Abstract
GABAergic interneurons synchronize network activities and monitor information flow. Post-mortem studies have reported decreased densities of cortical inter-neurons in schizophrenia (SZ) and bipolar disorder (BPD). The entorhinal cortex (EC) and the adjacent subicular regions are a hub for integration of hippocampal and cortical information, a process that is disrupted in SZ. Here we contrast and compare the density of interneuron populations in the caudal EC and subicular regions in BPD type I (BPD-I), SZ, and normal control (NC) subjects. Postmortem human parahippocampal specimens of 13 BPD-I, 11 SZ and 17 NC subjects were used to examine the numerical density of parvalbumin-, somatostatin- or calbindin-positive interneurons. We observed a reduction in the numerical density of parvalbumin- and somatostatin-positive interneurons in the caudal EC and parasubiculum in BPD-I and SZ, but no change in the subiculum. Calbindin-positive interneuron densities were normal in all brain areas examined. The profile of decreased density was strikingly similar in BPD-I and SZ. Our results demonstrate a specific reduction of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region in BPD-I and SZ, likely disrupting synchronization and integration of cortico-hippocampal circuits.
Keywords: Bipolar disorder, Schizophrenia, Parahippocampal gyrus, Entorhinal cortex, Subiculum, Somatostatin, Parvalbumin, Interneurons
Introduction
One of the most consistent observations in bipolar disorder (BPD) and schizophrenia (SZ) is a disturbance of GABA-ergic neurons and markers in neocortical regions and hippocampus [8, 10]. The GABA synthesizing enzymes GAD65 and GAD67 are markedly decreased in prefrontal cortex (PFC) and hippocampus [1, 29, 45, 50, 56], as are GABA neuron subtype-specific markers such as parvalbumin (PV) and somatostatin (SOM), [5, 19, 25, 35, 58]. The entorhinal cortex (EC), a crucial element in cortico-hippocampal communication, has a decreased density of PV neurons in BPD [42] as well as abnormal cytoarchitecture with disturbed neuronal migration in SZ [3, 31]. Imaging studies point to abnormal cortico-hippocampal integration in SZ [28, 51]. We examined the density of three inter-neuron populations, defined by the markers PV, SOM and calbindin (CB), in the EC, parasubiculum, presubiculum and subiculum, linked structures that connect hippocampus and cortical areas.
The subiculum is innervated by CA1 pyramidal axons from the hippocampus [41] and projects to the deep layers of the EC as well as the parasubiculum [54]. The parasubiculum is innervated by projections from subiculum and CA1 [49]. Parasubiculum and presubiculum project to the superficial layers of the EC [52]. Output of the EC is layer-specific: the superficial layers of the EC project to the dentate gyrus of the hippocampus, whereas the deep layers of the EC project to cortical regions [52].
The information flow through the EC is monitored by local GABA interneurons that synchronize network activity [11]. Interneurons can be classified histologically based on the presence of calcium-binding proteins such as PV and CB, or neuropeptides such as SOM [12, 38].
PV-positive interneurons terminate on the initial axon segments of pyramidal cells [18], are ‘fast-spiking’, and are connected to each other via chemical synapses as well as electrical gap junctions [22]. In SZ and BPD, a reduction in cortical PV mRNA levels and neurons has been reported [5, 26, 32, 35, 42, 47, 58]. This reduction has been postulated to cause gamma oscillation deficits and a disruption of synchronization of neural activity, and could explain some symptoms observed in BPD and SZ [4, 55].
CB-immunoreactive interneurons terminate on distal pyramidal cell dendrites and are non-fast-spiking [17, 57]. Studies of CB in BPD or SZ are inconsistent. In SZ, levels of CB mRNA and density of neurons have been reported as increased [15, 19], unchanged [46], and decreased [6, 13, 43], whereas studies in BPD reported non-significant reductions [6, 13, 43].
Neurons containing the neuropeptide SOM synapse onto dendrites of pyramidal cells [37]. SOM-positive cells are low-threshold-spiking and are connected via electrical gap junctions in addition to chemical synapses [7]. SOM mRNA levels and cell densities are reduced in SZ [19, 21, 24, 34, 39] as well as BPD [33, 35], with one report of upregulations in mRNA levels [40].
Here we examine PV, SST and CB interneuron populations in EC and subicular regions in BPD type 1 (BPD-I) and SZ, two disorders accompanied by psychosis [14], to test the hypothesis that a similar pattern of interneuron population changes may be shared by major psychoses.
Materials and methods
Sample collection
Brains were collected at the Harvard Brain Tissue Resource Center (HBTRC; McLean Hospital, Belmont, MA, USA). The HBTRC is funded by NIH and follows all regulations implemented by the Office for Human Research Protections.
For all the subjects included in this study, two psychiatrists established DSM-IV diagnoses based on the review of a questionnaire filled out by legal next of kin and a review of all available medical records. Normal control (NC) cases had sufficient information from next of kin and medical records to rule out major medical, neurologic, and psychiatric conditions. All brains underwent a neuro-pathological examination and cases with histopathological abnormalities were excluded from this study.
Three diagnostic groups, comprised of 17 NC cases, 13 BPD-I cases and 11 SZ cases were matched for gender, age, post-mortem interval, and hemisphere (Table 1). Because PV staining was crucial for the delineation of brain regions, SOM- and CB samples could only be used if PV staining as well as SOM or CB staining were of good quality in adjacent slices.
Table 1.
Demographic data of study subjects
| Diagnosis | Hemisphere | Gender | PMI | Age | Fresh brain weight (g) | Cause of death | Included in PV | Included in SOM | Included in CB |
|---|---|---|---|---|---|---|---|---|---|
| BPD | R | M | 32.9 | 83 | 1,220 | Congestive heart failure | x | x | |
| BPD | L | M | 30.8 | 40 | 1,385 | Suicide | x | x | |
| BPD | L | F | 13.4 | 62 | 1,210 | Congestive heart failure | x | x | x |
| BPD | R | F | 21.9 | 40 | 1,340 | Sepsis | x | x | x |
| BPD | L | M | 22.0 | 38 | 1,290 | Co poisoning | x | x | x |
| BPD | R | F | 33.3 | 77 | 1,400 | Pneumonia | x | x | |
| BPD | L | F | 24.2 | 23 | 1,340 | Suicide | x | x | x |
| BPD | L | F | 17.2 | 52 | 1,140 | Liver failure | x | x | x |
| BPD | R | F | 24.8 | 78 | 1,180 | Dehydration | x | x | x |
| BPD | L | F | 16.3 | 47 | 1,000 | Major systems failure | x | x | x |
| BPD | L | F | 35.1 | 51 | 1,260 | Ischemic heart disease | x | x | |
| BPD | L | F | 22.6 | 79 | 1,085 | Ovarian cancer | x | x | x |
| BPD | R | M | 17.9 | 18 | 1,490 | Motor vehicle accident | x | x | x |
| SZ | L | F | 23.0 | 49 | 1,240 | Pulmonary embolism | x | ||
| SZ | R | F | 22.0 | 44 | 1,265 | Cardiopulmonary arrest | x | x | x |
| SZ | R | F | 18.7 | 56 | 1,185 | Cardiopulmonary arrest | x | x | x |
| SZ | R | M | 33.3 | 41 | 1,610 | Cardiopulmonary arrest | x | x | |
| SZ | L | F | 28.6 | 74 | 1,325 | Pneumonia | x | x | x |
| SZ | L | F | 15.7 | 85 | 1,200 | Cardiopulmonary arrest | x | x | x |
| SZ | R | M | 14.8 | 52 | 1,280 | Cardiopulmonary arrest | x | x | x |
| SZ | R | M | 25.3 | 62 | 1,340 | Sepsis | x | x | x |
| SZ | R | M | 18.0 | 36 | 1,480 | Suicide (OD) | x | x | |
| SZ | L | M | 32.4 | 58 | 1,160 | Chronic obstruction pulmonary disease | x | x | x |
| SZ | R | M | 21.4 | 68 | 1,255 | Cardiopulmonary arrest | x | x | x |
| NC | R | M | 21.5 | 22 | 1,360 | Myocardial infarct | x | x | x |
| NC | R | F | 23.9 | 68 | 1,390 | Chronic obstruction pulmonary disease | x | x | x |
| NC | L | F | 12.5 | 60 | 1,160 | Breast cancer | x | x | x |
| NC | L | M | 24.6 | 77 | 1,190 | Cardiopulmonary arrest | x | x | |
| NC | L | F | 23.0 | 74 | 1,100 | Pneumonia | x | x | x |
| NC | R | M | 27.2 | 41 | 1,815 | Cardiopulmonary arrest | x | x | x |
| NC | L | M | 18.4 | 68 | 1,520 | Heart failure | x | x | x |
| NC | R | F | 23.1 | 51 | 1,375 | Cardiopulmonary arrest | x | x | x |
| NC | R | F | 27.5 | 55 | 1,245 | Cardiopulmonary arrest | x | x | x |
| NC | R | F | 17.4 | 81 | 1,135 | Colon cancer | x | x | x |
| NC | L | F | 20.3 | 42 | 1,480 | Myocardial infarct | x | x | x |
| NC | R | F | 18.1 | 36 | 1,390 | Cardiopulmonary arrest | x | x | x |
| NC | R | M | 14.8 | 30 | 1,570 | Suicide | x | x | x |
| NC | L | M | 13.1 | 52 | 1,450 | Heart disease | x | x | x |
| NC | R | M | 28.3 | 61 | 1,510 | Myocardial infarct | x | x | x |
| NC | R | F | 17.8 | 60 | 1,220 | Cardiac dysrhythmia | x | x | x |
| NC | L | M | 30.3 | 60 | 1,190 | Cardiopulmonary arrest | x | x | |
|
| |||||||||
| PV | |||||||||
| BPD | 5R/8L | 4M/9F | 24.0 ± 7.1 | 52.9 ± 21.6 | 1,256.9 ± 136.9 | n = 13 | |||
| SZ | 7R/4L | 6M/5F | 23.0 ± 6.3 | 56.8 ± 14.8 | 1,303.6 ± 134.4 | n = 11 | |||
| NC | 10R/7L | 8M/9F | 21.3 ± 5.4 | 55.2 ± 16.6 | 1,358.8 ± 189.2 | n = 17 | |||
| SOM | |||||||||
| BPD | 5R/8L | 4M/9F | 24.0 ± 7.1 | 52.9 ± 21.6 | 1,256.9 ± 136.9 | n = 13 | |||
| SZ | 6R/3L | 5M/4F | 23.6 ± 6.8 | 60.0 ± 14.1 | 1,291.1 ± 134.2 | n = 9 | |||
| NC | 10R/6L | 7M/9F | 21.1 ± 5.5 | 53.8 ± 16.2 | 1,369.4 ± 190.1 | n = 16 | |||
| CB | |||||||||
| BPD | 3R/6L | 2M/7F | 20.0 ± 3.9 | 48.6 ± 21.7 | 1,230.6 ± 150.1 | n = 9 | |||
| SZ | 6R/3L | 5M/4F | 21.9 ± 5.9 | 59.4 ± 15.0 | 1,276.7 ± 97.6 | n = 9 | |||
| NC | 10R/6L | 7M/9F | 20.7 ± 5.0 | 54.9 ± 17.1 | 1,369.4 ± 190.1 | n = 16 | |||
Cohorts for cell counts are identified in the last three columns. Mean (±SD) demographic data for cohorts are shown at the bottom of the table.
No significant differences were observed in any parameter
Tissue collection and processing
Tissue was immersion-fixed in 4.0% paraformaldehyde (0.1 M phosphate buffer (PBS), pH 7.4) at 4.0°C for 3 weeks and placed in cryoprotectant (0.1 M PBS, pH 7.4/0.1% sodium azide/30.0% ethylene glycol/30.0% glycerol), immersed in agar and cut into 2.5 mm thick coronal slabs using an antithetic tissue slicer. Sections were cut on a sliding microtome (American Optical Company, Buffalo, NY, USA), with a thickness of 100 μm for Nissl stain, or 50 μm for immunocytochemistry. Sections were mounted on gelatin-coated glass slides and consecutive slices within a tissue block were stained with 0.1% cresyl violet (Nissl stain) or used for PV, SOM and CB immunocytochemistry.
Immunocytochemical procedure
Sections were rinsed in 0.01 M phosphate buffered saline with 0.5% Triton-X100 (PBS-TX) for 3 × 5 min, boiled in Vector Antigen Unmasking Solution (1:100 in 0.1 M PBS; Vector laboratories, Burlingame, CA, USA) for 3 min, rinsed in PBS-TX for 3 × 10 min, incubated in 0.03% hydrogen peroxide solution in 0.01 M PBS for 30 min, rinsed in PBS-TX for 3 × 5 min, incubated in 2% bovine serum albumin (BSA; Fraction V; Sigma, MO, USA) in 0.01 M PBS for 1 h and rinsed in PBS-TX for 3 × 5 min. Sections were incubated with primary antibody in 0.01 M PBS with 1% BSA for 72 h at 4°C. Sections were rinsed in PBS-TX 3 × 5 min and incubated in biotinylated secondary antibody in 0.01 M PBS with 1% BSA for 2 h. After 3 × 5 min rinses in PBS-TX, sections were incubate with streptavidin horseradish peroxidase (HRP) conjugate (1:5,000; Zymed-Invitrogen; Carlsbad, CA, USA) in 0.01 M PBS with 1% BSA, rinsed 3 × 5 min, and incubated for 2–10 min in DAB solution (Sigma) in 0.1 M PBS with 0.003% hydrogen peroxide and nickel sulfate. Sections were transferred to PBS-TX and washed 3 × 5 min. Unless noted otherwise, all steps were carried out at room temperature [35].
Antibodies and dilutions: the PV antibody was diluted 1:10,000 (monoclonal mouse anti frog muscle parvalbumin, clone PARV-19; Sigma); the SOM antibody was diluted 1:500 (monoclonal rat anti synthetic cyclic somatostatin peptide corresponding to amino acids 1–14; Millipore, Billerica, MA, USA); the CB antibody was diluted 1:8,000 (monoclonal mouse anti-calbindin D-28K, clone CB-955; Sigma). Secondary antibodies were biotin-ylated, goat anti-mouse IgG for PV and CB (Vector laboratories), and goat anti-rat IgG for SOM. Secondary antibodies were diluted 1:500.
For each immunocytochemical labeling, all sections were processed simultaneously to avoid procedural differences. Each staining dish contained sections from SZ, BPD-I and NC cases and all dishes were treated for the same duration.
Cell counts
All cases were coded and data collection was performed without the knowledge of diagnosis. Morphometric analysis was carried out using a Zeiss Axioskop 2 Plus microscope (Germany) equipped with a LEP MAC 5000 automated stage (Ludl Electronic Products, Hawthorne, NY, USA). The microscope was interfaced with the Stereo Investigator stereological software (v 6.55, Microbrightfield, Colchester, VT, USA) via an Optronics DEI-750 video camera (Goleta, CA, USA). All regions were outlined using a 1.25× objective lens and cells were marked and counted with a 20× objective lens. All immunopositive cells were counted within each region on each slice. Three guidelines were applied to include a cell into the neuron count: size and shape of the body, presence of processes, and staining intensity. To be consistent across all samples, only slides with a particular PV staining pattern (as seen in Fig. 1b) were included in the analysis, amounting to one to three slices per case at 2.5 mm intervals, which were averaged for each case. Average number of slices per case was 1.8 for PV, 1.5 for SOM and 1.6 for CB. All data are presented as cells/mm2 in sections of 50 μm thickness. The entire dataset was counted by the same investigator who was blinded to the diagnosis. Cases were randomized for the counting.
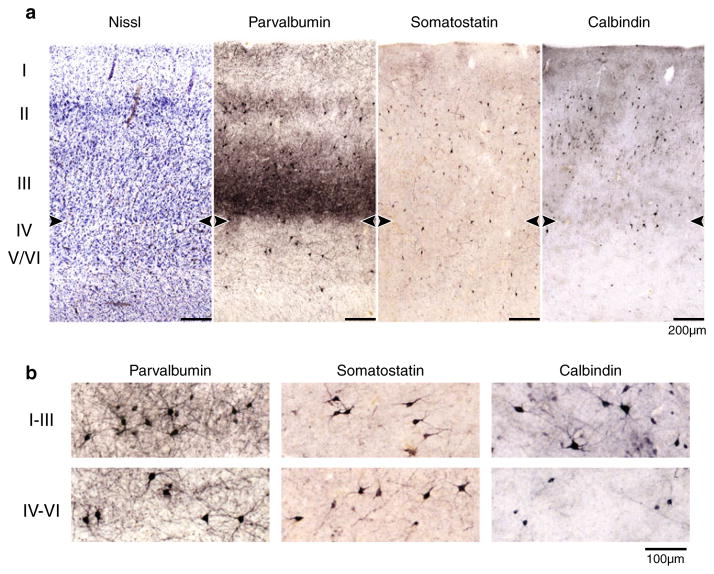
Fig. 1.
Overview of subiculum, presubiculum, parasubiculum and EC in a 42-year-old female NC subject. Regional borders are marked by arrowheads, boxes demarcate areas shown in subsequent figures. Neurons were counted within the entire region between two arrowheads. a Nissl stain; b PV immunocytochemistry, c SOM immunocytochemistry, d CB immunocytochemistry. Note the characteristic pattern in the PV stain. In the Nissl stain, a widening of the pyramidal cell layer between CA1 and subiculum defined the medial subicular border, whereas the narrowing of the pyramidal layer between subiculum and presubiculum defined the lateral subicular border
Delineation of brain regions
Interneuron numerical density in subicular regions and the EC was examined in the caudal part of the rostral–caudal axis [30]. In this area, the highest density of PV-positive neurons is observed in the EC in a very distinctive macroscopic pattern [42]. This pattern was used to ensure that all cases are counted in the same anatomical region.
To determine the borders between CA1/subiculum, subiculum/presubiculum, presubiculum/parasubiculum, parasubiculum/EC, and superficial layers EC/deep layers EC, we used a combination of structural markers and neuronal density, primarily based on Nissl stain and PV staining of the neuropil, with emphasis on reproducibility of markers across all cases (Fig. 1).
The border between CA1 and subiculum was determined in the Nissl-stained samples by the widening of the pyramidal cell layer in the subiculum and the loss of the stratum radiatum (Fig. 1a) [16]. Similar in structure to the hippocampus, the subiculum has a three-layered cortex (Fig. 2a). Darker stained neuropil patches were particularly visible in the PV and CB staining. These patches also contained groups of small CB-positive neurons (Fig. 2b). The border between the subiculum and the presubiculum was determined by the narrowing of the pyramidal cell layer and the increased density of small pyramidal cells in the superficial layer of the presubiculum (Lamina principalis externa), which frequently formed cellular islands that were densely innervated by PV-positive and CB-positive fibers (Figs. 1b, d, 3a), [16]. In preparations 5 mm caudal to the example used in Fig. 3a, the overlapping pattern between the dense cellular islands in the Nissl stain and the dark neuropil staining in the PV (Fig. 3b) as well as CB immunocytochemistries (not shown) was evident. Inter-neurons in the lamina principalis externa were generally smaller than interneurons in the lamina principalis interna (Fig. 3c). Due to the somewhat irregular borders between the two laminae and the changing pattern between adjacent slices (compare Fig. 1b–d), neuron counts were not separated for the two laminae.
Fig. 2.
a Comparison of Nissl stain, PV immunocytochemistry, SOM immunocytochemistry and CB immunocytochemistry in the subiculum. The pyramidal cell layer (II) is located between two distinct layers. Stars denote the same blood vessel in all tissue slices. b Representative neurons. Note the different populations of CB-positive neurons
Fig. 3.
a Comparison of Nissl stain, PV immunocytochemistry, SOM immunocytochemistry and CB immunocytochemistry in the presubiculum, a transition zone between 3-layered hippocampus and 6-layered neocortex. The presubiculum was divided into lamina principalis externa and interna. Stars denote the same blood vessels in all tissue slices. b A lower magnification of an adjacent slice of the same subject shows the overlap between cell-dense areas in the Nissl stain and darkly stained neuropil in the PV immunocytochemistry. Arrowheads denote boundaries of the presubiculum. c Representative neurons from each lamina
The parasubiculum was defined as the area between presubiculum and EC [16] (Fig. 4). The parasubiculum has a neocortical architecture with six layers, in which PV staining of the neuropil was lighter (Figs. 1b, 4a). Density of PV- and CB-positive neurons was higher in the superficial layers, and patches of lightly stained CB-positive neurons were interspersed with darker stained neurons (Fig. 4b).
Fig. 4.
a Comparison of Nissl stain, PV immunocytochemistry, SOM immunocytochemistry and CB immunocytochemistry in the parasubiculum. To improve contrast of layers II–VI, layer I has been overexposed in the Nissl stain. Note: the top torn part of the SOM sample was slightly rotated for better fit in the allocated space (compare to unaltered Fig. 1). b Representative neurons from layers II/III and IV–VI
In the six-layered EC [53], layers I, II, and III were delineated as superficial layers, and layers IV, V, and VI as deep layers [16]. Both layers were counted separately. Layer III, which contains medium-sized pyramidal cells [16], had strongly stained neuropil in the PV immunocytochemistry [48], (Fig. 5a); layer IV, the lamina dissecans, is marked by an absence of neurons [20]. The border between the superficial and deep layers of the EC was drawn based on darker PV neuropil staining in layer III, and the absence of cells in layer IV (Nissl stain). CB-positive neurons in the deep layers were smaller and lighter stained, while in the superficial layer darkly stained neurons with rich arborization were interspersed with lighter stained neurons (Fig. 5b). The PV staining observed in the EC was comparable to the literature [44].
Fig. 5.
a Comparison of Nissl stain, PV immunocytochemistry, SOM immunocytochemistry and CB immunocytochemistry in the EC. The border between layers III and IV was defined by the contrast in neuropil staining in the PV samples and the neuron-free layer IV in the Nissl stain, and is marked by arrowheads. b Representative neurons from layers I–III and IV–VI
The contours for SOM (Fig. 1c) and CB (Fig. 1c) staining were based predominantly on the contours of PV (Fig. 1b) staining and Nissl stain (Fig. 1a), which were from adjacent sections and adjusted for minor differences in cytoarchitecture. Cases in which regions could not be clearly delineated or immunostaining was insufficient were excluded from the analysis.
Statistical analysis
Study subjects were initially closely matched for demographic factors, but exclusion of poorly stained or otherwise damaged sections introduced some variability (Table 1). Although no group had statistically significant differences in demographic data, analyses of covariance (ANCOVA) were applied to all results, with diagnosis as the independent variable and age, gender and PMI as covariates. Initial analysis included all three groups (NC, BPD-I and SZ), followed by post-hoc ANCOVAs with diagnosis, age, gender and PMI as covariates.
Results
Pattern of PV-, SOM-, and CB-positive neurons in parahippocampal regions in NC cases
CB-positive neurons had the highest density in all regions examined (Fig. 6a, b, c). This was in large part due to CB-clusters scattered throughout all regions, predominantly in the superficial layers and frequently only lightly stained (Figs. 2, 3, 4, 5, 6). The density of PV-positive cells was higher than the density of SOM-positive cells in all brain regions except for the parasubiculum and the deep layers of the EC (Fig. 6a, b).
Fig. 6.
Box- and Whisker plots of interneuron counts/mm2 in BPD-I, SZ and NC subjects. a PV-positive interneuron density, BPD = 13, SZ = 11, NC = 17. b SOM-positive interneuron density, BPD = 13, SZ = 9, NC = 16; c CB-positive interneuron density, BPD = 9, SZ = 9, NC = 16. d Decrease in SOM neuron count with increasing age in subiculum, parasubiculum and EC in NC subjects. R2 values, F ratios and p values are presented in the right upper corner of each plot. Significant correlations were also seen in presubiculum and in the superficial as well as deep layers of the EC. Boxes in (a–c) cover the 25th to 75th quartiles; lower dashed line starts at the minimum sample number, upper dashed line ends at the maximum sample number. Outliers are shown in diamond shape. The horizontal line shows the median sample number. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 of two-group ANCOVAs with diagnosis as independent variable and age, gender and PMI as covariates (see “Results” for data)
The subiculum and deep layers of the EC were consistently among the areas with the lowest density of PV-, SOM-, and CB-positive neurons, while the superficial EC had the highest density in all immunocytochemical stainings (Fig. 6).
Density of PV-positive cells in subicular regions and EC of subjects with BPD-I, SZ, or NC
ANCOVA of the subiculum and the presubiculum with all three diagnostic groups showed no difference in PV cell density (subiculum: F 5,34 = 1.6, p = 0.218; presubicu-lum: F 5,35 = 0.4 p = 0.685; age, gender, PMI as covariates). PV cell density in the parasubiculum was significantly different between the diagnostic groups (F 5,35 = 7.2 p = 0.002; age, gender, PMI as covariates). Post-hoc ANCOVAs showed a reduction in cell density in BPD-I as well as SZ (Fig. 6a). The superficial layers, deep layers and combined layers (‘all’) of the EC had significant differences in PV cell density between the diagnostic groups (superficial EC: F 5,34 = 4.2, p = 0.024; deep EC: F 5,34 = 4.4, p = 0.020; all EC: F 5,34 = 4.6, p = 0.017; age, gender, PMI as covariates). PV cell density in the superficial layers of the EC was significantly reduced in SZ, with a trend in BPD-I, whereas in the deep layers of the EC BPD-I subjects showed a significant reduction. Both disorders showed a reduction when data for all layers were combined (Fig. 6a).
Density of SOM-positive cells in subicular regions and EC of subjects with BPD-I, SZ, or NC
ANCOVA of the subiculum for all three diagnostic groups showed no difference in SOM cell density (F 5,32 = 2.2, p = 0.122; age, gender, PMI as covariates). SOM cell density in the presubiculum, parasubiculum, superficial layers, deep layers and combined layers (‘all’) of the EC was significantly different between the diagnostic groups (presubiculum: F 5,32 = 3.8, p = 0.032; parasubiculum: F 5,32 = 7.6 p = 0.002; superficial EC: F 5,32 = 7.4 p = 0.002; deep EC: F 5,31 = 3.8 p = 0.035; all EC F 5,31 = 7.3 p = 0.003; age, gender, PMI as covariates). Post-hoc ANCOVAs showed a reduction in SOM cell density in BPD-I and SZ in parasubiculum, superficial layers of EC and combined layers of EC, while in presubiculum SZ subjects only, and in the deep layers of EC BPD-I subjects only showed a significant reduction (Fig. 6b). Interestingly, the density of SOM-positive neurons declined with age, a situation that was factored into our analysis (Fig. 6d). PV and CB densities were not affected by age, gender or PMI, and SOM densities were not affected by gender or PMI.
Density of CB-positive cells in subicular regions and EC of subjects with BPD-I, SZ, or NC
The density of CB neurons was similar in all diagnostic groups across all brain regions examined (Fig. 6c).
Discussion
In both psychiatric disorders, we observed a gradient of reduced neuron density, with no changes in the subiculum and significant reductions in the EC. The pattern of reduced density was similar for PV- and SOM stains, pointing to a pathological abnormality in psychotic disorders that affects both interneuron populations and is region-specific. The brain areas examined are tightly interconnected with each other, as well as with the hippocampus and the PFC [23, 41, 49, 52, 54]. Despite this interconnection, the subiculum showed normal interneuron density whereas hippocampus and EC had lower PV- and SOM densities in BPD-I and SZ [34, 35, 42]. Unlike PV and SOM-positive neurons, CB neurons had a normal density in all brain regions examined in both disorders, indicating that the reduction in inter-neuron density is not only specific for brain region, but also specific for particular interneuron populations.
BPD-I and SZ had similar patterns of reduced PV and SOM cell density, and no reduction in CB cell density, adding to the notion that these two disorders share significant pathological roots [9, 14]. Two exceptions to these similarities were observed: (1) the SOM staining in the presubiculum showed reduced neuron density exclusively in SZ, and (2) PV- and SOM-neuron density was reduced in the deep layers of the EC in BPD-I, but did not reach significance in SZ.
The decrease in PV-immunopositive neurons in the EC of BPD-I subjects is consistent with a previous report by Pantazopoulos et al. [42]. These authors observed a trend toward a decrease of PV neurons in the caudal EC of subjects with SZ, while the present study found a significant reduction [42]. Specifics of the subject cohort and the area of caudal EC investigated may account for this slight discrepancy between these reports.
The presubiculum connects to PFC and hippocampus [23, 52]. Loss of SOM-positive neurons in this area reduces synchronized network activity [11] and could be a central contributor to the disrupted cortico-hippocampal integration observed in SZ [28, 51]. However, interneuron deficits in the deep layers of EC, predominantly observed in BPD-I, could equally affect communication between EC and PFC and disrupt cortico-hippocampal integration in BPD-I [2, 52].
Like any experimental approach to examine the biological underpinnings of psychiatric disorders, postmortem studies have limitations. For example, we cannot answer the question if the reduced density of immuno-positive PV and SOM neurons is due to a lower density of neurons or due to the loss of GABA-specific markers synthesized by these neurons. Total cell counts within cortical areas capture predominantly the vast number of pyramidal neurons which are unchanged in BPD and SZ [27, 35], making it possible that a loss of the small population of interneurons remains undetected. This has been demonstrated in previous studies in which we counted subtype-specific interneurons throughout the entire hippocampus and compared them to stereological estimates of all neurons [34, 35]. While we found a large reduction of PV-and SOM-positive interneurons in BPD-I and SZ, the number of missing cells was smaller than the non-significant reduction in total neuron number estimates.
A second question inherent in post-mortem studies is the effect of drug treatment. Although we have carried out extensive studies in rats on the effect of chronic treatment with antipsychotic drugs or mood stabilizers [35, 36], the treatment durations are not comparable to the many years of treatment of patients. Thus, while there was no change of GABA markers with any treatment in rats, we cannot rule out a treatment effect in the present study.
Finally, density estimates are biased and affected by factors such as differences in tissue shrinkage. Unfortunately, extensive pilot testing showed that we could not use the optical disector or fractionator to quantify immunocytochemically stained neurons for unbiased sampling in the z axis. Three factors prevented us from doing this: (1) the relatively thin (50 μm) sections, chosen to maximize antibody penetration, collapsed to less than 10 μm, making it impossible to place a sufficiently large disector box; (2) precipitation of the immunoreaction product resulted in different somal shapes of immunoreactive cells; and (3) the intensity of the immunocytochemical stains made it often impossible to identify a unique structure for a disector count. However, we found that reduced cell density was specific to some brain regions and some interneuron subtypes within the same histological preparation, which substantially alleviates concerns about shrinkage artifacts.
The present study adds to the growing literature on pathological mechanisms in BPD. The patients in the study were diagnosed with BPD-I and all had presented with psychosis. The pattern of interneuron deficits in these patients was remarkably similar to the pattern seen in SZ, both in the brain areas affected as well as the interneuron subtypes affected. The overlap between both disorders suggests that measurable pathophysiological abnormalities map onto the dimension of psychosis rather than the categorical distinction of schizophrenia versus bipolar disorder.
Our findings also add a number of new aspects to the increasing literature on interneuron pathology in the cortex in SZ. We show that parahippocampal regions have reduced interneuron density and that the reduction does not affect all interneuron populations. The observed abnormalities indicate de-synchronization of network activity and abnormal cortico-hippocampal integration as observed in SZ and BPD-I.
Acknowledgments
This work was supported by the National Institute of Mental Health [MH67999 (SH) and MH068855 (Dr. Benes, HBTRC)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institute or the National Institutes of Health. The authors are indebted to Dr. Gary Van Hoesen who advised on the layers of the parahippocampal regions.
Abbreviations
- BPD
Bipolar disorder
- SZ
Schizophrenia
- NC
Normal control
- EC
Entorhinal cortex
- PFC
Prefrontal cortex
- PV
Parvalbumin
- SOM
Somatostatin
- CB
Calbindin
Contributor Information
Alice Y. Wang, Neuroscience Program for Undergraduates, Vanderbilt University, Nashville, TN, USA
Kathryn M. Lohmann, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA
C. Kevin Yang, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA.
Eric I. Zimmerman, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA
Harry Pantazopoulos, Translational Neuroscience Laboratory, McLean Hospital, Belmont, MA, USA. Department of Psychiatry, Harvard Medical School, Boston, USA.
Nicole Herring, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA.
Sabina Berretta, Translational Neuroscience Laboratory, McLean Hospital, Belmont, MA, USA. Department of Psychiatry, Harvard Medical School, Boston, USA.
Stephan Heckers, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA.
Christine Konradi, Email: christine.konradi@vanderbilt.edu, Department of Psychiatry, Vanderbilt University, Nashville, TN, USA. Department of Pharmacology, Vanderbilt University, MRB 3, Room 8160A, 465 21st Avenue South, Nashville, TN 37232-8548, USA.
References
- 1.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 2.Apergis-Schoute J, Pinto A, Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci. 2006;24:135–144. doi: 10.1111/j.1460-9568.2006.04894.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- 4.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 5.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 6.Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 7.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 9.Berrettini W. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet. 2003;123C:59–64. doi: 10.1002/ajmg.c.20014. [DOI] [PubMed] [Google Scholar]
- 10.Bird ED, Spokes EG, Barnes J, MacKay AV, Iversen LL, Shepherd M. Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet. 1977;2:1157–1158. doi: 10.1016/s0140-6736(77)91542-2. [DOI] [PubMed] [Google Scholar]
- 11.Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 12.Carboni AA, Lavelle WG, Barnes CL, Cipolloni PB. Neurons of the lateral entorhinal cortex of the rhesus monkey: a Golgi, histochemical, and immunocytochemical characterization. J Comp Neurol. 1990;291:583–608. doi: 10.1002/cne.902910407. [DOI] [PubMed] [Google Scholar]
- 13.Cotter D, Landau S, Beasley C, et al. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 14.Craddock N, O’Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daviss SR, Lewis DA. Local circuit neurons of the pre-frontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res. 1995;59:81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- 16.De Lacalle S, Lim C, Sobreviela T, Mufson EJ, Hersh LB, Saper CB. Cholinergic innervation in the human hippocampal formation including the entorhinal cortex. J Comp Neurol. 1994;345:321–344. doi: 10.1002/cne.903450302. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989;503:49–54. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- 18.DeFelipe J, Hendry SH, Jones EG. Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc Natl Acad Sci USA. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 20.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel SM, Davidson M, Haroutunian V, et al. Neuro-peptide deficits in schizophrenia vs. Alzheimer’s disease cerebral cortex. Biol Psychiatry. 1996;39:82–91. doi: 10.1016/0006-3223(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 22.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry. 1991;48:1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- 28.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 29.Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- 30.Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM. The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol. 1995;355:171–198. doi: 10.1002/cne.903550203. [DOI] [PubMed] [Google Scholar]
- 31.Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- 32.Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620. 544. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 33.Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 34.Konradi C, Yang CK, Zimmerman EI, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konradi C, Zimmerman EI, Yang CK, et al. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011;68:340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald ML, Eaton ME, Dudman JT, Konradi C. Antipsychotic drugs elevate mRNA levels of presynaptic proteins in the frontal cortex of the rat. Biol Psychiatry. 2005;57:1041–1051. doi: 10.1016/j.biopsych.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 38.Mikkonen M, Soininen H, Pitkanen A. Distribution of parvalbumin-, calretinin-, and calbindin-D28k-immunoreactive neurons and fibers in the human entorhinal cortex. J Comp Neurol. 1997;388:64–88. [PubMed] [Google Scholar]
- 39.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatani N, Hattori E, Ohnishi T, et al. Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum Mol Genet. 2006;15:1949–1962. doi: 10.1093/hmg/ddl118. [DOI] [PubMed] [Google Scholar]
- 41.O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61:640–652. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai T, Oshima A, Nozaki Y, et al. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. doi: 10.1111/j.1440-1789.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt S, Braak E, Braak H. Parvalbumin-immunoreactive structures of the adult human entorhinal and transentorhinal region. Hippocampus. 1993;3:459–470. doi: 10.1002/hipo.450030407. [DOI] [PubMed] [Google Scholar]
- 45.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase (67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Tooney PA, Chahl LA. Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:273–278. doi: 10.1016/j.pnpbp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Tunon T, Insausti R, Ferrer I, Sobreviela T, Soriano E. Parvalbumin and calbindin D-28K in the human entorhinal cortex. An immunohistochemical study. Brain Res. 1992;589:24–32. doi: 10.1016/0006-8993(92)91157-a. [DOI] [PubMed] [Google Scholar]
- 49.van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- 50.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 51.Weiss AP, Goff D, Schacter DL, et al. Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biol Psychiatry. 2006;60:1268–1277. doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 52.Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- 53.Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal–hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 55.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 57.Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]