Summary
Induction of prodynorphin gene expression by psychostimulant drugs may represent a compensatory adaptation to excessive dopamine stimulation and may contribute to the aversive aspects of withdrawal. We therefore investigated the molecular mechanisms by which dopamine psychostimulant drugs induce prodynorphin gene expression in vivo and in rat primary striatal cultures. We demonstrate that three recently described cAMP response elements (CREs), rather than a previously reported noncanonical AP-1 site, are critical for dopamine induction of the prodynorphin gene in striatal neurons. CRE-binding protein (CREB) binds to these CREs in striatal cell extracts and is phosphorylated on Ser-133 after dopamine stimulation in a D1 dopamine receptor-dependent manner. Surprisingly, following chronic administration of amphetamine, levels of phosphorylated CREB are increased above basal in rat striatum in vivo, whereas c-fos mRNA is suppressed below basal levels. D1 receptor-mediated CREB phosphorylation appears to mediate adaptations to psychostimulant drugs in the striatum.
Introduction
Acute administration of the psychostimulant drugs cocaine and amphetamine produces euphoria in humans and reward in animal models (Gawin and Ellinwood, 1988; Gawin, 1991). Repeated administration with adequate dose, frequency, and chronicity can produce significant behavioral changes, including sensitization, tolerance, and dependence. Although the psychostimulants do not produce a physical withdrawal syndrome in humans, drug discontinuation following chronic administration results in a psychological withdrawal syndrome characterized by dysphoria, anhedonia, and drug craving (Gawin and Ellin-wood, 1988; Gawin, 1991).
The mechanisms underlying the acute, reinforcing actions of the psychostimulants have been shown to depend on potentiation of dopaminergic neurotransmission in mesolimbic pathways, especially projections from the ventral tegmental area to a region of the ventral striatum, the nucleus accumbens (Butcher et al., 1988; Di Chiara and Imperato, 1988a; Carboni et al., 1989; Hurd et al., 1989). However, the mechanisms underlying the longer-term effects of psychostimulant administration are not yet understood. Altered release of dopamine has not been consistently observed in slices of striatum or nucleus accumbens derived from rats repeatedly exposed to cocaine (Kalivas and Duffy, 1988; Peris et al., 1990). In addition, no consistent changes have been observed in dopamine transporter levels or D1 dopamine receptor levels after chronic cocaine administration (Peris et al., 1990). The lack of consistent changes in dopamine release or in dopamine transporter or dopamine receptor protein levels following repeated psychostimulant administration raises the possibility that the alterations in synaptic function underlying drug dependence involve changes in postreceptor intracellular signaling. The requirement for repeated drug administration and the persistent nature of dependence suggest the involvement of drug-induced alterations in gene expression within critical neural circuits (Nestler, 1992; Nester et al., 1993; Hyman and Nestler, 1993).
D1 dopamine receptors play a critical role in the reinforcing properties of psychostimulant drugs. Pharmacological studies demonstrate that pretreatment with the dopamine receptor antagonist SCH-23390 alters cocaine self-administration in rat (Koob et al., 1987; Maldonado et al., 1993) and primate (Bergman et al., 1990). Although the SCH-23390 compound is selective for both D1 and D5 dopamine receptors, the lack of intrastriatal D5 dopamine receptors (Tiberi et al., 1991) makes it likely that D1 dopamine receptors within the striatum mediate this effect. We hypothesize that the powerful, prolonged activation of D1 dopamine receptor-mediated signaling pathways that occurs with chronic psychostimulant administration leads to compensatory adaptations downstream of D1 receptors that may contribute to the drug-dependent state.
In the dorsal and ventral striatum, the levels of dynorphin peptide significantly increase following repeated administration of the psychostimulants cocaine (Sivam, 1989; Smiley et al., 1990; Steiner and Gerfen, 1993) and methamphetamine (Hanson et al., 1988; Li et al., 1988). A significant increase in prodynorphin mRNA is observed after rats self-administer cocaine (Hurd et al., 1992), and in post-mortem studies of cocaine-dependent human drug abusers, there is a marked induction of prodynorphin, but not other peptide mRNAs, in the striatum (Hurd and Herkenham, 1993). Regulation of prodynorphin gene expression in the striatum has been shown to be dependent upon D1 dopamine receptor stimulation (Gerfen et al., 1990).
Dynorphin peptides are relatively selective for the κ opiate receptor and exert inhibitory actions in the nervous system (Chavkin et al., 1982; Corbett et al., 1982). Activation of κ receptors is associated with an aversive dysphoric syndrome in human (Pfeiffer et al., 1986) and rat (Bals-Kubik et al., 1993). Thus, increases in dynorphin peptides occurring with chronic cocaine or amphetamine use may potentially prove relevant to the motivational aspects of psychostimulant withdrawal, which in humans has been characterized by intense dysphoria, anhedonia, and drug craving (Gawin, 1991). We therefore performed a series of experiments to investigate the molecular mechanisms by which D1 dopamine receptor activation might be coupled to regulation of the prodynorphin gene.
The mechanisms by which the prodynorphin gene is regulated by Gs-coupled receptors and cyclic AMP (cAMP) are controversial. Until recently, studies assessing dynorphin gene regulation have focused on a noncanonical activating protein 1 (AP-1)–like site (TGACAAACA; −257/−249) in the prodynorphin promoter. This site has been reported to serve as a target for Fos/Jun trans-activation in NCB20 neuroblastoma cells (Naranjo et al., 1991). Moreover, c-Fos has been reported to be required for cAMP-mediated prodynorphin gene induction in spinal cord neurons (Lucas et al., 1993).
Recently, the prodynorphin promoter has been shown to contain three elements within its 5′ flanking sequences that are homologous to the consensus cAMP response element (CRE), termed DynCRE1 (−1660/−1553), Dyn-CRE2 (−1630/−1623), and DynCRE3 (−1546/−1539). These three elements have been characterized by deletion and single-base mutagenesis and are required for the positive regulation of the prodynorphin gene via cAMP pathways in CV-1 fibroblasts (Douglass et al., 1994). In addition, it has been demonstrated that DynCRE3 is involved in cAMP responsiveness of the prodynorphin gene in PC12 cells (Messersmith et al., 1994). Although these CREs have been shown to be functional in cell lines, a disagreement exists as to the relevant proteins binding these elements in vivo. A recent study found that purified CRE-binding protein (CREB), purified Jun protein, and purified Fos/Jun heterodimers can all bind with high affinity to DynCRE3 (Collins-Hicok et al., 1994). This study also reported that in unstimulated CV-1 fibroblasts CREB co-transfected with the rat prodynorphin gene acted as a repressor, whereas the cotransfected AP-1 proteins, Fos and Jun, were activators (Collins-Hicok et al., 1994).
As a first step in understanding the relevant signal transduction pathways regulating prodynorphin in response to psychostimulants and dopamine, it was necessary to determine which proteins actually interact with the prodynorphin gene in the relevant neurons. We report that the DynCRE1, DynCRE2, and DynCRE3 elements are required for full activation of the prodynorphin promoter in response to dopamine in striatal neurons and demonstrate that the AP-1 element, by itself, does not confer significant activation. In addition, we find that the induction of the prodynorphin gene by cAMP does not require new protein synthesis. We demonstrate that CREB binds to the DynCREs in extracts from striatal cultures, and that CREB, in a form that can bind the prodynorphin CREs, is phosphorylated in response to dopamine in a D1 dopamine receptor–dependent manner. We do not observe significant protein binding to the putative noncanonical AP-1-like site in our extracts, even under conditions that produce robust AP-1 binding to a consensus AP-1 sequence. Our data indicate that CREB but not AP-1 proteins serve to couple D1 dopamine receptor stimulation to prodynorphin gene expression in striatal neurons. We have previously shown that acute amphetamine administration induces CREB phosphorylation in striatum in vivo, and that CREB is required for amphetamine-induced c-fos gene expression (Konradi et al, 1994). Here we show that chronic amphetamine administration elevates levels of phosphoCREB immunoreactivity above basal levels, but suppresses c-fos mRNA expression below basal levels, demonstrating a dissociation between CREB phosphorylation and c-fos expression in the chronic state. Chronic elevation in levels of phosphorylated CREB is a potential mechanism for stimulant-induced adaptations in rat striatum.
Results
Dopamine Markedly Induces cAMP Levels and c-fos mRNA in Cultured Striatal Neurons
To elucidate the mechanisms of prodynorphin gene expression by dopamine in striatal neurons, we utilized neuron-enriched E19 rat embryonic primary striatal cultures. Immunohistochemical staining of the cultures with an antiserum directed against glial fibrillary acidic protein (GFAP) shows a large GFAP-positive astrocyte (Figure 1A) surrounded by smaller GFAP-negative cells, which are counterstained. Across multiple fields, the ratio of GFAP-negative cells to astrocytes is greater than 10:1. Histochemical staining in a parallel culture with an antiserum directed against neuron-specific enolase (NSE) demonstrates that a large percentage of the small GFAP-negative cells in Figure 1A are neurons (Figure 1B; no counterstain). A subpopulation of cells did not stain with either antiserum; overall, we estimate the striatal cultures to be greater than 80% neuronal.
Figure 1. Immunohistochemical Staining of Primary Striatal Cultures for GFAP and NSE.
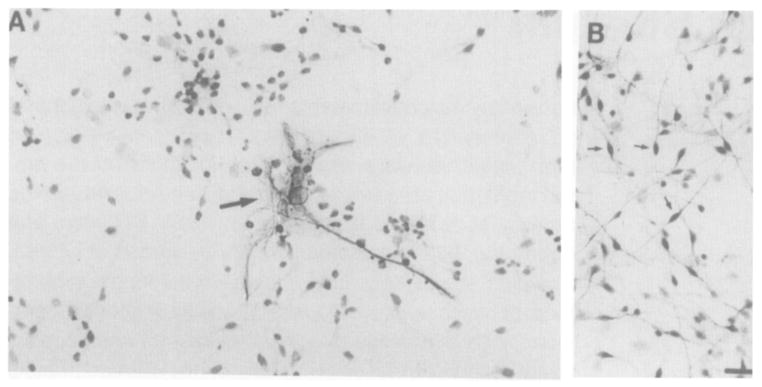
Arrow in (A) points to typical astrocyte stained with anti-GFAP; the surrounding smaller cells are counterstained. Small arrows in (B) point to typical neurons stained with anti-NSE; no counterstain was used. Bar, 20 μm.
In culture, it is not possible to use indirect dopamine agonists such as amphetamine or cocaine as a stimulus because their effects on dopamine concentrations require presynaptic innervation. Therefore, in our experiments we used dopamine itself. The D1 dopamine receptor, which is positively linked to adenylate cyclase, is required for psychostimulant induction of c-fos gene expression (Graybiel et al., 1990; Young et al., 1991; Nguyen et al., 1992) and CREB phosphorylation (Konradi et al., 1994) in striatum in vivo. To test for the intactness of the D1 dopamine receptor signaling pathway in our cultured neurons, we performed a time course of dopamine treatment and measured cAMP levels. Stimulation with dopamine and the phosphodiesterase inhibitor Ro20-1724 resulted in a 40-fold increase in cAMP levels, which was observable 5 min after treatment, peaked at 10–15 min, and was down to 10-fold after 1 hr (Figure 2A). In response to forskolin, the direct activator of adenylate cyclase, a greater than 100-fold increase in cAMP levels was observed, which peaked 10 min after treatment (data not shown). In addition, we observed a 10- to 30-fold induction of c-fos mRNA after dopaminergic stimulation (Figure 2B, lanes 5 and 6). This induction of c-fos was blocked by pretreatment with the D1 dopamine receptor antagonist SCH-23390 (Figure 2B, lanes 7 and 8). To rule out the possibility that glial cells contributed to the observed response, we compared the neuron-enriched cultures with glial-enriched cultures that were grown in parallel. The glial-enriched cultures, produced by mechanically shaking off the neurons, showed a 1.5-fold induction of c-fos by dopamine (data not shown), compared with the 10- to 30-fold induction observed in the neuron-enriched cultures.
Figure 2. Dopamine Stimulation Increases cAMP Levels and Induces Expression of c-fos mRNA in Primary Striatal Cultures.
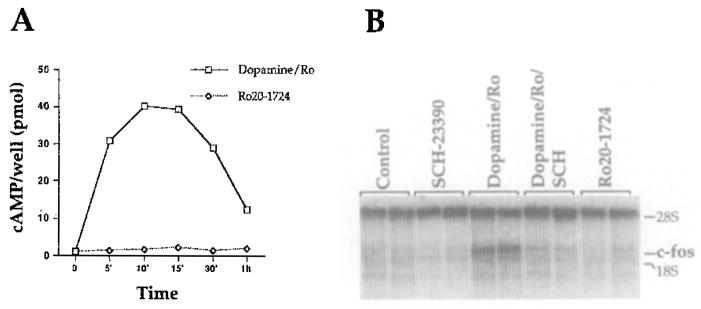
(A) Time course showing a 30- to 40-fold increase in cAMP levels 10 min after treatment with dopamine (50 μM) and Ro20-1724 (50 μM; open squares); Ro20-1724 alone (open diamonds) had no independent effects on cAMP levels (n = 3).
(B) Northern analysis with c-fos riboprobe showing a 10- to 30-fold increase in c-fos mRNA 45 min after stimulation by dopamine/Ro20-1724. Induction was blocked by 10 min pretreatment with the D1 dopamine receptor antagonist SCH-23390 (100 μM). SCH-23390 alone and Ro20-1724 alone had no independent effects on c-fos mRNA expression. Ribosomal RNA (28S) was used as an internal loading control (1 μg RNA per lane; n = 4).
Prodynorphin mRNA Is Induced in Primary Striatal Cultures by cAMP in a Protein Synthesis-Independent Manner
Northern analysis was used to confirm that prodynorphin mRNA is present in our striatal cultures. Prodynorphin mRNA levels were very low basally in the cultures and were induced 2- to 3-fold after acute stimulation by dopamine (Figure 3A). As the D1 dopamine receptor is positively coupled to adenylyl cyclase, we also utilized forskolin, a more potent stimulus than dopamine. In response to forskolin, a 5- to 7-fold induction of prodynorphin mRNA was observed (Figure 3B). Since prodynorphin mRNA is not present in glia, as determined by Northern analysis performed on glial-enriched cultures (data not shown), the forskolin effect is likely entirely neuronal.
Figure 3. Prodynorphin mRNA Is Induced by the cAMP Pathway in Primary Striatal Cultures in a Protein Synthesis–Independent Manner.
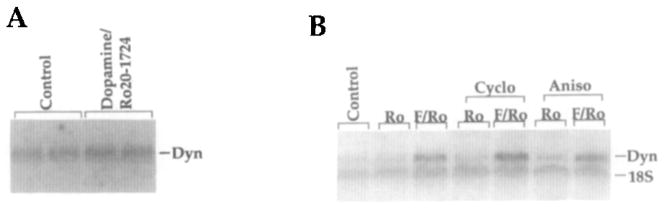
(A) Northern analysis with a prodynorphin riboprobe showing prodynorphin mRNA induced 2- to 3-fold 6 hr after dopamine/Ro20-1724 treatment.
(B) The 5- to 7-fold induction of prodynorphin mRNA 6 hr after stimulation by forskolin, a more potent stimulator of cAMP pathways, and Ro20-1724 (F/Ro) was not blocked by 15 min pretreatment with the protein synthesis inhibitors cycloheximide (Cyclo; 100 μM) or anisomycin (Aniso; 10 μM), indicating protein synthesis independence. Two different protein synthesis inhibitors were used to control for nonspecific pharmacological effects. Ro20-1724 (Ro) alone had no effect on prodynorphin mRNA levels. Control cultures received no drug treatment. Ribosomal RNA (18S) was used as an internal loading control (2–3 μg of RNA was loaded per lane). All results were replicated at least four times. In (A) only, the antioxidant sodium metabisulfite (0.01%; Sigma), which has no independent effects (data not shown), was included in the drug treatment.
To begin to investigate the mechanism of prodynorphin induction by cAMP in striatal neurons, we used short-term treatment with protein synthesis inhibitors. The effects of longer-term protein synthesis inhibition are not informative, owing to toxicity and the variable turnover time of critical signal transduction proteins. However, the relatively small inductions of prodynorphin mRNA observed in response to short-term dopamine treatment make any potential inhibitory effects of protein synthesis inhibitors difficult to assess. The larger forskolin-induced increase in prodynorphin mRNA levels was not inhibited by either of the protein synthesis inhibitors, cycloheximide and anisomycin (Figure 3B). Induction of prodynorphin mRNA independently of new protein synthesis is consistent with the hypothesis that constitutively expressed transcription factors are involved in the observed regulation.
Three CREs in the Prodynorphin Promoter Are Required for cAMP Responsiveness
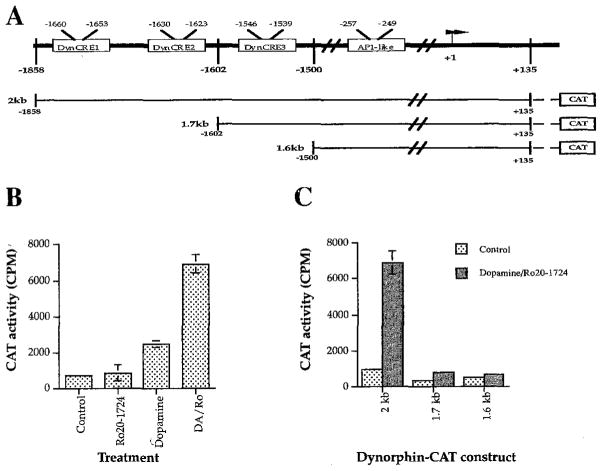
To ensure that the observed regulation has a transcriptional component and to determine whether the three CREs previously shown to be functional in transformed fibroblast cell lines are also functional in striatal neurons, we performed transfection studies in primary striatal cultures. Transfection with a construct containing the three upstream prodynorphin CREs as well as the putative non-canonical prodynorphin AP-1 element (Naranjo et al., 1991) fused to the CAT gene (2 kb; −1858/+135; Figure 4A) resulted in a 7- to 10-fold increase in CAT activity after dopamine stimulation (Figure 4B). Transfection with constructs containing deletions of the two 5′ DynCREs (1.7 kb; −1602/+135) or of all three DynCREs (1.6 kb; −1500/+135), but still containing the prodynorphin AP-1-like element, showed a dramatic reduction in CAT activity (Figure 4C). Parallel transfections were performed in glial-enriched cultures, and no dopamine response was detectable, indicating that the reported effects are likely neuronal.
Figure 4. Dopamine Stimulation Activates Expression of a Prodynorphin–CAT Fusion Construct.
(A) The prodynorphin promoter showing the three 5′ DynCRE elements and the noncanonical AP-1 element. The CAT fusion constructs used in transfection experiments are shown in the lower portion of (A). The full-length 2 kb construct contains all three DynCRE sites and the putative noncanonical DynAP-1 site. In the 1.7 kb construct, the two 5′ CREs are deleted. In the 1.6 kb construct, only the putative AP-1 element is preserved.
(B) After transfection with the 2 kb prodynorphin–CAT construct, an 8- to 10-fold increase in CAT activity followed stimulation with dopamine and Ro20-1724 (DA/Ro) as compared with control or Ro20-1724 alone (n ≥ 4 for each drug condition).
(C) Comparison of the induction of the 2 kb prodynorphin–CAT construct and the 1.7 and 1.6 kb constructs, which lack either two or all three of the DynCREs. In cells transfected with the 1.7 and 1.6 kb constructs, dopamine/Ro20-1724 treatment no longer produced significant induction. Background CAT activity determined from untransfected controls has been subtracted from the values shown. Data in (B) and (C) ± SEM.
A fine-grained promoter analysis could not be performed in our primary neuronal cultures because partial inactivation of the promoter produces expression that is very near the limits of reliable detection (Figure 4C). Thus, the relative contribution of each of the individual CREs in prodynorphin gene regulation cannot be readily assessed in striatal neurons. However, our results agree entirely with those obtained in CV-1 fibroblasts (Douglass et al., 1994), i.e., that these three upstream DynCRE elements are required for significant induction of the prodynorphin gene in response to stimuli that act via the cAMP pathway. Fine mapping in CV-1 cells has demonstrated that each of the DynCREs is functional, and that they act combinatorially to produce full transcriptional effects (Douglass et al., 1994).
Gel-Shift Competition Assays Demonstrate a CREB-Like Protein Binding to the Prodynorphin CREs and a Lack of Binding to the Putative Noncanonical Prodynorphin AP-1 Element in Extracts from Primary Striatal Cultures
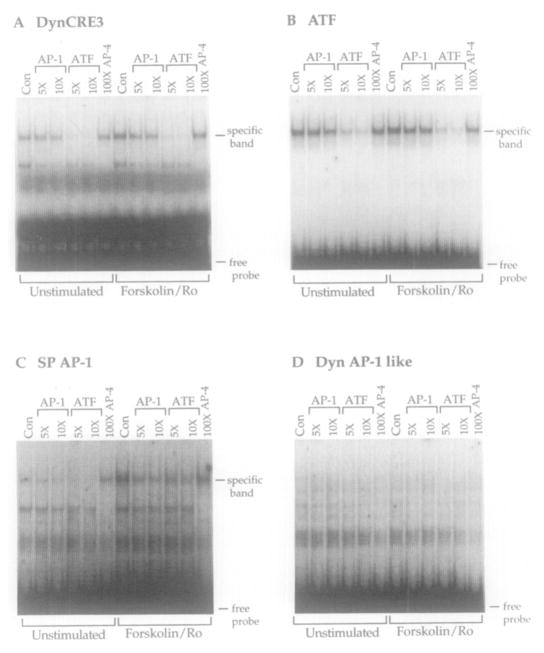
To investigate the proteins that bind to the DynCREs in extracts from striatal neurons, we performed gel-shift assays using oligonucleotides containing single DynCREs together with cell extracts from primary striatal cultures. The specificity of complexes formed with the DynCRE elements and the relative affinities of bound proteins for the DynCREs compared with consensus regulatory elements were examined using old competition. Complexes formed with striatal extracts were assessed using each of the three DynCREs (DynCRE3 is shown; Figure 5A), a consensus palindromic CREB/activating transcription factor (ATF) DNA binding site (Hai et al., 1989; Figure 5B), a consensus AP-1 element (Figure 5C), and the putative noncanonical prodynorphin AP-1-like element (Figure 5D). Cold competition was performed with oligonucleotides containing the ATF and consensus AP-1 elements and, for comparison, an unrelated AP-4 element.
Figure 5. Electrophoretic Mobility Shift Assay with Extracts from Primary Striatal Cultures.
The specificity of binding and the relative affinities of the complexes for consensus AP-1 and CREB binding sites were examined using cold competition. Binding to the DynCRE3 site (A), a consensus CREB binding site (B; ATF), a consensus AP-1 binding site derived from the substance P promoter (C; SP AP-1), and the putative noncanonical prodynorphin AP-1 element (D; Dyn AP-1 like) is shown. Specifically shifted bands were formed by striatal extracts with the DynCRE3, ATF, and SP AP-1 elements (A–C), but not with the noncanonical AP-1 element (D). When complexes were formed with the DynCRE3, ATF, and SP AP-1 elements with extracts from unstimulated cultures (no drug treatment), basal binding (left-most lane marked Con [no competitor]) in (A), (B), and (C) was observed. In each of these cases, unlabeled oligonucleotides containing the ATF element competed strongly for proteins bound to labeled oligonucleotide. In extracts made after 2 hr stimulation with forskolin/Ro20-1724, binding was markedly induced only with the SP AP-1 oligonucleotide (C; compare binding in the unstimulated and stimulated Con lanes). Stimulation also altered the pattern of competition to SP AP-1 by unlabeled oligonucleotides, such that the ATF oligonucleotide no longer fully competed. This is consistent with the observation that AP-1 proteins are induced. In contrast, stimulation with forskolin/Ro did not substantially alter binding to the DynCRE3 element or the ATF element (A and B) either quantitatively (Con lanes) or in terms of pattern of competition. In all cases, the unrelated AP-4 element did not compete for binding even at 100-fold molar excess. The numbers at the top signify molar excess of cold competitor oligonucleotides. Note that the competitions with the AP-1 oligonucleotide in (C) and the ATF oligonucleotide in (B) sites are self-competitions. All experiments were performed in probe excess. Each experiment was repeated at least four times.
In extracts from primary striatal cultures, the oligonucleotides containing the DynCRE elements (DynCRE3 is shown; Figure 5A) and the CREB/ATF element (Figure 5B) showed very similar patterns of binding and competition. In both cases, the ATF oligonucleotide competed strongly for protein binding, whereas an oligonucleotide containing the closely related consensus AP-1 site competed far less avidly (Figures 5A and 5B). An oligonucleotide containing an unrelated AP-4 site did not compete for binding with either labeled oligonucleotide. There was no quantitative change in binding to the DynCRE3 or ATF oligonucleotides following a 2 hr forskolin/Ro20-1724 treatment (Figures 5A and 5B, control), a time at which Fos and other AP-1 proteins were markedly induced in these cells (Figure 5C; compare unstimulated and forskolin/Ro). These results indicate that the specific band represents binding of a protein(s) with higher affinity for CREB/ATF binding sites than for AP-1 binding sites. DynCRE1 and DynCRE2 show a binding pattern essentially identical to DynCRE3 and ATF (data not shown).
In untreated striatal cultures, cold oligonucleotides containing an ATF element effectively competed for binding of proteins to oligonucleotides containing the AP-1 elements of the preprotachykinin (substance P; Figure 5C) and human metallothionein genes (data not shown), likely indicating that, in the unstimulated cultures, constitutively expressed CREB-like proteins are abundant enough compared with inducible AP-1 proteins that they comprise most of the protein interacting with AP-1 sites. However, 2 hr after stimulation with forskolin/Ro20-1724, binding to the AP-1 oligonucleotide was induced (Figure 5C; compare control lanes). Moreover, the ATF competitor oligonucleotide could no longer fully compete, consistent with induction of AP-1 proteins by cAMP. Nonetheless, 2 hr after forskolin/Ro20-1724 treatment, when binding to the consensus AP-1 element is induced, no specific binding to the putative noncanonical prodynorphin AP-1 element (Naranjo et al., 1991) was detectable (Figure 5D).
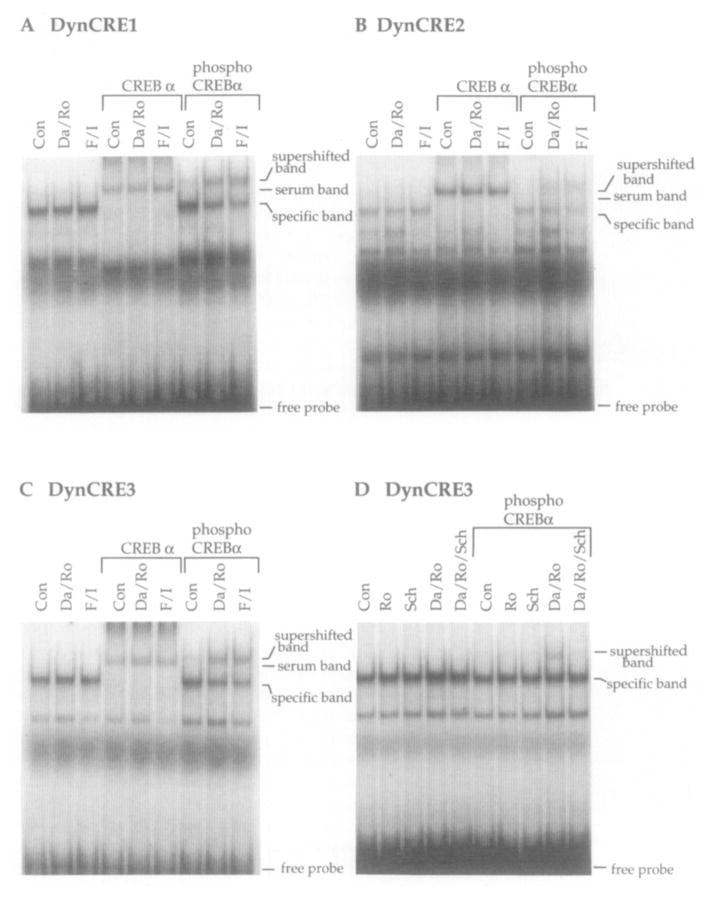
Gel Supershift Analysis Shows CREB Binding to the Three Prodynorphin CREs and CREB Phosphorylation after Treatment with Dopamine or Forskolin
We used antisera recognizing total CREB protein and CREB only when phosphorylated on Ser-133 (phosphoCREB; Ginty et al., 1993) to investigate the composition of the specific complexes observed in the electrophoretic mobility shift assays. As shown for the three prodynorphin CRE sites, the specific band is entirely supershifted by a CREB antiserum (Figures 6A–6C, lanes 4–6), indicating that CREB proteins bind these elements in striatal extracts. Whether the complex contains a CREB homodimer or whether CREB is heterodimerizing with another protein requires further analysis. Recognition of the binding complex by the CREB antiserum is consistent with the more avid competition of cold CREB/ATF oligonucleotides than of cold AP-1 oligonucleotides for the proteins bound to the DynCREs (Figures 5A–5C).
Figure 6. Supershift Analysis Demonstrating that CREB Binds to the Three Prodynorphin CRE Elements and Is Phosphorylated after cAMP Pathway Activation.
(A–C) Binding to the DynCRE site was not quantitatively altered in extracts from untreated primary striatal cultures compared with extracts from cultures treated for 15 min with dopamine/Ro20-1724 (Da/Ro) or forskolin/IBMX (F/l; [A]–[C], lanes 1–3). In the presence of a CREB antiserum, the band, which was shown to be specific by cold competition (Figure 5A), is entirely supershifted (A–C, lanes 4–6). A nonspecific band produced by serum components is present only in these three lanes and is also present if preimmune serum is used (data not shown). In the presence of the antiserum that only recognizes the phosphorylated form of CREB (phosphoCREBα), a supershifted band appeared only after drug stimulation (A–C; compare lane 7 with lanes 8 and 9; n = 4).
(D) As shown for DynCRE3, the phosphorylation of CREB after stimulation with dopamine and Ro20-1724 was blocked by 15 min pretreatment with the D1 dopamine receptor antagonist SCH-23390. RO20-1724 and SCH-23390 alone had no effects on CREB phosphorylation. Each experiment was repeated at least four times.
As CREB binds to the three prodynorphin CRE elements, we next investigated whether CREB is phosphorylated on Ser-133 after dopamine or forskolin stimulation. Using a phosphoCREB antiserum (Ginty et al., 1993), we observed induction of a supershifted band in response to stimulation of cells with dopamine/Ro20-1724 or forskolin/3-isobutyl-1-methylxanthine (IBMX; Figures 6A–6C, compare lane 7 with lanes 8 and 9). In response to dopamine and forskolin, the supershift is partial. However, in cultures stimulated in the presence of the phosphatase inhibitor okadaic acid (1 μM; GIBCO-BRL, Gaithersburg, MD), a nearly complete supershift was observed (data not shown). These results indicate that the three DynCRE sites bind CREB in extracts from primary striatal cultures and that CREB is phosphorylated in response to dopamine treatment.
Phosphorylation of CREB in Response to Dopamine Is Rapid and Dependent upon D1 Dopamine Receptor Activation
The induction of the phosphoCREB supershifted band by dopamine was blocked by pretreatment with SCH-23390 (Figure 6D), indicating that the phosphorylation of CREB in response to dopamine treatment is dependent upon D1 dopamine receptor activation. Pretreatment with the selective D2 dopamine receptor antagonist eticlopride (50 μM) had no effect on dopamine-induced CREB phosphorylation (data not shown). The selective D1 dopamine receptor agonists SKF-82938 (50 μM) and SKF-38393 (50 μM) both induced CREB phosphorylation, and this effect was not altered by eticlopride (50 μM) pretreatment. The D2/D3 dopamine receptor agonist quinpirole (50 μM) did not induce CREB phosphorylation in the cultures (data not shown). Together, these data indicate that D1 but not D2 family dopamine receptors are required for CREB phosphorylation in striatal culture.
A time course using electrophoretic mobility shift assays demonstrates that total CREB binding to the DynCRE elements is not quantitatively altered after dopamine stimulation (Figure 7A; DynCRE1 shown). However, utilizing the phosphoCREB antiserum, we show that CREB phosphorylation is apparent at 5 min, peaks at 15–30 min, and returns to baseline at 3 hr after dopamine stimulation (Figure 7B). This time course of CREB phosphorylation closely correlates with the time course of cAMP induction in response to dopamine (compare Figure 2). A similar time course of phosphorylation was observed using oligonucleotides containing the DynCRE2 and DynCRE3 elements (data not shown).
Figure 7. Time Course Showing Dopamine Induction of Phosphorylated CREB in a Form That Can Bind DNA in Primary Striatal Cultures.
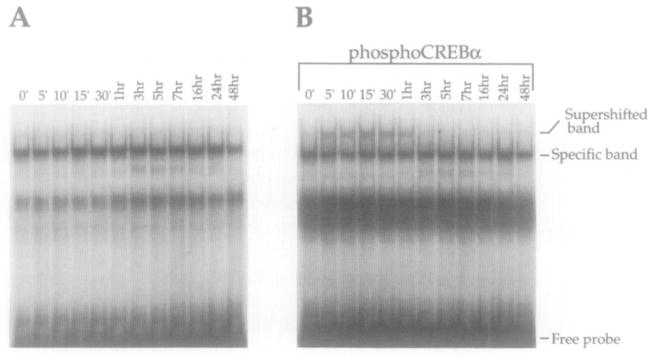
(A) CREB binding was not quantitatively altered after stimulation of cultures with dopamine and Ro20-1724.
(B) In the presence of the phosphoCREB antiserum, a phosphoCREB supershifted band was present 5 min after dopaminergic stimulation, peaked at 15 min, and was back to baseline levels by 3 hr. DynCRE1 is shown; comparable results were obtained with DynCRE2 and DynCRE3. Each experiment was repeated at least three times.
Following Chronic Amphetamine Administration, PhosphoCREB-Like Immunoreactivity Is Elevated and c-fos mRNA Is Suppressed in Rat Striatum
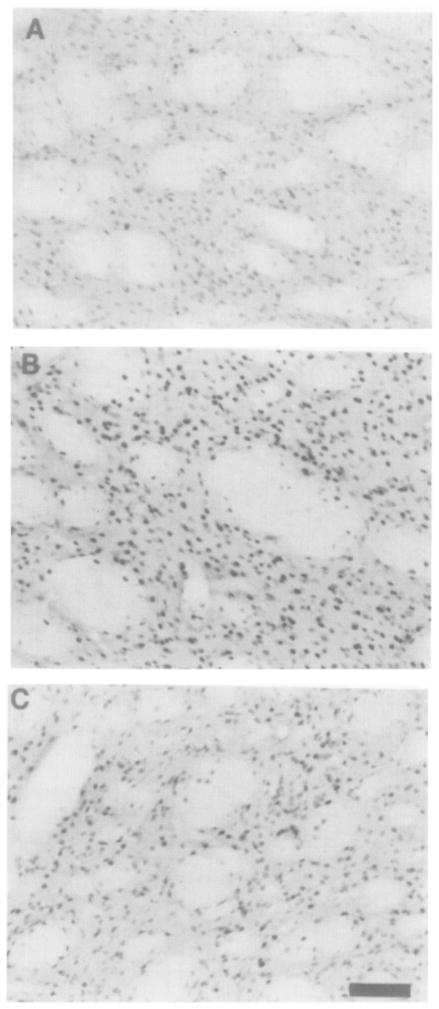
There are low basal level of phosphoCREB-like immunoreactivity (phosphoCREB-LI) in saline control rat striatum (Figure 8A). PhosphoCREB-LI was markedly induced, however, by acute amphetamine administration (Figure 8B). When chronically treated rats were given a final amphetamine injection 15 min prior to sacrifice (chronic/acute condition; see Experimental Procedures), phosphoCREB-Ll was significantly increased above control levels, but was less than that in acutely treated rats (data not shown). When the last amphetamine injection was replaced by saline (chronic condition; see Experimental Procedures), meaning that the rats were sacrificed 16 hr after the final injection of active drug, phosphoCREB-LI in the striatum was significantly increased over saline controls (Figure 8C), although not quite to the same extent as when a final amphetamine injection was administered. This increase in phosphoCREB-LI was observed even if no final saline injection was administered (data not shown), ruling out the possibility that this represents a conditioned response. This is in agreement with the demonstration that, following exposure to a cocaine paired environment, conditional neuronal activation does not occur in the dorsal striatum or nucleus accumbens (Brown et al., 1992). Levels of total CREB-LI are not altered by the different drug treatments (data not shown).
Figure 8. PhosphoCREB-Like Immunoreactivity after Acute and Chronic Amphetamine Administration in Rat Striatum.
(A) Basal levels of phosphoCREB-LI in unstimulated rat striatum are low.
(B) PhosphoCREB-LI was induced by acute amphetamine (6 mg/kg) administration.
(C) After chronic amphetamine administration, phosphoCREB-LI was significantly increased over unstimulated controls. ANOVA found a significant difference between the three groups (F(2,8) = 38.1; p = .0004). Post-hoc Fisher LSD tests found that both the chronic and acute treatment groups were significantly different from the control group (p < .05). Significance was also obtained with the nonparametric Kruskal-Wallis test (H = 7.2; p = .02), followed by the Mann–Whitney U test to compare drug treatment groups with control (Z = 1.96; p = .04). Bar, 100 μm.
Basal levels of c-fos mRNA are low in control rat striatum and could be robustly induced by acute amphetamine administration (Figure 9). Following chronic amphetamine treatment, c-fos mRNA was reproducibly suppressed below basal levels of expression, demonstrating a dissociation between c-fos mRNA levels and phosphoCREB-LI after chronic amphetamine administration.
Figure 9. Northern Blot Analysis Showing c-fos mRNA Regulation by Acute and Chronic Amphetamine Administration.
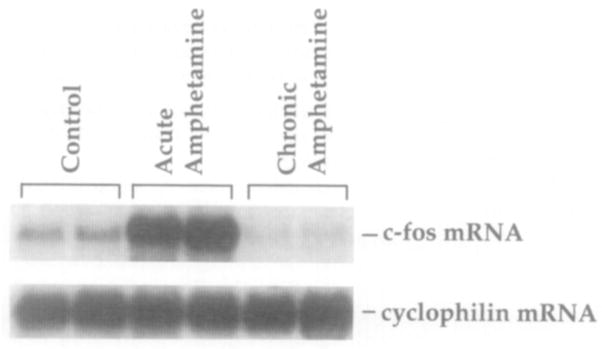
c-fos mRNA was induced in rat striatum following acute amphetamine (6 mg/kg) administration and was suppressed below basal levels following chronic drug administration in rat striatum. Cyclophilin mRNA was used as an internal loading control (8 μg of total striatal RNA per lane; n = 6 per treatment group).
Discussion
It has been well documented that the indirect dopamine agonists cocaine and amphetamine produce a selective increase in prodynorphin mRNA levels in dorsal and ventral striatum. As a model to investigate psychostimulant-mediated signal transduction, we have utilized rat primary embryonic striatal cultures to examine prodynorphin gene regulation by dopamine. We find, consistent with prior transfection studies examining cAMP regulation in CV-1 fibroblasts and PC-12 cells (Douglass et al., 1994; Messersmith et al., 1994), that the three upstream CREs within the prodynorphin promoter are necessary for full induction by dopamine in striatal neurons. Using gel-shift and supershift analyses, we find that CREB protein binds to these three functional CRE sites in striatal cell extracts and is phosphorylated after dopamine treatment in a D1 dopamine receptor-dependent manner. We do not observe binding in striatal cell extracts to the putative noncanonical AP-1 site (Naranjo et al., 1991), even under assay conditions that detect inducible binding to a consensus AP-1 site. It was recently demonstrated by cotransfection analysis in CV-1 cells that purified CREB, purified Jun protein, and purified Fos/Jun protein also do not bind this element (Collins-Hicok et al., 1994).
Our data are consistent with the hypothesis that CREB binds the three functional upstream CREs under both basal and stimulated conditions and is activated by phosphorylation. This is entirely congruent with models of CREB function in cell lines (Gonzalez and Montminy, 1989). It is therefore important to address a recent study reporting that CREB represses expression of a prodynorphin fusion construct when the two are cotransfected into unstimulated CV-1 fibroblasts (Collins-Hicok et al., 1994). In cotransfection studies, large amounts of the transcription factor, in this case CREB, are produced under the control of a strong, constitutively active promoter. Under such conditions, a large excess of unphosphorylated CREB may dimerize with basally phosphorylated CREB in the nucleus, or may similarly titrate out other necessary transcription factors. The physiological significance of repression under these conditions is challenging to sort out; cotransfection studies provide data on the range of possible interactions of transcription factors with targets, but cannot specify what actually occurs under more physiological conditions.
It is not surprising that both purified AP-1 proteins and CREB have been reported to bind the prodynorphin CREs in vitro (Collins-Hicok et al., 1994), as consensus AP-1 sites and CREB binding sites differ by only a single base (TGAC/GTCA versus TGACGTCA). The prodynorphin CREs are asymmetric CREs intermediate between consensus AP-1 and CREB binding sites. It should be noted, however, that they all contain the CGTCA core that has been shown to be relatively rigidly required for cAMP activation (Hyman et al., 1988), but not for AP-1 protein binding. Thus, for example, in the proenkephalin CRE2 site, which is identical to DynCRE3, a single base substitution mutation that converts the site from a CRE site (TGCGTCA) to a consensus AP-1 site (TGAGTCA) gives high affinity binding of purified AP-1 proteins, but is functionally unresponsive to cAMP (Comb et al., 1988).
Our results demonstrate that, in the complex mix of a striatal cell extract, it is CREB rather than AP-1 proteins that bind to all three DynCREs, even under conditions that induce c-Fos. We conclude that, even though AP-1 proteins can bind the prodynorphin CRE sites in vitro (Collins-Hicok et al., 1994), in striatal neurons it is likely that CREB-like proteins actually interact with the gene. The probable role of CREB-like proteins rather than inducible AP-1 proteins, such as c-Fos and c-Jun, in cAMP induction of prodynorphin gene expression is underscored by our finding that the induction occurs independently of new protein synthesis.
Rats chronically treated with amphetamine show increased phosphoCREB-like immunoreactivity 16 hr after the final amphetamine injection. This result indicates that, although in cultured cells CREB phosphorylation and dephosphorylation occur rapidly (Figure 7B), a moderate increase in steady-state phosphoCREB-LI is present in the striatum of rats chronically treated with amphetamine, even at a time at which c-fos mRNA is reduced below basal levels. Suppression of zif268 and c-fos mRNA levels below basal following chronic cocaine treatment has previously been demonstrated (Bhat et al., 1992; Ennulat and Cohen, 1995). This dissociation is also consistent with a role for CREB in mediating the increases in prodynorphin mRNA observed chronically. The chronic elevated levels of phosphoCREB-LI are also consistent with the up-regulation of cAMP pathways that occurs in vivo following chronic cocaine administration (Terwilliger et al., 1991).
In the striatum, there is evidence that dynorphin, released by recurrent collaterals, acts to control the response of striatal neurons to dopaminergic stimulation (Steiner and Gerfen, 1993). In addition, κ opioid receptor agonists attenuate the acute motor stimulatory effects and the stereotypy produced by cocaine administration, as well as cocaine-induced behavioral sensitization in the rat (Heidbreder et al., 1993). These prior studies suggest that dynorphin peptides, acting via κ opiate receptors, may negatively modulate cellular responses to dopaminergic stimulation.
κ receptor binding is present throughout the dorsal and ventral striatum (Tempel and Zukin, 1987; Mansour et al., 1987), but the precise location at which dynorphin peptides act remains unclear. The substantia nigra pars compacta expresses high levels of κ receptor mRNA, yet shows very little κ receptor binding, suggesting that the nigral κ receptors are transported to presynaptic terminals in the striatum (Mansour et al., 1994). Dynorphin peptides can potently and selectively inhibit the release of dopamine in slices from rat striatum (Mulder et al., 1984). In vivo microdialysis studies also demonstrate that dynorphin analogs and/or κ opiate receptor agonists inhibit dopamine release within the striatum and nucleus accumbens (Di Chiara and Imperato, 1988b; Spanagel et al., 1990).
Selective changes in dynorphin peptide levels after repeated psychostimulant administration may represent a compensatory inhibitory response to excessive stimulation mediated via D1 dopamine receptors. When unmasked by drug discontinuation, stimulant-induced increases in dynorphin may contribute to the motivational aspects of psychostimulant withdrawal, characterized in humans by intense dysphoria, anhedonia, and drug craving (Gawin, 1991). Here we have investigated the mechanism by which psychostimulant drugs may produce these compensatory adaptations.
Experimental Procedures
Animals and Drug Paradigm
Male Sprague–Dawley rats (200–250 g) were housed three per cage and maintained on a 12/12 hr light/dark cycle. Amphetamine sulfate (Research Biochemicals Inc., Natick, MA) was dissolved in saline and administered (6 mg/kg, free base) intraperitoneally. The experiments involve four groups of rats: control, acute, chronic, and chronic/acute. The control rats receive saline injections twice a day, 7 hr apart (10: 00 AM and 5:00 PM), for 6 days and a single saline injection on day 7. For the acute amphetamine paradigm, saline was administered for 6 days, and a single amphetamine injection was administered on day 7. In the chronic paradigm, rats were administered amphetamine twice daily for 6 days and given a single saline injection, or no final injection, on day 7. Therefore, the chronic rats are sacrificed 16–18 hr after their last amphetamine injection. In the chronic/acute condition, amphetamine was administered twice daily for 6 days, and a final amphetamine injection was given on day 7. For phosphoCREB and CREB immunohistochemistry, rats were perfused 15 min after the last injection on day 7. For Northern analysis, rats were perfused 45 min after the final injection.
Primary Striatal Cultures
Striata were dissected out under a stereo-microscope from 19-day-old Sprague–Dawley rat fetuses. Tissue was suspended in 2 ml of media (DMEM/F12 from GIBCO-BRL) with the following supplements: 4.5 g/l glucose, 1% penicillin-streptomycin liquid (GIBCO), and 10% Nuserum I (Collaborative Biomedical Products, Bedford, MA) with 0.2% DNase. The tissue was mechanically dissociated with a fire-narrowed Pasteur pipette, centrifuged, resuspended in media, and plated in 6-well plates (Costar, Cambridge, MA) at 2 × 106 cells per well. Plates were pretreated with a 1:500 diluted solution of polyethylenimine in 50 mM sodium borate (pH 7.4) for 24 hr and washed twice with PBS prior to plating. On day 2 in culture, cells were refed with defined media containing B27 supplement (GIBCO) to inhibit glial proliferation.
Experiments were performed with cells 6–8 days in culture. Drugs for treatment of cultures included dopamine (50 μM) or forskolin (10 μM) with a selective inhibitor of cAMP phosphodiesterase: Ro20-1724 (50 μM; RBI, Natick, MA) or IBMX (500 μM; Sigma, St. Louis, MO) and SCH-23390 (100 μM; RBI), a selective antagonist at dopamine D1 and D5 (also called D1a and D1b) receptors. cAMP levels were determined using a cAMP [3H] assay system (Amersham, Arlington Heights, IL) according to the provided protocol.
Northern Blot Analysis
Striatal cultures were washed once with PBS on ice, scraped into a microcentrifuge tube, and centrifuged at 4°C for 3 min at 14,000 rpm. The supernatant was aspirated and the cells lysed in 500 μl of Nonidet P-40 lysis buffer (50 mM Tris [pH 8.0], 100 mM NaCl, 5 mM MgCl2, 0.5% Nonidet P-40). After 5 min of incubation on ice, cells were centrifuged for 2 min at 14,000 rpm at 4°C, the supernatant was transferred, and SDS was added to a 0.2% final concentration. Cells were extracted twice with phenol-chloroform, followed by a chloroform extraction and ethanol precipitation. RNA (1–10 μg) was size-separated on a 1.2% denaturing agarose gel (1 M paraformaldehyde) in MOPS buffer (20 mM MOPS [pH 7.0], 5 mM sodium acetate, 1 mM EDTA), electroblotted onto a nylon membrane (GeneScreen, DuPont), and hybridized with a c-fos or prodynorphin riboprobe (Riboprobe system, Promega, Madison, WI). Hybridization of a cyclophilin cDNA probe (Danielson et al., 1988) was used as an internal loading control. For animal experiments, RNA was prepared as described (Berger and Chirgwin, 1989). Membranes were visualized with a Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Immunohistochemistry
Cell Culture
Cells were grown on 2-well glass chamber slides (Nunc, Inc., Naper-ville, IL) as described above. Prior to staining, cells were washed once with PBS and fixed for 15 min in 4% paraformaldehyde, then washed again in PBS until addition of primary antibody. Polyclonal anti-NSE (Incstar, Stillwater, MN) and monoclonal anti-GFAP (Sigma) antisera were each diluted 1:1000 and incubated for 16 hr at 4°C. The protocol used for subsequent steps was according to the Vectastain Elite ABC kit (Vector Labs., Burlingame, CA); the antibody complex was developed with 3,3′-diaminobenzidine (50 mg per 100 ml of TRIS [pH 7.6]) and 0.03% hydrogen peroxide. The GFAP and NSE immunostaining in Figure 1 was performed on cells from the same dissection grown in parallel slide wells.
Animals
Rats were perfused under deep pentobarbital (70 mg/kg) anesthesia with 300 ml of 4% paraformaldehyde in PBS. Brains were immersed in 30% sucrose for 24 hr, and 40 μm sections were cut on a freezing microtome. Tissue slices were incubated for 16–24 hr at 4°C with CREB and phosphoCREB primary antisera (diluted 1:1000; see Ginty et al., 1993) and processed with the Vectastain Elite ABC kit. An alternative blocking solution was used for phosphoCREB antiserum (3% bovine serum albumin [RIA grade; Sigma], 0.3% Triton X-100, 50 mM NaF in PBS).
Transfection and CAT Assay
Prodynorphin promoter constructs in pCAT-Basic vector (Douglass et al., 1994) were transfected into primary striatal cultures using Transfectam (Promega) according to the manufacturer’s protocol for serum-free transfection. Briefly, 5 μg of DNA per 25 μg of Transfectam (ratio = 1:5) was added to each 35 mm well for 4 hr, at which time cells were fed with fresh media. Drugs were applied 24 hr after transfection, and cells were harvested 12 hr after the drug treatment. Prior to harvesting, cells were washed twice with PBS, scraped into a microfuge tube, and spun for 5 min at 14,000 rpm. Cell pellet was resuspended in CAT–Tris buffer (250 mM Tris, 1 mM EDTA [pH. 8.0]), and cells were lysed by freeze–thaw. Resulting supernatant was stored at 4°C until CAT assay was performed. Standard CAT assay was performed according to Promega protocols and applications guide. CAT conversion was measured by liquid scintillation counting. Overall transfection efficiency ranged between 1% and 5%, determined by histochemistry following transfection of an RSV–β-galactosidase expression plasmid. While highly reproducible, overall levels of expression from the prodynorphin promoter constructs are low. Attempts to decrease the total amount of prodynorphin plasmid or to alter the DNA:transfectam ratio to permit cotransfection of an internal reference plasmid in the same assay reduced expression below reliably detectable limits. Thus, the equivalency of transfection efficiency for the 1.6, 1.7, and 2 kb prodynorphin constructs was established in CV-1 cells (Douglass et al., 1994) prior to transfection of the same plasmids from the same plasmid preps into striatal cultures.
Electrophoretic Mobility Shift Assays
Electrophoretic mobility shift assays were performed as described for primary striatal cultures (Konradi et al., 1994). Oligonucleotides were synthesized with partial BamHI sites or a two G overhang and annealed in the presence of 20 mM NaPO4, 1 mM EDTA, and 100 mM KCl. The overhangs of the double-stranded oligonucleotides were then filled in, using Superscript reverse transcriptase (GIBCO), with 32P-labeled dCTP. Sequence of oligonucleotides used in electrophoretic mobility shift assays were: DynCRE1, 5′-GGGTACCTGGTACGTCACAGA-CAT-3′; DynCRE2, 5′-GGTAGCCACATCCGTCATCTCGGT-3′; Dyn-CRE3, 5′-GGGTGGCTGCTGCGTCAGAGCATG-3′ (all prodynorphin oligonucleotides were derived from the rat prodynorphin gene); ATF, 5′-GATCGCTGACGTCAGGG-3′ (Hoeffler et al., 1988; Hai et al., 1989); AP-1 (human substance P promoter), 5′-GATCAGCATGAGTCACTTC-3′ (Konradi et al., 1993); AP-1 site (noncanonical; rat prodynorphin gene), 5′-GATCGAAGTGACAAACAGCG-3′ (Naranjo et al., 1991); AP-4 (human proenkephalin gene), 5′-GATCGTCAGCTGCAGGG-3′ (Comb et al., 1988). The double G overhang or partial BamHI site is shown in italics, and the core consensus sequences are in bold. The CREB and phosphoCREB antisera (Ginty et al., 1993) used in the gel-shift analysis immunoprecipitate a single protein of the expected molecular weight in extracts from [32P] orthophosphate-labeled striatal cultures, indicating specific recognition of CREB protein (Cole et al., 1994).
Data Analysis
For phosphoCREB immunohistochemical analysis, four to five sections from each rat (n = 3 per drug treatment group) were rated for phosphoCREB-like immunoreactivity by two independent observers, who were blinded to treatment condition, on the following scale: 2, strong; 1, modest; and 0, minimal or absent. Mean scores for the two raters were then analyzed. The utlility of this ordinal method has been previously reported (Cole et al., 1994). Drug treatment effects were analyzed by one-factor ANOVA, and Fisher LSD tests were performed post-hoc to compare the acute and chronic drug-treated rats with saline-treated controls. The nonparametric Kruskal-Wallis test was also performed. A Mann-Whitney U test was utilized post-hoc to compare different drug treatments. All statistical tests were two-tailed.
Acknowledgments
We thank Drs. David Ginty and Michael Greenberg for the gift of the CREB and phosphoCREB antisera, Dr. Alexia Pollack for advice on primary striatal cultures, and Dr. Lee Baer for statistical consultation. This work was supported by PHS grants DA07134 and MH44160 (to S. E. H.). R. L. C. is supported by DA07282 (to S. E. H.) and by a grant from the Sackler Foundation.
References
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Berger SL, Chirgwin JM. Isolation of RNA. Methods Enzymol. 1989;180:3–13. doi: 10.1016/0076-6879(89)80087-4. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D1 and D2 antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Cole AJ, Baraban JM. Chronic cocaine treatment suppresses basal expression of zif268 in rat forebrain: in situ hybridization studies. J Pharmacol Exp Ther. 1992;263:343–349. [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–659. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand for the k-opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Cole DG, Kobierski L, Konradi C, Hyman SE. 6-Hydroxydopamine lesions of rat substantia nigra up-regulate dopamine-induced phosphorylation of the cAMP-response element-binding protein in striatal neurons. Proc Natl Acad Sci USA. 1994;91:9631–9635. doi: 10.1073/pnas.91.20.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Hicok J, Lin L, Spiro C, Laybourn PJ, Tschumper R, Rapacz B, McMurray CT. Induction of the rat prodynorphin gene through Gs-coupled receptors may involve phosphorylation-dependent depression and activation. Mol Cell Biol. 1994;14:2837–2848. doi: 10.1128/mcb.14.5.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M, Mermod N, Hyman SE, Pearlberg J, Ross M, Goodman HM. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988;7:3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AD, Paterson SJ, McKnight AT, Magnan J, Kosterlitz HW. Dynorphin 1–8 and dynorphin 1–9 are ligands for the kappa-subtype of opiate receptor. Nature. 1982;299:79–81. doi: 10.1038/299079a0. [DOI] [PubMed] [Google Scholar]
- Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Pollock KM. Identification of multiple DNA elements regulating basal and protein kinase A-induced transcriptional expression of the rat prodynorphin gene. Mol Endocrinol. 1994;8:333–344. doi: 10.1210/mend.8.3.8015551. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, Babb SM, Cohen BM. Persistent reduction of immediate early gene mRNA in rat forebrain following single or multiple doses of cocaine. Brain Res. 1995 doi: 10.1016/0169-328x(94)90080-9. in press. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH. Cocaine and other stimulants: actions, abuse and treatment. N Engl J Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of methamphetamine effects on the striato-nigral dynorphin system. Eur J Pharmacol. 1988;155:11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Hai T, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Goldberg S, Shippenberg TS. The kappa opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:333–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Hoeffler JP, Meyer TE, Yun Y, Jameson JL, Habener JF. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental DNA. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, Anden NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498:199–206. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. The Molecular Foundations of Psychiatry. Washington, D. C: American Psychiatric Press; 1993. [Google Scholar]
- Hyman SE, Comb M, Lin YS, Pearlberg J, Green MR, Goodman HM. A common trans-acting factor is required for cyclic AMP-induction of multiple neurotransmitter-related genes. Mol Cell Biol. 1988;8:4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Konradi C, Kobierski LA, Nguyen TV, Heckers S, Hyman SE. The cAMP-response-element-binding protein interacts, but Fos protein does not interact, with the proenkephalin enhancer in rat striatum. Proc Natl Acad Sci USA. 1993;90:7005–7009. doi: 10.1073/pnas.90.15.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le HT, Creese I. The D1 receptor antagonist SCH23390 increases cocaine self-administration in the rat. Neurosci Lett. 1987;79:315–320. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- Li SJ, Sivam SP, McGinty JF, Jiang HK, Douglass J, Calavetta L, Hong JS. Regulation of the metabolism of striatal dynorphin by the dopaminergic system. J Pharmacol Exp Ther. 1988;246:403–408. [PubMed] [Google Scholar]
- Lucas JJ, Mellström B, Colado MI, Naranjo JR. Molecular mechanisms of pain: serotonin1A receptor agonists trigger transactivation by c-fos of the prodynorphin gene in spinal cord neurons. Neuron. 1993;10:599–611. doi: 10.1016/0896-6273(93)90163-l. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self administration in the rat. Pharmacol Biochem Behav. 1993;45:239–242. doi: 10.1016/0091-3057(93)90112-7. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa receptors in the rat forebrain and mid-brain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ. κ1, receptor mRNA distribution in the rat CNS: comparison to κ receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Messersmith DJ, Gu J, Dubner R, Douglass J, Iadarola MJ. Basal and inducible transcriptional activity of an upstream AP-1/CRE element (DynCRE3) in the prodynorphin promoter. Mol Cell Neurol. 1994;5:238–245. doi: 10.1006/mcne.1994.1028. [DOI] [PubMed] [Google Scholar]
- Mulder AH, Wardeh G, Hogenboom F, Frankhuyzen AL. κ- and δ-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature. 1984;308:278–280. doi: 10.1038/308278a0. [DOI] [PubMed] [Google Scholar]
- Naranjo JR, Mellström B, Achaval M, Sassone-Corsi P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron. 1991;6:607–617. doi: 10.1016/0896-6273(91)90063-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky B, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine and amphetamine. Proc Natl Acad Sci USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahnister NR. Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J Pharmacol Exp Ther. 1990;253:38–44. [PubMed] [Google Scholar]
- Pfeiffer A, Brandt V, Herz A. Psychotomimesis mediated by kappa opiate receptor. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;256:818–824. [PubMed] [Google Scholar]
- Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the μ, δ, and κ opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-protein and the cAMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT, Jr, Caron MC. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA. 1991;88:7491–7495. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]