Abstract
It has been known for many years that the endogenous neurotransmitter noradrenaline (NA) exerts anti-inflammatory and neuroprotective effects both in vitro and in vivo. In many cases the site of action of NA are beta-adrenergic receptors (βARs), causing an increase in intracellular levels of cAMP which initiates a broad cascade of events including suppression of inflammatory transcription factor activities, alterations in nuclear localization of proteins, and induction of patterns of gene expression mediated through activity of the CREB transcription factor. These changes lead not only to reduced inflammatory events, but also contribute to neuroprotective actions of NA by increasing expression of neurotrophic substances including BDNF, GDNF, and NGF. These properties have prompted studies to determine if treatments with drugs to raise CNS NA levels could provide benefit in various neurological conditions and diseases having an inflammatory component. Moreover, increasing evidence shows that disruptions in endogenous NA levels occurs in several diseases and conditions including Alzheimer’s disease (AD), Parkinson’s disease (PD), Down’s syndrome, posttraumatic stress disorder (PTSD), and multiple sclerosis (MS), suggesting that damage to NA producing neurons is a common factor that contributes to the initiation or progression of neuropathology. Methods to increase NA levels, or to reduce damage to noradrenergic neurons, therefore represent potential preventative as well as therapeutic approaches to disease.
Keywords: Alzheimer’s disease, amyloid, review, EAE, GFAP, locus coeruleus, multiple sclerosis, noradrenaline, transgenic mice, tyrosine hydroxylase.
INTRODUCTION
The noradrenergic system is comprised of a cluster of small brainstem nuclei in the pons and lateral reticular formation of the medulla. Of these nuclei, the Locus coeruleus (LC) is the major source of noradrenergic innervation, and the only source in certain areas of the forebrain such as the cortex and hippocampus [1]. Fibers from the LC are highly branched, mostly specialized for volume transmission release of noradrenaline (NA) from non-junctional varicosities. The released NA binds to and activates a variety of G-protein coupled adrenergic receptors (ARs) present on multiple cell types throughout the CNS, including glial cells (astrocytes and microglia) as well as neurons. The ARs are classified into several subclasses, namely α1A, α1B, α2A, α2B, β1, β2, and β3. The α1 subtypes are coupled to Gq and activation results in signaling through the inositol trisphosphate – protein kinase C (IP3-PKC) pathway. In contrast, the α2 and β subtypes are coupled to Gi and Gs proteins, respectively, and so their activity modulates the cyclic adenosine monophosphate – protein kinase A (cAMP-PKA) pathway.
Astrocytes have been shown to express α1A-, α2A-, β1-, and β2-adrenoceptors [2], hence the astrocyte response to NA is correspondingly diverse [3]. One of the earliest observations was that activation of b-ARs induced dramatic morphological changes in astrocytes [4] which can have multiple consequences that influence neuronal as well as glial functions. Numerous studies have demonstrated effects of NA on astrocyte metabolic functions, including increases in glycogen production mediated largely through α1 and α2-ARs [2] and involving increases in calcium fluxes. Activation of astrocyte α2-ARs activates various kinases and receptor tyrosine kinases, such as the EGF receptor [5]; increases astrocyte glutamine uptake and glutamate synthesis [6]; and increases calcium levels [7]. Glycogen metabolism is also greatly increased by stimulation of b-ARs [8, 9]. Selective β-AR agonists also increase the astrocyte ability to remove extracellular K+ [10] which helps to allow sustained neuronal activity. NA also has important effects on astrocyte inflammatory responses, and astrocyte derived neurotrophic support. The current review will focus on these latter aspects, and their role in neurodegenerative disease.
ANTI-INFLAMMATORY ACTIONS OF NA: IN VITRO STUDIES
Treatment with NA, b-adrenergic receptor (βAR) agonists, or direct elevation of intracellular cAMP suppresses microglial [11] and astroglial [12] inflammatory responses. NA reduces LPS-induced TNFα expression in microglia [13] and IFNγ induced expression of class II antigens in astrocytes [14]. NA reduces LPS and cytokine-dependent expression of the inducible form of nitric oxide synthase (NOS2) in both astrocytes [15] and microglia [16], mediated through activation of β2ARs [17,18], findings repeated by other labs in astrocytes as well as many other cell types (reviewed in [12]). The anti-inflammatory mechanisms of action of NA in glial cells are not fully understood, but our results [19] suggest that NA reduces activity of the inflammatory transcription factor NFκB, mediated by an increase in nuclear levels of inhibitory IκBa proteins. Though not as well characterized as in glial cells, NA also reduces induction of NOS2 in primary cortical neurons [20]. However, rather than having a direct effect on inflammatory transcription factors, it appears that NA reduces neuronal NOS2 expression by an indirect manner, involving a reduction in the release of stimulatory factors from microglial cells.
ANTI-INFLAMMATORY EFFECTS OF NORADRENALINE: IN VIVO STUDIES
In vitro results raised the possibility that increased NA levels in the CNS could provide benefit in certain neurological diseases or conditions by suppressing glial activation or by providing direct neuroprotective effects. NA can be increased in vivo by a variety of methods. Selective α2-adrenoceptor antagonists increase brain NA levels by blocking inhibitory pre-synaptic neuronal α2-autoreceptors that normally reduce NA release from noradrenergic neurons, primarily located in the Locus coeruleus (LC). The α2-adrenergic antagonists have neuroprotective effects in excitotoxicity models [21], improve functional recovery after cerebral ischemia [22], and promote neuronal survival in brain regions undergoing neurogenesis in the adult [23]. We showed that the highly selective α2-antagonist 5-fluoro-methoxyidazoxan reduced inflammatory responses and neuronal damage due to injection of aggregated amyloid beta (Ab1-42) into adult rat brain [24]. Interestingly, the α2-antagonist fluparoxan prevented the onset of pathology and cognitive deficits in a transgenic model of AD, but did not alter amyloid burden or gliosis [25]. Although several α2-antagonists have shown potential in treatment of depression and PTSD, their clinical use has been very limited.
NA levels can be increased by use of selective NA reuptake inhibitors (NARIs). For example, increasing NA levels using desipramine reduced CNS chemokine expression [26] and increased anti-inflammatory cytokine levels [27]. However, classical tricylic anti-depressants such as imipramine or desipramine block serotonin as well as NA reuptake. More recent non tricyclic antidepressants show highly selective effects on NA reuptake, and have anti-inflammatory effects on microglial cells [28]; increase BDNF expression in hippocampus [29]; and increase adult hippocampal neurogenesis [30]. We tested the NA selective NARI atomoxetine, which is currently used in the US for attention deficit hyperactivity disorder [31], for potential benefit in experimental autoimmune encephalomyelitis (EAE, a model of MS) studies. However, treatment of already ill mice with atomoxetine did not provide any recovery [32]. This suggests that although selective NARIs can increase extracellular NA levels, they may have limited effectiveness if endogenous NA levels are reduced (e.g. due to damage to noradrenaline producing neurons). Consistent with this, atomoxetine did not improve cognitive function in a clinical test in AD patients with mild to moderate disease [33].
NA levels can be directly increased by treatment with L-threo-3,4-dihydroxyphenylserine (L-DOPS, generic name Droxidopa) a synthetic catecholamino acid, which is given orally, and is converted to NA via decarboxylation by the ubiquitous enzyme L-aromatic-amino-acid decarboxylase (L-AAAD, also known as dopamine decarboxylase, or DDC) [34]. L-AAAD is the same enzyme that converts L-DOPA (levodopa) to dopamine used for treatment of PD. Peripheral conversion of L-DOPS to NA is blocked by co-treatment with a selective L-AAAD inhibitor such as carbidopa or benserazide which do not pass the blood brain barrier. The enzyme L-AAAD is expressed in glial cells as well as neurons [35], therefore L-DOPS may be more efficient at promoting NA effects in glial cells than NARIs. L-DOPS has been used in rodent models of neurological disease, and in dopamine beta hydroxylase (DBH) deficient mice restores central NA levels up to 26% of control values [36]. L-DOPS has been used in Europe and Japan for treatment of neurogenic orthostatic hypotension for about 15 years and was recently approved in the US for this indication.
In contrast to treatment with atomoxetine, administration of L-DOPS to EAE mice stabilized clinical scores and reduced CNS astrocyte activation [32]. Moreover, co-treatment with L-DOPS and atomoxetine has a synergistic effect since those mice showed clinical improvement. More recently, [37], we showed that treatment of 5xFAD mice (a transgenic mouse model of AD with robust amyloid deposition) with L-DOPS for 1 month reduced amyloid burden, CNS inflammation, and improved spatial learning in the Morris water maze test. The recent approval of L-DOPS in the US should facilitate clinical testing in neurological diseases.
An additional way by which NA levels can be raised is by treatment to reduce catecholamine breakdown. The 2 major pathways of NA metabolism in the CNS are mediated by monoamine oxidases (MAOs) and catechol-O-methyltransferase (COMT) [38]. COMT transfers a methyl group from S-adenosyl methionine to catecholamines including dopamine, epinephrine, and NA. COMT is expressed in the CNS, predominantly in astrocytes [39], microglia [40] and in some neurons [41]. COMT inhibitors have been in use for many years as a co-treatment for Parkinson’s disease [42], to reduce breakdown of levodopa [42]. COMT inhibitors may also provide benefit by reducing accumulation of homocysteine [43]. To our knowledge, COMT inhibitors have not been tested for benefit in neurological conditions where NA levels are reduced.
NA INDUCES ANTI-INFLAMMATORY FACTOR SECRETION FROM ASTROCYTES
In addition to having direct effects on inflammatory activation of glial cells, NA can act indirectly by regulating expression and secretion of anti-inflammatory factors from nearby cells or from the glial cells themselves. In mixed glial cultures, NA induced production of the IL-1 receptor antagonist (IL-1ra) as well as of IL-R2 which acts as a decoy receptor for IL1, thereby leading to an overall decrease in IL1 signaling [44]. The same authors showed that increasing NA levels in vivo by using reboxitine (a selective NARI) together with an α2-antagonist (idazoxan) also increased IL1-ra and IL-R2 [27], as well as IL-10 in the cortex and hippocampus, although other anti-inflammatory cytokines (IL-4, TGFbeta) were not induced.
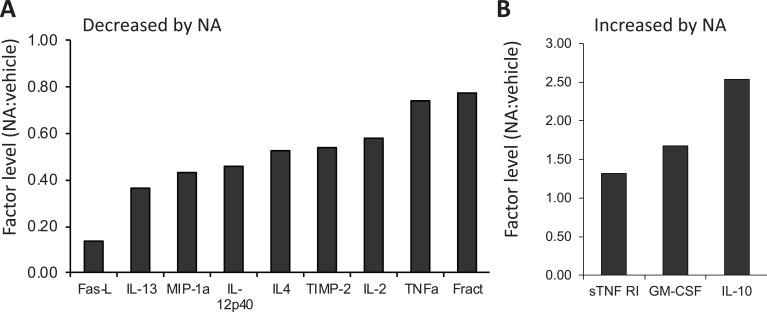
To obtain a better idea of which anti-inflammatory factors are induced by NA, we carried out studies using primary rat astrocytes robustly activated using bacterial endotoxin lipopolysaccharide (LPS) in the presence or absence of NA. After 24 hr, the conditioned media were used to screen low density cytokine arrays. As expected, NA reduced the secretion of several pro-inflammatory factors (Fig. 1A), including MIP1a (macrophage inflammatory protein 1a), TIMP2 (tissue inhibitor of metalloproteases-2), TNFa (tumor necrosis factor-α) and fractalkine. NA increased expression of several cytokines and chemokines, and we previously reported that NA increased astrocyte secretion of MCP-1 (monocyte chemotactic protein-1, also known as CCL2), which is anti-inflammatory as well as being neuroprotective [45]. Further analysis of the data reveals that NA also increased several other potential anti-inflammatory molecules (Fig. 1B), including IL-10 (interleukin-10, by 2.5-fold), GM-CSF (granulocyte-macrophage colony-stimulating factor, by 1.7 fold), and sTNFR1 (soluble TNF receptor-1, by 1.3 fold). Although GM-CSF is generally considered a pro-inflammatory factor, it also has anti-inflammatory and neuroprotective effects. For example, GM-CSF reduced dopaminergic cell loss in a Parkinsons’ mouse model [46], and reduced cognitive impairment and amyloid burden in a mouse model of AD [47]. The effects may be due in part to the ability of GM-CSF to induce maturation and proliferation of regulatory Tcells which attenuate ongoing Tcell inflammatory activities [48], and are known to play a role in attenuating auto-immune diseases such as MS [49] as well as in neurological conditions including stroke and glioma [50].
Fig. (1).
Effect of NA on factor secretion from astrocytes. Conditioned media from primary rat astrocytes activated overnight with LPS (1 µg/ml) in the presence or absence of 25 mM NA was used to screen Ray Biotech rat cytokine membranes. The relative levels of selected factors in CM-NA versus CM-vehicle is shown. (A) Secreted factors reduced by NA; (B) Secreted factors increased by NA. All samples were analyzed in duplicate.
NA REGULATION OF ASTROCYTE CHEMOKINE PRODUCTION
While the ability of NA to prevent the production of astrocyte pro-inflammatory mediators is well established, its regulation of astrocyte chemokine production has not been largely studied. Chemokines are a group of cytokines sharing similar structure, and best known for stimulating migration of cells that express chemokine receptors. However, chemokines exert other effects, most of which have been discovered over the last few years. Among these, CCL2 (also referred to as monocyte chemotactic protein 1, MCP-1) is one of the more versatile ones based on the number of actions that have been attributed to it [51]. We showed that NA stimulates CCL2 production from cultured astrocytes, and that the astrocyte derived CCL2 protected cortical neurons against excitotoxicity caused by direct treatment with NMDA or glutamate, as well as by exposure to ischemic injury [45]. CCL2 also induced microglia expression of the trophic factor insulin like growth factor 1 (IGF), which could provide neuroprotective actions [52]. This induction occurs in vivo, since treatment of mice with L-DOPS or desipramine increased brain levels of CCL2, primarily in astrocytes [53].
These above results are in agreement with reports that CCL2 reduced glutamate toxicity and neuronal damage caused by stimuli such as β-amyloid peptide [54] or ethanol [55]. In contrast, several studies demonstrate deleterious effects of CCL2, thought to contribute to progression of neurodegeneration in conditions where inflammatory responses occur [56]. The contrasting actions may be dependent upon CCL2 concentration: in mouse models of AD, CCL2 overexpression enhanced Aβ deposition and oligomer formation and accelerated memory impairments [57]; yet there was also an increase of neurodegeneration in CCL2 knockout mice [58]. These findings suggest that proper maintenance of adequate CCL2 levels are necessary to prevent neuronal damage. Accordingly, fluctuations in NA levels, as occurs in AD, could translate into dysregulation of astrocyte production of CCL2, and contribute to disease progression.
NA INCREASES TROPHIC FACTOR RELEASE FROM ASTROCYTES
In addition to effects on astrocyte cytokines and chemokines, a growing body of evidence shows that NA increases astrocytic production of trophic factors. In vitro, astrocytes express and secrete BDNF (brain derived neurotrophic factor), NGF (nerve growth factor), NT3 (neurotrophin-3) [59], GDNF (glial derived neuruotrophic factor) [60], CNTF (ciliary neurotrophic factor) [61], NTN (neurturin) [62], and NT4/5 (neurotrophin 4/5) [63]. Furthermore, changes in NA or cAMP regulate glial neurotrophin expression, including BDNF [64]; GDNF [65]; FGF-2 (fibroblast growth factor -2) [66], and NGF and NT3 [63]. With the exception of GDNF production, which appears to be primarily controlled by α2-receptors [67], NA-stimulated trophic factor elevations are largely mediated by activation of β- and/or α1-adrenoceptors. Astrocyte levels of NGF are increased by the nonselective β-adrenoceptor agonist isoproterenol [68], although a role for other receptors has not been ruled out. In the case of BDNF, both α1 and β receptors have been shown to independently contribute to NA-induced upregulation [69]. These results are similar for NA-induced elevation of FGF-2, since increases are diminished by pre-treatment with the α1 and β antagonists [66]. The adrenoceptor mediated elevation of NT-3 is blocked by the PKC inhibitor staurosporine as well as by the PKA inhibitor H-89 [70]. Although in vitro studies can be confounded by the presence of other factors in the media which can crosstalk with various second messenger systems (eg insulin, cortisol, growth hormone), the weight of evidence supports the noradrenergic control of astrocyte trophic factor secretion, via the major α1-, α2-, and β-adrenoceptor subtypes.
LOSS AND DAMAGE TO THE LC IN NEUROLOGICAL DISEASES
It is well known that noradrenergic neurons in the Locus coeruleus (LC) are damaged or lost in the majority of Alzheimer's disease patients [71,72] and reduced levels of NA [73,74] are well known hallmarks of several neurological diseases including AD and PD. The LC provides noradrenergic input to most regions of the brain including forebrain, cerebellum, brainstem, and spinal cord. AD patients have up to 60% fewer LC neurons compared to normal age matched controls [75] and LC neuronal loss has been correlated with plaque number and the duration and severity of dementia [72,76]. Although loss of cholinergic neurons has been a primary focus of AD research, in recent studies [77], the greatest loss of neurons in AD was found in the LC (83% loss).
Although loss of LC neurons has been known for many years to occur in AD and PD, the neuropathological consequences of that loss have until recently not been clear. In view of the important regulation of astrocyte function by NA, including providing metabolic and trophic support to neurons, it was hypothesized that LC neuronal loss could lead to metabolic impairment and disruption of neuronal: glial interactions, leading to a cascade of events culminating in increased neuropathology [78]. To experimentally address the role of the LC, we examined the effects of NA depletion on cortical responses in adult rats [79]. NA depletion was achieved by treatment with the selective neurotoxin N-(2-chloroethyl)-N-ethyl-2 bromobenzylamine (DSP4) [80]. In control animals, injection of aggregated forms of the amyloid beta 1-42 peptide (Aβ1-42) induced IL-1β and NOS2 expression in microglia, and increased astrocytic GFAP staining. Following LC lesion, there was increased expression of IL-1β and NOS2 in microglia and astrocytes, and increased astrocyte GFAP and IL-1β staining. While Aβ1-42 induced NOS2 in glia in controls, the site of NOS2 in DSP4-treated animals was also neuronal, a location observed in AD patients [81]. The increased inflammation due to DSP4 was prevented by increasing CNS NA levels using a selective α2-adrenergic antagonist, indicating that it is reduced NA and not other transmitters that are responsible for the increased responses [82]. We and others then went on to examine the consequences of LC damage in various transgenic mouse models of AD, and showed that LC lesion not only exacerbated glial inflammation but also increased amyloid burden, neuronal death, and cognitive deficits [83-87]. Together, these studies confirm that loss or dysregulation of CNS NA levels can have significant consequences on disease progression, suggesting that LC loss in AD contributes to neuropathology.
Several studies have now demonstrated that loss or damage to LC noradrenergic neurons occurs in certain transgenic mouse models of AD in the absence of exogenous chemical lesioning [83,88,89], an event which may recapitulate what occurs in AD. While the exact causes of LC damage in these mice (as in AD) remains to be determined; an accumulation of phosphorylated tau has been observed in LC neurons in several transgenic mouse lines [90], which recapitulates another aspect of AD disease and may be a target for intervention [91,92].
NEUROTROPHIC FACTORS IMPORTANT FOR LC
A likely contributing factor to LC damage is a reduction in necessary trophic factors and their cognate receptors, which are known to be critical for LC maturation and survival [93]. During normal development LC neurons express high levels of the neurotrophin receptor TrkB, TrkB deficient mice have 30% fewer LC neurons, and in vitro the TrkB ligands NT4 and BDNF provide neuroprotection [94,95]. In vivo studies show that BDNF heterozygous null mice have fewer LC neuronal cell bodies [96]; and that BDNF increases the density of noradrenergic axons [97] and LC neuronal activity [98]. As described above, a major source of neurotrophins are astrocytes; and in vivo, many neurotrophins have been localized to glial cells by in situ hybridization or immunochemical staining methods [99]. These findings suggest that changes in LC function, leading to reductions in NA levels could cause a feed-forward loop of increasing damage by reducing neurotrophic support.
IMPORTANCE OF THE LC:NA AXIS IN MS
Changes in NA levels also occur in MS and its animal model EAE. Several studies reported alterations in peripheral NA levels [100,101] in MS patients versus healthy controls; and changes in β-AR expression on leukocytes [102]. Experimental lesion of the sympathetic nervous system augmented symptoms of EAE [103]; as did depletion of splenic NA [104]. Conversely, treatment with βAR agonists suppressed disease severity and decreased immune activation [105,106]. In contrast to peripheral effects, which to a large extent involve alterations of adoptive immune responses, the role of central NA during EAE and MS has not been as well characterized. In one study, CSF and white matter NA levels were found increased at early times, and reduced during the later clinical period [107]. Other studies reported reduced levels of NA in brainstem and cords of EAE rats [108,109]. In MS patients, it was reported that NA levels are increased in CSF [110], and in astrocytes in white matter there are reduced levels of β2-ARs compared to controls [111].
The above findings led us to speculate that, as for AD, LC damage may also be a feature of MS and EAE and similarly contribute to disease progression [112]. We first measured NA levels in CNS samples from EAE mice, and confirmed that at day 60 after immunization (during the chronic phase of disease), NA levels were significantly reduced in the frontal cortex (about 35% reduction) and spinal cord (about 50% reduction). Immunostaining of the LC from those mice showed an increase in GFAP staining associated with a slight decrease in tyrosine hydroxylase (TH - the rate limiting enzyme in NA synthesis) staining, and a significant atrophy of the TH+ neurons, suggesting stress and damage occurring in or around LC noradrenergic neurons. Staining of sections from MS patients and controls also revealed a significant increase in GFAP staining around the area of the LC. However, in contrast to neuronal atrophy, we detected significant hypertrophy of the LC TH+ neurons, which we hypothesize is due to the large difference in ages (4-5 month old mice as compared to 70-80 year old MS patients).
Relatively few studies have examined possible beneficial effects of selectively increasing CNS NA in EAE or MS. Early studies reported that destruction of noradrenergic neurons attenuated the course of EAE in rats [113] suggesting an exacerbating role for central NA. However more recently, it was shown that chronic treatment with venlafaxine, a bicyclic, serotonin-noradrenaline reuptake inhibitor ameliorated clinical disease in adoptive transfer EAE [114]. Similarly a combination of the phosphodiesterase (PDE) inhibitor rolipram with lovastatin provided synergistic benefit in an inflammatory EAE model [115]. Our studies [32] confirm that increasing CNS NA levels, by use of L-DOPS alone or with atomoxetine, provide clinical benefit in EAE.
CAN WE PREVENT LC DAMAGE AND NA LOSS?
Despite the potential benefit of several methods which raise CNS NA levels, they do not address the root problem, namely LC damage and reduced function; drugs or interventions which reduce LC neuronal damage or increase LC neuronal activity could potentially reduce underlying pathology and disease progression. Vindeburnol (Fig. 2), a semi-synthetic derivative of the plant alkaloid vincamine, was shown to exert some of these properties [116]. Vindeburnol increased TH protein expression and activity in noradrenergic neurons in the LC, but not in dopaminergic neurons in the substantia nigra or ventral tegmental area. In rats, a single i.p. dose of 20 mg/kg vindeburnol increased TH protein levels about 2-fold measured 3 days later. Vindeburnol also accelerated maturation of LC TH activity during normal development [117] and increased TH mRNA levels (Marcel et al. 1998). Both immunostaining and in situ data [118] suggest that vindeburnol re-activates TH expression in a sub-population of LC neurons that transiently express TH during development, but lose expression in the adult while maintaining expression of transcription factor Phox2a which is necessary for noradrenergic maturation [119].
Fig. (2).
Chemical structure of vindeburnol, a semi-synthetic derivative of the plant alkaloid vincamine.
The reported actions of vindeburnol on LC physiology prompted us to test this compound in mouse models of neurodegenerative diseases. Our initial studies were done using the EAE model of MS [120]. We reported that vindeburnol not only reduced the clinical signs of EAE disease, but also reduced glial inflammation in the area of the LC, reduced LC TH+ neuronal stress, and increased NA levels in the spinal cord. The mechanisms by which vindeburnol reduced LC damage in EAE mice are currently under investigation. However, measurement of mRNA levels for several genes involved in LC maturation or function showed reductions in EAE mice compared to controls, and restoration of those levels due to vinderburnol treatment.
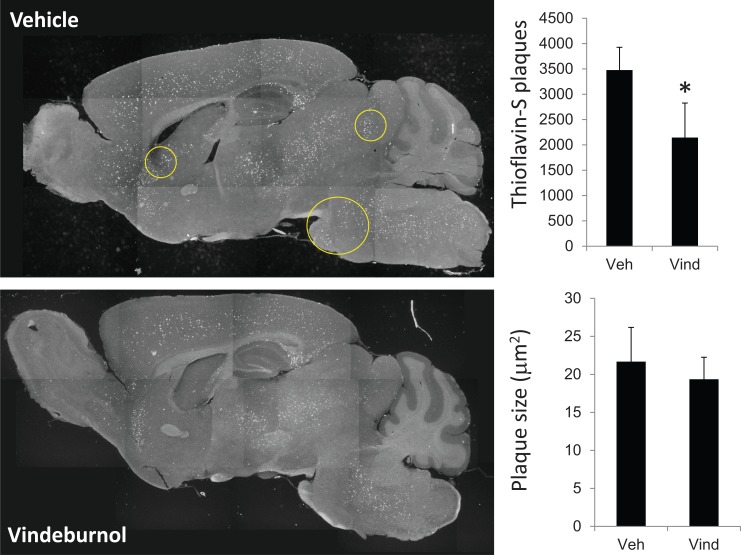
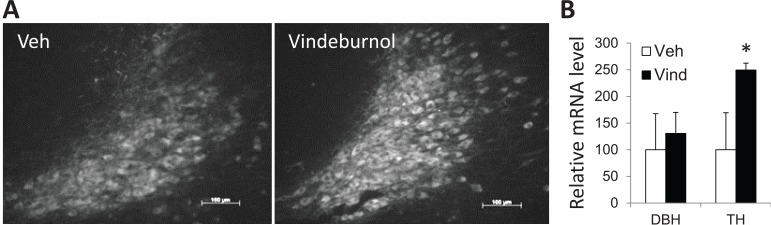
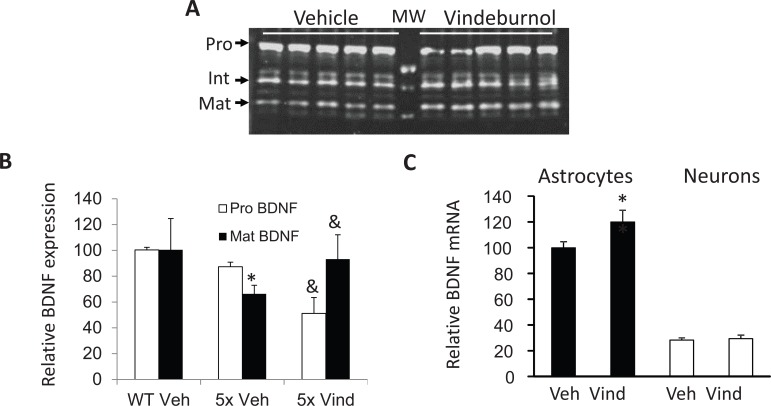
More recently, we’ve been testing if vindeburnol would provide benefit in the transgenic mouse models of AD. Our initial findings suggest that vinderburnol treatment of 5xFAD mice, beginning at age 5 months and continued for 4 weeks, reduced amyloid burden throughout the brain (Fig. 3), with some regions (encircled) showing greater attenuation than others. Initial examination of the LC shows a trend towards increased TH expression and staining (Fig. 4), suggesting induction of neuronal maturation. We also observed that in the hippocampus, levels of neurotrophin BDNF were reduced in 5xFAD mice, and those levels were restored in the vindeburnol treated mice (Fig. 5A,B). In vitro studies using enriched glial and neuronal cultures suggests that vindeburnol selectively induces BDNF expression in astrocytes (Fig. 5C), suggesting that astrocytes may be a direct target of vindeburnol actions.
Fig. (3).
Vindeburnol reduces thioflavin-S stained plaques in 5xFAD mice. 5-month old male 5xFAD mice [124] were treated with vindeburnol (20 mg/kg, i.p., 3 times per week) for 4 weeks. Serial sagital sections were stained with thioflavin-S, and then total staining quantified in 5-6 sections per mouse (n=6 mice per group). Representative images show areas where thioflavin-S staining was reduced by treatment. Quantitative analysis shows that vindeburnol significantly reduces the number of plaques, but did not affect average plaque size.
Fig. (4).
Vindeburnol induces LC maturation in 5xFAD mice. (A) Sagital sections through vehicle and vindeburnol treated 5xFAD mice treated as in Fig. 3 were stained for the presence of TH+ neurons. Images are representative of 4 to 6 mice in each group, and show an increase of TH+ staining due to vindeburnol treatment. (B) Total cytosolic RNA was purified from the LC, and used for qPCR analysis of DBH (dopamine beta hydroxylase) and TH. Values are mean±se of n=6 (vehicle) and n=9 (vindeburnol) samples per group, and are normalized to values measured for tubulin in the same samples. The values for the vehicle treated samples are normalized to 100%. *, P < 0.05. Scale bars are 100 mm.
Fig. (5).
Vindeburnol induces BDNF expression. (A) Tissue lysates were prepared from vehicle and vindeburnol treated 5xFAD mice (n=5 per group) as described in Fig. 3, and used for western blot analysis of BDNF. The antibody used (Santa Cruz sc-546, rabbit polyclonal) detects pro-BDNF (Pro), intermediate forms (Int), and mature BDNF (Mat). (B) Bands were quantified using NIH Image J software, normalized to total Commassie Blue staining obtained for the same samples run on a second gel, and are presented relative to values measured in wildtype littermates (WT). Vindeburnol did not alter BDNF levels in WT mice (not shown). *, P <0.05 versus WT-Vehicle; &, P<0.05 versus 5xFAD-Vehicle. (C) Primary enriched cultures of mouse astrocytes and cortical neurons were prepared, kept in culture for 1 (neurons) or 2 weeks (astrocytes), then treated with 0 or 20 mM vindeburnol. After overnight incubation, total RNA was analyzed by qPCR for BDNF4 (the major BDNF mRNA isotype present in 5xFAD hippocampus, data not shown). BDNF values are normalized to b-tubulin levels measured in the same samples, and are presented relative to values measured in vehicle treated astrocytes. Data is mean ± se of n=4 samples per group, *, P<0.05 versus vehicle treated astrocytes.
ACKNOWLEDGEMENTS
This work was supported in part by the National Multiple Sclerosis Society (DLF), a Research Career Scientist award from the Department of Veterans Affairs (DLF), and a grant from the Spanish Ministry of Science and Innovation (SAF2010-21948) to JLMM.
SUMMARY/CONCLUSIONS
Although damage to LC noradrenergic neurons, and associated decreases in CNS NA levels, have been known for almost 40 years, dating back to early studies in PD and AD patients, the causes of that damage, its consequences on glial activation and neuropathology, and development of methods to prevent LC damage have been largely limited. LC damage now appears to be a common feature of several neurological diseases and conditions, including not only AD and PD but also MS, posttraumatic stress disorder [121,122], and Down’s syndrome [123]. Lesion studies indicate that LC neuronal damage and associated reductions or fluctuations in NA levels in LC projection areas (i.e. the hippocampus in AD; the spinal cord in MS) are permissive for increased inflammatory action of glial cells in those target areas, which can lead to a feed forward cycle in which reduced neurotrophic support increases LC neuronal damage. Findings that increasing NA levels by use of α2-antagonists, NA reuptake inhibitors, and NA precursors reduces glial inflammation, as well as improving neuropathology, offers several therapeutic options for treatment, some of which are currently being considered for design of early stage clinical trials in AD and MS. In contrast, findings using agents such as vindeburnol which can reduce LC damage, possibly by increasing the differentiation of quiescent noradrenergic neurons, offers a novel means to potentially limit the initial events of a the feed-forward mechanism of damage.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Szabadi E. Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 2013;27:659–693. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- 2.Hertz L, Lovatt D, Goldman S A, Nedergaard M. Adrenoceptors in brain cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem. Int. 2010;57:411–420. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vardjan N, Kreft M, Zorec R. Dynamics of beta-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia. 2014;62:566–579. doi: 10.1002/glia.22626. [DOI] [PubMed] [Google Scholar]
- 5.Du T, Li B, Liu S, Zang P, Prevot V, Hertz L, Peng L. ERK phosphorylation in intact, adult brain by alpha(2)-adrenergic transactivation of EGF receptors. Neurochem. Int. 2009;55:593–600. doi: 10.1016/j.neuint.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Huang R, Hertz L. Clonidine enhances astrocytic glutamine uptake by authentic alpha 2-adrenoceptor stimulation. Neuroreport. 1994;5:632–634. doi: 10.1097/00001756-199401000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Code W E, Hertz L. Dexmedetomidine, a potent and highly specific alpha 2 agonist, evokes cytosolic calcium surge in astrocytes but not in neurons. Neuropharmacology. 1992;31:1077–1079. doi: 10.1016/0028-3908(92)90111-2. [DOI] [PubMed] [Google Scholar]
- 8.Magistretti P J, Sorg O, Yu N, Martin J L, Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev. Neurosci. 1993;15:306–312. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- 9.Subbarao K V, Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990;536:220–226. doi: 10.1016/0006-8993(90)90028-a. [DOI] [PubMed] [Google Scholar]
- 10.Pesce L, Guerrero C, Comellas A, Ridge K M, Sznajder J I. beta-agonists regulate Na,K-ATPase via novel MAPK/ERK and rapamycin-sensitive pathways. FEBS Lett. 2000;486:310–314. doi: 10.1016/s0014-5793(00)02298-5. [DOI] [PubMed] [Google Scholar]
- 11.Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G. Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia. 1997;19:152–160. [PubMed] [Google Scholar]
- 12.Galea E, Feinstein D L. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 1999;13:2125–2137. doi: 10.1096/fasebj.13.15.2125. [DOI] [PubMed] [Google Scholar]
- 13.Szabo C, Hasko G, Zingarelli B, Nemeth Z H, Salzman A L, Kvetan V, Pastores S M, Vizi E S. Isoproterenol regulates tumour necrosis factor, interleukin-10, interleukin-6 and nitric oxide produciton and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohman E M, Vayuvegula B, Gupta S, van den N S. Norepinephrine inhibits gamma-interferon-induced major histo-compatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc. Natl. Acad. Sci. U. S. A. 1988;85:1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinstein D L, Galea E, Reis D J. Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J. Neurochem. 1993;60:1945–1948. doi: 10.1111/j.1471-4159.1993.tb13425.x. [DOI] [PubMed] [Google Scholar]
- 16.Dello R C, Boullerne A I, Gavrilyuk V, Feinstein D L. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J. Neuroinflammation. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Keyser J, Laureys G, Demol F, Wilczak N, Mostert J, Clinckers R. Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis. Neurochem. Int. 2010;57:446–450. doi: 10.1016/j.neuint.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, Kooijman R, Smolders I, Michotte Y, De Keyser J. Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog. Neurobiol. 2010;91:189–199. doi: 10.1016/j.pneurobio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Gavrilyuk V, Horvath P, Weinberg G, Feinstein D L. A 27-bp region of the inducible nitric oxide synthase promoter regulates expression in glial cells. J. Neurochem. 2001;78:129–140. doi: 10.1046/j.1471-4159.2001.00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Madrigal J L, Feinstein D L, Dello R C. Norepinephrine protects cortical neurons against microglial-induced cell death. J. Neurosci. Res. 2005;81:390–396. doi: 10.1002/jnr.20481. [DOI] [PubMed] [Google Scholar]
- 21.Martel J, Chopin P, Colpaert F, Marien M. Neuroprotective effects of the alphα2-adrenoceptor antagonists, (+)-efaroxan and (+/-)-idazoxan, against quinolinic acid-induced lesions of the rat striatum. Exp. Neurol. 1998;154:595–601. doi: 10.1006/exnr.1998.6942. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson I, Westerberg E, Wieloch T. Protection against ischemia-induced neuronal damage by the alpha 2-adrenoceptor antagonist idazoxan influence of time of administration and possible mechanisms of action. J Cereb. Blood Flow Metab. 1990;10:885–894. doi: 10.1038/jcbfm.1990.145. [DOI] [PubMed] [Google Scholar]
- 23.Veyrac A. A, Didier A, Colpaert F, Jourdan F, Marien M. Activation of noradrenergic transmission by alphα2-adrenoceptor antagonists counteracts deafferentation-induced neuronal death and cell proliferation in the adult mouse olfactory bulb. Exp. Neurol. 2005;194:444–456. doi: 10.1016/j.expneurol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kalinin S, Polak P E, Madrigal J L, Gavrilyuk V, Sharp A, Chauhan N, Marien M, Colpaert F, Feinstein D L. Beta-amyloid-dependent expression of NOS2 in neurons prevention by an alphα2-adrenergic antagonist. Antioxid. Redox. Signal. 2006;8:873–883. doi: 10.1089/ars.2006.8.873. [DOI] [PubMed] [Google Scholar]
- 25.Scullion G A, Kendall D A, Marsden C A, Sunter D, Pardon M C. Chronic treatment with the alphα2-adrenoceptor antagonist fluparoxan prevents age-related deficits in spatial working memory in APPxPS1 transgenic mice without altering beta-amyloid plaque load or astrocytosis. Neuropharmacology. 2011;60:223–234. doi: 10.1016/j.neuropharm.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan J B, Ryan K M, Harkin A, Connor T J. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J. Neuroimmunol. 2010;220:34–42. doi: 10.1016/j.jneuroim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.McNamee E N, Griffin E W, Ryan K M, Ryan K J, Heffernan S, Harkin A, Connor T J. Noradrenaline acting at beta-adrenoceptors induces expression of IL-1beta and its negative regulators IL-1ra and IL-1RII, and drives an overall anti-inflammatory phenotype in rat cortex. Neuropharmacology. 2010;59:37–48. doi: 10.1016/j.neuropharm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Hashioka S, Klegeris A, Monji A., Kato T., Sawada M., McGeer P L, Kanba S. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp. Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Russo-Neustadt A, Beard R C, Cotman C W. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 30.Malberg J E, Eisch A J, Nestler E J, Duman R S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince J B. Pharmacotherapy of attention-deficit hyperactivity disorder in children and adolescents update on new stimulant preparations, atomoxetine, and novel treatments. Child Adolesc. Psychiatr. Clin. N. Am. 2006;15:13–50. doi: 10.1016/j.chc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Simonini M V, Polak P E, Sharp A, McGuire S, Galea E, Feinstein D L. Increasing CNS noradrenaline reduces EAE severity. J. Neuroimmune Pharmacol. 2010;5:252–259. doi: 10.1007/s11481-009-9182-2. [DOI] [PubMed] [Google Scholar]
- 33.Mohs R C, Shiovitz T M, Tariot P N, Porsteinsson A P, Baker K D, Feldman P D. Atomoxetine augmentation of cholinesterase inhibitor therapy in patients with Alzheimer disease: 6-month, randomized, double-blind, placebo-controlled, parallel-trial study. Am. J. Geriatr. Psychiatry. 2009;17:752–759. doi: 10.1097/JGP.0b013e3181aad585. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein D S. L-Dihydroxyphenylserine (L-DOPS) a norepinephrine prodrug. Cardiovasc. Drug Rev. 2006;24:189–203. doi: 10.1111/j.1527-3466.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Ahmed M, Barr E, Leiden J M, Kang U J. The localization and functional contribution of striatal aromatic L-amino acid decarboxylase to L-3,4-dihydroxyphenylalanine decarboxylation in rodent parkinsonian models. Cell Transplant. 2000;9:567–576. doi: 10.1177/096368970000900502. [DOI] [PubMed] [Google Scholar]
- 36.Rommelfanger K S, Edwards G L, Freeman K G, Liles L C, Miller G W, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalinin S, Polak P E, Lin S X, Sakharkar A J, Pandey S C, Feinstein D L. The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2012;33:1651–1663. doi: 10.1016/j.neurobiolaging.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenhofer G, Finberg J P. Different metabolism of norepinephrine and epinephrine by catechol-O-methyltransferase and monoamine oxidase in rats. J. Pharmacol. Exp. Ther. 1994;268:1242–1251. [PubMed] [Google Scholar]
- 39.Huang G, Dragan M, Freeman D, Wilson J X. Activation of catechol-O-methyltransferase in astrocytes stimulates homo-cysteine synthesis and export to neurons. Glia. 2005;51:47–55. doi: 10.1002/glia.20185. [DOI] [PubMed] [Google Scholar]
- 40.Helkamaa T, Reenila I, Tuominen R K, Soinila S, Vaananen A, Tilgmann C, Rauhala P. Increased catechol-O-methyltrans-ferase activity and protein expression in OX-42-positive cells in the substantia nigra after lipopolysaccharide microinfusion. Neurochem. Int. 2007;51:412–423. doi: 10.1016/j.neuint.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Myohanen T T, Schendzielorz N, Mannisto P T. Distribution of catechol-O-methyltransferase (COMT) proteins and enzymatic activities in wild-type and soluble COMT deficient mice. J. Neurochem. 2010;113:1632–1643. doi: 10.1111/j.1471-4159.2010.06723.x. [DOI] [PubMed] [Google Scholar]
- 42.Muller T. Levodopa/carbidopa and entacapone in the treatment of Parkinson's disease efficacy, safety and patient preference. Patient. Prefer. Adherence. 2009;3:51–59. doi: 10.2147/ppa.s4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller T, Kuhn W, Przuntek H. Therapy with central active catechol-O-methyltransferase (COMT)-inhibitors: is addition of monoamine oxidase (MAO)-inhibitors necessary to slow progress of neurodegenerative disordersκ. J. Neural Transm. Gen. Sect. 1993;92:187–195. doi: 10.1007/BF01244877. [DOI] [PubMed] [Google Scholar]
- 44.McNamee E N, Ryan K M, Kilroy D, Connor T J. Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1beta-induced neuro-toxicity. Eur. J. Pharmacol. 2010;626:219–228. doi: 10.1016/j.ejphar.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 45.Madrigal J L, Leza J C, Polak P, Kalinin S, Feinstein D L. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J. Neurosci. 2009;29:263–267. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangano E N, Peters S, Litteljohn D, So R, Bethune C, Bobyn J, Clarke M, Hayley S. Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson's disease. Neurobiol. Dis. 2011;43:99–112. doi: 10.1016/j.nbd.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Boyd T D, Bennett S P, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash G W, Potter H. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers. Dis. 2010;21:507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kosloski L M, Kosmacek E A, Olson K E, Mosley R L, Gendelman H E. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J. Neuroimmunol. 2013;265:1–10. doi: 10.1016/j.jneuroim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buc M. [Immunogenetic mechanisms in autoimmune processes: disorders of immune regulatory mechanisms, genetic determination of autoimmunity, effector mechanisms of autoimmune processes and their therapy]. Bratisl. Lek. Listy. 1996;97:660–668. [PubMed] [Google Scholar]
- 50.Lowther D E, Hafler D A. Regulatory T cells in the central nervous system. Immunol. Rev. 2012;248:156–169. doi: 10.1111/j.1600-065X.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- 51.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity a review. Clin. Chim. Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Hinojosa A E, Garcia-Bueno B, Leza J C, Madrigal J L. CCL2/MCP-1 modulation of microglial activation and proliferation. J. Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madrigal J L, Garcia-Bueno B, Hinojosa A E, Polak P, Feinstein D L, Leza J C. Regulation of MCP-1 production in brain by stress and noradrenaline-modulating drugs. J. Neurochem. 2010;113:543–551. doi: 10.1111/j.1471-4159.2010.06623.x. [DOI] [PubMed] [Google Scholar]
- 54.Bruno V, Copani A, Besong G, Scoto G, Nicoletti F. Neuroprotective activity of chemokines against N-methyl-D-aspartate or beta-amyloid-induced toxicity in culture. Eur. J. Pharmacol. 2000;399:117–121. doi: 10.1016/s0014-2999(00)00367-8. [DOI] [PubMed] [Google Scholar]
- 55.Bray J G, Reyes K C, Roberts A J, Ransohoff R M, Gruol D L. Synaptic plasticity in the hippocampus shows resistance to acute ethanol exposure in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Neuropharmacology. 2013;67:115–125. doi: 10.1016/j.neuropharm.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bose S, Cho J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 2013;36:1039–1050. doi: 10.1007/s12272-013-0161-z. [DOI] [PubMed] [Google Scholar]
- 57.Kiyota T, Gendelman H E, Weir R A, Higgins E E, Zhang G, Jain M. CCL2 affects beta-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2013;34:1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furukawa S, Furukawa Y, Satoyoshi E, Hayashi K. Regulation of nerve growth factor synthesis/secretion by catecholamine in cultured mouse astroglial cells. Biochem. Biophys. Res. Commun. 1987;147:1048–1054. doi: 10.1016/s0006-291x(87)80176-6. [DOI] [PubMed] [Google Scholar]
- 60.Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie E T, Vivien D, Buisson A. Neuroprotection mediated by glial cell line-derived neurotrophic factor involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J. Neurosci. 2001;21:3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudge J S, Alderson R F, Pasnikowski E, McClain J, Ip N Y, Lindsay R M. Expression of Ciliary Neurotrophic Factor and the Neurotrophins-Nerve Growth Factor, Brain-Derived Neuro-trophic Factor and Neurotrophin 3-in Cultured Rat Hippocampal Astrocytes. Eur. J. Neurosci. 1992;4:459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 62.Remy S, Naveilhan P, Brachet P, Neveu I. Differential regulation of GDNF, neurturin, and their receptors in primary cultures of rat glial cells. J. Neurosci. Res. 2001;64:242–251. doi: 10.1002/jnr.1072. [DOI] [PubMed] [Google Scholar]
- 63.Condorelli D F, Salin T, Dell' A P, Mudo G, Corsaro M, Timmusk T, Metsis M, Belluardo N. Neurotrophins and their trk receptors in cultured cells of the glial lineage and in white matter of the central nervous system. J. Mol. Neurosci. 1995;6:237–248. doi: 10.1007/BF02736783. [DOI] [PubMed] [Google Scholar]
- 64.Seki M, Tanaka T, Sakai Y, Fukuchi T, Abe H, Nawa H, Takei N. Muller Cells as a source of brain-derived neurotrophic factor in the retina noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Muller cells. Neurochem. Res. 2005;30:1163–1170. doi: 10.1007/s11064-005-7936-7. [DOI] [PubMed] [Google Scholar]
- 65.Koyama Y, Egawa H, Osakada M, Baba A, Matsuda T. Increase by FK960, a novel cognitive enhancer, in glial cell line-derived neurotrophic factor production in cultured rat astrocytes. Biochem. Pharmacol. 2004;68:275–282. doi: 10.1016/j.bcp.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 66.Kajitani N, Hisaoka-Nakashima K, Morioka N, Okada-Tsuchioka M, Kaneko M, Kasai M, Shibasaki C, Nakata Y, Takebayashi M. Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors differential regulation of FGF-2 by noradrenaline. PLoS. One. 2012;7:e51197. doi: 10.1371/journal.pone.0051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan M, Dai H, Ding T, Dai A, Zhang F, Yu L, Chen G, Chen Z. Effects of dexmedetomidine on the release of glial cell line-derived neurotrophic factor from rat astrocyte cells. Neurochem. Int. 2011;58:549–557. doi: 10.1016/j.neuint.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Nakagawa T, Schwartz J P. Gene expression profiles of reactive astrocytes in dopamine-depleted striatum. Brain Pathol. 2004;14:275–280. doi: 10.1111/j.1750-3639.2004.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juric D M, Miklic S, Carman-Krzan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006;1108:54–62. doi: 10.1016/j.brainres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Mele T, Carman-Krzan M, Juric D M. Regulatory role of monoamine neurotransmitters in astrocytic NT-3 synthesis. Int. J. Dev. Neurosci. 2010;28:13–19. doi: 10.1016/j.ijdevneu.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Tomlinson B E, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J. Neurol. Sci. 1981;49:419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- 72.Bondareff W, Mountjoy C Q, Roth M, Rossor M N, Iversen L L, Reynolds G P, Hauser D L. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1987;1:256–262. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- 73.Adolfsson R, Gottfries CG, Roos B E, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br. J. Psychiatry. 1979;135:216–223. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- 74.Mann D M, Lincoln J, Yates P O, Stamp J E, Toper S. Changes in the monoamine containing neurones of the human CNS in senile dementia. Br. J. Psychiatry. 1980;136:533–541. doi: 10.1192/bjp.136.6.533. [DOI] [PubMed] [Google Scholar]
- 75.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash D C, Mesulam M M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer's disease. Neurobiol. Aging. 2006;28:327–35. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash D C, Mesulam M M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer's disease. Neurobiol. Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Zarow C, Lyness S A, Mortimer J A, Chui H C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 78.Hertz L. Is Alzheimer's disease an anterograde degeneration, originating in the brainstem, and disrupting metabolic and functional interactions between neurons and glial cellsκ. Brain Res. Brain Res. Rev. 1989;14:335–353. doi: 10.1016/0165-0173(89)90017-9. [DOI] [PubMed] [Google Scholar]
- 79.Heneka M T, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O'Banion M K, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation implications for Alzheimer's disease. J. Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fritschy J M, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons are there pharmacologically different types of noradrenergic axons in the central nervous systemκ. Prog. Brain Res. 1991;88:257–268. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- 81.Vodovotz Y, Lucia M S, Flanders K C, Chesler L, Xie Q W, Smith T W, Weidner J, Mumford R, Webber R, Nathan C, Roberts A B, Lippa C F, Sporn M B. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J. Exp. Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalinin S, Gavrilyuk V, Polak P E, Heneka M T, Feinstein D L. Noradrenaline deficiency in brain increases beta-amyloid plaque burden in an animal model of Alzheimer's disease. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Pugh P L, Vidgeon-Hart M P, Ashmeade T, Culbert A A, Seymour Z, Perren M. J., Joyce F., Bate S. T., Babin A., Virley D J, Richardson J C, Upton N, Sunter D. Repeated administration of the noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) modulates neuroinflammation and amyloid plaque load in mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. J. Neuroinflammation. 2007;4:8. doi: 10.1186/1742-2094-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heneka M T, Ramanathan M, Jacobs A H, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss W D, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J. Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heneka M T, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch U K, Kummer M P. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalinin S, Gavrilyuk V, Polak P E, Vasser R, Zhao J, Heneka M T, Feinstein D L. Noradrenaline deficiency in brain increases beta-amyloid plaque burden in an animal model of Alzheimer's disease. Neurobiol. Aging. 2007;28:1206–1214. doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Jardanhazi-Kurutz D, Kummer M P, Terwel D, Vogel K, Dyrks T, Thiele A, Heneka M T. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem. Int. 2010 doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 88.O'Neil J. N. Mouton P R, Tizabi Y. Ottinger M A, Lei D. L. Ingram D K, Manaye K F. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J. Chem. Neuroanat. 2007;34:102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guerin D, Sacquet J, Mandairon N. Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 90.Li L, Cheung T, Chen J, Herrup K. A comparative study of five mouse models of Alzheimer's disease cell cycle events reveal new insights into neurons at risk for death. Int. J. Alzheimers. Dis. 2011;2011:171464. doi: 10.4061/2011/171464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braak H, Del T K. Where, when, and in what form does sporadic Alzheimer's disease beginκ. Curr. Opin. Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 92.Attems J, Thal D R, Jellinger K A. The relationship between subcortical tau pathology and Alzheimer's disease. Biochem. Soc. Trans. 2012;40:711–715. doi: 10.1042/BST20120034. [DOI] [PubMed] [Google Scholar]
- 93.Arenas E, Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994;367:368–371. doi: 10.1038/367368a0. [DOI] [PubMed] [Google Scholar]
- 94.Holm P C, Rodriguez F J, Kresse A, Canals J M, Silos-Santiago I, Arenas E. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development. 2003;130:3535–3545. doi: 10.1242/dev.00565. [DOI] [PubMed] [Google Scholar]
- 95.Counts S E, Mufson E J. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J. Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luellen B A, Bianco L E, Schneider L M, Andrews A M. Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes Brain Behav. 2007;6:482–490. doi: 10.1111/j.1601-183X.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 97.Matsunaga W, Shirokawa T, Isobe K. BDNF is necessary for maintenance of noradrenergic innervations in the aged rat brain. Neurobiol. Aging. 2004;25:341–348. doi: 10.1016/S0197-4580(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 98.Nakai S, Matsunaga W, Ishida Y, Isobe K, Shirokawa T. Effects of BDNF infusion on the axon terminals of locus coeruleus neurons of aging rats. Neurosci. Res. 2006;54:213–219. doi: 10.1016/j.neures.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Friedman W J, Black I B, Kaplan D R. Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain an immuno-cytochemical study. Neuroscience. 1998;84:101–114. doi: 10.1016/s0306-4522(97)00526-5. [DOI] [PubMed] [Google Scholar]
- 100.Rajda C, Bencsik K, Fuvesi J, Seres E, Vecsei L, Bergquist J. The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Mult. Scler. 2006;12:265–270. doi: 10.1191/135248506ms1269oa. [DOI] [PubMed] [Google Scholar]
- 101.Cosentino M, Zaffaroni M, Marino F, Bombelli R, Ferrari M, Rasini E, Lecchini S, Ghezzi A, Frigo G. Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients effect of cell stimulation and possible relevance for activation-induced apoptosis. J. Neuroimmunol. 2002;133:233–240. doi: 10.1016/s0165-5728(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 102.Zoukos Y, Leonard J P, Thomaides T, Thompson A J, Cuzner M L. beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis a regulatory role for cortisol and interleukin-1. Ann. Neurol. 1992;31:657–662. doi: 10.1002/ana.410310614. [DOI] [PubMed] [Google Scholar]
- 103.Chelmicka-Schorr E, Checinski M, Arnason B G. Chemical sympathectomy augments the severity of experimental allergic encephalomyelitis. J. Neuroimmunol. 1988;17:347–350. doi: 10.1016/0165-5728(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 104.Leonard J P, MacKenzie F J, Patel H A, Cuzner M L. Hypothalamic noradrenergic pathways exert an influence on neuroendocrine and clinical status in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 1991;5:328–338. doi: 10.1016/0889-1591(91)90028-9. [DOI] [PubMed] [Google Scholar]
- 105.Chelmicka-Schorr E, Wollmann R L, Kwasniewski M N, Kim D H, Dupont B L. The beta 2-adrenergic agonist terbutaline suppresses acute passive transfer experimental autoimmune myasthenia gravis (EAMG). Int. J. Immunopharmacol. 1993;15:19–24. doi: 10.1016/0192-0561(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 106.Wiegmann K, Muthyala S, Kim D H, Arnason B G, Chelmicka-Schorr E. Beta-adrenergic agonists suppress chronic/relapsing experimental allergic encephalomyelitis (CREAE) in Lewis rats. J. Neuroimmunol. 1995;56:201–206. doi: 10.1016/0165-5728(94)00153-f. [DOI] [PubMed] [Google Scholar]
- 107.Khoruzhaia T A, Saakov B A. [Change in monoamine content and monoamine oxidase activity in brain structures during experimental allergic encephalomyelitis]. Biull. Eksp. Biol. Med. 1975;79:80–82. [PubMed] [Google Scholar]
- 108.Krenger W, Honegger C G, Feurer C, Cammisuli S. Changes of neurotransmitter systems in chronic relapsing experimental allergic encephalomyelitis in rat brain and spinal cord. J. Neurochem. 1986;47:1247–1254. doi: 10.1111/j.1471-4159.1986.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 109.White S R, Bhatnagar R K, Bardo M T. Norepinephrine depletion in the spinal cord gray matter of rats with experimental allergic encephalomyelitis. J. Neurochem. 1983;40:1771–1773. doi: 10.1111/j.1471-4159.1983.tb08156.x. [DOI] [PubMed] [Google Scholar]
- 110.Barkhatova V P, Zavalishin I A, Askarova L S, Shavratskii V K, Demina E G. Changes in neurotransmitters in multiple sclerosis. Neurosci. Behav. Physiol. 1998;28:341–344. doi: 10.1007/BF02464784. [DOI] [PubMed] [Google Scholar]
- 111.Zeinstra E, Wilczak N, De Keyser J. [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain. Neurosci. Lett. 2000;289:75–77. doi: 10.1016/s0304-3940(00)01254-4. [DOI] [PubMed] [Google Scholar]
- 112.Polak P E, Kalinin S, Feinstein D L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2011;134:665–677. doi: 10.1093/brain/awq362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jovanova-Nesic K, Nikolic V, Jankovic B D. Locus ceruleus and immunity.II. Suppression of experimental allergic encephalomyelitis and hypersensitivity skin reactions in rats with lesioned locus ceruleus. Int. J. Neurosci. 1993;68:289–294. doi: 10.3109/00207459308994284. [DOI] [PubMed] [Google Scholar]
- 114.Vollmar P, Nessler S, Kalluri S R, Hartung H P, Hemmer B. The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int. J. Neuropsychopharmacol. 2008:1–12. doi: 10.1017/S1461145708009425. [DOI] [PubMed] [Google Scholar]
- 115.Paintlia A S, Paintlia M K, Singh I, Skoff R B, Singh A K. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2008;57:182–93. doi: 10.1002/glia.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Labatut R, Richard F, Milne B, Quintin L, Lecestre D, Pujol J F. Long-term effects of RU24722 on tyrosine hydroxylase of the rat brain. J. Neurochem. 1988;51:1367–1374. doi: 10.1111/j.1471-4159.1988.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 117.Zyzek E, Richard F, Bouilloux J P, Pujol J F. Ontogeny of tyrosine hydroxylase concentration in locus coeruleus of newborn rats: long-term effects of RU24722. J. Neurochem. 1990;55:849–853. doi: 10.1111/j.1471-4159.1990.tb04569.x. [DOI] [PubMed] [Google Scholar]
- 118.Ginovart N, Marcel D, Bezin L, Gagne C, Pujol J F, Weissmann D. Tyrosine hydroxylase expression within Balb/C and C57black/6 mouse locus coeruleus.II. Quantitative study of the enzyme level. Brain Res. 1996;719:45–55. doi: 10.1016/0006-8993(96)00075-3. [DOI] [PubMed] [Google Scholar]
- 119.Bezin L, Marcel D, Desgeorges S, Pujol J F, Weissmann D. Singular subsets of locus coeruleus neurons may recover tyrosine hydroxylase phenotype transiently expressed during development. Brain Res. Mol. Brain Res. 2000;76:275–281. doi: 10.1016/s0169-328x(00)00007-3. [DOI] [PubMed] [Google Scholar]
- 120.Polak P E, Kalinin S, Braun D, Sharp A, Lin S X, Feinstein D L. The vincamine derivative vindeburnol provides benefit in a mouse model of multiple sclerosis effects on the Locus coeruleus. J. Neurochem. 2012;121:206–216. doi: 10.1111/j.1471-4159.2012.07673.x. [DOI] [PubMed] [Google Scholar]
- 121.Vanderheyden W M, Poe G R, Liberzon I. Trauma exposure and sleep using a rodent model to understand sleep function in PTSD. Exp. Brain Res. 2014;232:1575–1584. doi: 10.1007/s00221-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 122.Pietrzak R H, Gallezot J D, Ding Y S, Henry S, Potenza M N, Southwick S M, Krystal J H, Carson R E, Neumeister A. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry. 2013;70:1199–1205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Salehi A, Faizi M, Colas D, Valletta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner S L, Aisen P, Shamloo M, Mobley W C. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci. Transl. Med. 2009;1:7ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- 124.Oakley H, Cole S L, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van E L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neuro-degeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]