evaluated clinical and pathologic characteristics of 486 patients undergoing surgical treatment for stage III melanoma and compared outcomes for those given adjuvant treatment with IFN with those who had surgery alone. A particular focus was on the effect of IFN therapy on RFS and overall survival (OS) among those with stage IIIA disease.
RESULTS
Median follow-up for the entire cohort was 5.2 years; 5-year RFS and OS for the entire group were 41% and 53%, respectively. Adjuvant IFN was given to 141 patients (29%). In multivariate analysis, IFN was the only independent predictor for RFS in stage IIIA disease (hazard ratio 0.4, 95% confidence interval 0.2–0.9, P = 0.02). IFN was not associated with increased RFS in patients with more advanced nodal disease (stage IIIB and IIIC). IFN had no effect on OS in any patient with stage III disease.
CONCLUSIONS
Adjuvant treatment with IFN improves RFS in melanoma patients with early stage III disease. These results should help guide management when considering adjuvant treatment for these patients.
Keywords: High-dose interferon, adjuvant therapy, stage III melanoma
Introduction
At least 10% of the estimated 59,940 new cases of cutaneous malignant melanoma diagnosed in 2007 will present as regional/nodal (stage III) disease.1–3 The outcome for patients with stage III melanoma varies significantly, with 5-year overall survival (OS) rates ranging from 26% to 67% depending on the extent of metastatic disease (number of positive nodes and whether the metastasis is microscopic vs macroscopic)2, 3 and primary tumor characteristics (such as the presence of ulceration). In general, surgical treatment has proven inadequate for stage III melanoma, and several adjuvant regimens have been investigated. Although no apparent benefit with regard to OS has been demonstrated, high-dose interferon-α (IFN) has been shown to increase relapse-free survival (RFS) in patients with high-risk disease,4–11 and this is the only adjuvant treatment approved by the U.S. Food and Drug Administration (FDA) for malignant melanoma. Nevertheless, IFN is not always recommended because of its significant toxicity profile,10 its effects on quality of life,12, 13 and its cost.14
Recommendations for the use of IFN are derived primarily from the findings of two randomized trials (Eastern Cooperative Oncology Group [ECOG] 1684 and Intergroup E1690)4, 5 in which this regimen was compared directly with observation only for patients with thick primary tumors (i.e., > 4 mm Breslow thickness) in the absence of nodal disease (stage IIB) and for patients with primary or recurrent metastatic nodal disease (stage III).15 The participants in these studies were accrued from 1984 to 1995, primarily before sentinel lymph node biopsy (SLNB) had been introduced; thus microscopic nodal disease was identified in few patients following elective lymph node dissection, which was performed either as a study prerequisite (ECOG 1684) or after selective lymphadenectomy for patients with high-risk disease (Intergroup E1690).
Since that time, SLNB has become the recommended method for pathologic staging of all primary melanomas more than 1 mm thick and in selected patients with thin (< 1 mm) melanomas.16–18 As a result of the adoption of SLNB19 and the use of enhanced tissue processing (e.g., immunohistochemical staining),16, 20, 21,22 patients with early stage III disease, specifically those with micrometastatic nodal involvement, are being identified more frequently. This shift is now reflected in the most recent (2002) version of the American Joint Committee on Cancer (AJCC) staging system (6th edition),2, 3, 23 in which nodal stage is defined not only by the number of positive lymph nodes but also by the extent of nodal involvement (microscopic vs. macroscopic), as derived from tissue evaluation that includes serial sectioning and immunohistochemical analysis of SLNs. Although both of the randomized trials noted above4, 5 included patients with microscopic nodal disease identified by elective lymph node dissection, few such patients were studied (34 in ECOG 1684 and 68 in the Intergroup study) and surgical specimens were not subjected to serial sectioning or immunohistochemical staining, which raises the question of whether the outcomes associated with adjuvant IFN can reasonably be extrapolated to patients with micrometastatic nodal disease as defined by more contemporary methods.
Given the lack of information about the role of adjuvant high-dose IFN therapy for patients with early stage III disease, we conducted a retrospective review to identify the impact of IFN therapy on survival outcomes for patients with metastatic nodal cutaneous melanoma, with the primary objective of examining the subgroup of patients with stage IIIA disease (micrometastatic disease with non-ulcerated primary tumor).
MATERIALS AND METHODS
Patient Selection
The study was approved by the Institutional Review Board of the University of Texas M. D. Anderson Cancer Center (MDACC). Initially, we identified a total of 804 consecutive patients with melanoma metastatic to one or more regional nodal basins (i.e., stage III disease) who had been treated at MDACC between 1990 and 2001. From this group, we selected only patients treated after 1995 (the time at which high-dose IFN was FDA approved as adjuvant therapy for melanoma), yielding the 486 patients who constitute the cohort for this study. Medical records from all patients were reviewed and demographic, clinical, pathologic, treatment, and outcome variables were extracted and recorded. Disease was restaged at the time of review according to the 6th edition of the AJCC staging system.2, 3 All 486 patients had undergone therapeutic lymph node dissection (LND) of the regional nodal basin or basins at MDACC. Two groups were subsequently identified: those given IFN as adjuvant therapy (IFN group) and those who underwent surgery without adjuvant treatment (observation group) based on recommendations from the treating physician and individual patient preference according to standard protocols described elsewhere.4–6 Patients were followed every 3 months for the first 2 years after LND, at 6-month intervals until year 5, and yearly thereafter.
Outcomes
RFS time, defined as the period from therapeutic LND to disease recurrence (local/regional or distant) or death, was the primary outcome of interest; OS time, calculated from the date of therapeutic LND to the date of death, was considered as a secondary endpoint. Distant disease-free survival (DDFS) was also calculated as the time from therapeutic LND to time of distant recurrence or death. Patients without recurrence or death were censored by using the date of last follow-up for each outcome. Median RFS and OS, as well as 5-year RFS and OS, were calculated for both the IFN and the observation groups. The association between IFN and RFS was further evaluated for subgroups with pathologic stage IIIA, IIIB, and IIIC disease. Pathologic stage IIIA disease includes patients with up to three microscopic nodal metastases arising from a non-ulcerated primary melanoma. Stage IIIB disease consists of patients with up to three macroscopic nodal metastases arising from a non-ulcerated primary melanoma, or up to three microscopic nodal metastases arising from an ulcerated primary, or intralymphatic metastases without nodal metastases. Stage IIIC disease consists of patients with clinically apparent nodal metastasis (macroscopic) arising from an ulcerated primary, or those with four or more nodal metastasis, matted nodal metastasis or combined nodal and in-transit metastasis in the absence of distant disease.24
Statistical Analysis
Demographic and clinicopathologic characteristics were compared for patients in the IFN and No-IFN groups by using the chi-square test or Fisher’s exact test, as appropriate. The Kaplan-Meier method was used to estimate RFS and OS in all patients with stage III disease as well as for subsets of patients with IIIA, IIIB, or IIIC disease. The Cox proportional hazards model was used to assess the prognostic significance of demographic, clinicopathologic, and treatment variables. A P value of 0.05 was considered statistically significant. All computations were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The median follow-up time for the 486 patients with stage III melanoma was 5.2 years. Of these patients, 141 (29%) received adjuvant therapy with IFN. The distribution of demographic and clinicopathologic variables for both the IFN and observation groups are listed in Table 1. Patients in the IFN group were more likely to be younger than those in the observation group (54% vs. 43% patients < 50 years, P = 0.02) and less likely to have extracapsular nodal extension in the LND specimens (12% vs. 21%, P = 0.02). Disease stage was classified according to the 6th edition of the AJCC system in 462 patients; 110 patients (24%) had stage IIIA disease, 192 (42%) had stage IIIB disease, and 160 (35%) had stage IIIC disease. Disease stage in the remaining 24 patients could not be determined, primarily because of unknown primary tumor characteristics.
TABLE 1.
Clinicopathologic Characteristics of Patients with Stage III Melanoma Treated With or Without Adjuvant Interferon
| Clinicopathologic Factors | No. of Patients (%) | P Value | |
|---|---|---|---|
| IFN (n=141) | Observation (n=345) | ||
| Age (years) | 0.02 | ||
| < 50 | 76 (54) | 147 (43) | |
| ≥ 50 | 65 (46) | 198 (57) | |
| Sex | NS | ||
| Female | 67 (48) | 141 (41) | |
| Male | 74 (52) | 204 (59) | |
| Primary Tumor Site | NS | ||
| Head & neck | 3 (2) | 8 (2) | |
| Trunk | 62 (44) | 132 (38) | |
| Extremities | 65 (46) | 183 (53) | |
| Unknown | 11 (8) | 22 (7) | |
| Primary Tumor Histology | NS | ||
| Superficial spreading | 33 (24) | 89 (26) | |
| Nodular | 40 (28) | 82 (24) | |
| Acral lentiginous | 9 (6) | 24 (7) | |
| Other | 48 (34) | 128 (37) | |
| Unknown | 11 (8) | 22 (6) | |
| Primary Tumor Stage | NS | ||
| T1 | 14 (10) | 46 (13) | |
| T2 | 30 (21) | 83 (24) | |
| T3 | 43 (30) | 99 (29) | |
| T4 | 36 (26) | 62 (18) | |
| Unknown | 18 (13) | 55 (16) | |
| Clark Level | NS | ||
| I–III | 37 (26) | 84 (24) | |
| IV–V | 81 (57) | 194 (56) | |
| Unknown | 23 (17) | 67 (20) | |
| Primary Tumor Ulceration | NS | ||
| Yes | 38 (27) | 99 (29) | |
| No | 92 (65) | 219 (63) | |
| Unknown | 11 (8) | 27 (8) | |
| Nodal Basin Involved | NS | ||
| Axilla | 74 (52) | 172 (50) | |
| Groin | 61 (43) | 154 (45) | |
| Other | 6 (5) | 19 (5) | |
| Nodal Stage | NS | ||
| N1 | 74 (52) | 168 (49) | |
| N2 | 38 (27) | 87 (25) | |
| N3 | 29 (21) | 90 (26) | |
| Extracapsular Nodal Extension | 0.02 | ||
| Yes | 17 (12) | 71 (21) | |
| No/Unknown | 124 (88) | 274 (79) | |
NS, not statistically significant.
Distant Disease-Free Survival
The median DDFS time for the entire cohort (n=486) was 4.07 years (95% confidence interval [CI] 2.75–5.2), and the Kaplan-Meier estimated 5-year DDFS for the entire group was 47% (95% CI 42–52). The median DDFS time for the IFN group was 5.06 years (95% CI 3.75–not reached) and that for the observation group was 3.02 years (95% CI 2.22–5.2). Kaplan-Meier estimated 5-year DDFS was 51% (95% CI 44–61) for the IFN group and 45% (95% CI 40–51) for the observation group (P = 0.042).
Recurrence-Free Survival
The median RFS time for the entire cohort (n=486) was 2.27 years (95% confidence interval [CI] 1.84–3.76), and the Kaplan-Meier estimated 5-year RFS for the entire group was 41% (95% CI 36–46). The median RFS time for the IFN group was 3.82 years (95% CI 2.21–not reached) and that for the observation group was 2.06 years (95% CI 1.54–2.96). Kaplan-Meier estimated 5-year RFS was 44% (95% CI 36–54) for the IFN group and 39% (95% CI 34–45) for the observation group (P = 0.08). Independent prognostic factors for RFS in the multivariate analyses were age ≥ 50 (hazard ratio [HR] 1.4, 95% CI 1.1–1.8, P = 0.01), N2 disease (HR 1.5, 95% CI 1.1–2.1, P = 0.01), N3 disease (HR 3, 95% CI 2.2–4.1, P < 0.001), and acral lentigenous tumor histology (HR 1.8, 95% CI 1.1–2.9, P = 0.01). Receiving adjuvant IFN was not an independent prognostic factor for RFS for the combined group of patients with stage III disease (HR 0.8, 95% CI 0.6–1.1, P = 0.09).
Overall Survival
The median OS time for the entire stage III cohort was 5.6 years (95% CI 4.9–not reached), and the Kaplan-Meier estimated 5-year OS was 53% (95% CI 49–58). The median 5-year OS for the IFN group was 60% (95% CI 52–69) and 50% (95% CI 45–56) in the observation group (P = 0.04). However, when controlling for additional prognostic factors, adjuvant IFN therapy was not found to be an independent prognostic factor of OS for the entire stage III cohort (data not shown).
Effect of Adjuvant IFN According to Disease Substage
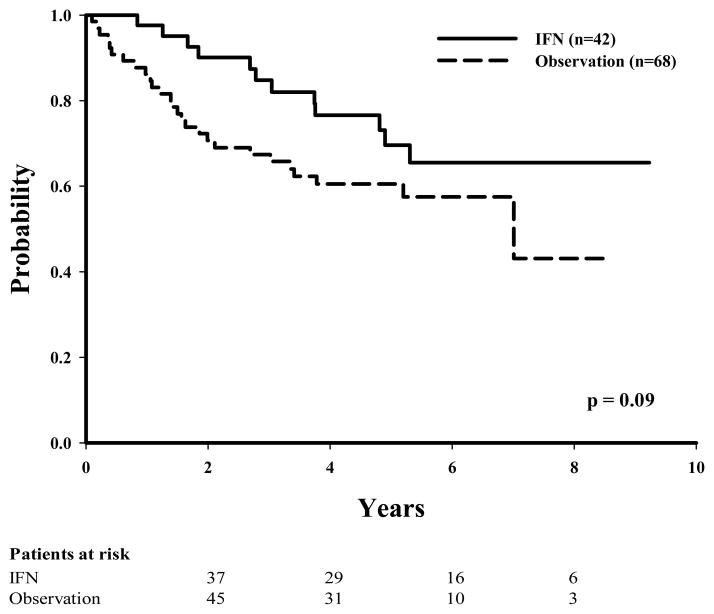
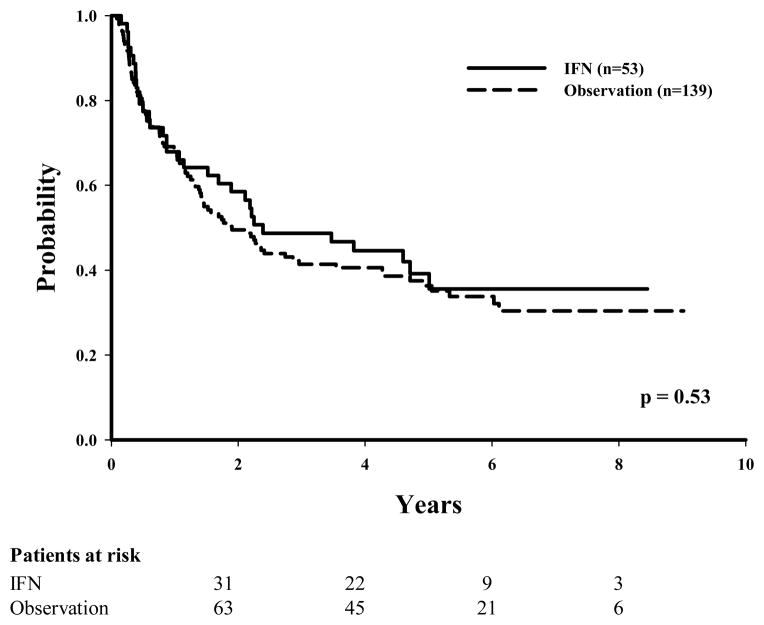
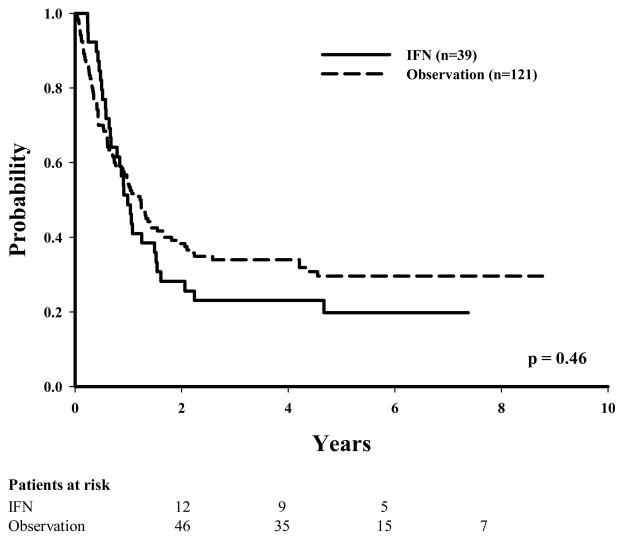
Of the 110 patients with stage IIIA disease, 42 (38%) had been given IFN as adjuvant therapy. Of these 42 patients, 12 (28%) had disease recurrence as compared with 27 (39%) in the observation group (P = 0.09 in univariate analysis). The numbers of recurrences between the IFN and the observation groups were more evenly distributed among patients with more advanced stage (60% IFN vs. 61% observation for stage IIIB disease and 79% IFN vs. 74% observation for stage IIIC disease). Unadjusted Kaplan-Meier estimates for DDFS, RFS, and OS were initially examined to evaluate the effect of IFN therapy according to disease substage (Table 2). The 5-year RFS was found to be slightly lower, although not statistically significant, in the observation group (61%) than in the IFN group (70%) for those with stage IIIA disease (P = 0.09 [Fig. 1]), whereas the 5-year RFS times were similar among patients with stage IIIB disease (36% observation vs. 39% IFN [Fig. 2]) or stage IIIC disease (30% observation vs. 20% IFN [Fig. 3]).
Table 2.
Comparison of survival outcomes for patients treated with IFN vs. No IFN stratified by substage
| Substage | Outcomes | IFN
|
No IFN
|
||
|---|---|---|---|---|---|
| 5-Year | 95% CI | 5-Year | 95% CI | ||
| IIIA | DDFS | 0.73 | (0.64, 0.91) | 0.66 | (0.55, 0.8) |
| RFS | 0.7 | (0.56, 0.87) | 0.61 | (0.50, 0.74) | |
| OS | 0.76 | (0.63, 0.91) | 0.71 | (0.6, 0.84) | |
| IIIB | DDFS | 0.49 | (0.37, 0.65) | 0.42 | (0.35, 0.52) |
| RFS | 0.39 | (0.28, 0.56) | 0.36 | (0.29, 0.46) | |
| OS | 0.64 | (0.52, 0.79) | 0.46 | (0.38, 0.56) | |
| IIIC | DDFS | 0.29 | (0.17, 0.48) | 0.35 | (0.27, 0.45) |
| RFS | 0.2 | (0.1, 0.38) | 0.3 | (0.22, 0.39) | |
| OS | 0.37 | (0.24, 0.56) | 0.44 | (0.35, 0.54) | |
Abbreviations: IFN: Interferon; DDFS: Distant disease-free survival; DFS: Disease-free survival; OS: Overall survival
FIGURE 1.
Recurrence-free survival by treatment (IFN vs. observation) for patients with stage IIIA melanoma.
FIGURE 2.
Recurrence-free survival by treatment (IFN vs. observation) for patients with stage IIIB melanoma.
FIGURE 3.
Recurrence-free survival by treatment (IFN vs. observation) for patients with stage IIIC melanoma.
When clinicopathologic covariates were accounted for in multivariate analyses, adjuvant IFN therapy was identified as the only independent predictor of RFS for patients with stage IIIA disease with a hazard ratio of 0.4 (95% CI 0.2–0.9, P = 0.02). Adjuvant IFN therapy was not an independent predictor for RFS for patients with stage IIIB or IIIC disease (Table 3). Similarly, after controlling for prognostic factors, IFN was not found to be an independent predictor of OS in any of the three disease substages (IIIA, IIIB, or IIIC) (data not shown).
TABLE 3.
Independent Predictors of Recurrence Free Survival from Multivariate Analysis
| Predictor* | Hazard Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Stage IIIA | |||
| IFN vs observation | 0.4 | 0.2–0.9 | 0.02 |
| Stage IIIB | |||
| Acral lentiginous vs superficial spreading | 4.1 | 1.7–9.8 | 0.002 |
| T2 vs T1 | 2.7 | 1.2–6 | 0.01 |
| T4 vs T1 | 4.5 | 1.9–10.9 | 0.001 |
| IFN vs observation | 0.8 | 0.5–1.3 | 0.4 |
| Stage IIIC | |||
| N3 vs N1 | 4.3 | 2–9.3 | < 0.001 |
| IFN vs observation | 1 | 0.6–1.6 | 0.9 |
| All Stage III | |||
| Age > 50 vs < 50 | 1.4 | 1.1–1.8 | 0.01 |
| Acral lentiginous vs superficial spreading | 1.8 | 1.1–2.9 | 0.01 |
| N2 vs N1 | 1.5 | 1.1–2.1 | 0.01 |
| N3 vs N1 | 3 | 2.2–4.1 | < 0.001 |
| IFN vs observation | 0.8 | 0.6–1.1 | 0.1 |
Note: Variables were included in the multivariate analysis if P < 0.25 in univariate analysis. Table includes effect of IFN for each group and significant predictors after multivariate analysis (P < 0.05).
Additional covariates adjusted for in the model included: for stage IIIA, T stage and N stage; for stage IIIB, N stage and ulceration; for stage IIIC, histologic subtype, T stage, and ulceration; and for all stage III, sex, T stage, and ulceration.
DISCUSSION
In our covariate-adjusted analysis of 110 melanoma patients with stage IIIA disease, patients who had been given adjuvant IFN showed significant improvement in RFS relative to patients who had not been given adjuvant IFN. At 5 years, the use of IFN was associated with lower rates of disease recurrence, with a cumulative absolute benefit of 9%. However, these results did not translate into significant differences in OS. Moreover, this benefit was not evident for patients with more advanced nodal disease (stages IIIB and IIIC). Patients with IIIB disease experienced similar 5-year RFS regardless of whether they had received IFN or not. Similarly, no benefit was found for patients treated with IFN who had bulky nodal disease (stage IIIC), which likely can be explained by the presence of a higher burden of distant micrometastatic disease which may not derive clinical benefit from IFN.25
Our study population was a relatively homogeneous group of patients with high-risk, node-positive melanoma; patients who traditionally are considered likely to benefit from adjuvant IFN. The IFN and observation subgroups were relatively balanced with respect to known prognostic factors, except that patients with pathologic evidence of extracapsular nodal extension and older patients were less likely to have received IFN (P = 0.02). Because our multivariate model adjusted for these factors in addition to all other known prognostic factors, the effects of IFN were unlikely to have been confounded by these two variables. In addition, the current study population was homogeneous relative to the techniques used for pathologic nodal staging (SLNB) including immunohistochemical analysis. The most recent revision of the AJCC staging system for melanoma (2002) identified both ulceration and micro- vs. macrometastatic nodal disease as important prognostic factors,2, 3, 23 factors that were not part of the staging classification at the time of the previous IFN trials (ECOG 1684, Intergroup E1690).4, 5 In the current analysis, patients with micrometastatic nodal disease and no primary tumor ulceration (Stage IIIA) were identified and examined as a separate cohort, thereby allowing us to evaluate the effect of IFN therapy in a specific subgroup that had not been previously studied.
Several points merit comment when comparing our findings with those of published randomized trials of high-dose IFN therapy,4–7 a few systematic reviews and a meta-analysis on the topic.9–11 First, the randomized trials included heterogeneous populations of patients with high-risk disease with respect to prognosis. Second, the previously published trials included only relatively small numbers of patients with microscopic-only disease, and the relative accuracy with which this condition was diagnosed was lower compared to the current report. For example, in the ECOG 1684 trial, microscopic-only disease was identified after mandatory LND,4 which is recognized to be less accurate for pathologic staging than are current SLNB techniques.16, 20, 21 The Intergroup E1690 trial, LND was not required for patients without clinically apparent nodal metastasis and was performed electively only in a minority of these patients (24%-selection criteria not specified) allowing for an even greater gap in the accuracy of the diagnosis of microscopic-only disease based on current standards.5 The Intergroup E1694 trial, which compared high-dose IFN therapy vs. the GMK vaccine for patients with high-risk melanoma, used the same guidelines for selective lymphadenectomy as those used in the E1690 trial.5, 6 Together, these three trials constitute the largest numbers of patients with high-risk disease (280, 608, and 774, respectively) randomized to high-dose IFN vs. observation (ECOG 1684 and E1690) or to high-dose IFN vs. other treatment (E1694). However, the numbers of patients identified with microscopic nodal disease in each trial and in a pooled analysis of ECOG 1684 and E16907 were significantly less than the numbers in our report (Table 4). Furthermore the presence of micrometastatic disease in relation to ulcerated and non-ulcerated primary tumors is not well delineated, limiting the interpretation of their results in different prognostic groups stratified with current standards (stage IIIA specifically).
TABLE 4.
Published Analyses of High-Dose Interferon Therapy and Relapse-Free Survival in Patients with High-Risk Melanoma
| Study | AJCC Disease Stage | Total No. of Patients | No. of Patients with Micrometastatic Nodal Disease | Impact of IFN on 5-year RFS | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total | IFN | Observation Only | Effect on Total Study Population | Primary Finding | |||
| ECOG 1684* | IIB, III | 280 | 34 | 20 | 14 | Absolute increase in RFS of 11% (P = 0.002); P = 0.001 after adjustment for multiple variables | Major impact on patients with clinically evident node-positive disease |
| Intergroup E1690 | IIB, III | 608 | 68† | 18 | 29 | Absolute increase in RFS of 9% (HR 1.28, P = 0.05); P = 0.03 after adjustment for multiple variables | Major impact on patients with node-positive disease, particularly those with 2–3 positive nodes |
| Pooled analysis (E1684/1690) | IIB, III | 713 | NR | NR | NR | Increased RFS (HR 1.3, P = 0.006); P = 0.01 after adjustment for multiple variables | No data on subset analysis |
| Intergroup E1694‡ | IIB, III | 774 | NA§ | NA§ | NA | Absolute increase in 2-year RFS of 13% (HR 1.49, P = 0.0004); P = 0.0007 after adjustment for multiple variables | High-dose IFN was of the most benefit for patients with no nodal involvement (P = 0.01). |
| Current Study | III | 486 | 110 | 42 | 68 | Absolute increase in RFS of 5% (P = 0.08); P = 0.1 after adjustment for multiple variables | Stage IIIA absolute increase in RFS of 9% (P = 0.09); P = 0.02 after adjustment for multiple variables |
Abbreviations: AJCC, American Joint Committee on Cancer; IFN, interferon; RFS, relapse-free survival; HR, hazard ratio; NR, not reported; NA, not applicable
All patients with stage III disease underwent lymphadenectomy
Includes patients given high-dose IFN, low-dose IFN, and observation
Groups were randomized between high-dose IFN and GMK vaccine; no observation group was included. Effect of IFN based on comparison with vaccine group.
Micrometastases were defined according to the 5th edition of the AJCC staging system (i.e., nodes < 3 cm) and cannot be compared with the current definition (which implies clinically negative disease).
The effects of adjuvant high-dose IFN therapy for patients with microscopic-only nodal disease, and stage IIIA specifically, are inconsistent in the published literature. For example, in ECOG 1684, the benefit with regard to RFS was seen in all patients with node-positive disease, although the effect was more pronounced in those with clinically positive nodes.4 In E1690, patients with 2 or 3 positive lymph nodes were noted to derive the most benefit with respect to RFS following treatment with high-dose IFN.5 In contrast, in E1694 patients with high-risk node-negative disease (stage IIB) benefited the most from adjuvant IFN therapy.6 This group of high risk patients, which likely included a significant proportion of patients with unidentified micrometastatic disease, may well represent the most similar group to our contemporary cohort. These differences in survival outcomes have previously been explained by the heterogeneity of each study population and by the differences in patient characteristics between trials. Furthermore, the different distribution and small numbers of patients with microscopic-only disease in these trials may also contribute to these seemingly contradictory results.
Although the patient population in the current analysis was homogeneous and the analyses were adjusted for known prognostic factors, interpretation of these findings must take into consideration that the study size was relatively small and that patients were not randomized between the IFN and the observation groups. Although the Cox model can adjust for known clinicopathologic and treatment factors, we cannot exclude the possibility that the effects of IFN arose as a consequence of an imbalance in unknown confounding factors. Also, the retrospective nature of this analysis did not allow us to evaluate the toxicity of IFN and whether or not dose adjustments may have influenced the final results.
In summary, this retrospective analysis of patients with node-positive melanoma suggests that the use of adjuvant IFN may confer a benefit in terms of RFS and that such a benefit is most striking for those patients with early –micrometastatic- nodal disease. Despite the ongoing debate on the risk-benefit ratio of adjuvant IFN, our data support that patients with stage IIIA melanoma be informed that IFN may have better clinical outcomes than those reported in previous trials, which had included patients with greater metastatic nodal tumor burden and more advanced stage III disease.
Acknowledgments
Acknowledgment of research support: National Cancer Institute Clinical Oncology Research Development Program Award 5-K12-CA088084 and American Society of Clinical Oncology Career Development Award 88202.
The authors would like to acknowledge Christine Wogan for editorial assistance and Debbie Dunaway for manuscript preparation.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB–III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 8.Creagan ET, Dalton RJ, Ahmann DL, et al. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol. 1995;13:2776–2783. doi: 10.1200/JCO.1995.13.11.2776. [DOI] [PubMed] [Google Scholar]
- 9.Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 10.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818–1825. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Quirt I, McCready D, Bak K, Charette M, Iscoe N. Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer. 2006;106:1431–1442. doi: 10.1002/cncr.21760. [DOI] [PubMed] [Google Scholar]
- 12.Kilbridge KL, Cole BF, Kirkwood JM, et al. Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. J Clin Oncol. 2002;20:1311–1318. doi: 10.1200/JCO.2002.20.5.1311. [DOI] [PubMed] [Google Scholar]
- 13.Kilbridge KL, Weeks JC, Sober AJ, et al. Patient preferences for adjuvant interferon alfa-2b treatment. J Clin Oncol. 2001;19:812–823. doi: 10.1200/JCO.2001.19.3.812. [DOI] [PubMed] [Google Scholar]
- 14.Cormier JN, Xing Y, Ding M, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol. 2007;25:2442–2448. doi: 10.1200/JCO.2007.10.7284. [DOI] [PubMed] [Google Scholar]
- 15.Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15:1039–1051. doi: 10.1200/JCO.1997.15.3.1039. [DOI] [PubMed] [Google Scholar]
- 16.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 17.McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: controversy despite widespread agreement. J Clin Oncol. 2001;19:2851–2855. doi: 10.1200/JCO.2001.19.11.2851. [DOI] [PubMed] [Google Scholar]
- 18.McMasters KM, Swetter SM. Current management of melanoma: benefits of surgical staging and adjuvant therapy. J Surg Oncol. 2003;82:209–216. doi: 10.1002/jso.10216. [DOI] [PubMed] [Google Scholar]
- 19.Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol. 2005;23:6054–6062. doi: 10.1200/JCO.2005.21.360. [DOI] [PubMed] [Google Scholar]
- 20.Morton DL, Thompson JF, Cochran AJ, Essner R, Elashoff R. Interim results of the multicenter selective lymphadenectomy trial (MSLR-I) in clinical stage I melanoma. Paper presented at: ASCO; 2005. [Google Scholar]
- 21.Morton DL, Hoon DS, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238:538–549. doi: 10.1097/01.sla.0000086543.45557.cb. discussion 549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 24.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging handbook: from the AJCC cancer staging manual. 6. New York: Springer; 2002. Melanoma of the Skin; p. 239. [Google Scholar]
- 25.Eggermont AM. The role of interferon-alpha in malignant melanoma remains to be defined. Eur J Cancer. 2001;37:2147–2153. doi: 10.1016/s0959-8049(01)00272-6. [DOI] [PubMed] [Google Scholar]