Abstract
Brain edema is a serious complication in ischemic stroke because even relatively small changes in brain volume can compromise cerebral blood flow or result in compression of vital brain structures on account of the fixed volume of the rigid skull. Literature data indicate that administration of either antagonists of the V1 vasopressin (AVP) receptor or the β1-adrenergic receptor are able to reduce edema or infarct size when administered after the onset of ischemia, a key advantage for possible clinical use. The present review discusses possible mechanisms, focusing on the role of NKCC1, an astrocytic cotransporter of Na+, K+, 2Cl- and water and its activation by highly increased extracellular K+ concentrations in the development of cytotoxic cell swelling. However, it also mentions that due to a 3/2 ratio between Na+ release and K+ uptake by the Na+,K+-ATPase driving NKCC1 brain extracellular fluid can become hypertonic, which may facilitate water entry across the blood-brain barrier, essential for development of edema. It shows that brain edema does not develop until during reperfusion, which can be explained by lack of metabolic energy during ischemia. V1 antagonists are likely to protect against cytotoxic edema formation by inhibiting AVP enhancement of NKCC1-mediated uptake of ions and water, whereas β1-adrenergic antagonists prevent edema formation because β1-adrenergic stimulation alone is responsible for stimulation of the Na+,K+-ATPase driving NKCC1, first and foremost due to decrease in extracellular Ca2+ concentration. Inhibition of NKCC1 also has adverse effects, e.g. on memory and the treatment should probably be of shortest possible duration.
Keywords: Astrocyte, β1-adrenoceptor, brain edema, MCAO, memory, Na+, K+-ATPase, NKCC1, vasopressin.
1. INTRODUCTION
Ischemic stroke is a major contributor to human death and functional impairment. Besides the direct adverse effects energy deprivation has on brain cells, brain ischemia followed by re-oxygenation often leads to brain edema. A major reason for this is ‘cytotoxic’ swelling, mainly of astrocytes. However, redistribution of water from extracellular to intracellular fluid cannot on its own cause expansion of total water content in the brain, so in addition water distribution across the blood-brain barrier must also be affected. Brain edema is an important event, because even relatively small changes in total brain volume can compromise cerebral blood flow or result in compression of vital brain structures on account of the fixed volume of the rigid skull. This review deals mainly with the ‘cytotoxic’ astrocytic swelling and the ability of antagonists of either the V1 vasopressin receptor or the β1-adrenergic receptor to prevent the swelling, at least in experimental stroke. A major reason for the swelling is the increase in extracellular K+ concentration occurring during brain ischemia [1, 2] and its effect on the Na+, K+, and 2 Cl- transporter NKCC1, which co-transports not only ions but also water [3, 4]. However, when necessary for understanding of the mechanisms involved in the creation of brain edema hypotonicity-induced cell swelling and aquaporin-mediated water fluxes will also be discussed.
2. NKCC1 PATHWAY FOR K+-INDUCED STIMULA-TION AND K+ EFFECT ON AQUAPORIN-MEDIATED WATER FLUXES
2.1. K+effects on NKCC1 and the Aquaporin AQP4
Experiments in cultured astrocytes have shown that extracellular K+ concentrations ≥15 mM stimulate the Na+,K+,Cl-, water cotransporter NKCC1 [5]. This co-transporter [3, 4, 6] is in the adult brain mainly expressed in astrocytes [7-10] and at the blood-brain barrier [11, 12]. In contrast to cultured astrocytes, cultured neurons show no increase in the rate of K+ uptake at highly elevated K+ concentrations [13, 14]. Astrocytes are the cells that show most swelling in brain tissue after exposure to pathologically elevated extracellular K+ concentrations [15, 16]. In experimental stroke the specific NKCC1 inhibitor bumetanide inhibits edema [17, 18]. Moreover, knock-out of NKCC1 in mice reduces brain swelling by one half following 2 hr of MCAO and 24 hr of reperfusion [19]. During exposure to highly elevated K+ concentrations brain slices show pronounced swelling [20], localized mainly in astrocytes [21], and the swelling increases with increasing extracellular K+ concentration up to ~50 mM [22]. This is important because extracellular K+ concentrations of this magnitude (and even higher) rapidly occur during brain ischemia [1, 2], where they stimulate NKCC1. During ischemia there is also a massive decrease in extracellular Ca2+ to ~0.1 mM and a more moderate decrease in extracellular Na+ and Cl- [2].
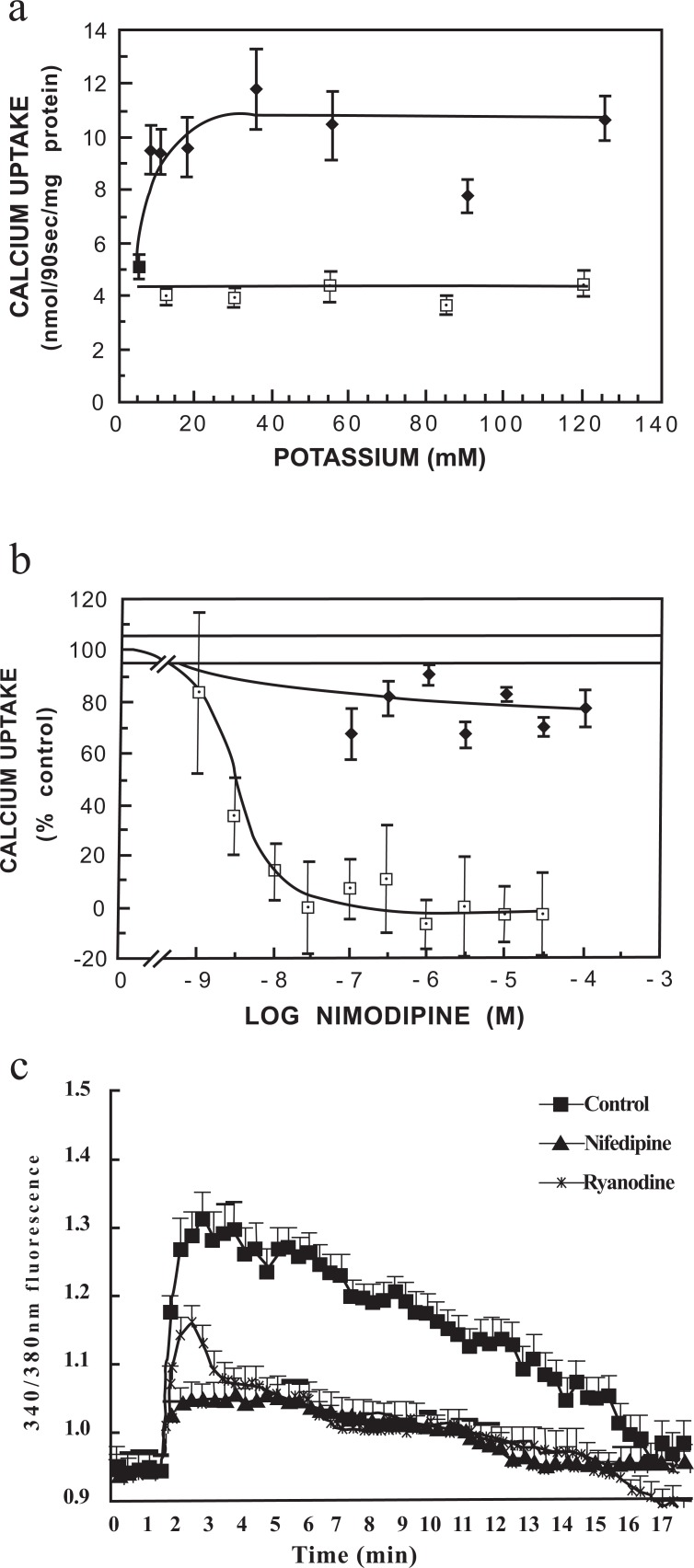
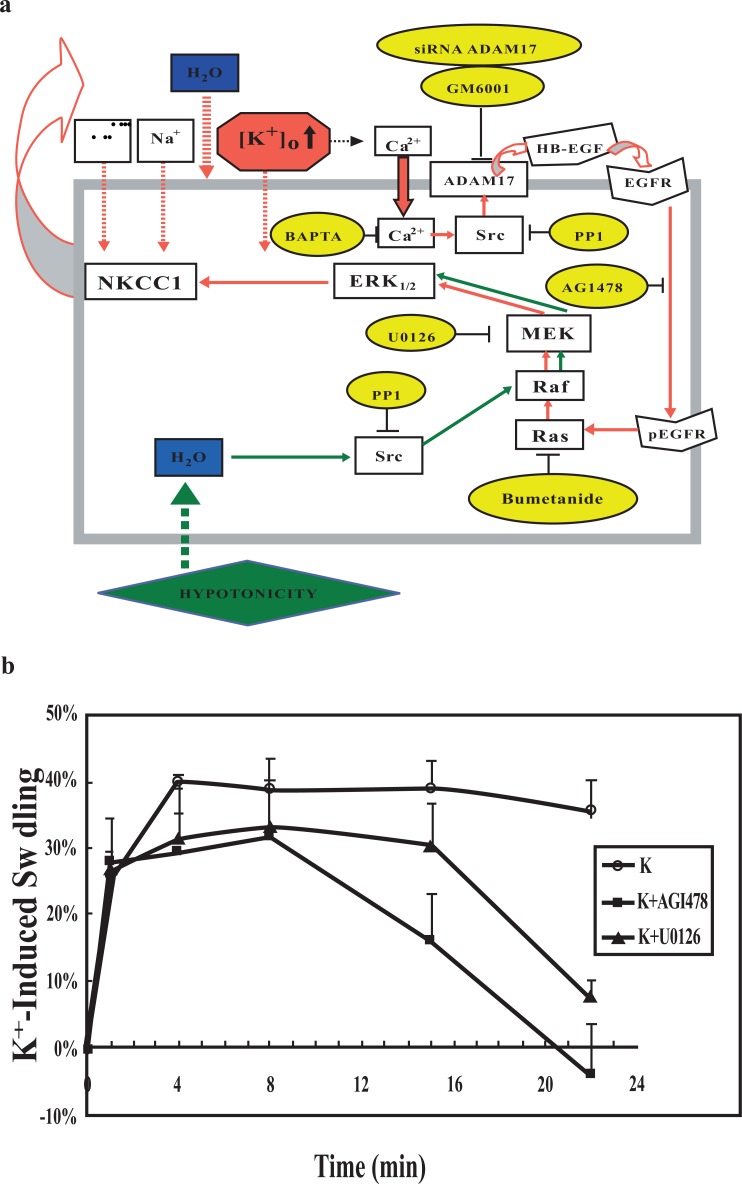
Parts of the signaling pathway by which highly elevated K+ concentrations stimulate NKCC1 are shown in Figs. 1 and 2. Addition of 8-10 mM K+ causes an increase in astrocytic uptake of pre-bound Ca2+, which has reached its maximum around 20 mM K+ (Fig. 1a). This response is completely inhibited by nimodipine, a dihydropyridine inhibitor of L-channel-mediated Ca2+ uptake (Fig. 1b). The increase in [Ca2+]i is reinforced by stimulation of the ryanodine receptor by accumulated Ca2+ (Fig. 1c) as shown by the inhibition by the ryanodine receptor inhibitor ryanodine of all but the initial increase in [Ca2+]i caused by addition of 45 mM KCl [24]. The latter Fig. also shows that the K+-induced increase in Ca2+ uptake is reflected by a nifedipine-inhibited increase [Ca2+]i, although at least at highly elevated extracellular K+ concentrations a minor [Ca2+]i increase is resistant to this dihydropyridine. These observations are consistent with neuroprotective and edema-reducing effect of L-channel inhibitors during ischemia when given early [25-27], because K+-mediated Ca2+ entry initiates a signaling pathway that leads to NKCC1 activation. This pathway is shown in Fig. 2a until phosphorylation of extracellular regulated kinases 1 and 2 (ERK1/2) together with the inhibitors used for its identification, shown in yellow ovals [28]. An important point is Src-activation of the metalloproteinase ADAM 17, releasing a growth factor causing transactivation (phosphorylation) of the epidermal growth factor (EGF) receptor leading to Ras-Raf-MEK-induced phosphorylation of ERK1/2. This in turn causes swelling that can be prevented by inhibitors of this pathway (Fig. 2b). Fig. 2a also shows that hypo-osmotic swelling activates ERK1/2 via a different pathway and therefore is not an appropriate model for swelling induced by ischemia/re-oxygenation. Since this pathway bypassed Ras and was not inhibited by bumetanide, it was tentatively concluded that bumetanide inhibited Ras. It is consistent with this conclusion that regulatory volume increase caused by hypertonic medium exposure is inhibited in NIH 3T3 cells by furosemide and bumetanide, but only in cells expressing Ras [29]. The further pathway between ERK phosphorylation and NKCC1 activation was not investigated by Cai et al. [28] but NKCC1 is directly regulated by phosphorylation [30, 31], mediated by the specific kinases SPAK (STE20/SPS1-related proline/alanine-rich kinase) and OSR1 (oxidative stress-responsive kinase-1 [32, 33].
Fig. (1).
(a) Uptake of 45Ca2+ (90 sec) into astrocytes in primary cultures as a function of the final extracellular K+ concentration. Excess K+ (2.5 to 125 mM KCl) was added either simultaneously with 45Ca2+ (☐) or 60 sec after 45Ca2+ (♦). The difference between the two conditions suggests that the channel transports pre-bound Ca2+. (b) Effect of calcium channel blocker nimodipine on basal unstimulated 45Ca2+ uptake (5.4 mM potassium, ♦) and potassium-stimulated 45Ca2+ uptake (55.4 mM potassium, ☐) into cultured astrocytes. Methodologies for a and b were as in Hertz et al. [23]. (c) Effects of nifedipine or ryanodine on the increase of [Ca2+]i by addition 45 mM KCl to normal medium (to a total K+ concentration of 50 mM), determined as described by Yan et al. [24]. After loading with fura-2 AM for 30 min, 45 mM KCl was added with or without nifedipine (100 nM), or ryanodine (1 μM), which at this concentration inhibits the ryanodine receptor. Results are averages from 60 cells on three individual coverslips. S.E.M. values are indicated by vertical bars. *Statistically significant (p<0.05) difference from control group at the same time period. From Hertz et al. [23] and Yan et al., 2013 [24].
Fig. (2).
(a) Diagram showing signaling pathways towards ERK1/2 phosphorylation activated by elevation of [K+]o (red arrows) or hypotonicity (green arrows) and inhibition of these pathways by specific inhibitors (yellow ovals). Elevation of [K+]o depolarizes the cell membrane and thereby leads to Ca2+ entry through voltage-dependent L-channels. The increase in [Ca2+]i is necessary for ERK1/2 phosphorylation, which is inhibited by BAPTA-AM, and it leads to a Src-dependent (and PP1-inhibited) release of HB-EGF from its membrane-bound precursor by the metalloproteinase ADAM 17 (inhibited by GM6001 and by siRNA against ADAM 17). The released HB-EGF activates (phosphorylates) the EGF receptor (inhibited by AG1478), leading to activation of the MAP kinase cascade, Ras (inhibited by bumetanide), Raf and MEK (inhibited by U0126), with activation of MEK causing ERK1/2 phosphorylation. ERK1/2 phosphorylation activates (phosphorylates) the cotransporter NKCC1 through pathways that were not studied and are only partly known. This leads to influx of Na+ and K+ together with 2 Cl- and water. Accordingly K+-induced swelling is contingent upon ERK1/2 phosphorylation. In contrast hypotonicity-induced swelling is independent of ERK1/2 phosphorylation, since it is not inhibited by U0126, which inhibits swelling induced by high extracellular K+ concentrations. From Cai et al., 2011[28]. (b) Effect of high [K+]o on cell swelling in astrocytes requires EGF receptor stimulation and ERK1/2 phosphorylation. Astrocytes were treated with isotonic phosphate buffered saline containing 60 mM K+ with concomitant reduction of the Na+ concentration to maintain iso-osmolarity (○), in some experiments the cells were treated with 1 μM tyrphostin AG1478, the inhibitor of the EGF receptor tyrosine kinase (■) or 10 μM U0126, the inhibitor of MEK (▲) at the same time high K+ was added. Means ± SEM were calculated for 3–5 individual experiments from the fluorescence ratios at selected times after medium change and converted to change in water space relative to that in the corresponding isotonic media at time zero. Two-way ANOVA using GraphPad showed drug effects which initially were non-significant but rapidly became significant at P < 0.05. From Cai et al. 2011 [28].
Smaller increase in extracellular K+ concentration (to ~10 mM) do not increase swelling but they stimulate the Na+,K+-ATPase, which on its own is the transporter responsible for most extracellular K+ clearance during normal brain activity [5, 34]. Since excitation causes Na+ increase in neurons, its neuronal operation needs no stimulation of Na+ uptake, whereas that in astrocytes does [5, 34, 35]. Experiments in cultured astrocytes have demonstrated activation of a ouabain signaling pathway initiated by small increases in extracellular K+, which mediates Na+ uptake by opening of Na+ channels. In contrast to the pathway mediating the effect of highly elevated K+ concentrations activation of the IP3 receptor is necessary in the ouabain pathway [5]. In spite of not leading to cell swelling these small increases in extracellular K+ concentration augment water fluxes through AQP4 [36]. These authors showed in cultured astrocytes that the water permeability increase occurred via increased cAMP and that no significant increase in specific AQP4 water permeability occurred in AQP4-negative cells. They also showed that larger elevation of extracellular K+ (to 35 mM) abolished the K+-induced increase in AQP4-mediated water flux. This is consistent with the observation by Peng et al. [37] that astrocytes in which AQP4 was knocked out by siRNA treatment show absent or reduced hypotonicity-induced swelling. Na+,K+-ATPase activity is also needed to create the ion gradients providing the driving force for NKCC1, which is a secondary active transporter [6]. The regulation of the astrocytic Na+,K+-ATPase will be discussed in section 4.
2.2. Summary
Highly elevated extracellular K+ concentrations (≥ 15 mM) stimulate the ion/water co-transporter NKCC1, which in brain parenchyma is localized in astrocytes. Smaller K+ increases stimulate Na+,K+-ATPase activity and aquaporin-mediated water flux in astrocytes, but do not cause edema. Both transporters are also likely to influence AQP4-mediated water flow across the blood-brain barrier, but this aspect of brain edema is not discussed in detail in this review.
3. VASOPRESSIN (AVP)
3.1. AVP Targets Include Astrocytes
AVP is not only a peptide hormone with a major effect on the kidney but also acts as a neurotransmitter/neuromodulator. Thus i) it is synthesized not only in hypothalamo-neurohypophysial cells, but also in other neurons, whose axon projects to the limbic system, the brainstem and the spinal cord; ii) it can be released from central axons similar to classical neurotransmitters; and iii) specific binding sites, i.e., membrane receptors with high affinity for AVP are present in the central nervous system [38]. It has long been known that AVP receptors are expressed not only on neurons but also on astrocytes [39-41].
AVP acts on different receptors in the kidney and in the brain. Its antidiuretic effect occurs via activation of the Gs- and cAMP-linked V2 receptor in the kidney, whereas astrocytes in the brain express the V1 receptor [41, 42]. The Gq/11-coupled, V1a receptor stimulates phospholipase C, PLC [43] and is thus linked to intracellular Ca2+ release, although some of the different effects of V1a stimulation require the presence of extracellular Ca2+ [44]. The V1b (also called V3) receptor is also PLC- and Ca2+-linked, but it has a pharmacological profile distinct from that of the human V1a receptor [45].
3.2. Inhibition of V1 Receptors Reduces Edema after Ischemia/Reperfusion
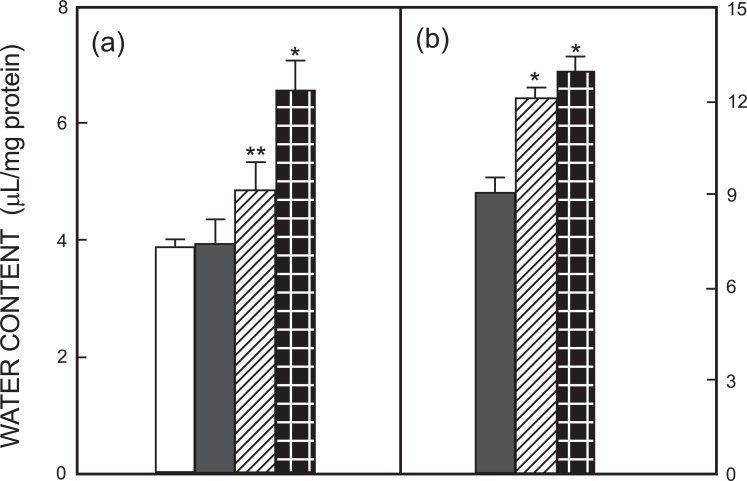
Vakili et al. [46] in their study of mice subjected to 60-min middle cerebral artery occlusion (MCAO) followed by 23 hr of reperfusion found that inhibition of V1 receptors reduced infarct volume in a dose-dependent manner by 54% to 70%, and brain edema formation by 67%, while V2 receptor inhibition had no effect. The V1-specific effect has been confirmed by Liu et al. [47]. Reduction of infarct size and edema in an embolic focal ischemia model in rats by a V1 receptor antagonist had previously been shown by Shuaib et al [48]. Kleindienst et al. [49] also found a reduction of brain water content by a V1 antagonist after a 2-hr MCAO followed by 2 hr of reperfusion in the rat. Na+ and water increase in the brain was prevented regardless whether the antagonist was administered 60 min before or 60 min after MCAO while K+ loss was inhibited only by pretreatment. The authors attributed all effects to inhibition of AQP4, an inhibition which has been shown experimentally [47, 50], but the difference between the effects on Na+ and K+ must indicate an additional effect on NKCC1. It has been mentioned that ischemia causes a massive and relatively early increase in extracellular K+ in the brain by cellular release together with a decrease in extracellular Na+ and a 90% reduction in extracellular Ca2+ [1, 2]. AQP4 may be involved during some of these alterations. However, the ability to prevent cellular increase in Na+ by administration of V1 receptor antagonist 1 hr after the insult can only be explained by an additional effect on either the Na+,K+-ATPase or NKCC1. Unfortunately Kleindienst et al. [49] did not measure Cl- content, which would have been important to distinguish between these two transporters. Ion and water uptake occurs after re-supply of metabolic energy as shown by the finding that 3 hr after MCAO there is no significant edema in the ipsilateral hemisphere, whereas the increase is considerable (and statistically significant) after 8 hr of re-perfusion (Table 1). This finding differs from a previous report by Matsuoka and Hossmann [51], but blood flow was not completely abolished in their experiments. However it is in good agreement with the observation by Nielsen et al. [52] that recirculation usually precedes malignant edema in middle cerebral artery infarcts.
Table 1.
Brain water content in MCAO model with and without reperfusion.
| No Reperfusion | 8 hr Reperfusion | |||
|---|---|---|---|---|
| Left Hemisphere | Right Hemisphere | Left Hemisphere | Right Hemisphere | |
| Control | 77.99±0.12 (n=2) | 77.61±0.09 (n=2) | 77.34±0.18 (n=3) | 77.32±0.14 (n=3) |
| Ischemia 3 h | 77.31±0.13 (n=3) | 78.14±0.32(n=3) | 77.97±0.17 (n=8) | 81.28±0.34* (n=8) |
Contents of dry matter (as percent) were calculated as the ratio between wet and dry weights, and water content determined as 100% minus % dry matter. In control animals no change occurred with or without reperfusion. In animals with MCAO in the right hemisphere a small apparent increase in water content in this hemisphere after 3 hr of ischemia was not statistically significant, whereas the much larger increase after re-perfusion marked with *was significant (p<0.05). No changes occurred in control animals. From D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International.
The ischemia/reperfusion-mediated edema described in Table 1 is abolished by intraventricular injection of the NKCC1 inhibitor furosemide (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International) suggesting that both the Na+ and water uptake found by Kleindienst et al. [49] depend upon this co-transporter. It is important and consistent with the Kleindienst results [49] that edema following complete MCAO occlusion does not occur until after reperfusion, because this should allow administration of a V1 antagonist. That administration of a V1 antagonist can reduce edema following an intracerebral hemorrhagic stroke in mice when administered after the onset of the ischemic insult has also been observed by Manaenko et al., [53]. These results are obviously of key clinical importance. The potential advantage of using a V1 antagonist in stroke patients is further supported by observations of increased plasma levels of AVP in patients with ischemic stroke [54]. Moreover, post-ischemic brain edema is exacerbated after exogenous AVP application [55] and ischemia-evoked cerebral edema is attenuated in AVP-deficient rats [56].
3.3. AVP Increases Water Content only in Astrocytes that are Already Swollen
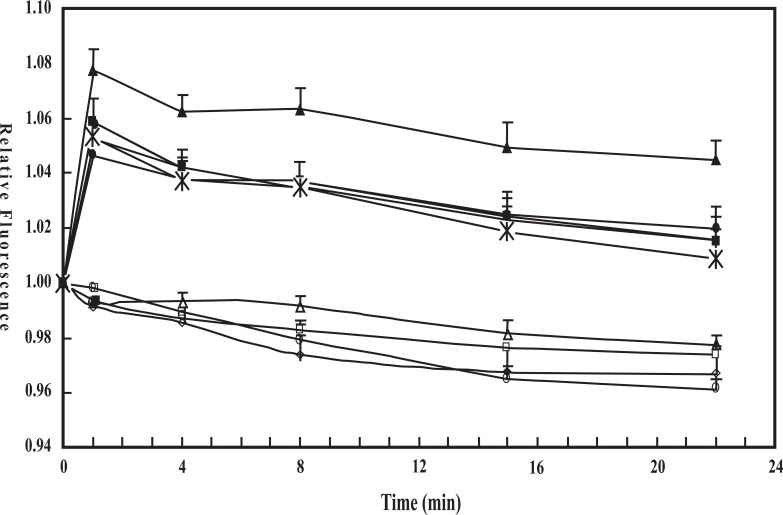
That astrocytes possess a mechanism for volume regulation which depends on both AVP and NKCC1, making it furosemide-sensitive, was first shown by Chen et al. [13]. However, before that del Bigio and Fedoroff [57] had demonstrated an AVP-induced swelling in cultured astrocytes exposed to either elevated K+ concentrations or hypotonic surroundings. Fig. 3 (from [13]) shows that exposure to 60 mM extracellular K+ causes a significant increase in water content both in cultured mouse astrocytes and neurons, but only the response in astrocytes shows an additional, large increase in the presence of 10-12 M AVP. In the absence of an increase in extracellular K+ AVP had no effect on astrocytic volume, and AVP does not increase the rate of astrocytic K+ uptake at non-increased K+ concentrations [13]. Swelling of astrocyte cultures during exposure to a hypo-osmotic medium (reduction of Na+ concentration) is also greatly enhanced by AVP [58], but these authors did not report whether AVP had any effect under isotonic conditions. That this is not the case in cultured astrocytes was, however, shown by Du et al. [59], confirming the results of Chen et al. [13]. Like Sarfaraz and Fraser [58] they found an increased swelling in the presence of AVP during exposure to hypotonic media (Fig. 4).
Fig. (3).
Changes of water content (determined as [14C] urea space) in astrocytes (a) and neurons (b) in primary cultures by an elevated K+ concentration and/or addition of AVP. The following incubation conditions were used during a 30-min period: control (5 mM potassium) (□); AVP 10-12 M added to control (■); 60 mM potassium ( ); 60 mM potassium plus 10-12 M AVP ( ). SEM values are indicated by vertical bars. *Significantly (p<0.05) different from the control; **Significantly different from value obtained at the same K+ concentration (60 mM) in the presence of AVP. From Chen et al., 1992 [13].
Fig. (4).
Fluorescence of calcein relative to the initial fluorescence during incubation in isotonic drug-free medium as an indication of tonicity- and/or AVP-induced changes in astrocytes. After the cells had been loaded with calcein, they were incubated for 2.5 min in 200 ml isotonic solution in the absence of any drug. Subsequently either 200 ml isotonic medium or 200 ml de-ionized water was added to each well, without any drug, together with 10 nM AVP; together with 1 mM AG1478 (inhibitor of EGF receptor); together with AVP and AG1478; or together with AVP and 10 mM U0126 (inhibitor of MAP kinase/ERK kinase (MEK) and thus of ERK1/2 phosphorylation), and the incubation was continued for another 22.5 min. Fluorescence was recorded at 15 s intervals and is shown at selected times as means±S.E.M with n = 5-7. Results obtained in isotonic media are shown by open symbols and results obtained in hypotonic media by filled symbols. Squares indicate no addition of drugs, triangles addition of vasopressin, circles addition of vasopressin plus AG1478, diamonds addition of AG1478 alone, and stars addition of AVP plus U0126. Note that all cultures in the isotonic groups are significantly different from all in the hypotonic groups but not from each other, indicating no drug effects under isotonic conditions. Among the cultures in the hypotonic groups those treated with AVP alone was significantly different from the 3 other groups (except at 22min), and no significant difference was found among the 3 other groups. Thus both AG1478 and U0126 completely inhibit the effect of AVP under hypotonic conditions. A small decrease in fluorescence with time may be a methodological artifact or indicate slight cell shrinkage. From Du et al., 2008 [59].
3.4. AVP Signaling
AVP has consistently been found to increase [Ca2+]i in astrocytes [14, 60], pituicytes, a bona fide astrocyte-like cell [41] and non neuronal cells from dorsal root ganglia [61]. The highest potency (threshold 10-10 M) was reported by Chen et al. [14] and Moriya et al. [61], whereas the other authors found a threshold effect at 10-8 M. However this effect can, on its own not explain the effect of AVP on astrocyte swelling induced by exposure to high extracellular K+ concentrations or hypotonic medium, since it occurs under normal conditions. All authors found the response to be independent of the presence of extracellular Ca2+ and thus caused solely by intracellular release of Ca2+.
The ability of AVP to increase hypotonic astrocyte swelling depends on a pathway, which like that activated by elevated K+ concentrations (Fig. 2a) leads to phosphorylation of ERK1/2 via metalloproteinase-mediated release of a growth factor that stimulates EGF receptors and leads to phosphorylation of ERK1/2 [59]. It is noteworthy that the metalloproteinase involved in both the K+-activated and the AVP-activated pathway is ADAM 17, a metalloproteinase not involved in transactivation of astrocytes by any other transmitter studied [37]. It is therefore feasible, but not proven, that AVP increases swelling by high extracellular K+ concentrations simply by additional stimulation of the pathway involved. AVP alone may cause too little stimulation to activate NKCC1, although it does stimulate ERK1/2 phosphorylation, inhibited by the same inhibitors in cells incubated in isotonic medium [59]. This does not explain why AVP also increases hypotonicity-induced swelling. However, this effect can probably be explained by the demonstration by Gunnarson et al. [62] that the AQP4 residue serine 111 is a molecular target for dynamic, short term regulation of water permeability and that Ca2+-dependent phosphorylation of this residue augments water flux. The role of serine 111 was confirmed by Song and Gunnarson [36] by water permeability measurements with the mutant AQP4 S111A as well as in vitro phosphorylation experiments on AQP4 S111. Although the study by Gunnarson et al. [62] used glutamate-induced [Ca2+]i increase and phosphorylation it is likely that [Ca2+]i increase evoked by AVP will have a similar effect. Thus, not only are the mechanisms for brain edema evoked by exposure to hypotonic media and highly elevated K+ concentrations different, but the AVP stimulation is also evoked on NKCC1 in one situation (high K+) and on AQP4 in another (hypotonicity). Both effects are likely to be important for the edema-reducing effect by antagonists of the V1 receptor and an additional effect on blood-brain barrier NKCC1 and AQP4 activity cannot be excluded.
The studies by Gunnarson and coworkers, by Du et al. and by Xu et al. were all carried out using cultured astrocytes. At least part of the findings are however in agreement with a study by Niermann et al. [63] in which neuronal stimulation increased extracellular K+ concentration to ~ 9 mM. Although this study was performed in animals too young (15 days old) to reflect all function of the mature brain and the ionic composition of the extracellular fluid was abnormal (drastic reduction in Cl- content) it did show that neuronal excitation increased water flux. A rapid onset and high capacity of this flux suggested that it was mediated through the AQP4-containing astrocytic syncytium spanning the entire cortex. AVP and receptor V1 agonists facilitated the flux whereas the effect of AVP was blocked by a V1 antagonist. The antagonist even reduced the flux in the absence of AVP suggesting endogenous AVP function. V2 agonists or antagonists had no effect. The effect of a minor increase in extracellular K+ concentration is important. It may seem peculiar that no stimulation is exerted at normal K+ concentrations but that a minor increase has profound effects. However, this situation is similar to that of K+ effects on glycogenolysis, which both in brain tissue [64] and in cultured astrocytes [65] is stimulated by minor K+ increases. The studies in the cultured cells showed that the glycogenolytic effect of the small increases in K+ is due to K+/Na+,K+-ATPase-mediated stimulation of the signaling pathway for endogenous (and non-endogenous) ouabains.
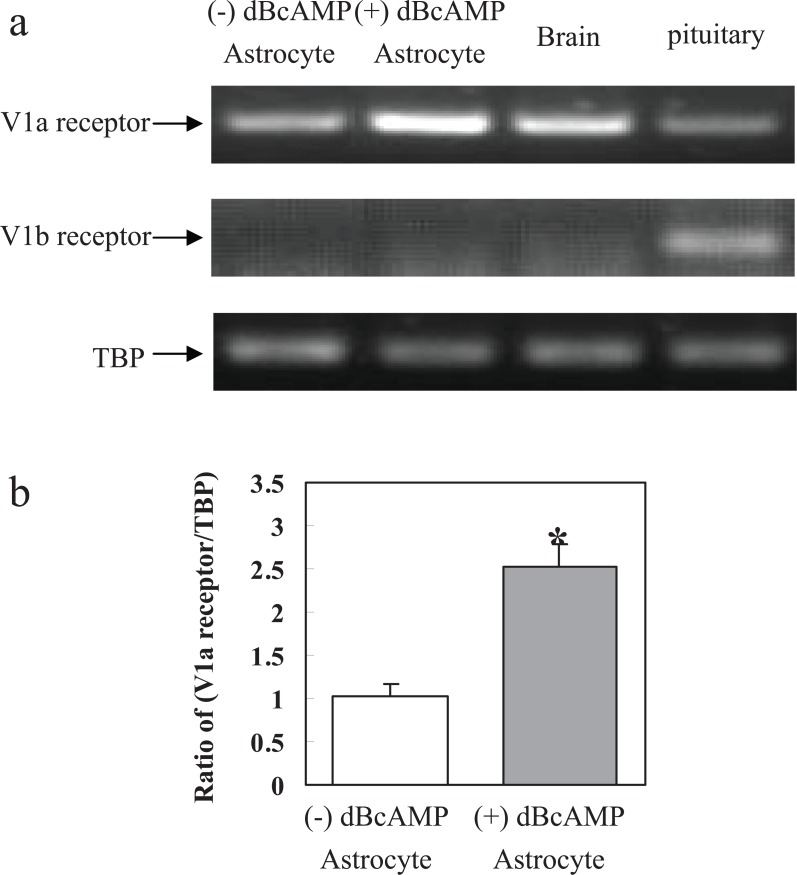
Whether the AVP effects are mainly exerted on V1a or V1b receptors has not been discussed. Previous conclusions by Chen et al. [14] that the effects on NKCC1 are exerted on V1b receptors were based exclusively on their independence of extracellular Ca2+, but this is not a reliable criterion, and should be disregarded. Most studies in cultured astrocytes regard the effects of AVP as V1a-mediated in cerebral cortex, whereas V1b receptors are expressed in other regions [66]. Exclusive V1a expression in the presently used astrocytes was confirmed as shown in Fig. 5, which also suggests that it is the only or major subtype in brain tissue. However V1b expression was found in the pituitary. Its expression in the cultured astrocytes was enhanced after differentiation with dibutyl cyclic AMP (dBcAMP), an indication of its expression in mature cells.
Fig. (5).
Expression of mRNA of V1a receptor and V1b receptor in primary cultures of astrocytes, in brain in vivo and in the pituitary. Astrocytes were cultured for 3 weeks with or without addition of 0.25 mM dibutyryl cAMP to the medium from the age of 2 weeks. (a). A representative experiments showing mRNAs for V1a receptor, V1b receptor and for TBP, used as a house-keeping gene. The first lane represents the PCR product from primary cultures of astrocytes without dBcAMP, the second lane that from primary cultures of astrocytes cultured with dBcAMP, the third lane that from brain of adult mouse, and the last lane that from the pituitary of an adult mouse. The size of PCR product of V1a receptor is 332 bp, that of V1b receptor 110 bp, and that of TBP 236 bp. (b) Average mRNA expression was quantitated as scanned ratios between V1a receptor expression in 4 individual experiments and that of TBP. S.E.M. values are indicated by vertical bars. *Statistically significant (P < 0.05) difference from astrocytes without dBcAMP. (Previously unpublished experiments by Ting Du and Liang Peng).
3.5. Summary
Antagonists of the AVP1 receptor are capable of reducing experimental brain edema after MCAO/reperfusion. AVP may enhance the effect of elevated extracellular K+ concentrations due to the similarities between the signaling pathway activated by the high K+ concentrations and by AVP, perhaps especially the use of the same metallo-proteinase (ADAM 17) for release of the growth factor causing transactivation of the EGF receptor and ultimately NKCC1 stimulation. The V1 antagonists also inhibit water flux into the brain by an effect on AQP4, but NKCC1 in endothelial cells might also be affected.
4. β1-adrenergic ANTAGONISTS
4.1. MCAO/Reperfusion-Induced Edema and Infarct are Reduced by β1-adrenergic Antagonists
Administration of subtype-specific β1-adrenergic antagonists before experimental brain ischemia has been shown to provide neuroprotection against transient focal cerebral ischemia in rats [67]. Unfortunately, although the β1-adrenergic antagonists provided long-term improvement of histological outcome, they had no effect on neurological outcome and spatial memory retention 14 days later when administration began 30 min before the onset of ischemia and continued for 24 hrs [68]. Nevertheless, the same group has shown that administration 30 min after the onset of a 2-hour-long ischemic period drastically reduced infarct size (Fig. 6) and improved neurological deficit score after 7 days (Table 2) [69]. Unfortunately they did not measure edema. Similarly Iwata et al. [70] found that administration of antagonists specifically of the β1-adrenoceptor beginning 60 min after an 8-min bilateral carotid artery occlusion combined with hypotension reduced neuronal injury after forebrain ischemia, although motor activity was not improved. However, motor deficit index scores were significantly lower and neuronal survival better in rats treated with β1-adrenoceptor antagonists beginning 30 min before 10 min of spinal cord ischemia and continued for 24 hr [71]. Recently Song et al. (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International) found that intraventricular injection of any of several inhibitors of the β1-adrenergic pathway as well as the β1-adrenoceptor antagonist betaxolol abolished cerebral edema in rats exposed to 3 hr of MCAO followed by 8 hr of reperfusion. In contrast a single selective inhibitor of the b2-adrenergic pathway or the b2-adrenoceptor antagonist ICI118555 had no effect.
Fig. (6).
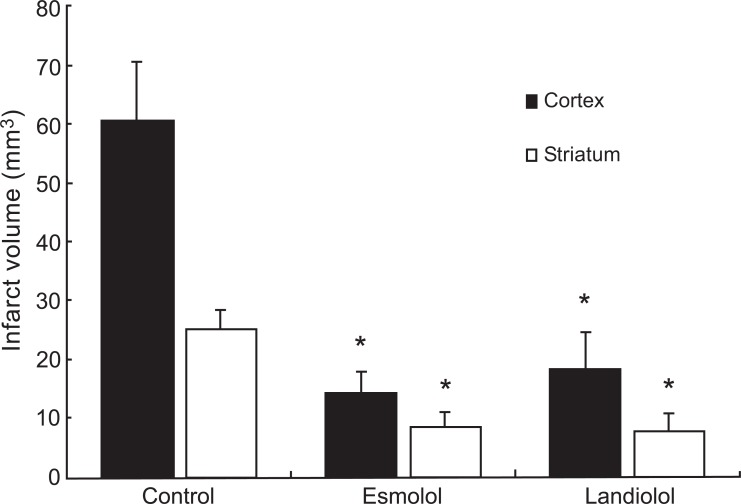
Infarct volumes (determined by staining with triphenyltetrazolium chloride) 7 days after reperfusion of the cortex and striatum in rats exposed to 2 hr MCAO followed by 24 hr re-perfusion and treated with saline (control), esmolol, or landiolol, shown as means ± SEM. Drug (or saline) treatment was started 30 min after the onset of MCAO and continued during 24 hr of reperfusion. *p<0.05 versus saline treatment group. From Goyagi et al., 2010 [69].
Table 2.
Neurological deficit scores determined as described by Goyagi et al. [69] in control animals (injected with saline) and exposed to 2 hr of MCAO followed by reperfusion and in rats treated with either of the β1-adrenergic antagonists esmolol or landiolol, beginning 30 min after the onset of ischemia and continued for 24 hr.
| Control (n=8) | Esmolol (n=8) | Landiolol (n=8) | |
|---|---|---|---|
| 1 day | 18 (10-45) | 7.5 (0-20)* | 0 (0-16)* |
| 4 days | 4 (0-24) | 0 (0-4)* | 0 (0-6)* |
| 7 days | 2 (0-13) | 0 (0-2)* | 0 (0-0)* |
Values are expressed as median and range). The neurological deficit scores in the esmolol- or landiolol-treated rats were significantly lower than the saline-treated control rats 1, 4, and 7 days after focal ischemia. *P<0.05 versus control group. From Goyagi et al, 2010 [69].
4.2. β1-adrenergic Stimulation of NKCC1-Mediated Regulatory Volume Increase
Most cells shrink during exposure to a hypertonic medium and NKCC1 activity is important for a subsequent regulatory volume increase [6]. This also happens in cultured astrocytes, where the regulatory volume increase after addition of 100 mM sucrose to the incubation medium is inhibited by bumetanide, showing the involvement of NKCC1 [72]. The volume regulation is slow in cultured astrocytes under control conditions, but accelerated by the β-adrenergic drug isoproterenol (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International). The pathway for both β1- and b2-mediated signaling is known in astrocytes [73]. In well-differentiated astrocyte cultures this pathway reminds of that shown in Fig. 2a for the effect of highly elevated K+ concentrations. Although β-adrenergic receptors are Gs-linked, a Gs to Gi switch mediated by protein kinase A (PKA) leads to an increase in [Ca2+]i and a subsequent growth factor release, which transactivates the EGF receptor and stimulates ERK1/2 phosphorylation [73]. Two important differences between the two signaling pathways are that Src and the metallopropteinase ADAM 17 are not involved in the pathway activated by stimulation of β1-adrenergic receptors, although Src constitutes part of the b2-adrenoceptor-mediated pathway. Song et al. (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International) found that inhibitors of Gi, metalloproteinases, EGF receptor phosphorylation and ERK1/2 phosphorylation as well as the β1-adrenergic antagonist betaxolol all abolished the ability of isoproterenol to enhance regulatory volume increase in the astrocytes exposed to a medium supplemented with 100 mM sucrose, whereas an inhibitor of b2-adrenergic signaling and the b2-adrenergic antagonist ICI118555 had no effect. Moreover it was also shown that glycogenolysis, which is essential for K+-stimulated activation of both the Na+,K+-ATPase and NKCC1 [5] is needed for β-adrenergic stimulation of the Na+,K+-ATPase/NKCC1 transport system during regulatory volume increase. These observations are in excellent agreement with stimulation of the astrocytic, but not the neuronal Na+,K+-ATPase with isoproterenol [74]. This ATPase, which provides the driving force for NKCC1 operation (see above) is ubiquitously expressed in both neurons and astrocytes [75, 76].
4.3. Abrogation of Na+,K+-ATPase Stimulation by Elevated K+ Due to Extracellular Ca2+ Deficiency Allows Exclusive β1-adrenergic Stimulation of NKCC1
The stimulation of regulatory volume increase by β1-adrenergic stimulation triggered the experiments by Song and her colleagues showing that the same antagonists inhibited edema formation after MCAO/reperfusion. However, there is one important difference between the two situations. During regulatory volume increase the extracellular K+ concentration is unaltered, whereas it is increased after ischemia. This difference should be important, because a rise in extracellular K+ concentrations above its normal level provides an important stimulus for the astrocytic Na+,K+-ATPase [77, 78]. In cultures of cerebral cortical astrocytes Hajek et al. [74] found Km value for K+ of 1.9 mM, which is high enough for an increase in extracellular K+ concentration in the interval 5-12 mM on its own to allow a significant increase in the activity of the enzyme. In cerebral cortical neurons, the affinity was higher (Km 0.43 mM), showing that there is no stimulation of enzyme activity when the extracellular K+ concentration is increased beyond control levels. Astrocytes are a major target for noradrenaline released from locus coeruleus [79]. It is likely that under normal, non ischemic conditions most of the K+ released to the extracellular space during action potential propagation is initially accumulated into astrocytes, and that this uptake stops due to cessation of K+-stimulated glycogenolysis when extracellular K+ normalizes [5]. This will allow Kir4.1-mediated K+ release from astrocytes [80] and re-uptake by the neuronal Na+,K+-ATPase. This raises the question why K+-mediated stimulation of Na+,K+-ATPase did not support NKCC1 operation after ischemia when energy became available after reperfusion.
The profound decrease in extracellular Ca2+ caused by brain ischemia is combined with an increase in [Ca2+]i, causing cell damage and even death [81]. Cellular increase in Ca2+ promotes NADH hyperoxidation and electrical dysfunction after anoxia in hippocampal slices [82]. Not only neurons are affected by the Ca2+ overload, since cultured astrocytes rapidly die following anoxia and re-perfusion when the gaseous and interstitial ionic changes of transient brain ischemia are simulated, and their death requires external Ca2+ [83]. Nevertheless, our own results (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International) have shown that in astrocyte cultures exposed to 3 hr anoxia the increase in swelling in response to 50 mM K+ occurred more slowly than normally, but it was not abolished. This may shift the focus to the 90% reduction in extracellular Ca2+ concentration shown by Hansen and Nedergaard [2].
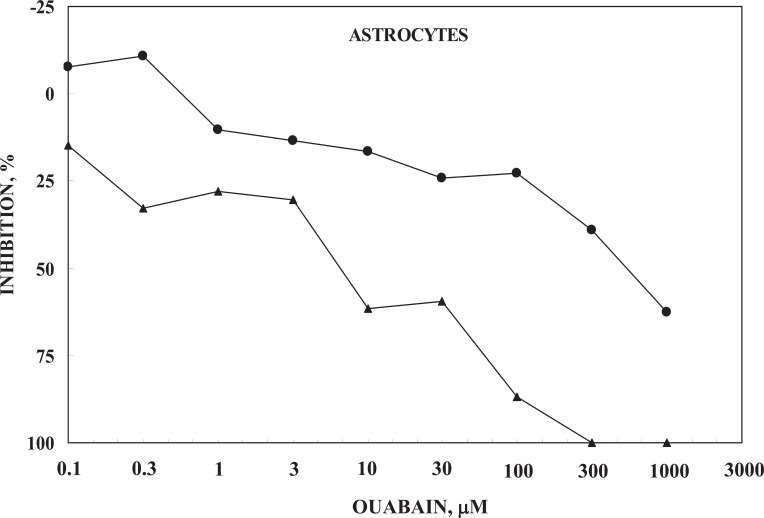
It was pointed out above i) that stimulation of the astrocytic Na+-K+-ATPase requires ouabain signaling in order to allow entry of Na+, needed for co-stimulation at the intracellular Na+-sensitive site, and ii) that NKCC1 function requires Na+-K+-ATPase activity. It is therefore of interest that Ca2+ deficiency in the medium (0 Ca2+ added, but no chelating agent) leads to a large decrease in potency of ouabain-induced inhibition of 42K uptake in cultured astrocytes, expressed as percentage of the uninhibited rate in a medium containing1.8 mM CaCl2 (Fig. 7) (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International). Since no Ca2+ chelator was added, the Ca2+-deficient medium probably contained traces of Ca2+released from the cells. The K+ concentration in the medium was at control level (5.4 mM) but addition of 42K provided a small increase of ~0.3 mM. In both types of media a minor part of the uptake is inhibited by the lower ouabain concentrations, with relatively little dependence on percentage changes in ouabain concentration, and a major part of the uptake is inhibited by higher ouabain concentrations with a greater effect of relative changes in ouabain concentration. This biphasic effect can be explained by binding to two different Na+,K+-ATPase subunits [84]. However, at all concentrations the potency of ouabain is ≥10 times lower in the absence of added Ca2+ to the medium. Accordingly, the normally occurring endogenous ouabain concentrations may not suffice to stimulate the Na+,K+-ATPase when the extracellular Ca2+ concentration is drastically reduced. The apparent slight stimulation at low concentration of ouabain (100 and 300 ìM) seen in Fig. 7 suggests that in the Ca2+-deficient medium ouabain may be stimulatory at concentrations which are normally inhibitory, although in a medium containing sufficient Ca2+ stimulatory ouabain concentrations are much lower [5].
Fig. (7).
Ouabain induced inhibition of net influx of 42K into primary cultures of mouse astrocytes determined during a 1.00 min incubation, which provides initial uptake rates. The graph marked by triangles was obtained in a slightly modified Dulbecco's medium containing 1.8 mM Ca2+ and that marked by circles in a corresponding medium containing no CaCl2. The inhibition was calculated from the difference between uptake rates in the absence of ouabain and that at the ouabain concentration in question. Without ouabain and at all lower ouabain concentrations the S.E.M. values of these measurements were below 5%, but at the highest ouabain concentration they were slightly higher, although always below 10%. For both graphs 6 cultures were routinely used from at least two different batches. The apparent stimulation at low ouabain concentrations in the absence of Ca2+ was not significant, but might suggest a ouabain-mediated stimulation of uptake, normally occurring at low nanomolar concentrations, as described by Xu et al. [5]. No similar increase was seen in neurons, which also showed a decrease in ouabain potency in the absence of Ca2+, although of somewhat smaller magnitude (not shown). Results from D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International.
The large decrease in ouabain potency in the absence of added Ca2+ in the medium is consistent with an abolishment of K+-mediated uptake into cultured astrocytes in the absence of Ca2+ in the incubation medium (D. Song, J. Xu, L. Hertz, W. Walz and L. Peng, in press, BioMed Research International). It is also in agreement with the observation by Wang et al. [35] that a transmitter-induced rise in [Ca2+]i triggers an increase in ouabain-sensitive K+ uptake in cultured astrocytes, which was abolished by the Na+/Ca2+ exchange inhibitors SEAO4000 or SN-6. The same astrocyte-specific transmitter also evoked a transient decrease in extracellular K+ concentration in hippocampal slices. Finally an increased utilization of glucose in the striatum in freely behaving rats in the presence of elevated extracellular K+ concentrations [85] confirms a previously observed stimulation of oxygen consumption rate in brain slices [86, 87]. In the slices the threshold concentration of K+ evoking this effect (20 mM) coincides with that causing swelling [22] and the stimulation is inhibited by ethacrynic acid [88], suggesting that it is a metabolic manifestation of the normally occurring NKCC1-stimulated K+ uptake. In the present context it is important that the metabolic stimulation was abolished when Ca2+ was excluded from the medium. In vivo the stimulation probably also reflects stimulation of Na+,K+-ATPase activity, which in the slices may be supported exclusively by glycolysis, the rate of which in brain slices is greatly enhanced as indicated by a large lactate release [86].
Since brain swelling also requires additional fluid uptake across the blood-brain barrier, which similarly expresses NKCC1 [12], it is possible that Ca2+ deficiency at the blood-brain barrier also may have played a role for the prevention of edema after MCAO/reperfusion. The NKCC1 in endothelial cells is expressed at their luminal side [89, 90], facilitating uptake from the circulation. Ca2+ deficiency also reduces the potency of ouabain in neuronal cultures, but to a lesser degree. Consistent with neuronal Na+ uptake during excitation abolishing the need for additional Ca2+ entry there is no evidence suggesting stimulation at the lowest concentrations (L. Hertz and W. Walz, unpublished).
4.4. Which Ionic Consequences can be Expected after Complete NKCC1 Inhibition?
Even when NKCC1 is completely inhibited the large increase in extracellular K+ is likely to be re-accumulated, but exclusively into neurons and considerably more slowly than normally. This is both because no NKCC1 activity contributes to the uptake and because the neuronal Na+,K+-ATPase has a lower Vmax than the astrocytic enzyme and is not stimulated by elevated K+ [74]. However, it may be stimulated by excess intraneuronal Na+. Hossmann et al [1] have shown that uptake of the increased extracellular K+ concentration after a non-complete MCAO under normal conditions is completed within one hr, and it would be very important also to obtain information how rapidly excess extracellular K+ is normalized during inhibition of β1-adrenergic activity. NKCC1 inhibition beyond that point in time may be damaging brain function rather than protecting it. Normalization of Ca2+ distribution between extra- and intracellular spaces is equally important. Although it is known that extracellular Ca2+ normalizes more slowly than K+ after shortlasting anoxia [91], similar information is lacking after a longer insult, let alone in a situation where Na+,K+-ATPase function is inhibited. Such information would also be crucial.
4.5. Adverse Effect of NKCC1 Inhibition: Impairment of Learning
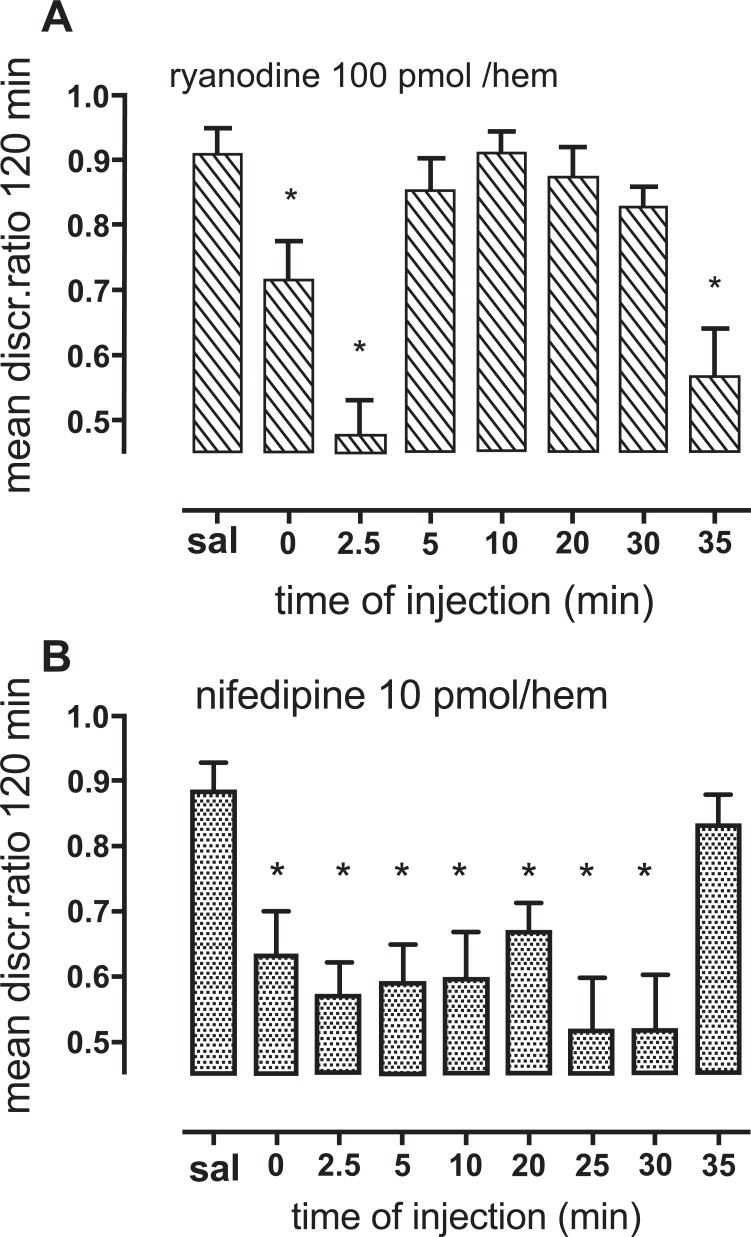
Frieder and Allweis [92] showed that ethacrynic acid, a less specific inhibitor of NKCC1 than bumetanide, inhibits memory formation in a rat model. Since nifedipine inhibits the Ca2+ uptake which after reinforcement by Ca2+ release by stimulation of the ryanodine receptor leads to the initiation of the pathway that ultimately stimulates NKCC1 (Figs. 1, 2) we tested effects of nifedipine and the ryanodine receptor antagonist ryanodine on learning. In Figs. 8a and b the discrimination ratio (DR) between pecking at beads of a neutral color and a color previously associated with an aversive taste during earlier 10-sec training is an indication of learning by day-old chicks [93]. Ethacrynic acid also inhibits learning in this model [94]. Fig. 8a shows that injection of a high, inhibitory concentration of ryanodine into the hippocampus at two different times, 2.5 and 35 min inhibits learning, as indicated by almost similar pecking at beads of the two colors (DR close to 0.5), whereas ryanodine injection at other times have little or no effect (DR 0.8-0.9). These two time periods are known to be very sensitive to inhibitors of learning, and glutamate release causing excitation with cellular release of K+ occurs immediately before these times [95, 96]. That the effect of ryanodine is associated with deficient Ca2+ entry leading to stimulation of NKCC1 is supported by the effect of nifedipine to inhibit learning. This is seen in Fig. 8b, which shows that in contrast to earlier administration of nifedipine injection at 35 min does not inhibit learning. That this is slightly earlier than the shown sensitivity to ryanodine is consistent with the uptake of Ca2+ preceding Ca2+ release from the ryanodine receptor. It is likely, but not proven that the shown effects, like many others [97], are exerted on astrocytes. An effect on astrocytes is supported by a memory-enhancing effect of thrombin at the same times and its inhibition by fluoroacetate, an astrocyte-specific toxin [98]. However, even if this should not be the case, Fig. 8 demonstrates that inhibition of the first steps of the pathway leading to NKCC1-mediated uptake of ions and water inhibits memory. Although this effect is likely to be reversible it strongly suggests that inhibition of NKCC1 function after MCAO/reperfusion should be of as short duration as possible.
Fig. (8).
Effects of the ryanodine receptor antagonist ryanodine and the L-channel inhibitor nifedipine, compared to injection of saline only, on learning in day-old chickens. As described in the text the DR between pecking at a bead of a previously neutral color and a bead of a color that during training was aversive is an indication of learning, with a high DR (~0.9) indicating normal learning and a low DR (close to 0.5) indicating failure to learn. A: Intrahippocampal injection of ryanodine inhibits learning when injected at two different time periods, 2.5 and 35 min after training. B: Intraventricular injection of nifedipine inhibits learning at several injection times. It does not inhibit learning when injected at 35 min, although ryanodine was inhibitory at this time. This difference is consistent with L-channel activation and Ca2+ entry (Fig. 1) occurring slightly earlier than the effect of accumulated Ca2+ on the ryanodine receptor. Previously unpublished results by M.E. Gibbs.
5. CONCLUDING REMARKS
The present review has focused on the role of cytotoxic swelling primarily or exclusively in astrocytes and the ways in which antagonists of the V1 receptor or the β1-adrenergic receptor can counteract edema formation. Since the β1-adrenergic receptor antagonists in principle can completely prevent the development of cytotoxic edema they may be the more powerful agents, whereas V1 receptor antagonists only prevent the additive effect of AVP on cell swelling. However, AVP inhibition of AQP may be very important to inhibit water uptake across the blood-brain barrier as discussed below. Like other recent review papers it points out that a volume shift from the extracellular to intracellular space in itself does not cause brain swelling, which depends upon water influx across the blood-brain barrier. Mechanisms leading to this influx have recently been discussed by Khanna et al. [99]. We would like also to emphasize that due to the 3/2 ratio between Na+-K+-ATPase-mediated Na+ efflux and K+ uptake [100, 101] the intense operation of the Na+-K+-ATPase/NKCC1 system during removal of extracellular K+ concentrations as high as 80 mM might lead to considerable hypertonicity in brain extracellular fluid that would facilitate AQP4 mediated water uptake across the blood-brain barrier. A small increase in osmolality during a non-complete MCAO has been demonstrated by Matsuoka and Hossmann [51]. The same group [102] also concluded that early edema following a non-complete ischemia is cytotoxic and develops at flow rates below 10-15 ml/100g tissue per min and that the blood-brain barrier remains intact for at least 4 hr. These conclusions seem to be in agreement with the observations cited in this review. Also, although ischemic and traumatic brain injury are widely different diseases it is highly relevant that the successful Lund project for treatment of post-trauma brain edema recommended use of a β1-adrenergic antagonist and that AVP should be omitted unless absolutely necessary [37, 103]. However, the effect of β1-adrenergic antagonists may seem at odds with the finding by de Raedt et al. [104] that stroke patients already under treatment with β1-adrenergic inhibitors have no improved outcome after stroke. In this connection it may be important that the human hypothalamus and hippocampus have a much lower β1-/β2-adrenoceptor ratio than the corresponding tissues in the rat [105, 106]. On the other hand a higher level of total β-adrenergic receptor binding was found in the human than in the rat hippocampus in the regions investigated [106]. Also, Russo-Neustadt and Cotman observed high levels of β1 receptors in layers I and II, low levels in layers III–V, and intermediate levels in layer VI of the human orbitofrontal cortex, while confirming the low β1-/β2-adrenoceptor ratio in hypothalamus [107]. Since β1-adrenergic receptor expression has been determined with certainty in freshly isolated astrocytes (from the mouse) but not in corresponding neurons [108], the species difference may mainly or exclusively apply to astrocytes. Finally, human white matter contains no β1-adrenergic receptors [107, 109], suggesting a difference between gray and white matter astrocytes and inability of β1-adrenergic inhibitors to counteract white matter ischemic damage. In addition there may be a question of drug dosage in patients treated chronically with β1-adrenergic inhibitors. The doses used to completely inhibit brain edema may be much higher than those used for continuous treatment, where they would have intolerable side effects, including learning difficulties. As previously mentioned, clinical effect of V1 or β1-adrenergic antagonists would probably require use of high doses during the shortest possible time period.
ACKNOWLEDGEMENTS
Supported by Grant No. 31300883 to T.D. from the National Natural Science Foundation of China.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hossmann KA, Sakaki S, Zimmermann V. Ion activities in reversible ischemia of the cat brain. Acta Neurol. Scand. . 1977;64(Suppl. ):108–109. [PubMed] [Google Scholar]
- 2.Hansen AJ, Nedergaard M. Brain ion homeostasis in cerebral ischemia. Neurochem. Pathol. 1988;9:195–209. doi: 10.1007/BF03160362. [DOI] [PubMed] [Google Scholar]
- 3.Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ. Cotransport of water by the Na+-K+-2Cl- cotransporter NKCC1 in mammalian epithelial cells. J. Physiol. 2010;588(Pt 21):4089–4101. doi: 10.1113/jphysiol.2010.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeuthen T, Macaulay N. Cotransport of water by Na+-K+-2Cl- cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J. Physiol. 2012;590(Pt 5):1139–1154. doi: 10.1113/jphysiol.2011.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes potential implications for K+ homeostasis and glycogen usage in brain. Neurochem. Res. 2013;38(3):472–485. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SF, O'Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na+/H+ exchange and Na+ -K+ -2Cl- cotransport in the heart, brain, and blood. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(1):R1–25. doi: 10.1152/ajpregu.00782.2005. [DOI] [PubMed] [Google Scholar]
- 7.Marty S, Wehrlé R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C. Postnatal maturation of Na+, K+, 2Cl- cotransporter expression and inhibitory synaptogenesis in the rat hippocampus an immunocytochemical analysis. Eur. J. Neurosci. 2002;15(2):233–245. doi: 10.1046/j.0953-816x.2001.01854.x. [DOI] [PubMed] [Google Scholar]
- 8.Deisz RA, Lehmann TN, Horn P, Dehnicke C, Nitsch R. Components of neuronal chloride transport in rat and human neocortex. J. Physiol. 2011;589(Pt 6):1317–1347. doi: 10.1113/jphysiol.2010.201830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanaka C, Ohno K, Okabe A, et al. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104(4):933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 10.Yan Y, Dempsey RJ, Sun D. Expression of Na+-K+-Cl- cotransporter in rat brain during development and its localization in mature astrocytes. Brain Res. 2001;911(1):43–55. doi: 10.1016/s0006-8993(01)02649-x. [DOI] [PubMed] [Google Scholar]
- 11.Abbruscato TJ, Lopez SP, Roder K, Paulson JR. Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J. Pharmacol. Exp. Ther. 2004;310(2):459–468. doi: 10.1124/jpet.104.066274. [DOI] [PubMed] [Google Scholar]
- 12.Yuen N, Lam TI, Wallace BK, Klug NR, Anderson SE, O'Donnell ME. Ischemic factor-induced increases in cerebral microvascular endothelial cell Na/H exchange activity and abundance evidence for involvement of ERK1/2 MAP kinase. Am. J. Physiol. Cell Physiol. 2014;306(10):C931–942. doi: 10.1152/ajpcell.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, McNeill JR, Hajek I, Hertz L. Effect of vasopressin on brain swelling at the cellular level: do astrocytes exhibit a furosemide vasopressin sensitive mechanism for volume regulationκ. Can. J. Physiol. Pharmacol. 1992;70(Suppl ):S367–373. doi: 10.1139/y92-285. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zhao Z, Hertz L. Vasopressin increases [Ca2+]i in differentiated astrocytes by activation of V1b/V3 receptors but has no effect in mature cortical neurons. J. Neurosci. Res. 2000;60(6):761–766. doi: 10.1002/1097-4547(20000615)60:6<761::AID-JNR8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Zadunaisky JA, Curran PF. Sodium fluxes in isolated frog brain. Am. J. Physiol. 1963;205(5):949–956. doi: 10.1152/ajplegacy.1963.205.5.949. [DOI] [PubMed] [Google Scholar]
- 16.Bourke RS, Nelson KM. Further studies on the K+-dependent swelling of primate cerebral cortex in vivo the enzymatic basis of the K+-dependent transport of chloride. J. Neurochem. 1972;19(3):663–685. doi: 10.1111/j.1471-4159.1972.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 17.Kahle KT, Barnett SM, Sassower KC, Staley KJ. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na+-K+-2Cl- cotransporter NKCC1. J. Child Neurol. 2009;24(5):572–576. doi: 10.1177/0883073809333526. [DOI] [PubMed] [Google Scholar]
- 18.Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM. Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Transl. Stroke Res. 2014;5(1):3–16. doi: 10.1007/s12975-013-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25(1):54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- 20.Bourke RS, Kimelberg HK, Nelson LR. The effects of temperature and inhibitors on HCO3-stimulated swelling and ion uptake of monkey cerebral cortex. Brain Res. 1976;105(2):309–323. doi: 10.1016/0006-8993(76)90427-3. [DOI] [PubMed] [Google Scholar]
- 21.Moller M, Mollgård K, Lund-Andersen H, Hertz L. Concordance between morphological and biochemical estimates of fluid spaces in rat brain cortex slices. Exp. Brain Res. 1974;21(3):299–314. doi: 10.1007/BF00235749. [DOI] [PubMed] [Google Scholar]
- 22.Lund-Andersen H, Hertz L. Effects of potassium and of glutamate on swelling and on sodium and potassium content in brain-cortex slices from adult rats. Exp. Brain Res. 1970;11(2):199–212. doi: 10.1007/BF00234323. [DOI] [PubMed] [Google Scholar]
- 23.Hertz L, Bender AS, Woodbury DM, White HS. Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J. Neurosci. Res. 1989;22(2):209–215. doi: 10.1002/jnr.490220215. [DOI] [PubMed] [Google Scholar]
- 24.Yan E, Li B, Gu L, Hertz L, Peng L. Mechanisms for L-channel-mediated increase in [Ca2+]i and its reduction by anti-bipolar drugs in cultured astrocytes combined with its mRNA expression in freshly isolated cells support the importance of astrocytic L-channels. Cell Calcium. 2013;54(5):335–342. doi: 10.1016/j.ceca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Hara H, Nagasawa H, Kogure K. Nimodipine attenuates both ischaemia-induced brain oedema and mortality in a rat novel transient middle cerebral artery occlusion model. Acta Neurochir. Sppl.(Wien) 1990;51:251–253. doi: 10.1007/978-3-7091-9115-6_84. [DOI] [PubMed] [Google Scholar]
- 26.Campbell CA, Mackay KB, Patel S, King PD, Stretton JL, Hadingham SJ, Hamilton TC. Effects of isradipine, an L-type calcium channel blocker on permanent and transient focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 1997;148(1):45–50. doi: 10.1006/exnr.1997.6611. [DOI] [PubMed] [Google Scholar]
- 27.Deguchi K, Yamashita T, Abe K. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–150. doi: 10.1016/j.brainres.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Du T, Song D, Li B, Hertz L, Peng L. Astrocyte ERK phosphorylation precedes K+-induced swelling but follows hypotonicity-induced swelling. Neuropathology. 2011;31(3):250–264. doi: 10.1111/j.1440-1789.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- 29.Lang F, Ritter M, Wöll E, et al. Altered cell volume regulation in ras oncogene expressing NIH fibroblasts. Pflugers Arch. 1992;420(5-6):424–427. doi: 10.1007/BF00374615. [DOI] [PubMed] [Google Scholar]
- 30.Lytle C, Forbush B. The Na-K-Cl cotransport protein of shark rectal gland.II. Regulation by direct phosphorylation. J. Biol. Chem. 1992;267(35):25438–25443. [PubMed] [Google Scholar]
- 31.Kurihara K, Moore-Hoon ML, Saitoh M, Turner RJ. Characterization of a phosphorylation event resulting in upregulation of the salivary Na+-K+-2Cl- cotransporter. Am. J. Physiol. 1999;277(6 Pt 1):C1184–1193. doi: 10.1152/ajpcell.1999.277.6.C1184. [DOI] [PubMed] [Google Scholar]
- 32.Vitari AC, Thastrup J, Rafiqi FH, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem. J. 2006;397(1):223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delpire E, Gagnon KB. SPAK and OSR1 STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem. J. 2008;409(2):321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 34.Hertz L, Xu J, Song D, Yan E, Gu L, Peng L. Astrocytic and neuronal accumulation of elevated extracellular K+ with a 2/3 K+/Na+ flux ratio-consequences for energy metabolism, osmolarity and higher brain function. Front. Comput. Neurosci. 2013;7:114. doi: 10.3389/fncom.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Smith NA, Xu Q, et al. Astrocytes modulate neural network activity by Ca2+ -dependent uptake of extracellular K+. Sci. Signal. 2012;5(218):ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Y, Gunnarson E. Potassium dependent regulation of astrocyte water permeability is mediated by cAMP signaling. PLoS One. 2012;7(4):e34936. doi: 10.1371/journal.pone.0034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng L, Du T, Xu J, et al. Adrenergic and V1-ergic Agonists/Antagonists Affecting Recovery from Brain Trauma in the Lund Project Act on Astrocytes. Curr. Signal. Transduct. Ther. 2012;7:43–55. [Google Scholar]
- 38.Raggenbass M. Vasopressin- and oxytocin-induced activity in the central nervous system electrophysiological studies using in-vitro systems. Prog. Neurobiol. 2001;64(3):307–326. doi: 10.1016/s0301-0082(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 39.Hösli E, Hösli L. Autoradiographic localization of binding sites for second messengers on neurones and astrocytes of cultured rat cerebellum. Neurosci. Lett. 1991;125(1):49–52. doi: 10.1016/0304-3940(91)90128-g. [DOI] [PubMed] [Google Scholar]
- 40.Hösli E, Hösli L. Autoradiographic localization of binding sites for arginine vasopressin and atrial natriuretic peptide on astrocytes and neurons of cultured rat central nervous system. Neuroscience. 1992;51(1):159–166. doi: 10.1016/0306-4522(92)90480-p. [DOI] [PubMed] [Google Scholar]
- 41.Hatton GI, Bicknell RJ, Hoyland J, Bunting R, Mason WT. Arginine vasopressin mobilises intracellular calcium via V1-receptor activation in astrocytes (pituicytes) cultured from adult rat neural lobes. Brain Res. 1992;588(1):75–83. doi: 10.1016/0006-8993(92)91346-g. [DOI] [PubMed] [Google Scholar]
- 42.Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1--receptor physiology. Crit. Care. 2003;7(6):427–434. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem. 1994;269(5):3304–3310. [PubMed] [Google Scholar]
- 44.Briley EM, Lolait SJ, Axelrod J, Felder CC. The cloned vasopressin V1a receptor stimulates phospholipase A2, phospholipase C, and phospholipase D through activation of receptor-operated calcium channels. Neuropeptides. 1994;27(1):63–74. doi: 10.1016/0143-4179(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 45.Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997;138(10):4109–4122. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- 46.Vakili A, Kataoka H, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005;25(8):1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Arginine-vasopressin V1 but not V2 receptor antago nism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit. Care. 2010;12(1):124–131. doi: 10.1007/s12028-009-9277-x. [DOI] [PubMed] [Google Scholar]
- 48.Shuaib A, Xu Wang C, Yang T, Noor R. Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke. 2002;33(12):3033–3037. doi: 10.1161/01.str.0000039405.31526.06. [DOI] [PubMed] [Google Scholar]
- 49.Kleindienst A, Fazzina G, Dunbar JG, Glisson R, Marmarou A. Protective effect of the V1a receptor antagonist SR49059 on brain edema formation following middle cerebral artery occlusion in the rat. Acta Neurochir. . 2006;96(Suppl. ):303–306. doi: 10.1007/3-211-30714-1_65. [DOI] [PubMed] [Google Scholar]
- 50.Kleindienst A, Dunbar JG, Glisson R, Marmarou A. The role of vasopressin V1A receptors in cytotoxic brain edema formation following brain injury. Acta Neurochir (Wien). 2013;155(1):151–164. doi: 10.1007/s00701-012-1558-z. [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka Y, Hossmann KA. Cortical impedance and extracellular volume changes following middle cerebral artery occlusion in cats. J. Cereb. Blood Flow Metab. 1982;2(4):466–474. doi: 10.1038/jcbfm.1982.53. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen T H, Ståhl N, Schalén W, Reinstrup P, Toft P, Nordström C-H. Recirculation usually precedes malignant edema in middle cerebral artery infarcts. Acta Neurol. Scand. 2012;126(6):404–410. doi: 10.1111/j.1600-0404.2012.01664.x. [DOI] [PubMed] [Google Scholar]
- 53.Manaenko A, Fathali N, Khatibi NH, et al. Post-treatment with SR49059 improves outcomes following an intracerebral hemorrhagic stroke in mice. Acta Neurochir. . 2011;111(Suppl. ):191–196. doi: 10.1007/978-3-7091-0693-8_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barreca T, Gandolfo C, Corsini G, et al. Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc. Dis. 2001;11(2):113–118. doi: 10.1159/000047622. [DOI] [PubMed] [Google Scholar]
- 55.Liu XF, Shi YM, Lin BC. Mechanism of action of arginine vasopressin on acute ischemic brain edema. Chin Med (Engl). 1991;104(6):490–493. [PubMed] [Google Scholar]
- 56.Dickinson LD, Betz AL. Attenuated development of ischemic brain edema in vasopressin-deficient rats. J. Cereb. Blood Flow Metab. 1992;12(4):681–690. doi: 10.1038/jcbfm.1992.93. [DOI] [PubMed] [Google Scholar]
- 57.Del Bigio MR, Fedoroff S. Swelling of astroglia in vitro and the effect of arginine vasopressin and atrial natriuretic peptide. Acta Neurochir. . 1990;51(Suppl Wien. ):14–16. doi: 10.1007/978-3-7091-9115-6_5. [DOI] [PubMed] [Google Scholar]
- 58.Sarfaraz D, Fraser CL. Effects of arginine vasopressin on cell volume regulation in brain astrocyte in culture. Am. J. Physiol. 1999;276(3Pt1):E596–601. doi: 10.1152/ajpendo.1999.276.3.E596. [DOI] [PubMed] [Google Scholar]
- 59.Du T, Song D, Li H, Li B, Cai L, Hertz L, Peng L. Stimulation by vasopressin of ERK phosphorylation and vector-driven water flux in astrocytes is transactivation-dependent. Eur. J. Pharmacol. 2008;587(1-3):73–77. doi: 10.1016/j.ejphar.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Jurzak M, Müller AR, Gerstberger R. AVP-fragment peptides induce Ca2+ transients in cells cultured from rat circumventricular organs. Brain Res. 1995;673(2):349–355. doi: 10.1016/0006-8993(95)00017-k. [DOI] [PubMed] [Google Scholar]
- 61.Moriya T, Kayano T, Kitamura N, et al. Vasopressin-induced intracellular Ca2+ concentration responses in non-neuronal cells of the rat dorsal root ganglion. Brain Res. 2012;1483:1–12. doi: 10.1016/j.brainres.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 62.Gunnarson E, Zelenina M, Axehult G, et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia. 2008;56(6):587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- 63.Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP. A novel role of vasopressin in the brain modulation of activity-dependent water flux in the neocortex. J. Neurosci. 2001;21(9):3045–3051. doi: 10.1523/JNEUROSCI.21-09-03045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hof PR, Pascale E, Magistretti PJ. K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. J. Neurosci. 1988;8(6):1922–1928. doi: 10.1523/JNEUROSCI.08-06-01922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Song D, Bai Q, Cai L, Hertz L, Peng L. Basic mechanism leading to stimulation of glycogenolysis by isoproterenol, EGF, elevated extracellular K+ concentrations, or GABA. Neurochem Res. 2014;39(4):661–667. doi: 10.1007/s11064-014-1244-z. [DOI] [PubMed] [Google Scholar]
- 66.Syed N, Martens CA, Hsu WH. Arginine vasopressin increases glutamate release and intracellular Ca2+ concentration in hippocampal and cortical astrocytes through two distinct receptors. J. Neurochem. 2007;103(1):229–237. doi: 10.1111/j.1471-4159.2007.04737.x. [DOI] [PubMed] [Google Scholar]
- 67.Goyagi T, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Beta-adrenoreceptor antagonists attenuate brain injury after transient focal ischemia in rats. Anesth. Analg. 2006;103(3):658–663. doi: 10.1213/01.ane.0000228859.95126.69. [DOI] [PubMed] [Google Scholar]
- 68.Goyagi T, Tobe Y, Nishikawa T. Long-term and spatial memory effects of selective ß1-antagonists after transient focal ischaemia in rats. Br. J. Anaesth. 2012;109(3):399–406. doi: 10.1093/bja/aes134. [DOI] [PubMed] [Google Scholar]
- 69.Goyagi T, Horiguchi T, Nishikawa T, Tobe Y. Post-treatment with selective ß1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Res. 2010;1343:213–217. doi: 10.1016/j.brainres.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 70.Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Posttreatment but not pretreatment with selective beta-adrenoreceptor 1 antagonists provides neuroprotection in the hippocampus in rats subjected to transient forebrain ischemia. Anesth. Analg. 2010;110(4):1126–1132. doi: 10.1213/ANE.0b013e3181d278f7. [DOI] [PubMed] [Google Scholar]
- 71.Umehara S, Goyagi T, Nishikawa T, Tobe Y, Masaki Y. Esmolol and landiolol, selective beta1-adrenoreceptor antagonists, provide neuroprotection against spinal cord ischemia and reperfusion in rats. Anesth. Analg. 2010;110(4):1133–1137. doi: 10.1213/ANE.0b013e3181cdb06b. [DOI] [PubMed] [Google Scholar]
- 72.Hertz L, Peng L, Song D. Ammonia, like K+, stimulates the Na+, K+, 2 Cl- cotransporter NKCC1 and the Na+,K+-ATPase and interacts with endogenous ouabain. Neurochem. Res. 2014 doi: 10.1007/s11064-014-1352-9. [DOI] [PubMed] [Google Scholar]
- 73.Du T, Li B, Li H, Li M, Hertz L, Peng L. Signaling pathways of isoproterenol-induced ERK1/2 phosphorylation in primary cultures of astrocytes are concentration-dependent. J. Neurochem. 2010;115(4):1007–1023. doi: 10.1111/j.1471-4159.2010.06995.x. [DOI] [PubMed] [Google Scholar]
- 74.Hajek I, Subbarao KV, Hertz L. Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem. Int. 1996;28(3):335–342. doi: 10.1016/0197-0186(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 75.McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. 1991;11(2):381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Hertz L, Peng L. Cell-specific mRNA alterations in Na+, K+-ATPase a and ß isoforms and FXYD in mice treated chronically with carbamazepine, an anti-bipolar drug. Neurochem. Res. 2013;38(4):834–841. doi: 10.1007/s11064-013-0986-3. [DOI] [PubMed] [Google Scholar]
- 77.Henn FA, Haljamäe H, Hamberger A. Glial cell function active control of extracellular K+ concentration. Brain Res. 1972;43(2):437–443. doi: 10.1016/0006-8993(72)90399-x. [DOI] [PubMed] [Google Scholar]
- 78.Grisar T, Frere JM, Franck G. Effect of K+ ions on kinetic properties of the (Na+, K+)-ATPase (EC 3..1.3) of bulk isolated glialells perikarya and synaptosomes from rabbit brain cortex. . Brain Res. 1979;165(1):87–103. doi: 10.1016/0006-8993(79)90047-7. [DOI] [PubMed] [Google Scholar]
- 79.Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb. Cortex. 2008;18(12):2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bay V, Butt AM. Relationship between glial potassium regulation and axon excitability a role for glial Kir4. channels. . Glia. 2012;60(4):651–660. doi: 10.1002/glia.22299. [DOI] [PubMed] [Google Scholar]
- 81.Siesjö BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression a unifying hypothesis. J. Cereb. Blood Flow Metab. 1989;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- 82.Pérez-Pinzón MA, Mumford PL, Carranza V, Sick TJ. Calcium influx from the extracellular space promotes NADH hyperoxidation and electrical dysfunction after anoxia in hippocampal slices. J. Cereb. Blood Flow Metab. 1998;18(2):215–221. doi: 10.1097/00004647-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 83.Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxia-related acute astrocyte death. Glia. 2005;49(1):143–152. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- 84.Peng L, Huang R, Zhang S, Hertz L. Ouabain binding kinetics and FXYD7 expression in astrocytes and neurons in primary cultures: implications for cellular contributions to extracellular K+ homeostasisκ. Neuron Glia Biol. 2010;6(2):127–135. doi: 10.1017/S1740925X10000013. [DOI] [PubMed] [Google Scholar]
- 85.Darbin O, Carre E, Naritoku D, Risso JJ, Lonjon M, Patrylo PR. Glucose metabolites in the striatum of freely behaving rats following infusion of elevated potassium. Brain Res. 2006;1116(1):127–131. doi: 10.1016/j.brainres.2006.06.095. [DOI] [PubMed] [Google Scholar]
- 86.Ashford CA, Dixon KC. The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochm J. 1935;29(1):157–168. doi: 10.1042/bj0290157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hertz L, Schou M. Univalent cations and the respiration of brain-cortex slices. Biochem. J. 1962;85:93–104. doi: 10.1042/bj0850093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hertz L, Peng L, Kjeldsen CC, O'Dowd BS, Dienel GA, Hertz L, editors. In Non-Neuronal Cells of the Nervous System Function and Dysfuntion. Elsevier: Amsterdam; 2004. Ion, Transmitter and Drug Effects on Energy Metabolism in Astrocytes. pp. 435–460. [Google Scholar]
- 89.Keep RF, Xiang J, Ennis SR, Beer ME, Betz AL. Brain volume regulation during development the role of blood-brain barrier potassium transport. Adv. Exp. Med. Biol. 1993;331:65–69. doi: 10.1007/978-1-4615-2920-0_11. [DOI] [PubMed] [Google Scholar]
- 90.Kawai N, Yamamoto T, Yamamoto H, McCarron RM, Spatz M. Endothelin 1 stimulates Na+,K+-ATPase and Na+-K+-Cl- cotransport through ETA receptors and protein kinase C-dependent pathway in cerebral capillary endothelium. J. Neurochem. 1995;65(4):1588–1596. doi: 10.1046/j.1471-4159.1995.65041588.x. [DOI] [PubMed] [Google Scholar]
- 91.Siemkowicz E, Hansen AJ. Brain extracellular ion composition and EEG activity following 10 minutes ischemia in normo- and hyperglycemic rats. Stroke. 1981;12(2):236–240. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- 92.Frieder B, Allweis C. Memory consolidation: further evidence for the four-phase model from the time-courses of diethyldithiocarbamate and ethacrynic acid amnesias. Physiol. Behav. 1982;29(6):1071–1075. doi: 10.1016/0031-9384(82)90300-6. [DOI] [PubMed] [Google Scholar]
- 93.Gibbs ME, Bowser DN, Hutchinson DS, Loiacono RE, Summers RJ. Memory processing in the avian hippocampus involves interactions between beta-adrenoceptors, glutamate receptors, and metabolism. Neuropsychopharmacology. 2008;33(12):2831–2846. doi: 10.1038/npp.2008.5. [DOI] [PubMed] [Google Scholar]
- 94.Gibbs ME, Ng KT. Psychobiology of memory Towards a model of memory formation. Biobehav. Rev. 1977;1:113–136. [Google Scholar]
- 95.Daisley JN, Gruss M, Rose SP, Braun K. Passive avoidance training and recall are associated with increased glutamate levels in the intermediate medial hyperstriatum ventrale of the day-old chick. Neural Plast. 1998;6(3):53–61. doi: 10.1155/NP.1998.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daisley JN, Rose SP. Amino acid release from the intermediate medial hyperstriatum ventrale (IMHV) of day-old chicks following a one-trial passive avoidance task. Neurobiol. Learn Mem. 2002;77(2):185–201. doi: 10.1006/nlme.2001.4011. [DOI] [PubMed] [Google Scholar]
- 97.Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci. Biobhav.Rev. 2008; 32(5):927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Gibbs ME, Shleper M, Mustafa T, Burnstock G, Bowser DN. ATP derived from astrocytes modulates memory in the chick. Neuron Glia Biol. 2011;7(2-4):177–86. doi: 10.1017/S1740925X12000117. [DOI] [PubMed] [Google Scholar]
- 99.Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM. Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Transl. Stroke Res. 2014;5(1):3–16. doi: 10.1007/s12975-013-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas RC. Electrogenic sodium pump in nerve and muscle cells. Physiol. Rev. 1972;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- 101.Clarke RJ, Apell HJ, Läuger P. Pump current and Na+/K+ coupling ratio of Na+/K+-ATPase in reconstituted lipid vesicles. Biochim. Biophys. Acta. 1989;981(2):326–336. doi: 10.1016/0005-2736(89)90044-8. [DOI] [PubMed] [Google Scholar]
- 102.Schuier FJ, Hossmann KA. Experimental brain infarcts in cats.II. Ischemic brain edema. . Stroke. 1980;11(6):593–601. doi: 10.1161/01.str.11.6.593. [DOI] [PubMed] [Google Scholar]
- 103.Asgeirsson B, Grände PO, Nordström C-H. A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation. Intens. Care Med. 1994;20(4):260–267. doi: 10.1007/BF01708961. [DOI] [PubMed] [Google Scholar]
- 104.De Raedt S, Haentjens P, De Smedt A, et al. Pre-stroke use of beta-blockers does not affect ischaemic stroke severity and outcome. Eur. J. Neurol. 2012;19(2):234–240. doi: 10.1111/j.1468-1331.2011.03475.x. [DOI] [PubMed] [Google Scholar]
- 105.Duncan GE, Little KY, Koplas PA, Kirkman JA, Breese GR, Stumpf WE. Beta-adrenergic receptor distribution in human and rat hippocampal formation marked species differences. Brain Res. 1991;561(1):84–92. doi: 10.1016/0006-8993(91)90752-h. [DOI] [PubMed] [Google Scholar]
- 106.Little KY, Duncan GE, Breese GR, Stumpf WE. Beta-adrenergic receptor binding in human and rat hypothalamus. Biol Psychiatry. 1992;32(6):512–522. doi: 10.1016/0006-3223(92)90219-p. [DOI] [PubMed] [Google Scholar]
- 107.Russo-Neustadt A, Cotman CW. Adrenergic receptors in Alzheimer's disease brain selective increases in the cerebella of aggressive patients. J Neurosci. 1997;17(14):5573–5580. doi: 10.1523/JNEUROSCI.17-14-05573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem. Int. 2010;57(4):411–420. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeinstra E, Wilczak N, De Keyser J. [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain. Neurosci. Lett. 2000;289(1):75–77. doi: 10.1016/s0304-3940(00)01254-4. [DOI] [PubMed] [Google Scholar]