Abstract
The causal role of ammonium in hepatic encephalopathy was identified in 1930s. Astroglial cells are primary cellular elements of hepatic encephalopathy which conceptually, can be considered a toxic astrogliopathology. Previously we have reported that acute exposure to ammonium activated ouabain/Na,K-ATPase signalling pathway, which includes Src, EGF receptor, Raf, Ras, MEK and ERK1/2. Chronic incubation of astrocytes with ammonium increased production of endogenous ouabain-like compound. Ouabain antagonist canrenone abolished effects of ammonium on astrocytic swelling, ROS production, and upregulation of gene expression and function of TRPC1 and Cav1.2. However, ammonium induces multiple pathological modifications in astrocytes, and some of them may be not related to this signalling pathway. In this review, we focus on the effect of ammonium on ouabain/Na,K-ATPase signalling pathway and its involvement in ammonium-induced ROS production, cell swelling and aberration of Ca2+ signals in astrocytes. We also briefly discuss Na,K-ATPase, EGF receptor, endogenous ouabain and ouabain antagonist.
Keywords: Ammonium, astrocytes, canrenone, Na, K-ATPase, ouabain.
1. INTRODUCTION
Hyperammonemia resulting from urea cycle deficiencies, Reye's syndrome or liver failure causes polymorphic mental and behavioral manifestations such as confusion, forgetfulness, irritability as well as alterations of consciousness represented by lethargy, somnolence and, in the terminal stages, coma associated with brain oedema that causes death [1, 2]. Brain oedema is linked to delayed stimulation of NKCC1, a cotransporter of Na+, K+, 2Cl- and water (for review, see [3]). The causal role of ammonium in hepatic encephalopathy was identified in 1930s [4] and it is now generally accepted that characteristic symptomatology in experimental fulminant hepatic failure results from an increase in brain concentration of ammonium to 3 - 5 mM [5]. In hepatic encephalopathy and acute liver failure these concentrations are generally lower [6].
Astroglial cells are primary cellular targets of hepatic encephalopathy, and the astroglia-specific glutamine synthetase [7] is a central enzyme providing for ammonium detoxification [8-10]. Increased activity of glutamine synthetase in the presence of elevated ammonium in the brain affects major homeostatic functions of astroglia, and fundamentally hepatic encephalopathy can be considered a toxic astrogliopathy.
Our previous studies identified enhanced Na,K-ATPase activity in cultured mouse astrocytes exposed to chronic treatment with ammonium [11]. This was linked to an increase in expression of α2 subunit of Na,K-ATPase (NKA), which arguably results from activation of a signalling cascade comprised of Src, EGF receptor, Raf, Ras, MEK and ERK1/2. Phosphorylation of the ERK1/2 induces up-regulation of the α2 gene expression [12, 13]. Incidentally, this signalling sequence is almost identical to the pathway initiated by a low concentration of ouabain in a kidney cell line [14]. The ouabain antagonist, canrenone is able to abolish ammonium-induced astrocytic swelling [13]. Recently, we have found that canrenone also inhibits upregulation of gene expression and function of TRPC1 and Cav1.2 in astrocytes after chronic treatment with 3 mM ammonium [15, 16]. In this paper, we will focus on ouabain signal pathway and its relevance in ammonium pathology in astrocytes.
2. ENDOGENOUS DIGITALIS
Endogenous digitalis, also known as endogenous cardiotonic steroids (CTS) were discovered in 1990s [17]. These compounds contain steroid structure and have inhibitory effect on Na/K-ATPase at high concentrations, but stimulate ouabain/Na,K-ATPase signalling pathway at very low concentrations. There are two classes of endogenous digitalis, endogenous cardenolides and endogenous bufadienolides.
2.1. Endogenous Cardenolides
Endogenous cardenolides are represented by endogenous ouabain and endogenous digoxin. Their chemical structures are identical to plant-derived compounds. Endogenous ouabain was the first endogenous cardenolide found in human serum [17]. The adrenal medulla and hypothalamus contain high level of endogenous ouabain [18-20]. The role of endogenous ouabain in human hypertension has been established [21]. The level of endogenous ouabain was increased in amygdala, hippocampus and hypothalamus in response to salt-loading in rats [22]. Endogenous digoxin was purified from human urine in 1990 [23]. It remains controversial whether endogenous digoxin is an antagonist of endogenous ouabain (for review, see [24]).
2.2. Endogenous Bufadienolides
Bufadienolides are different from cardenolides by a doubly unsaturated six-membered lactone ring in their structure. Marinobufagenin (MBG) in mammalian plasma and urine was identified by using different techniques, including immunoassays and mass spectrometry. Similarly to endogenous ouabain, the role of MBG in hypertension has been extensively studied (for review, see [24]). Telocinobufagin is another bufadienolide, which may be a precursor of MBG [25].
2.3. Synthesis of Endogenous Digitalis in Brain
On account of their steroidal structure, biosynthesis of endogenous digitalis was studied in adrenocortical tissue or in endocrine cells according to the classic scheme of steroidgenesis. Cholesterol is converted to pregnenolone catalyzed by the cytochrome P450 side chain cleavage (P450 scc), and pregnenolone is converted to progesterone catalyzed by the 3β-hydroxy-steroid dehydrogenase (3β-HSD). The enzymes converting progesterone into endogenous digitalis are unknown [24]. Bufadienolides are derived from cholesterol, but cholesterol side chain cleavage is probably not involved.
In the brain, steroidogenesis seems to occur mainly in astrocytes. Astrocytes express P450 scc and 3b-HSD, as well as other enzymes involved in steroidogenesis [26]. Astrocytes in culture produce pregnenolone, progesterone and, other neurosteroids [27, 28]. Astrocytes also express steroid acute regulating protein (StAR) and peripheral-type benzodiazepine receptor (PBR), which transfer cholesterol into mitochrondrial [29-31]. Upregulation of PBR was detected in human hyperammonemic disorders, in experimental hyperammonemic syndromes and in astrocytes exposed to ammonium in vitro (for review, see [32]). In addition, PBR agonists induce mitochrondria swelling, oxidative damage and steroidogenesis [32]. In pour previous studies, we had found that incubation of astrocytes with 3 mM ammonia for 4 days increased an endogenous compound with ouabain-like activity by 50% [11]. The released endogenous “ouabain” during 4 days reached 3.7 µg/mg protein in control (part of which, in principle might originate from the serum added to the incubation media), and 5.4 µg/mg in ammonium-treated cells, which was a significantly increase.
3. OUABAIN SIGNALLING PATHWAY
3.1. Na,K-ATPase
Na/K-ATPase might be a primary target for ammonium toxicity due to similarities between K+ and NH4+ [33]. Ammonium increases Na/K-ATPase activity in cultured mouse astrocytes due to the enhanced production of ouabain-like compounds [11]. The Na/K-ATPase is composed of two essential subunits, α and β. The α subunits are catalytic, they span the membrane multiple times and contain the binding sites for Na+, K+, ATP and the specific inhibitor ouabain and thus also the ouabain antagonist canrenone [34]. The β subunit is a single span glycoprotein with most of its mass exposed to the extracellular space [35]. There are four isoforms of α subunit, namely α1, α2, α3 and α4. In adult brain and in cultured CNS cells, the α1 isoform is expressed in both neurones and astrocytes, α2 is a virtually astrocyte-specific isoform, and α3 is only expressed in neurones [36, 37]. The α1 isoform also functions as a receptor ligand for signalling, mediated by nanomolar concentrations of ouabain or endogenous ouabain-like compounds.
3.2. EGF Receptor (EGFR)
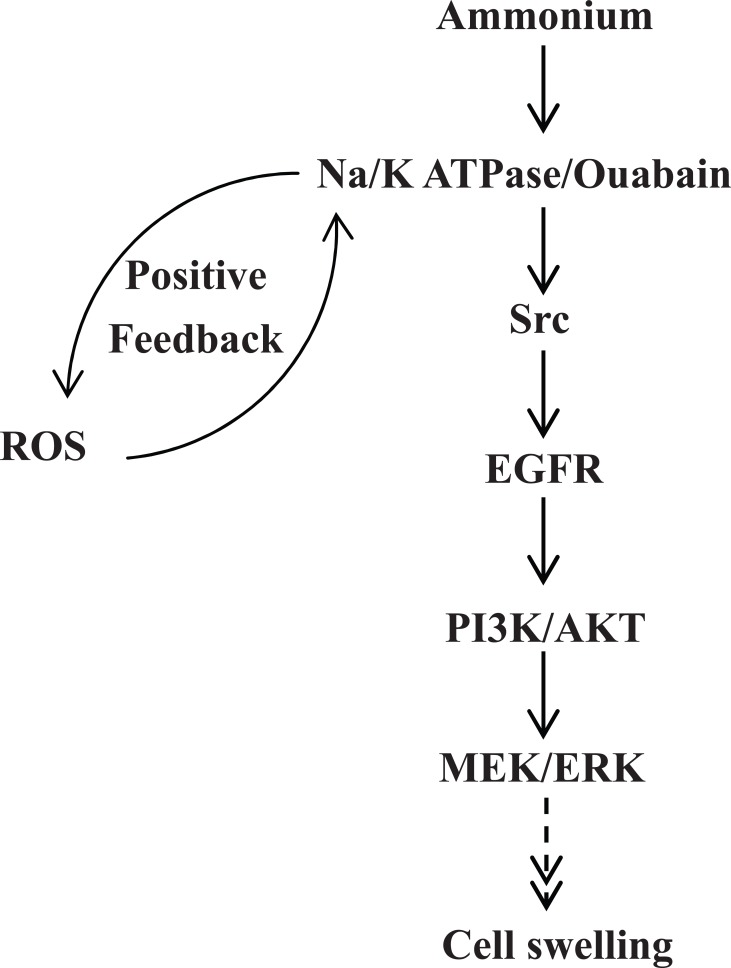
The activation of EGFRs activates two major intracellular signalling cascades, represented by the MAPK/ERK and PI3K/AKT pathways. EGF can induce phosphorylation of all five known tyrosine phosphorylation sites of EGFR [38]. EGFRY992, EGFRY1173 and EGFRY1045 are autophosphorylation sites, with EGFRY1173 being the major one and EGFRY992 being the minor one. EGFRY845 is known to be the major Src phosphorylation site [39-41].EGFRY1068 is not phosphorylated in the brain in vivo [42] and in cultured astrocytes, unless stimulated by EGF addition [38] or following production of an EGFR ligand, as indicated by its phosphorylation by ammonium treatment, which stimulates EGFR (Fig. 1).
Fig. (1).
Diagram showing signal pathways for EGFR transactivation in response to 3 mM NH4Cl. Ammonium acts on the Na,K-ATPase to activate both its activity and Na,K-ATPase/ouabain signalling. The latter proceeds via Src to the EGF receptor (EGFR). We have previously shown that this occurs via MEK and therefore probably also via Ras and Raf [70]. We now have shown that also AKT needs to be activated upstream of ERK, but where this occurs in relation to Ras and Raf is not known with certainty. We have also previously shown that ERK phosphorylation can lead to NKCC1 activation, but not determined the intermediate steps [47]. The swelling caused by 3 mM ammonium is delayed, beginning after 12 hr, a delay caused by the requirement for ROS and its action on NKCC1. Since we have shown that ROS does not operate directly via the EGF receptor it is suggested that ouabain-mediated ROS stimulation, confirmed in the present study, in a feed-forward reaction re-inforces the effect of ouabain, as suggested by Liu et al. [46]. Literature evidence suggests that higher concentrations of ammonium may also act directly on NKCC1. Such concentrations are generally not encountered in hepatic encephalopathy, but occur in other hypeammonemic conditions.From Dai et al. [13].
3.3. Ouabain Signalling Pathway
The ouabain signalling pathway has been well established in kidney cell lines. Binding of ouabain to α1 isoform recruits Src which in turn phosphorylates EGFR and initiates its conventional intracellular signalling pathways, MAPK/ERK and PI3K/AKT (Fig. 1) [43]. This process is independent of shedding of growth factor(s) and at least partly occurs in lipid rafts, where it depends on the presence of caveolin, the major component of the lipid raft [44].
The Na/K-ATPase/ouabain signalling pathway is involved in the intracellular signalling of ammonium in primary cultures of astrocytes. Ammonium-induced Na/K-ATPase/Src/EGFR interaction occurs instantly. A study by Dai et al. [13] shows that twenty minutes of incubation with 3 mM ammonium induced an increase of phosphorylation at Y845 and Y1068 of EGF receptor. The phosphorylation levels at Y992, Y1045 and Y1173 were however unchanged. Ammonium induced EGFR activation can be inhibited by the EGFR inhibitor AG1478 and Src inhibitor PP1, but not by zinc-dependent metalloproteinase GM6001, indicating that ammonium induced EGFR activation is ligand-independent [13]. The process of interaction induced by ammonium among α1 isoform, Src, EGF receptor, ERK1/2, AKT (Fig. 1) and caveolin-1 occurs in lipid raft. Crosstalk between MAPK/ERK and PI3K/AKT induced by ammonium is shown by inhibition of ammonium-induced phosphorylation of ERK1/2 by both the MAPK inhibitor U0126, and the AKT inhibitor LY294002. This confirms that PI3K and AKT are likely to operate upstream of MAPK/ERK1/2 [45]. Canrenone, a ouabain antagonist can inhibit ERK phosphorylation induced by ammonium [13].
In cardiac cells ouabain can induce production of reactive oxygen species, ROS (Fig. 1) (for review, [46]). The involvement of ROS in ammonium-induced ERK1/2 phosphorylation in astrocytes is indicated by the inhibitory effect on the phosphorylation by superoxide dismutase (SOD), an inhibitor of free radicals (Fig. 2A). In contrast with the effect of ammonium, H2O2-induced ERK1/2 phosphorylation in astrocytes can not be inhibited by AG 1478, indicating that this effect is not mediated via the EGF receptor (Fig. 2B). Nevertheless, the observation that the effects of ouabain on ammonium-induced ERK phosphorylation, ROS production and cell swelling all can be inhibited by an ouabain antagonist indicates that enhanced ouabain signalling is responsible for all three phenomena [13]. This raises the question how ROS can increase brain swelling in the absence of any effect on the EGFR, which is involved in the pathway activating NKCC1 [47]. A possible answer to this question is a suggested feed-back increase of Na,K-ATPase signalling by ROS [46]. As illustrated in Fig. 1, the well established increase in ROS production resulting from ouabain signalling may therefore further increase Na,K-ATPase/ouabain signalling and all its down-stream effects. This time-consuming sensitizing process probably explains why ammonium-induced edema in most cases is delayed as discussed below.
Fig. (2).
Ammonium -inducedERK1/2 phosphorylation can be inhibited by SOD, and ROS production can be inhibited by canrenone, but H2O2-induced ERK1/2 phosphorylation does not require EGFR activity. (A) Cells were incubated with 0 or 3 mM NH4Cl in the absence or presence of 25 U/ml SOD for 2 h. (B) Cells were incubated with 0, 250 or 500 μM H2O2 in the absence or presence of 1 μM AG1478 for 8 min. Thereafter, cells were harvested, and the level of ERK1/2 phosphorylation was measured by Western blotting. (A1 and B1) Immunoblots from a representative experiment. Similar results were obtained from three independent experiments. (A2 and B2) Average ERK phosphorylation was quantitated as ratios between p-ERK1/2 and ERK1/2. The ratio between p-ERK1/2 and ERK1/2 in control group was designated a value of one. (A2) *Statistically significant (P<0.05) different from all other groups. (B2) *Statistically significant (P<0.05) different from 0 and 250 μM H2O2 with or without AG1478 groups. D Song, H Dai and L Peng, unpublished results. (C) Ammonium-induced ROS production. Cells were incubated with 0 or 3 mM NH4Cl in the absence or presence of 100 mM canrenone for 2 h. ROS was determined as fluorescence intensity of oxidized carboxy-H2DCFDA. *Statistically significant (P<0.05) different from all other groups. From Dai et al. [13].
4. EFFECTS OF OUABAIN INHIBITOR
4.1. Cell Swelling
Astrocytic swelling that occurs in hepatic brain edema and can be induced by administration of ammonium into primary cultures of astrocytes [48, 49]. Ammonium-induced astrocytic swelling develops slowly, and swelling did not become apparent until 8 h of exposure. The swelling could be completely prevented by EGF receptor inhibitor AG1478, the Src inhibitor PP1, the MAPK inhibitor U0126 and the AKT inhibitor LY294002 [13], indicating the role for ouabain/Na,K-ATPase signalling pathway. The mechanisms of the astrocytic swelling are not clear. However, ammonium can replace K+ in stimulation of Na,K-ATPase activity in mammals [50-53], and Na,K-ATPase activity provides the driving force for NKCC1 [54].
4.2. Aberrat Ca2+ Signals
Up-regulation of TRPC1: Treatment of cultured astrocytes with ammonium for 3 days increased expression of TRPC1 mRNA in a concentration-dependent manner; with 1 mM NH4+ showing no effect, whereas 3 and 5 mM causing a significant upregulation of mRNA presence [15]. The highest level of expression was observed at 3 mM being 180 ± 9.5% of control group. Changes in expression of TRPC1 protein expression mirrored those of mRNA [15]. The increase in mRNA became significant after 3 and 4 days of treatment and declined after 5 days of treatment (Fig. 3). In addition, [Ca2+]i responses to the β-adrenergic agonist isoproterenol, the α2-adrenergic agonist dexmedetomidine, the InsP3 receptor (InsP3R) agonist adenophostin A and ryanodine receptor (RyR) agonist 4- chloro-m-cresol (4-CMC) are significantly increased in astrocytes chronically exposed to ammonium [15]. Similarly, the store-operated Ca2+ entry (SOCE) meditated by the transient receptor potential channel 1 (TRPC1), is significantly augmented. Increases in TRPC1 expression and in SOCE were both prevented by the ouabain antagonist canrenone [15]. Up-regulation of TRPC1 gene expression was also found in the brain of adult mice treated with intraperitoneal injections of urease for 3 days and in astrocytes, but not in neurones freshly isolated from similarly treated transgenic mice tagged with an astrocyte-specific or a neurone-specific markers [15].
Fig. (3).
Up-regulation expression of TRPC1 mRNA and protein in primary cultures of mouse astrocytes chronically treated with ammonium. Cells were treated with PBS (Control) or with 3 mM NH4Cl for 1-5 days. Thereafter, cells were harvested, and mRNA expression was measured by RT-PCR. (A) Southern blot from a representative experiment. Similar results were obtained from three independent experiments. (B) Average mRNA expression was quantified as a ratio between TRPC1 and TBP, as a housekeeping gene. *Statistically significant (P<0.05) different from all other groups. T Du, F Wang and L Peng, unpublished results.
Up-regulation of Cav1.2: The ammonium-induced increase in Ca2+ influx in astrocytes resulted from an up-regulation of Cav1.2 channels expression identified at mRNA and protein levels (T Du, F Wang and L. Peng, unpublished results). Increase in Cav1.2 expression was also prevented by ouabain antagonist canrenone (T Du, F Wang and L. Peng, unpublished results).
Astrocytes are non-excitable neural cells that utilise dynamic fluctuations of intracellular Ca2+ and Na+ concentrations for their excitability [55]. Regulated release of Ca2+ from endoplasmic reticulum (ER) through intracellular Ca2+ release channels, such as the InsP3 receptors (InsP3R) and ryanodine receptors (RyRs) (for review, see [55, 56]) have a leading role in glial Ca2+ signalling. According to contemporary views, SOCE can be mediated either by ORAI channels that mediate calcium release activated Ca2+ current (ICRAC) or by some members of the extended family of transient receptor potential (TRP) cationic channels [57-59]. In astrocytes the major and essential component of SOCE is represented by "canonical" TRPC1 channels [60]. Another source of increase in [Ca2+]i are astroglial L-type calcium channels. We have demonstrated that activation of these channels results in a relatively modest Ca2+ entry, which however triggers substantial [Ca2+]i increases due to a consequent Ca2+-induced Ca2+ release (CICR) form the ER mediated by RyRs [61, 62]. Ammonium-induced deregulation of astroglial Ca2+ signalling should be considered as an important pathogenic step, which in turn may trigger gene expressions, contribute to cell damage, mitochondrial permeability transition (MPT) and increased glutamate exocytosis. Ammonium-induced aberration of Ca2+ homeostasis and Ca2+ signalling can define the disease progression, and its potential inhibition might be used for specific therapy.
4.3. Ouabain Inhibitors
The prodrug of canrenone, spironolactone is the first antialdosteronic drugs developed in 1960s. It is used for the treatment of hypertension, primary hyperaldosteronism and peripheral oedema related to the cardiac failure and other pathologies associated with aldosteronism (for review, [63]). Spironolactone is not well tolerated since it also binds to progesterone and androgen receptors. Canrenone competes with ouabain for the same binding site, and reverses the inhibition of Na,K-ATPase by ouabain and ouabain-like factor [64, 65]. It has also been reported that canrenone has anti-MBG effect [65]. After i.v. infusion of the water soluble derivative of spironolactone, 3H-canrenoate-K, 3H-activity in white matter of the brain was 36X and in brain cortex 27X the activity in plasma [66]. In addition, canrenone accounted for 72% of the total concentration in CSF. This finding suggests canrenoate exerts pharmacological effects on the CNS and can be used in hyperammonemia in clinic.
Rostafuroxin (PST 2238) is derived from digistoxigenin. This coumpound antagonizes all ouabain functional effects by specifically displacing ouabain binding from the high affinity Na,K-ATPase isoform present in the caveolae [67]. Digibind is a commercial preparation containing the purified Fab fragment of the sheep antidigoxin antibody, which cross-reacts with other cardiotonic steroids.
5. CONCLUSION
We have suggested that 3 mM ammoniun, a concentration relevant for cell culture studies of hepatic encephalopathy, exerts ouabain-like effects on Na,K-ATPase’s signalling, which lead to EGFR transactivation, PI3K/AKT and MAPK/ERK phosphorylation and ROS formation. This could possibly result from the ability of ammonium to stimulate the Na,K-ATPase in the same manner as K+. Although astrocytes synthesize endogenous ouabain, it is unlikely that ouabain concentration would increase fast enough to have an immediate effect. However, cerebral ammonium toxicity is undoubtedly multifactorial. At least some of ammonium effects are probably not related to ouabain signalling, such as ammonium-induced increase in glutamine formation from glutamate [68]. Some ammonium effects (reduction of pyruvate/lactate ratio) are due to a resulting decrease in glutamate and can in cultured astrocytes be prevented by addition of excess glutamate [69]. On the other hand prevention of glutamine production by inhibition of glutamine synthetase can for unknown reasons reduce or prevent edema formation [reviewed in 33] in the brain in vivo, where the situation may be even more complicated. Experiments are in progress to evaluate potential therapeutic effects of canrenone during ammonium toxicity in animal experiments.
ACKNOWLEDGEMENTS
This work was supported by Grant No. 31171036 and No. 30711120572 from the National Natural Science Foundation of China.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ede RJ, Williams RW. Hepatic encephalopathy and cerebral edema. Semin. Liver Dis. 1986;6:107–118. doi: 10.1055/s-2008-1040594. [DOI] [PubMed] [Google Scholar]
- 2.Link A, Kaplan BT, Böhm M. 21-year-old woman with Reye's syndrome after influenza. Dtsch. Med. Wochenschr. 2012;137(38):1853–1856. doi: 10.1055/s-0032-1305311. [DOI] [PubMed] [Google Scholar]
- 3.Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab. Brain Dis. 2010;25(1):31–38. doi: 10.1007/s11011-010-9180-3. [DOI] [PubMed] [Google Scholar]
- 4.van Caulaert C, Deviller C. Ammoniémie expérimentale après ingestion de chlorure d’ammonium chez l’homme à l’état normal et pathologique. Compt. Rend. Soc. Biol. (Paris) 1932;111:50–52. [Google Scholar]
- 5.Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–453. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- 6.Bosoi CR, Rose CF. Oxidative stress a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab. Brain Dis. 2013;28(2):175–178. doi: 10.1007/s11011-012-9351-5. [DOI] [PubMed] [Google Scholar]
- 7.Norenberg MD. Distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem. 1979;27(3):756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- 8.Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Astrocyte glutamine synthetase importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7(4):452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth RF. Altered glial-neuronal crosstalk cornerstone in the pathogenesis of hepatic encephalopathy. Neurochem. Int. 2010;57(4):383–388. doi: 10.1016/j.neuint.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Rose CF, Verkhratsky A, Parpura V. Astrocyte glutamine synthetase pivotal in health and disease. Biochem. Soc. Trans. 2013;41(6):1518–1524. doi: 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- 11.Kala G, Kumarathasan R, Peng L, Leenen FH, Hertz L. Stimulation of Na+,K+-ATPase activity, increase in potassium uptake, and enhanced production of ouabain-like compounds in ammonia-treated mouse astrocytes. Neurochem. Int. 2000;36(3):203–211. doi: 10.1016/s0197-0186(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 12.Xue Z, Li B, Gu L, Hu X, Li M, Butterworth RF, Peng L. Increased Na, K-ATPase alpha2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem. Int. 2010;57(4):395–403. doi: 10.1016/j.neuint.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Dai H, Song D, Xu J, Li B, Hertz L, Peng L. Ammonia-induced Na,K-ATPase/ouabain-mediated EGF receptor trans-activation, MAPK/ERK and PI3K/AKT signaling and ROS formation cause astrocyte swelling. Neurochem. Int. 2013;63(6):610–625. doi: 10.1016/j.neuint.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell. 2006;17(1):317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang C, Du T, Zhou J, Verkhratsky A, Peng L. Ammonium increases Ca2+ signalling and up-regulates expression of TRPC1 gene in astrocytes in primary cultures and in the in vivo brain. Neurochem.Res.under revision. doi: 10.1007/s11064-014-1406-z. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Du T, Liang C, Verkhratsky A, Peng L. Ammonium increases Ca2+ signalling and up-regulates expression of Cav1. gene in astrocytes in primary cultures and in the in vivo brain. Cell Res submitted [Google Scholar]
- 17.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. U. S. A. 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komiyama Y, Nishimura N, Munakata M, Mori T, Okuda K, Nishino N, Hirose S, Kosaka C, Masuda M, Takahashi H. Identification of endogenous ouabain in culture supernatant of PC12 cells. J. Hypertens. 2001;19(2):229–236. doi: 10.1097/00004872-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 19.el-Masri MA, Clark BJ, Qazzaz HM, Valdes R. Human adrenal cells in culture produce both ouabain-like and dihydroouabain-like factors. Clin. Chem. 2002;48(10):1720–1730. [PubMed] [Google Scholar]
- 20.Murrell JR, Randall JD, Rosoff J, Zhao JL, Jensen RV, Gullans SR, Haupert GT. Endogenous ouabain upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation. 2005;112(9):1301–1308. doi: 10.1161/CIRCULATIONAHA.105.554071. [DOI] [PubMed] [Google Scholar]
- 21.Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids and salt-sensitive hypertension. Biochim. Biophys. Acta. 2010;1802(12):1230–1236. doi: 10.1016/j.bbadis.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorova OV, Zhuravin IA, Agalakova NI, Yamova LA, Talan MI, Lakatta EG, Bagrov AY. Intrahippocampal microinjection of an exquisitely low dose of ouabain mimics NaCl loading and stimulates a bufadienolide Na/K-ATPase inhibitor. J. Hypertens. 2007;25(9):1834–1844. doi: 10.1097/HJH.0b013e328200497a. [DOI] [PubMed] [Google Scholar]
- 23.Goto A, Ishiguro T, Yamada K, Ishii M, Yoshioka M, Eguchi C, Shimora M, Sugimoto T. Isolation of a urinary digitalis-like factor indistinguishable from digoxin. Biochem. Biophys. Res. Commun. 1990;173(3):1093–1101. doi: 10.1016/s0006-291x(05)80898-8. [DOI] [PubMed] [Google Scholar]
- 24.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005;38(1):36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Melcangi RC, Azcoitia I, Galbiati M, Magnaghi V, Garcia-Ovejero D, Garcia-Segura LM. Hertz L., editor. Non-neuronal cells in the nervous system sources and targets of neuroactive steroids.In Non-neuronal cells of the nervous system: function and dysfuntion. Elsevier Amsterdam. 2004;Part II:535–559. [Google Scholar]
- 27.Zwain IH, Yen SS. Dehydroepiandrosterone: biosynthesis and metabolism in the brain. Endocrinology. 1999;140(2):880–887. doi: 10.1210/endo.140.2.6528. [DOI] [PubMed] [Google Scholar]
- 28.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140(8):3843–352. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- 29.Ritta MN, Campos MB, Calandra RS. Effect of GABA and benzodiazepines on testicular androgen production. Life Sci. 1987;40(8):791–798. doi: 10.1016/0024-3205(87)90307-9. [DOI] [PubMed] [Google Scholar]
- 30.Yanagibashi K, Ohno Y, Nakamichi N, Matsui T, Hayashida K, Takamura M, Yamada K, Tou S, Kawamura M. Peripheral-type benzodiazepine receptors are involved in the regulation of cholesterol side chain cleavage in adrenocortical mitochondria. J. Biochem. 1989;106(6):1026–1029. doi: 10.1093/oxfordjournals.jbchem.a122958. [DOI] [PubMed] [Google Scholar]
- 31.Sierra A, Lavaque E, Perez-Martin M, Azcoitia I, Hales DB, Garcia-Segura LM. Steroidogenic acute regulatory protein in the rat brain: cellular distribution, developmental regulation and overexpression after injury. Eur. J. Neurosci. 2003;18(6):1458–1467. doi: 10.1046/j.1460-9568.2003.02872.x. [DOI] [PubMed] [Google Scholar]
- 32.Bélanger M, Ahboucha S, Desjardins P, Butterworth RF. Upregulation of peripheral-type (mitochondrial) benzodiazepine receptors in hyperammonemic syndromes Consequences for neuronal excitability. Adv. Mol. Cell Biol. 2004;31(3):983–997. [Google Scholar]
- 33.Hertz L, Peng L, Song D. Ammonia, like K+, stimulates the Na+, K+, 2 Cl- cotransporter NKCC1 and the Na+,K+-ATPase and interacts with endogenous ouabain. Neurochem. Res. 2014 doi: 10.1007/s11064-014-1352-9. [DOI] [PubMed] [Google Scholar]
- 34.Tal DM, Karlish SJ. Do canrenone and 6,7-dihydroxylated derivatives compete with ouabain at the same site on Na,K-ATPaseκ. Mol. Pharmacol. 1988;34(3):245–249. [PubMed] [Google Scholar]
- 35.Sweadner KJ. Overlapping and diverse distribution of Na-K ATPase isozymes in neurons and glia. Can. J. Physiol. Pharmacol. 1992;70(Suppl ):S255–S259. doi: 10.1139/y92-269. [DOI] [PubMed] [Google Scholar]
- 36.Peng L, Martin-Vasallo P, Sweadner KJ. Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J. Neurosci. 1997;17(10):3488–3502. doi: 10.1523/JNEUROSCI.17-10-03488.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Hertz L, Peng L. Cell-specific mRNA alterations in Na+,K+-ATPase a and ß isoforms and FXYD in mice treated chronically with carbamazepine, an anti-bipolar drug. Neurochem. Res. 2013;38(4):834–841. doi: 10.1007/s11064-013-0986-3. [DOI] [PubMed] [Google Scholar]
- 38.Peng L, Li B, Du T, Kong EK, Hu X, Zhang S, Shan X, Zhang M. Astrocytic transactivation by a2A-adrenergic and 5-HT2B serotonergic signaling. Neurochem. Int. 2010;57(4):421–431. doi: 10.1016/j.neuint.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 1999;274(12):8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 40.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. U. S. A. 1999;96(4):1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu W, Graves LM, Gill GN, Parsons SJ, Samet JM. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation. J. Biol. Chem. 2002;277(27):24252–24257. doi: 10.1074/jbc.M200437200. [DOI] [PubMed] [Google Scholar]
- 42.Du T, Li B, Liu S, Zang P, Prevot V, Hertz L, Peng L. ERK phosphorylation in intact, adult brain by a2-adrenergic transactivation of EGF receptors. Neurochem. Int. 2009;55(7):593–600. doi: 10.1016/j.neuint.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(3):R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67(5):1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 45.Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J. Biol. Chem. 1996;271(21):12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Kennedy DJ, Yan Y, Shapiro JI. Reactive oxygen species modulation of Na/K-ATPase regulates fibrosis and renal proximal tubular sodium handling. Int. J. Nephrol. 2012:381320. doi: 10.1155/2012/381320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai L, Du T, Song D, Li B, Hertz L, Peng L. Astrocyte ERK phosphorylation precedes K+-induced swelling but follows hypotonicity-induced swelling. Neuropathology. 2011;31(3):250–264. doi: 10.1111/j.1440-1789.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- 48.Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem. Res. 1991;16(7):833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- 49.Norenberg MD, Bender AS. Astrocyte swelling in liver failure role of glutamine and benzodiazepines. Acta. Neurochir. 1994;60(Suppl Wien. ):24–27. doi: 10.1007/978-3-7091-9334-1_6. [DOI] [PubMed] [Google Scholar]
- 50.Robinson JD. Interactions between monovalent cations and the (Na+ + K+)-dependent adenosine triphosphatase. Arch. Biochem. Biophys. 1970;139(1):17–27. doi: 10.1016/0003-9861(70)90040-8. [DOI] [PubMed] [Google Scholar]
- 51.Rossi B, Gache C, Lazdunski M. Specificity and interactions at the cationic sites of the axonal (Na+, K+)-activated adenosinetriphosphatase. Eur. J. Biochem. 1978;85(2):561–570. doi: 10.1111/j.1432-1033.1978.tb12271.x. [DOI] [PubMed] [Google Scholar]
- 52.Skou JC, Esmann M. The Na,K-ATPase. J. Bioenerg. Biomembr. 1992;24(3):249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- 53.Wall SM. Ammonium transport and the role of the Na,K-ATPase. Miner Electrolyte Metab. 1996;22(5-6):311–317. [PubMed] [Google Scholar]
- 54.Pedersen SF, O'Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na+/H+ exchange and Na+ -K+ -2Cl- cotransport in the heart, brain, and blood. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(1):R1–R25. doi: 10.1152/ajpregu.00782.2005. [DOI] [PubMed] [Google Scholar]
- 55.Verkhratsky A, Rodríguez JJ, Parpura V. Calcium signalling in astroglia. Mol. Cell Endocrinol. 2012;353(1-2):45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 56.Parpura V, Grubišic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta. 2011;1813(5):984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 58.Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium. 2007;42(2):103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salido GM, Sage SO, Rosado JA. Biochemical and functional properties of the store-operated Ca2+ channels. Cell Signal. 2009;21(4):457–461. doi: 10.1016/j.cellsig.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Verkhratsky A, Parpura V. Store-operated calcium entry in neuroglia. Neurosci. Bull. 2014;30(1):125–133. doi: 10.1007/s12264-013-1343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan E, Li B, Gu L, Hertz L, Peng L. Mechanisms for L-channel-mediated increase in [Ca2+]i and its reduction by anti-bipolar drugs in cultured astrocytes combined with its mRNA expression in freshly isolated cells support the importance of astrocytic L-channels. Cell Calcium. 2013;54(5):335–342. doi: 10.1016/j.ceca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Du T, Liang C, Li B, Hertz L, Peng L. Chronic fluoxetine administration increases expression of the L-channel gene Cav1. in astrocytes from the brain of treated mice and in culture and augments K+-induced increase in [Ca2+]i. Cell Calcium. 2014;55(3):166–174. doi: 10.1016/j.ceca.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Hargovan M, Ferro A. Aldosterone synthase inhibitors in hypertension: current status and future possibilities. JRSM Cardiovasc. Dis. 2014;3:2048004014522440. doi: 10.1177/2048004014522440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balzan S, Nicolini G, Bellitto L, Ghione S, Biver P, Montali U. Effect of canrenone on the digitalis site of Na+/K+-ATPase in human placental membranes and in erythrocytes. J. Cardiovasc. Pharmacol. 2003;42(1):32–36. doi: 10.1097/00005344-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gupta S, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54(6):1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmiedek P, Sadée W, Baethmann A. Cerebral uptake of a 3H-labelled spirolactone compound in the dog. Eur. J. Pharmacol. 1973;21(2):238–241. doi: 10.1016/0014-2999(73)90232-x. [DOI] [PubMed] [Google Scholar]
- 67.Ferrari P. Rostafuroxin an ouabain-inhibitor counteracting specific forms of hypertension. Biochim. Biophys. Acta. 2010;1802(12):1254–1258. doi: 10.1016/j.bbadis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Huang R, Kala G, Murthy RK, Hertz L. Effects of chronic exposure to ammonia on glutamate and glutamine interconversion and compartmentation in homogeneous primary cultures of mouse astrocytes. Neurochem. Res. 1994;19(3):257–265. doi: 10.1007/BF00971573. [DOI] [PubMed] [Google Scholar]
- 69.Kala G, Hertz L. Ammonia effects on pyruvate/lactate production in astrocytes-interaction with glutamate. Neurochem. Int. 2005;47(1-2):4–12. doi: 10.1016/j.neuint.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem. Res. 2013;38(3):472–485. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]