Abstract
The hallmark of Alzheimer's disease (AD) is declarative memory loss, but deficits in semantic fluency are also observed. We assessed how semantic fluency relates to cortical atrophy to identify specific regions that play a role in the loss of access to semantic information. Whole-brain structural magnetic resonance imaging (MRI) data were analyzed from 9 Normal Control (NC)(M=76.7, SD=5.6), 40 Mild Cognitive Impairment (MCI) (M=74.4, SD=8.6), and 10 probable AD (M=72.4, SD=8.0) subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI). They all were administered the Category Fluency (CF) animals and vegetables tests. Poorer semantic fluency was associated with bilateral cortical atrophy of the inferior parietal lobule (Brodman areas (BA) 39 and 40) and BA 6, 8, and 9 in the frontal lobe, as well as BA 22 in the temporal lobe. More diffuse frontal associations were seen in the left hemisphere involving BA 9, 10, 32, 44, 45, and 46. Additional cortical atrophy was seen in the temporoparietal (BA 37) and the right parastriate (BA 19, 18) cortices. Associations were more diffuse for performance on vegetable fluency than animal fluency. The permutation-corrected map-wise significance for CF animals was pcorrected=0.01 for the left hemisphere, and pcorrected=0.06 for the right hemisphere. The permutation-corrected map-wise significance for CF vegetables was pcorrected=0.009 for the left hemisphere, and pcorrected=0.03 for the right hemisphere. These results demonstrate the profound effect of cortical atrophy on semantic fluency. Specifically, tapping into semantic knowledge involves the frontal lobe in addition to the language cortices of the temporoparietal region.
Keywords: Alzheimer's Disease, MRI, imaging, semantic fluency, language, Category Fluency, atrophy, biomarkers
1. Introduction
Alzheimer's disease (AD) pathology is characterized by loss of neurons and synapses in the cerebral cortex and a build-up of amyloid plaques and neurofibrillary tangles in the brain. Cortical atrophy is one of the hallmark features of AD. The typical progression begins in the mesial temporal region and spreads to the rest of the temporal and parietal lobes and eventually to the frontal lobes (Braak and Braak 1995; Thompson, Hayashi et al. 2003). In the early stages of AD, the most common symptom is declarative memory loss; however, deficits in language, executive, and visuospatial function are also frequently reported.
Verbal fluency tests are commonly used to assess language and can be categorized as measuring phonetic fluency (generation of words specific to certain letters) and semantic fluency (generation of words specific to certain categories). Semantic fluency is a sensitive measure for distinguishing between normal subjects and those with early cognitive decline who proceed to AD (Canning, Leach et al. 2004; Grundman, Petersen et al. 2004; Clark, Gatz et al. 2009). There is growing interest in the prodromal stages of AD and how best to predict conversion to AD from the intermediate cognitive state known as mild cognitive impairment (MCI). MCI is characterized by early AD symptoms with intact daily functioning; in the ADNI cohort patients diagnosed with MCI progressed to AD at a rate of 16.5% per year (Petersen, Aisen et al. 2010). Given the pattern of cortical atrophy beginning in the temporal region in AD, semantic fluency deficits may play a key role in identifying MCI, and as such, it is vital to understand the neuroanatomical correlates of semantic fluency loss.
Many investigations into the linguistic correlates of AD have been conducted with functional neuroimaging(Welsh, Hoffman et al. 1994; Hirono, Mori et al. 2001; Teipel, Willoch et al. 2006; Schonknecht, Hunt et al. 2011). Fewer studies of the linguistic correlates of AD have been conducted using structural neuroimaging. A study evaluating language networks in clinical and preclinical AD found associations between performance on the Category Fluency (CF) animals test and lower grey matter density in the posterior superior and middle frontal gyri, the somatomotor cortex (SMA), the anterior cingulate, and the posterior left temporal lobe association areas (Apostolova, Lu et al. 2008). A second study of regional atrophy rates and cognitive decline over a 2-year period found associations between performance on the CF animals and vegetables tests and higher cortical atrophy rates within the left lateral temporal, right lateral temporal, left anterior cingulate, and left prefrontal lobar regions (McDonald, Gharapetian et al. 2012).
We examined the relationship between cortical atrophy and semantic fluency. We analyzed 1.5 T structural magnetic resonance imaging (MRI) data using a cortical pattern matching technique to control for inter-subject anatomical variability. This method uses sulcal-based cortical alignment to identify disease specific cortical atrophy and analyze the associations between structure and, in this study, one key aspect of language function. This tool has been validated through the efforts of several disciplines that include neurodegenerative, psychiatric, and developmental research (Sowell, Thompson et al. 2001; Sowell, Peterson et al. 2003; Sowell, Thompson et al. 2003; Thompson, Hayashi et al. 2003; Ballmaier, O'Brien et al. 2004; Ballmaier, Sowell et al. 2004; Thompson, Hayashi et al. 2004; Apostolova, Lu et al. 2006; Apostolova, Steiner et al. 2007). We hypothesized that we would find associations between verbal fluency and cortical atrophy in several brain regions known to sub-serve language processing. The hypothesized regions included the temporal and parietal cortices, which are associated with phonological and semantic representations and retrieval of word forms (Bookheimer 2002; Martin 2003).
2. Subjects and Methods
Subjects
We examined MRI data from a cohort of 9 Normal Control (NC), 40 Mild Cognitive Impairment (MCI), and 10 probable AD subjects who were enrolled and scanned as part of the Alzheimer's Disease Neuroimaging Initiative (ADNI). Although ADNI assessed 818 subjects, all with MRI and neuropsychiatric data, we focused on these specific subjects as they also had available Pittsburg compound B (PiB)-PET scans. PiB-PET was administered in only a subset of those scanned with MRI. Initially, this PIB data set was comprised of 101 MRI scans with time-matched PIB-PET scans. This subset of 59 subjects represents the first in a series of analyses to be conducted on the larger data set that will include PIB analyses in the future. ADNI inclusion criteria can be examined in detail at http://www.adniinfo.org/Scientists/ADNIGrant/ProtocolSummary.aspx. To summarize, all ADNI subjects are between 55-90 years of age and must have a study partner who is capable of providing information regarding the subjects' daily functioning. NC subjects have a Mini Mental State Exam (MMSE) score between 24 and 30 (inclusive) and a global clinical Dementia Rating (CDR) of 0. MCI subjects have MMSE scores between 20 and 30 (inclusive), a subjective memory complaint, objective memory loss as determined by the Wechsler Memory Scale Logical Memory II, a global CDR of 0.5, preserved activities of daily living, and an absence of dementia. AD subjects have MMSE scores between 20 and 26 (inclusive), a global CDR between 0.5 and 1.0, and meet the NINCDS/ADRDA criteria for probable AD. Written informed consent was obtained from all participants.
Neuropsychological Testing
All subjects were administered the CF animals and vegetables tests within one month of the MRI. For these tests, subjects are asked to name as many animals or vegetables as possible within one minute, respectively (Benton 1989). These tests are commonly used to measure semantic fluency and tap into semantic representation, semantic judgment, and semantic retrieval.
Image Acquisition and Processing
Subjects were scanned with a standardized high-resolution MRI protocol on scanners from one of three manufacturers (General Electric Healthcare, Siemens Medical Solutions, or Philips Medical Systems) with protocols optimized for the best contrast to noise in a feasible acquisition time (Leow, Klunder et al. 2006; Jack, Bernstein et al. 2008). Raw data with an acquisition matrix of 192 × 192 × 166 and voxel size 1.25 × 1.25 × 1.2 mm3 in the x-, y-, and z-dimensions was subjected to in-plane, 0-filled reconstruction (i.e., sinc interpolation) resulting in a 256 × 256 matrix and voxel size of 0.9375 × 0.9375 × 1.2 mm3. Image quality was inspected at the ADNI MRI quality control center at the Mayo Clinic (in Rochester, MN, USA) (Jack, Bernstein et al. 2008). Phantom-based geometric corrections were applied to ensure that spatial calibration was kept within a specific tolerance level for each scanner involved in the ADNI study (Gunter 2006). Additional image corrections included GradWarp correction for geometric distortion due to gradient nonlinearity (Jovicich, Czanner et al. 2006), a “B1-correction” for image intensity non-uniformity (Jack, Bernstein et al. 2008), and an “N3” bias field correction, for reducing intensity inhomogeneity (Sled, Zijdenbos et al. 1998).
A computational anatomy-based cortical thickness technique was applied to the structural MRI scans. To do this, the scans were aligned to International Consortium on Brain Mapping 53 (ICBM53) space with a 9-parameter linear transformation method (Collins, Neelin et al. 1994). The MRI images were skull stripped automatically using Brainsuite (Shattuck, Sandor-Leahy et al. 2001), visually inspected, and manually corrected as needed. Following 3D hemispheric reconstruction, 38 sulci per hemisphere were manually traced and averaged across all 59 subjects. Cortical surfaces were parameterized, flattened, and warped; allowing for explicit matching of cortical topography prior to averaging across subjects. Cortical thickness, defined as the 3D distance from the gray/white matter to the gray matter/cerebrospinal fluid interfaces, was computed at each hemispheric surface point andmapped onto the corresponding hemispheric model in exact spatial correspondence. Individual test scores were entered as covariates in a general linear model that predicted cortical thickness at each cortical point for individual subjects. The results of these regression analyses were then presented as significance (p-value) and correlation or beta coefficient maps. The overall significance of the statistical maps was corrected for multiple comparisons using permutation methods with a threshold p<0.01. These methods have been used in many other investigations and have become standard practice in imaging analysis.
3. Results
One-way analysis of variance (ANOVA) was conducted to test for any significant differences in age and education in our sample. The NC, MCI, and AD groups did not differ significantly in age [F(2, 56)=0.65, p=0.53] or education [F(2, 56)=1.28, p=0.29]. The groups did not differ significantly in sex distribution [X2 (2, N=59) = 2.68, p =0.26]. These demographic data are displayed in Table 1.
Table 1. Demographic information.
| Variable (SD) | NC (N=9) | MCI (N=40) | AD (N=10) | p-value, ANOVA/Chi-Square |
|---|---|---|---|---|
| Age, yr | 76.7 (5.6) | 74.4 (8.6) | 72.4 (8.0) | 0.53 |
| Education, yr | 16.3 (2.9) | 16.0 (2.6) | 14.6 (2.8) | 0.29 |
| Gender, M:F | 5:4 | 27:13 | 4:6 | 0.27 |
ANOVA was conducted to examine differences between diagnostic categories in performances on the CF animals and vegetables tasks. There were statistically significant differences at the p<0.01 level in the CF animals scores [F(2, 56)=8.15, p=0.001]. Post-hoc comparison using a Tukey post-hoc test indicated that the mean score for NC (M=22, SD=7.68), MCI (M=16.45, SD=5.48), and AD (M=11.5, SD=4.09) groups were all significantly different from one another. As expected, AD subjects performed worse on semantic fluency than both the MCI and NC groups, and the MCI subjects performed worse than the NC subjects. There were also statistically significant differences at the p<.01 level in the CF vegetables test [F(2, 56)=7.33, p=0.001]. Once again as expected, AD subjects performed worse on semantic fluency than both the MCI and NC groups, and the MCI subjects performed worse than the NC subjects. However, post hoc comparisons indicated that while the mean scores for the NC (M=14.67, SD=5.96) and MCI (M=11.1, SD=3.81) groups were significantly different from the AD (M=7.5, SD=3.03), the MCI group mean did not differ significantly from the NC group mean (p=0.055). These data are displayed in detail in Table 2.
Table 2.
ANOVA-Diagnostic group comparison with animal and vegetable fluency (Top). Post-hoc test results of all significant diagnostic group comparisons (Bottom).
| Variable | Diagnostic Groups | F | η2 | ||
|---|---|---|---|---|---|
| NC | MCI | AD | |||
| Animal Fluency, Mean (SD) | 22 (7.7) | 16.5 (5.5) | 11.5 (4.1) | 8.15*** | 0.23 |
| Vegetable Fluency, Mean (SD) | 14.67 (6.0) | 11.1 (3.8) | 7.5 (3.0) | 7.33*** | 0.21 |
| Variable | Diagnostic Comparison | Mean Difference | SE | p-value |
|---|---|---|---|---|
| Animal Fluency | NC vs. MCI | 5.550* | 2.09 | 0.027 |
| NC vs. AD | 10.500* | 2.6 | 0.000 | |
| MCI vs. AD | 4.950* | 2 | 0.043 | |
| Vegetable Fluency | NC vs. MCI | 3.57 | 1.5 | 0.055 |
| NC vs. AD | 7.167* | 1.87 | 0.001 | |
| MCI vs. AD | 3.567* | 1.44 | 0.041 |
= The mean difference is significant at the .05 level;
= The mean difference is significant at the .001 level; SD= Standard Deviation; SE= Standard Error; NC= Normal Control; MCI= Mild Cognitive Impairment; AD = Alzheimer's disease
The global permutation-corrected map-wise significance for CF animals was pcorrected=0.01 for the left hemisphere, and pcorrected=0.06 for the right hemisphere. The global permutation-corrected map-wise significance for CF vegetables was pcorrected=0.009 for the left hemisphere, and pcorrected=0.03 for the right hemisphere.
As demonstrated in the significance and correlation maps of Figure 1, poor semantic fluency measured on the animals test was associated with cortical atrophy of the inferior parietal lobule (approximately corresponding to Brodmann's areas (BA) 39 and 40), the superior temporal gyrus (BA 22), and the premotor and dorsolateral prefrontal cortices (BA 4, 6, 8 and 9), bilaterally. More diffuse associations were detected in the left hemisphere involving the lateral (BA 10, 44, 45 and 46) and medial (BA 9,10 and 32) frontal cortices. Cortical atrophy was also detected bilaterally in the temporo-occipital (BA 37) region of the right hemisphere.
Figure 1.
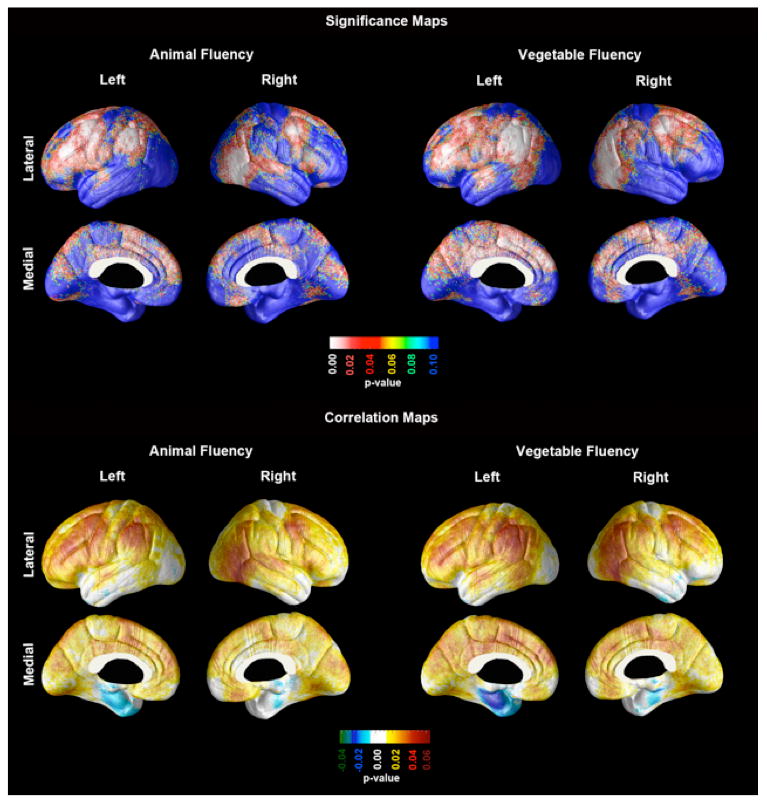
Statistical (top) and Correlation (bottom) maps demonstrating the associations between the Category Fluency Animals and Category Fluency Vegetables test scores to cortical thickness in a pooled sample of subjects diagnosed as Normal Controls, Mild Cognitive Impairment, or Probable Alzheimer's disease.
Poor semantic fluency on the vegetables test was associated with cortical atrophy of the inferior parietal lobule (BA 39 and 40) and the premotor and dorsolateral prefrontal cortices (BA 4, 6, 8, 9 and 46) bilaterally (Figure 1). More diffuse associations were seen in the lateral frontal (BA 10, 44, 45), medial frontal (BA 4,6, 8, 9 and 32), lateral temporal (BA 22), medial parietal (BA 31 and 7), and peristriate (BA 18,19) cortices on the left. Additional cortical associations were seen in the temporoparietal region (BA 37), and in the lateral visual association cortices (BA 19, 18) of the right hemisphere. Associations between cortical atrophy and CF performance were more diffuse for the vegetable fluency test than they were for animal fluency test scores.
4. Discussion
Alzheimer's disease is the most prevalent neurodegenerative disorder worldwide. It manifests with cognitive decline in multiple domains, including language, and provides us with an opportunity to study the relevance of various cortical areas to cognitive processing. In this study, we examined correlations between semantic fluency, as measured by the CF animals and vegetables tests, and cortical atrophy in normal control, MCI, and probable AD diagnosed subjects.
Correlations Between Semantic Fluency and the Left Hemisphere
Poor performance on both the CF animals and vegetables tests was associated with cortical atrophy in the left posterior temporal, parietal, cingulate, and prefrontal cortices. These cortical areas are linked to semantic and phonologic processing (McGraw, Mathews et al. 2001). Functional neuroimaging investigations into the linguistic correlates of AD have found fluency performance correlated with metabolism in the inferior parietal lobule (Schonknecht, Hunt et al. 2011), left temporal and prefrontal cortices (Welsh, Hoffman et al. 1994), temporoparietal and prefrontal cortices (Teipel, Willoch et al. 2006), and the left anterior cingulate (Hirono, Mori et al. 2001). These results correlate nicely with our own. In another study relating CERAD language tests to More specifically, the literature has implicated the left premotor areas (BA 6, 44), the inferior frontal gyrus or Broca's area (BA 44, 45, and 47), and the supplementary motor cortex (BA 8, 9) in language and semantic processing (Thompson-Schill, D'Esposito et al. 1997; Grabowski 2000; Bookheimer 2002; Gold and Buckner 2002). In addition to Broca's area, we found associations between poor semantic fluency and cortical atrophy in Wernicke's area (BA 22) and the angular and supramarginal gyri (BA 39, 40), which comprise the classic language network.
Correlations between Semantic Fluency and the Right Hemisphere
Poor performance on the CF animals and vegetables tests was associated with cortical atrophy in the right posterior temporal, temporo-occipital, parietal and prefrontal cortices. The right frontal lobe supports verbal episodic memory retrieval, as well as retrieval of semantic information (Grady 1999). Our results find less support in functional neuroimaging studies, however past studies have found fluency performance correlated to right middle and medial frontal gyri (Schonknecht, Hunt et al. 2011) and as well as the right parietal lobe (Welsh, Hoffman et al. 1994). Our additional findings may be the result of the sensitivity of the analytical methods used. Atrophy of the superior and middle temporal gyri, medial temporal region, middle and inferior frontal gyri, and the superior parietal lobule was found to be associated with poor semantic fluency in a cohort of Korean patients with AD or MCI (Ahn, Seo et al. 2011).
CF Animals and Vegetables Variability
Interestingly, verbal fluency for vegetables showed more diffuse associations than for animals. This may be a function of the size of the animals category versus the vegetables category. The category “animals” has a greater number of items and multiple levels of semantic organization (e.g., primates, reptiles, etc.). As such, it may be easier to generate information from this category than vegetables - a subordinate semantic category with fewer items and levels of semantic organization (Hodges, Salmon et al. 1992; Azuma, Bayles et al. 1997; Diaz, Sailor et al. 2004). AD patients show a disproportionate reduction in the generation of exemplars from lower order categories. This may be the result of storage degradation (Hodges, Salmon et al. 1992). With regard to the difference between cortical atrophy results in animals versus vegetables, our findings correspond to some degree with those of Ahn et al. (Ahn, Seo et al. 2011) who also found associations of semantic fluency with cortical atrophy in both hemispheres with a more diffuse association pattern for the supermarket category (more closely related to the vegetable category) as compared to the animals category. However, in our study, perhaps as a result of the more advanced analytic methods, we also find strong parietal and frontal cortices associations that were not reported by Ahn et al.
Our new findings on animal fluency also agree well with prior results from our group in a cohort of 19 AD and 5 MCI subjects who later converted to probable AD (Apostolova, Lu et al. 2008). While the previous research was based on similar cortical extraction methods, the stronger associations in the current study are likely due to both the larger sample size as well as the inclusion of cognitively normal subjects. Providing a greater range of cognitive performance strengthened the previously observed associations in the animal fluency results. We further extended our previous work by examining cortical associations with vegetable fluency - a task that imposes greater cognitive demands than animal fluency, and therefore more diffuse patterns of cortical atrophy than our previous results.
5. Conclusion
Studies of neurodegenerative disorders are useful for testing our theoretical models of brain networks. Overall, our results show that poor semantic fluency is associated with cortical atrophy in brain regions previously thought to process lexical, phonologic, and semantic representations as well as those responsible for selection and retrieval of semantic and phonological knowledge. However, our study has some limitations. Our sample size was moderate but large enough to find significant associations between verbal fluency and cortical atrophy. Additional plausible limitation lies in the fact that in AD, there is also a global atrophy pattern that is disease-specific rather than task-specific, which might pose a challenge when investigating the anatomic correlates of specific cognitive processes. If that were the case however one would expect to see strong associations with the entorhinal/perihippocampal cortex – the earliest cortical area that succumbs to cortical atrophy in AD. It is also important to note that the CF animals and vegetables tasks are language fluency measures. A task that more clearly differentiates semantic knowledge from basic language ability may further improve our understanding of the cortical areas responsible for semantic categorization and therefore the semantic component of verbal fluency. Our findings build on the data from other structural and functional imaging studies investigating the linkage between language and AD, and our results further the understanding of the neural correlates of semantic fluency. Our research demonstrates that cortical thickness is related to language performance in old age; preservation of gray matter is clearly a priority for future therapeutic research in AD.
Acknowledgments
Data used in preparing this article were obtained from the Alzheimer's Disease Neuroimaging Initiative database (www.loni.ucla.edu/ADNI). Many ADNI investigators have therefore contributed to the design and implementation of ADNI or provided data but did not participate in the analysis or writing of this report. A complete list of ADNI investigators is available at http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
We thank the members of the ADNI Imaging Core for their contributions to the image pre-processing.
The analyses reported in this manuscript were funded by the Easton Consortium for Alzheimer's Drug Discovery and Biomarker Development, NIA R01 AG040770, NIA P50 AG16570. Algorithm development was also supported, in part, by NIMH R01 MH097268 and NIA R01 AG040060 (to P.T.). O.K. was supported, in part, by a UCLA Dissertation Year Fellowship, and by the UCLA Medical Scientist Training Program.
References
- Ahn HJ, Seo SW, et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49(14):3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Ahn HJ, Seo SW, et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: A surface-based morphometric analysis. Neuropsychologia. 2011;49:3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Lu P, et al. 3D mapping of language networks in clinical and pre-clinical Alzheimer's disease. Brain and language. 2008;104(1):33–41. doi: 10.1016/j.bandl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Lu PH, et al. 3D mapping of mini-mental state examination performance in clinical and preclinical Alzheimer disease. Alzheimer disease and associated disorders. 2006;20(4):224–231. doi: 10.1097/01.wad.0000213857.89613.10. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Steiner CA, et al. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Archives of neurology. 2007;64(10):1489–1495. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T, Bayles KA, et al. Comparing the difficulty of letter, semantic, and name fluency tasks for normal elderly and patients with Parkinson's disease. Neuropsychology. 1997;11(4):488–497. doi: 10.1037//0894-4105.11.4.488. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, O'Brien JT, et al. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects. NeuroImage. 2004;23(1):325–335. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Sowell ER, et al. Mapping brain size and cortical gray matter changes in elderly depression. Biological psychiatry. 2004;55(4):382–389. doi: 10.1016/j.biopsych.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Benton AL, d HK. Multilingual apahasia examination. Iowa: AJA Associations; 1989. [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual review of neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiology of aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-284. [DOI] [PubMed] [Google Scholar]
- Canning SJ, Leach L, et al. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology. 2004;62(4):556–562. doi: 10.1212/wnl.62.4.556. [DOI] [PubMed] [Google Scholar]
- Clark LJ, Gatz M, et al. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer's disease. American journal of Alzheimer's disease and other dementias. 2009;24(6):461–468. doi: 10.1177/1533317509345154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, et al. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of computer assisted tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Diaz M, Sailor K, et al. Category size effects in semantic and letter fluency in Alzheimer's patients. Brain and language. 2004;89(1):108–114. doi: 10.1016/S0093-934X(03)00307-9. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Grabowski TD, AR . Investigating Language with Functional Neuroimaging. In: Toga AWM, JC, editors. Brain Mapping : The Systems. London, UK: Academic Press; 2000. pp. 425–461. [Google Scholar]
- Grady C. Structural and functional asymmetris of the human frontal lobes. In: Miller BL, Cummings JL, editors. The human frontal lobes: functions and disorders. New York, USA: Guilford press; 1999. pp. 196–230. [Google Scholar]
- Grundman M, Petersen RC, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of neurology. 2004;61(1):59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Gunter J, Bernstein M, Borowski B, Flemlee J, Blezek D, Mallozzi R. Validation testing of the MRI calibration phantom for the Alzheimer's Disease Neuroimaging Initiative Study. ISMRM 14th Scientific Meeting and Exhibition (2006) 2006 [Google Scholar]
- Hirono N, Mori E, et al. Neuronal substrates for semantic memory: a positron emission tomography study in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2001;12(1):15–21. doi: 10.1159/000051231. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, et al. Semantic memory impairment in Alzheimer's disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30(4):301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of magnetic resonance imaging : JMRI. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Leow AD, Klunder AD, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. NeuroImage. 2006;31(2):627–640. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC. Language processing: functional organization and neuroanatomical basis. Annual review of psychology. 2003;54:55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Gharapetian L, et al. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiology of aging. 2012;33(2):242–253. doi: 10.1016/j.neurobiolaging.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw P, Mathews VP, et al. Approach to functional magnetic resonance imaging of language based on models of language organization. Neuroimaging clinics of North America. 2001;11(2):343–353. x. [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonknecht OD, Hunt A, et al. Bihemispheric cerebral FDG PET correlates of cognitive dysfunction as assessed by the CERAD in Alzheimer's disease. Clinical EEG and neuroscience : official journal of the EEG and Clinical Neuroscience Society. 2011;42(2):71–76. doi: 10.1177/155005941104200207. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, et al. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, et al. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, et al. Mapping cortical change across the human life span. Nature neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Willoch F, et al. Resting state glucose utilization and the CERAD cognitive battery in patients with Alzheimer's disease. Neurobiology of aging. 2006;27(5):681–690. doi: 10.1016/j.neurobiolaging.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, et al. Dynamics of gray matter loss in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, et al. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. NeuroImage. 2004;23(Suppl 1):S2–18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, et al. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Hoffman JM, et al. Neural correlates of dementia: regional brain metabolism (FDG-PET) and the CERAD neuropsychological battery. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 1994;9(5):395–409. [PubMed] [Google Scholar]


