Abstract
It is generally assumed that the neuropathology of sporadic (late-onset or nonfamilial) Alzheimer’s disease (AD) is driven by the overproduction and spreading of first Amyloid-βx-42 (Aβ42) and later hyperphosphorylated (hp)-Tau oligomeric “infectious seeds”. Hitherto, only neurons were held to make and spread both oligomer types; astrocytes would just remove debris. However, we have recently shown that exogenous fibrillar or soluble Aβ peptides specifically bind and activate the Ca2+-sensing receptors (CaSRs) of untransformed human cortical adult astrocytes and postnatal neurons cultured in vitro driving them to produce, accrue, and secrete surplus endogenous Aβ42. While the Aβ-exposed neurons start dying, astrocytes survive and keep oversecreting Aβ42, nitric oxide (NO), and vascular endothelial growth factor (VEGF)-A. Thus astrocytes help neurons’ demise. Moreover, we have found that a highly selective allosteric CaSR agonist (“calcimimetic”), NPS R-568, mimics the just mentioned neurotoxic actions triggered by Aβ●CaSR signaling. Contrariwise, and most important, NPS 2143, a highly selective allosteric CaSR antagonist (“calcilytic”), fully suppresses all the Aβ●CaSR signaling-driven noxious actions. Altogether our findings suggest that the progression of AD neuropathology is promoted by unceasingly repeating cycles of accruing exogenous Aβ42 oligomers interacting with the CaSRs of swelling numbers of astrocyte-neuron teams thereby recruiting them to overrelease additional Aβ42 oligomers, VEGF-A, and NO. Calcilytics would beneficially break such Aβ/CaSR-driven vicious cycles and hence halt or at least slow the otherwise unstoppable spreading of AD neuropathology
Keywords: Alzheimer’s disease, amyloid-beta oligomers, astrocyte-neuron teams, calcium-sensing receptor, calcilytics, calcimimetics.
PREAMBLE
Previous reviews from our Group have covered either the origins and diffusion of the “infectious Aβ and Tau seeds” advancing Alzheimer’s disease (AD) or more restricted views on the links between the calcium-sensing receptor (CaSR) and the overproduction of nitric oxide (NO) by cortical normofunctioning adult human astrocytes (NAHAs) exposed to mixtures of microglial proinflammatory cytokines and/or to Amyloid (A)β peptides (Aβs) [1-4]. The present work is based on recently gained evidences [5,6] revealing the manifold critical roles the Aβ-binding CaSRs of human astrocyte-neuron teams play to promote AD progression and the possibility of effectively interfering with the pathological consequences of Aβ●CaSR signaling through the administration of selective allosteric CaSR antagonists (“calcilytics”).
ALZHEIMER’S DISEASE SLOW CIRCUIT-BREAKING MARCH THROUGH THE BRAIN
Late-onset (sporadic or nonfamilial) Alzheimer’s disease (AD) patients (about 95% of all AD cases) are older than 65 years of age, whereas the infrequent younger cases (e.g., in their 40’s) suffer from the early onset (or familial, i.e. genetic) form of AD [7,8]. AD most likely subclinically starts its ∼30-year-long march through the brain in neurons of the parahippocampal region and the layer II of entorhinal cortex [8-10]. The neuropathology begins when such neurons groups increasingly lose their ability to rid themselves (via the action of proteases such as neprilysin or expulsion into the extracellular space and out of the brain) of the non-toxic Amyloid-βx-42 (Aβ42) peptide monomers they produce during their physiological activity [11]. The accumulating Aβ42 monomers rapidly aggregate into neurotoxic oligomers [12]. The Aβ42 oligomers start the full cytopathological cycle leading to the production of as well neurotoxic hyperphosphorylated (hp)-Tau oligomers, which impair axonal transport and cause synaptic failure by damaging dendritic spines [13,14]. Alternatively, the hp-Tau oligomers can by themselves trigger the hp-Tau production and/or hp-Tau “infection” [9,15]. Thus, the long march of the AD neuropathology begins when Aβ42 and hp-Tau oligomers, both “AD infectious seeds”, are released from “infected” entorhinal neurons and disseminated to “infect” neurons of the dentate gyrus and the CA3 region of the memory-encoding and -retrieving hippocampal formation (reviewed in [1]). The neuropathology driven by the two kinds of “AD infectious seeds” slowly yet progressively spreads disconnecting neuronal circuits along the pathways going to the brain’s upper areas of the cognition machinery. It leaves in its wake neurons jammed by neurofibrillary tangles (NFTs) and vulnerable to being further damaged if not killed by proinflammatory cytokines released from a microglia activated by a trail of extracellular Aβ42 fibrillar deposits and their associated oligomers and of “ghost” NFTs [8,14,15]. Because of the limbic sites of origin and subsequent upward trajectory of the AD neuropathology, it is a person’s increasingly failing memory and growing mental confusion that eventually attract the clinician’s attention.
ASTROCYTE-NEURON TEAMWORKS AND AD
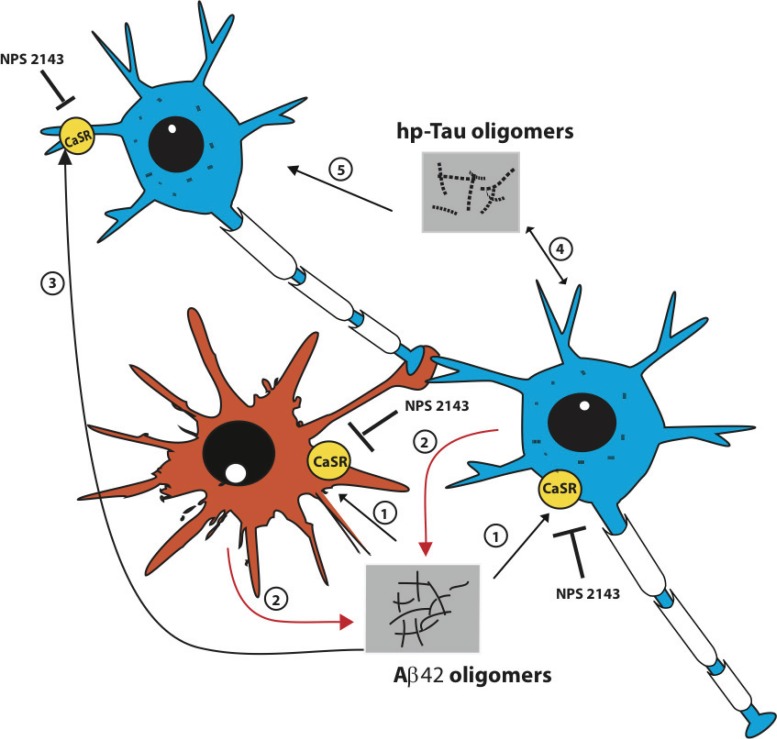
The conventional view of AD pathology is a “neuronocentric” one, in which the neurons are the producers, transmitters, and at the same time the victims of the Aβ42 and hp-Tau toxic oligomeric “seeds” [16]. But what about the astrocytes, of which in the AD-targeted regions there are least as many as, or maybe even several fold more than, neurons [17-19]? Astrocytes have traditionally been regarded as neuron-supporting cells and/or janitorial bystanders working with the activated microglia to sweep up neuronal debris [20]. But by experimentally using cultures of functionally normal adult human astrocytes (NAHAs) isolated from the cerebral cortex we have recently gained evidences that send a new message, that is “along with the neurons, the Aβ oligomer-exposed astrocytes become makers and spreaders of neurotoxic “AD infectious seeds” thereby remarkably advancing the transmission of the AD neuropathology” [5,21] (Fig. 1).
Fig. (1).
The interactions between exogenous Aβs and the CaSRs located on the plasma membrane of astrocyte-neuron teams advance the extracellular release and spread of Aβ42 and hp-Tau oligomers, the “AD infectious seeds”. Two neurons and one astrocyte of the same team are schematically depicted here. The neuropathology begins when for aging-related causes Aβ42 monomers accumulate in the extracellular space, oligomerize, and bind the CaSRs inserted in the plasma membranes of both cell types (1). The engendered Aβ●CaSR signaling induces the intracellular accrual (not shown) and oversecretion of de novo produced Aβ42 monomers from both neurons and astrocytes (2). The Aβ42 monomers oligomerize, spread, bind, and activate the CaSRs of a further neuron of the team (3). By doing this the Aβ42 oligomers cause the additional release of surplus Aβ42 moieties from this and other neurons (not shown). These vicious cycles can be unceasingly repeated and hence recruit ever-increasing numbers of astrocyte-neuron teams and thus inexorably advance the progression of AD. Hp-Tau oligomers formation is also triggered in the Aβ42-exposed neurons by still unclear mechanisms that might be at least partially driven by pathological Aβ●CaSR signaling (4). Secreted hp-Tau oligomers are next either taken up by the secreting cells or transferred to contiguous neurons (and maybe astrocytes too) to hinder microtubule functions and help destroy synaptic spines (5). Once produced, hp-Tau oligomers are also capable of an independent self-induction and spreading. A highly selective allosteric CaSR antagonist (calcilytic) like NPS 2143 can completely suppress the manifold noxious effects driven by pathological Aβ42●CaSR signaling both in neurons and in astrocytes thereby restoring conditions close if not identical to physiological ones [5,6]. Other relevant effects of the pathological Aβ42●CaSR signaling, like the surplus production and secretion of NO and VEGF-A from the astrocytes, which are also suppressed by calcilytic NPS 2143 [6,38], and the extracellular accrual of Aβ42 oligomers, which activate microglia, damage oligodendrocytes, and cause cerebral amyloid angiopathy, have been omitted from the picture for the sake of clarity.
It is well established that each astrocyte can embrace several “client” neurons with its mobile processes to form a working team [22-24]. For example, a single human astrocyte domain in the hippocampus can contain from 27 x 104 to 2.0 x 106 synapses vs. the 2.0 x 104 - 12 x 104 synapses of a rodent astrocyte domain [25-28]. Thus, human cortical astrocytes have from 1.4-to-16.7-fold more chances to directly trade with their neuron partners a variety of factors influencing, amongst other processes, memory formation and retrieval. And to add to the impact of astrocytes on neuronal networks, several astrocytes may embrace the synapses of a single neuron. Under normal conditions, astrocytes induce synapses and stabilize their neuron partners’ synapses by sweeping up glutamate and K+ spillovers and by modulating signal transmission via so-called “gliotransmitters” [22-24,29-31]. Regional astrocyte heterogeneity may be crucial to refine neural circuits postnatally, e.g., via the release of Semaphorin 3a (Sema3a) in the spinal cord [30,31]. An important consequence of this astrocyte-neuron physical and functional embrace is the astrocytes’ ability to promote or reduce the release of neurotransmitters into the synapses they envelop with the Ca2+ they expel or take up during their Ca2+ waves [32]. Moreover, the working astrocyte-neuron teams are fueled with glucose and oxygen when signalers secreted from the astrocytes’ vessel-touching end-feet open the arteriolar “taps” and increase the local blood flow [33].
But this constructive astrocyte-neuronal teamwork in the circuits of the cortical cognitive regions can become disruptive if aging or mutant neurons start producing, accruing, and releasing a surplus of Aβ peptides (Aβs). When neurons and the brain tissue become increasingly unable to get rid of the accumulating Aβ monomers, toxic Aβ oligomers are generated that trigger the formation, oligomerization, and release of pathological hp-Tau oligomers. Thus, neurons are likely to spill out both kinds of “AD infectious seeds” onto their astrocyte team partners [13] with consequences that are now beginning to emerge (Fig. 1).
An example of the destructive potential of an Aβ42-attacked astrocyte-neuron team is provided by Talantova et al. [34]. Aβ42 oligomers released from a neuron can bind to its astrocyte partner’s α-7-nicotinic acetylcholine receptors (α-7-nAChRs). Next, the signals from these receptors induce the astrocyte to exocytose the glutamate spillover from the neuronal synapses it has been sweeping up. The astrocyte-released glutamate activates the partner neuron’s extrasynaptic glutamate N-methyl-D-aspartate receptors (NMDARs). This in turn triggers intraneuronal Ca2+ surges inducing a cascade of events that include dysfunctional mitochondria pumping out reactive oxygen species (ROS). In sequence, ROS inflict oxidative damage that destroys neuronal synapses thereby cutting communications between the client neurons of the astrocyte’s network [14,34].
Amyotrophic lateral sclerosis (ALS) may be another example of astrocytes actually being in the driver’s seat of a major motor neuron disease. Re et al. [35] set up a model system in which astrocytes isolated from sporadic ALS patients are co-cultured with human embryonic motor neuron stem cells. They found that the ALS astrocytes secreted neurotoxic factors that killed the motor neurons via necroptosis. In other words, as stated by Pirooznia et al. [36], the motor neurons in sporadic ALS may be surrounded by killer astrocytes, which therefore should be the targets of therapeutic efforts. Thus, it seems that astrocytes might be the sole drivers of sporadic ALS, while both astrocytes and neurons are the co-drivers of AD.
In addition, astrocytes can express enough prion protein (PrPC) to support progressive neurodegeneration in prion disease [37] and are also likely to act essential parts in neurodevelopmental diseases such as Rett syndrome and fragile X mental retardation [30].
Some of the roles astrocytes play in AD were brought to light by our demonstration that when cultured NAHAs isolated from the cerebral cortex were exposed to exogenous Aβs, both their β-secretase (BACE1) and γ-secretase (γ-S) activities were stimulated to produce increased amounts of Aβ42 out of the endogenous amyloid precursor protein (APP) [5,21]. The astrocytes then intracellularly accrued and oversecreted the endogenous oligomerizing Aβ42 monomers into the culture medium together with a surplus of nitric oxide (NO) and vascular endothelial growth factor (VEGF)-A [5,6,38]. If in vivo surplus Aβ oligomers, VEGF-A, and NO are released from the astrocytes’ end-feet, they will also impair the supply of oxygen and glucose by distorting and eventually destroying the local vasculature [39,40]. Thus can start the astrocyte-neuronal engine that widely disseminates cognition-destroying neurotoxic “AD infectious seeds” [13-15]. And notably, while the AD pathology is driven by the toxic secretions from both team members, it appears that human astrocytes are far stronger and abler to survive than their teams’ client neurons because they appear to be unaffected by their several neurotoxic secretions. Consequently, as the astrocytes can keep producing and releasing their neurotoxins, they become the chief murderers of the neurons [5,6].
Thus, our in vitro observations strongly suggest that Aβ42 released from aging or mutant human neurons partaking in astrocyte-neuron teams in vivo would likely stimulate the teams’ astrocytes to make and secrete additional Aβ42 oligomers. These would spread and recruit even more astrocyte-neuron teams to produce and secrete surplus Aβ42 oligomers. The involved neurons would also synthesize and release hp-Tau oligomers (whether the astrocytes too play any part in this process remains to be ascertained). Therefore, both kinds of “AD seeds” would advance the inexorable progression of AD [5,13].
Here, the obvious question that arises is how to stop or at least significantly slow AD’s development. Ideally, inhibiting the overproduction of the pivotal Aβ42 and its toxic oligomers by both the astrocytes and neurons would halt the disease. But preventing the astrocytes and their client neuron partners from oversecreting their endogenous Aβ42 oligomers could also effectively hinder AD spreading and progression (Fig. 1).
Therefore, we set out to answer this question through the experimental use of NAHAs and human cortical postnatal (HCN-1A) neurons. This allowed us to find a way to stop the exogenous Aβ-treated NAHAs and neurons from oversecreting their endogenous Aβ42 and concurrently the NAHAs from releasing toxic amounts of NO and VEGF-A [5,6,38]. The clue that led us to this discovery was the knowledge that Aβ oligomers bind several membrane receptors harbored by both astrocytes and neurons, namely those for ApoE, insulin, NMDA, cellular prion protein, advanced glycation end products, and in addition the p75 neurotropin receptor, α-7-nAChR, frizzled receptor, formyl peptide receptor-like 1, and the CaSR (see [5] for references). The CaSR was of particular interest to us, as we had previously shown that it is involved in the overproduction of NO by NAHAs exposed to a mixture of proinflammatory cytokines or to Aβ25-35,an established proxy of Aβ42 [5,41]. But before proceeding further it is worth recalling here the salient features of the CaSR to get a better glimpse of the complex roles it plays in AD.
STRUCTURE, SIGNALING, AGONISTS AND ANTAGONISTS OF THE CaSR
Structure
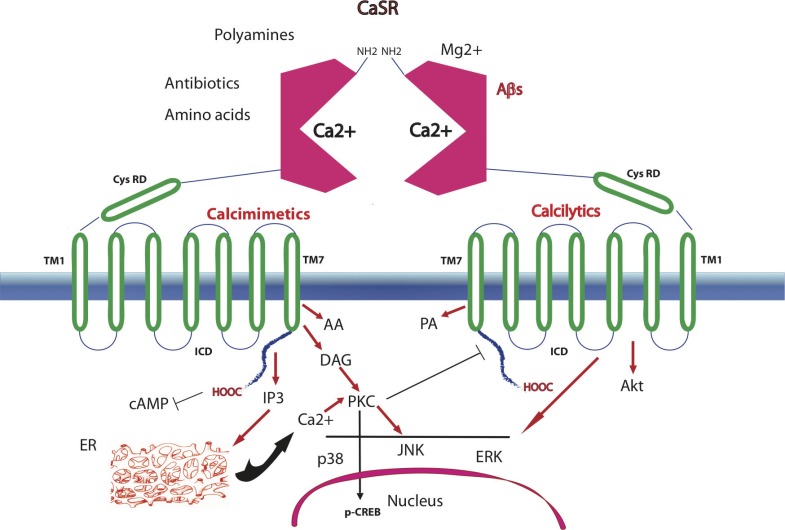
The CaSR belongs to family 3 of G protein-coupled receptors (GPCRs), also named seven transmembrane spanning receptors (7TMRs), and plays regulatory roles in Ca2+ homeostasis and cellular signaling [42]. It was first cloned from the bovine and later the human parathyroid gland [43,44]. In humans, the CaSR’s structure is comprised of an aminoterminal extracellular domain (ECD; 612 amino acids (aa)), seven α-helical transmembrane domains (TMD with TM1-TM7; 250 aa), which are typical of the GPCRs, and an intracellular domain with a carboxyterminal tail (ICD; 216 aa). A cysteine rich domain (CysRD) links the ECD with TM1 and is important for signals transmission to the TMD upon binding of a ligand (Fig. 2). The ECD’s surface is glycosylated and can form CaSR●CaSR homeodimers via a disulphide-link involving Cys129 and Cys131 [45,46] or CaSR●mGluR1α heterodimers. The conformational arrangement of each monomer, determined by molecular modeling, indicates that the CaSR’s ECD has a bilobed Venus flytrap (VFT)-like structure. The orthosteric Ca2+-binding site is located between the two lobes of the VFT [46] (Fig. 2). The cleft formed by the orthosteric Ca2+-binding site is thought to be open when the agonist is missing and closed after binding the Ca2+ or any other orthosteric (type I) agonist. A second putative Ca2+-binding site is placed in the TMD, because an ECD-lacking CaSR still responds to a Ca2+ signal [46]. A binding site for aromatic L-amino acids is located near the orthosteric Ca2+-binding site. Several putative binding pockets are located in the seven extracellular loops of the TMD [47]. Ca2+ binding triggers changes in the conformational structure of the TMD and ICD that allow the ICD’s carboxyterminal tail to interact with various G proteins (e.g., G11α, Gi/o, Gqα), which in turn mediate the activation/inhibition of manifold signaling pathways [48].
Fig. (2).
A CaSR homeodimer inserted in the plasma membrane. Each extracellular domain (ECD) has a bilobed Venus fly-trap (VFT; enclosing between its lobes the orthosteric binding site of the Ca2+ or of divalent or trivalent cations, aminoglycoside antibiotics and polyamines. A cysteine-rich domain (CysRD) connects the VFT with the first (TM1) of the seven transmembrane helices, being important for signal transmission. Aromatic L-α-amino acids, calcimimetics, and calcilytics are among the allosteric (type II) CaSR modulators and bind overlapping but not identical sites of the receptor, i.e. calcilytics between TM3 and TM5, and calcilytics and calcimimetics between TM6 and TM7. The last transmembrane helix (TM7) is joined to the intracellular domain (ICD). Ligand binding changes the CaSR conformation (not shown), and causes the interaction of the ICD’s tail with various G-proteins (not shown), which then activate/inhibit several signaling pathways (only a few of them are depicted here; see the text for more details and relevant references). By phosphorylating the ICD tail Ca2+/diacyl glycerol (DAG)-activated protein kinase Cs (PKCs) attenuate the signaling of the ligand bound CaSR. AA, arachidonic acid; Akt, protein kinase B; cAMP, cyclic AMP; p-CREB, phosphorylated cAMP response element-binding protein; ER, endoplasmic reticulum; IP3, inositol triphosphate; p38, JNK, and ERK are members of the mitogen-activated protein kinases (MAPKs); PA, phospholipase A.
Signaling
CaSR signaling is triggered and modulated by several factors and the kind(s) of response(s) it evokes depend(s) on the cell type considered and the pathways involved. Different ligands besides Ca2+ trigger CaSR’s activity, such as divalent and trivalent cations (e.g., Mg2+, Sr2+, La3+, Gd3+), polycations (e.g., polylysine, polyarginine), polyamines (spermine, spermidine, protamine), and aminoglycoside antibiotics (tobramycin, neomycin, gentamycin) [49-51]. These type I agonists bind the orthosteric Ca2+-binding site even in concert with the Ca2+ itself. The levels of these agonists vary in the tissues expressing the CaSR; hence the signaling differs accordingly. In tissues where the Ca2+ abounds, like the bone, CaSR’s activity is affected by the constitutive presence of high Ca2+ levels. On the other hand, the gastrointestinal mucosa cells are exposed to changeable Ca2+ concentrations depending on the variable composition of meals and their content of other cations [52,53]. Multivalent cationic proteins like Aβs also bind the CaSR [6,38,54,55] (Fig. 3B). Moreover, CaSR’s signaling activity is modulated by the extracellular pH and ionic strength, both of which alter the EC50 for Ca2+, as they do in the kidney tubules [56,57]. CaSR signaling is affected also by factors modifying its level of mRNA and protein expression at a given Ca2+ concentration, e.g., cellular proliferation or mitotic quiescence, and agents like interleukin (IL)-1β, IL-6, vitamin D3, and Ca2+ itself [39,58-60]. On the other hand, stromal derived factor (SDF)-1 and macrophage chemotactic protein (MCP)-1 and some ligands (see below) regulate CaSR’s traffic from the endoplasmic reticulum to the plasma membrane [61]. Various studies have identified a panoply of G-protein-mediated CaSR signal transducing pathways besides the TRPC6-encoded Ca2+ channels. They include: (A) the inhibition of adenylyl cyclase preventing the synthesis of cyclic AMP (cAMP) [62]; (B) the activation of lipid kinases including several phospholipases with the production of (i) inositol triphosphate (IP3) eliciting the release of Ca2+ from intracellular stores [48]; and of (ii) diacyl glycerol (DAG) activating conventional protein kinase C (PKC) isoforms that phosphorylate at Ser133 the CREB transcription factor [63] and CaSR’s Thr888 in the ICD that has been recognized as a critical negative regulator of CaSR signaling [64]; and (C) the stimulation of other protein kinases, e.g., AKT, and filamin-regulated mitogen-activated protein kinases (MAPKs), including MEK, ERK, and JNK [41,55,56]. In turn, the various second messengers involved activate downstream-placed signaling cascades, which are likely to amplify the noxious effects of a pathological Aβ●CaSR signaling [41,48,65] (Fig. 2).
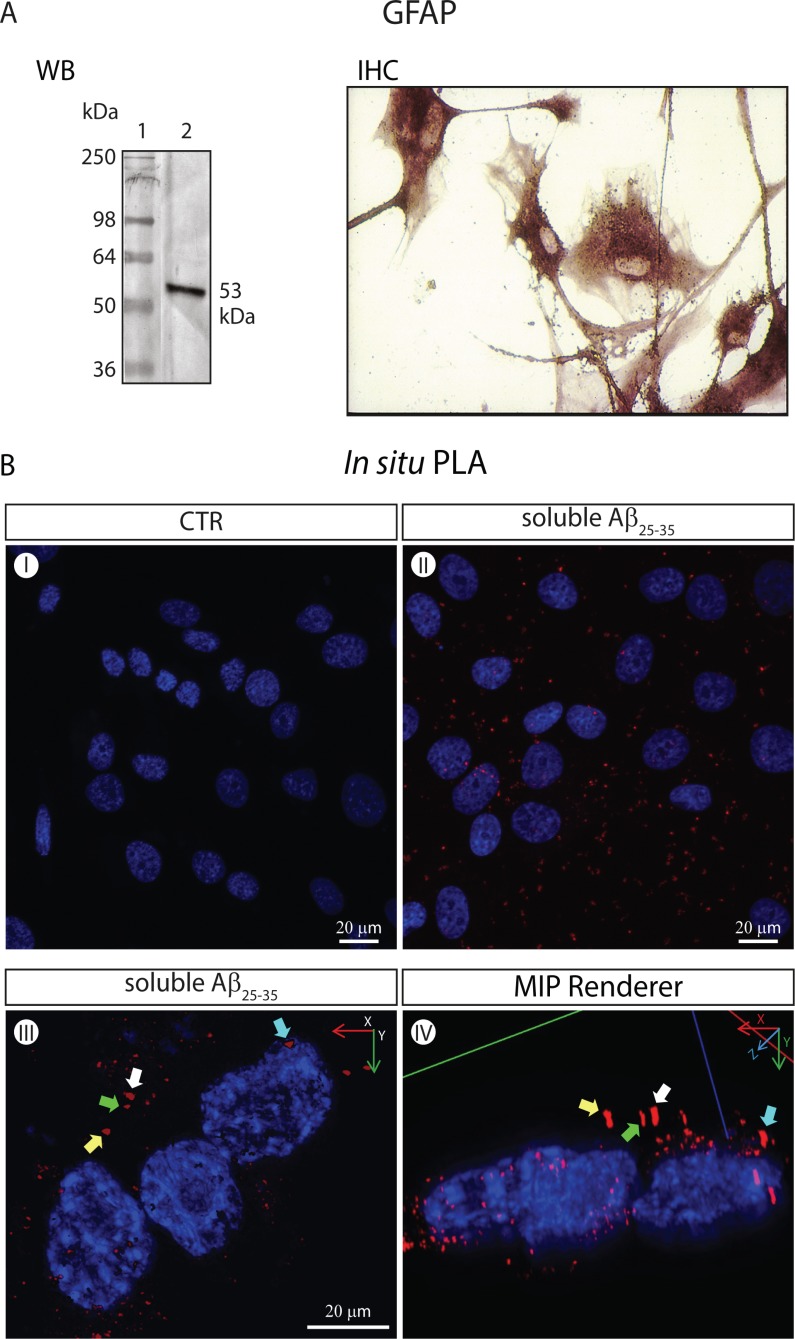
Fig. (3).
The CaSRs of GFAP-expressing NAHAs form specific complexes with soluble Aβ25-35 moieties visualized via the in situ PLA (isPLA) method. (A). Untransformed proliferatively quiescent NAHAs set in culture strongly express the GFAP marker. WB. GFAP Western immunoblotting was carried out according to Chiarini et al. [108] on NAHAs protein lysates using an anti-human GFAP rabbit polyclonal antiserum followed by an alkaline phosphatase (AP)-conjugated secondary antibody. Lane 1, molecular weight markers. Lane 2, specific GFAP protein band (53 kDa). IHC. GFAP immunohistochemistry on NAHAS using an anti-human GFAP mouse IgG1 followed by an AP-conjugated secondary antibody (for technical details see [108]). (B) Imaging of specific Aβ25-35●CaSR complexes via the in situ Proximity Ligation Assay (isPLA) according to Söderberg et al. [105] and 3D digital renderings. Proliferatively quiescent NAHAs were exposed for 1 h at 37 °C to soluble biotinylated Aβ25-35 (5.0 mM, Anaspec, CA, USA) dissolved in complete growth medium prior to fixation in 4 %v/v paraformaldehyde (PFA). Notably, after fixation NAHAs were not permeabilized, which restricted the antigen-antibody interactions to the outside of their plasma membrane. A mouse anti-CaSR monoclonal and a rabbit anti-biotin polyclonal were the two primary antibodies. The PLUS and MINUS isPLA probes were donkey anti-rabbit IgG (heavy + light chains) and donkey anti-mouse IgG (heavy + light chains), respectively (Olink Bioscience, Uppsala, Sweden). Once challenged with antibodies and isPLA probes, the samples were sequentially incubated with the Duolink Hybridization Solution, Ligation Mix, and Amplification Mix (all from Olink Bioscience) to produce single-stranded rolling-circle amplification products that were hybridized to oligonucleotide probes labeled with the red fluorophore Tye624 (λex = 594 nm and λem = 624 nm) by incubating with the Duolink Detection Reagents (Olink Bioscience). Next, nuclear DNA was stained with 1.0 µg ml−1 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich). Pictures were acquired under a Leica TCS SP5 AOBS Laser confocal microscope (Leica-Microsystems, Wezlar, Germany). A 40X/1.25 NA oil-immersion objective (HCX PL APO 40x 1.25 OIL UV, Leica-Microsystem) was used. The Maximum Intensity Projection (MIP) renderingsof original isPLA pictures were obtained using the Image Pro Plus 3D Viewer (Image-Pro Plus TM, version 7.0, Media Cybernetics, Bethesda, MD). (I) Untreated (i.e. not exposed to Aβ25-35) NAHAS show no specific isPLA signal or background. Only the DAPI-stained nuclei are detectable. (II) Aβ25-35-incubated NAHAs exhibit isPLA signals (red dots) corresponding to specific Aβ25-35●CaSR complexes that are in part aggregating in patches. Nuclear DNA is blue (DAPI). (III) A higher magnification of Aβ25-35-exposed adult human cortical astrocytes showing specific Aβ25-35●CaSR complexes (in red) visualized via the isPLA method. Nuclear DNA is blue (DAPI). The complexes have started aggregating in patches, an event preceding their internalization. Some of the patches have been purposedly marked by differently colored arrows to allow the identification of the same patches in a different projection shown in panel (IV). (IV) The 3D MIP-rendering of the picture shown in panel (III) seen in an oblique lateral projection. The colored arrows indicate the same specific Aβ25-35●CaSR complexes (in red) as in panel (III). The location of the Aβ25-35●CaSR complexes at the periphery of the cytoplasm can be appreciated here somewhat better than in the top-bottom 2D image in panel (III).
CaSR’s Allosteric Agonists (Calcimimetics) and Antagonists (Calcilytics)
The allosteric or type II and agonists and antagonists bind the CaSR at sites different from the orthosteric one and sensitize the receptor to activation by the Ca2+ in opposite directions. The best characterized classes of type II CaSR agonists are aromatic L-amino acids and two highly selective and CaSR-specific synthetic polyalkylamines, i.e. NPS R-568 and Cinacalcet HCl, which were named “calcimimetics” because their effects mimic those elicited by extracellular Ca2+ [66,67]. Conversely, highly selective synthetic allosteric antagonists or “calcilytics”, i.e. NPS 89636 and NPS 2143, diminish the CaSR response to Ca2+ and other type I agonists [66,67]. Indeed, calcilytics, which increase PTH secretion, and calcimimetics, which decrease PTH secretion, were first developed by NPS Pharmaceuticals [66,67]. As revealed by the results of point mutation studies, these quite interesting pharmacological agents bind to partially overlapping but not identical sites of the CaSR, the calcilytics between TM3 and TM5, and both the calcimimetics and calcilytics between TM6 and TM7 [68,69]. Calcimimetics are right now used to treat primary or secondary hyperparathyroidism conditions and to rescue loss-of function CaSR mutants. Conversely, calcilytics were initially meant (but till now not clinically used) to treat osteoporosis and may mitigate the effects of gain-of-function CaSR mutants [66-73]. Finally, it is worth recalling that the CaSR exhibits several distinct conformational states, each of which is induced and stabilized by a different ligand, allosteric agonists and antagonists included, and is linked to a particular set of intracellular signaling pathways—a property defined “ligand-biased signaling” [74]. And, it is the specific cell type considered that determines the preferential activation of a particular set of CaSR signaling pathways by the same ligand [75].
HUMAN ASTROCYTES, CaSR, AND AD
In recent years, a growing body of novel evidences has led to realize that human cortical astrocytes substantially differ from rodent ones. The former are much bulkier, emit 10-fold more primary processes, exhibit novel morphological subtypes, e.g., the interlaminar one, control broader synaptic domains, and are capable of performing more complex and intense functional tasks, e.g., faster Ca2+ waves propagation, than the latter [22,24,76-83]. The new evolutionary features acquired by the astrocytes have significantly impacted on human brain physiology. This is indirectly confirmed by recent findings of Han et al. [84], who showed that human astrocytes engrafted on the brains of mice increase the learning abilities and activity-dependent plasticity of the animals. Obviously, astrocytes’ evolutionary changes have also impacted on human neurodegenerative diseases, AD included. Perhaps, this is the reason why animal AD models fail to fully reproduce the human disease and why pharmacological findings gained in animal AD models cannot be successfully translated to human clinical settings [85-89]. Various authors had previously shown that astrocytes are able to engulf and degrade exogenously accruing Aβs [90,91]. However, more recent findings prove that astrocytes can also significantly contribute to the Aβs overload of an AD brain. In fact, unstimulated (control) adult human cortical astrocytes exhibit a discrete level of activity of β-site APP-cleaving enzyme 1/β-secretase (BACE1/β-S) and γ-secretase (γ-S), and under chronic stress or during AD or after an exposure to exogenous Aβs both these enzymatic activities surge remarkably thereby leading to the de novo production of larger amounts of Aβs [5,21,92].
From a neuropathological standpoint, a diffuse astrogliosis is detected in both AD-model animals [92] and postmortem human AD brains [93]. Generally, the astrocytes become hypertrophied, keep their spatial domains, and overexpress S100β and GFAP proteins while partially losing their complement of glutamate metabolizing enzymes [80,83,92]. Notably, an activation of the human astrocytes can be detected via Positron Emission Tomography (PET) after administering the inflammation-revealing tracer (11)C-D-deprenyl to mild cognitive impairment (MCI) patients, being more intense in them than in patients at later AD stages and in healthy individuals [94]. Alterations in astrocyte signaling as revealed by intercellular Ca2+ waves and synchronous hyperactivity were reported to occur in transgenic AD-model animals [95]. In addition, Aβ-exposed astrocytes exhibited increases in their intracellular Ca2+ levels, yet at variance with neurons they did not die [5,96]. Hence, as a Ca2+ dyshomeostasis befalls in the activated astrocytes of AD brains (reviewed in [97-99]), what role(s) would play the CaSRs jutting from the plasma membranes of the astrocytes and of their client neurons?
The CaSR is expressed ubiquitously in the brain, though more intensely in some regions, e.g., the hippocampus, than in others [100]. In primary cultures of rat embryo brains, neurons and oligodendrocyte progenitor cells expressed CaSR’s mRNA more intensely than astrocytes did. In addition, CaSR expression tended to decline from postnatal to adult age likely in relation to oligodendrocyte and astrocyte differentiation [101]. Chattopadhyay et al. [102] were the first to show that a functional CaSR is expressed in cultured human embryo astrocytes, besides human astrocytoma and meningioma cells. On their own part, Dal Prà et al. [41] showed that NAHAs cultured from surgical left-overs of the cerebral cortex also express a functional CaSR, at lower levels while proliferating and at higher ones while mitotically quiescent, levels that were little affected by the actual Ca2+ concentration in the medium.
In tissues like the brain, where the cells are not involved in the maintenance of systemic Ca2+ homeostasis, the extracellular Ca2+ acts as the first messenger to regulate through the CaSR a variety of cellular functions; (i) during the developmental stages, in which controls proliferation, migration, and differentiation of oligodendrocytes and neurons; (ii) in the postnatal life, in which modulates neurotransmission and synaptic plasticity [51,82,103]; and (iii) in the course of diseases affecting the central nervous system [104].
Concerning AD, Ye et al. [54] showed that exogenous Aβs bind and activate the CaSR causing the opening of a Ca2+-permeable non-selective cation channel that elicited a sustained surge of intracellular Ca2+ in cultures of hippocampal pyramidal neurons from wild-type mice and rats with resultant neuronal dysfunction. By contrast, exogenous Aβs could not evoke these intracellular Ca2+ surgesin CaSR-/- mice [54]. In addition, exogenous Aβs activated the same cationic channel in human embryo kidney cells overexpressing the CaSR (HEK293-CaSR), but failed to do so in wild-type HEK293 cells [54]. Moreover, using a sensitive luciferase-reporter gene assay, Conley et al. [55] demonstrated that in CaSR-transfected Cos1 cells exogenous Aβ1-42 activated CaSR signaling in a dose-dependent fashion. Recently, by applying to GFAP-expressing NAHAs in cultures (Fig. 3A) the in situ Proximity Ligation Assay (isPLA) method, which reveals through a fluorescent sharp signal the very close (≤30 nm) and definite interaction between two proteins [105], we showed that soluble Aβ oligomers do specifically bind the CaSRs at the plasma membrane [5,6] (Fig. 3B) to be subsequently endocytosed (unpublished observations). That exogenous Aβs not only bind but also activate the human astrocytes’ CaSR is supported by our findings that (i) either soluble or fibrillar Aβ25−35,an established Aβ42 functional surrogate [5,106], stimulates the excess production, accrual, and release of endogenous Aβ42 in the NAHAs and in human cortical postnatal HCN-1A neurons in parallel with the astrocytes’ surplus synthesis and secretion of NO and VEGF-A [5,38]; (ii) a highly selective calcimimetic, NPS R-568 also stimulates the synthesis, accrual, and release of surplus Aβ42 besides of NO and VEGF-A from the NAHAs thereby mimicking the effects of exogenous Aβs [5,6,38]; (iii) conversely, in the presence of exogenous Aβs, calcilytic NPS 2143 effectively blocks the oversecretion of Aβ42 by the cortical astrocytes and neurons, and of NO and VEGF-A by the NAHAs [5,6,38]; and (iv) last but not least, calcilytic NPS 2143 fully preserves the viability of the Aβs-exposed human cortical postnatal HCN-1A neurons, which would otherwise have progressively died [5]. Therefore, the extracellularly accruing Aβ42 oligomers do bind and activate the CaSRs of both members of the astrocyte-neuron teams. Thus, they excite the further release and spreading of a set of neurotoxins, including Aβ42, NO, and VEGF-A, which would advance AD progression. Importantly, the addition of a calcilytic agent like NPS 2143 fully prevents these noxious effects driven by the pathological Aβ●CaSR signaling [5].
Interestingly, an exposure of the NAHAs to exogenous Aβ oligomers also induced by 48 hours a significant albeit transient increase in astrocytes’ total CaSR proteins; under the same respect calcimimetic NPS R-568 was ineffective, calcilytic NPS 2143 given by itself caused an early, but transient decrease of the total CaSR protein, whereas in the presence of exogenous Aβs NPS 2143 elicited an intense and persistent fall of the total CaSR levels and likely of the Aβ●CaSR signaling intensity [5]. These are examples of alterations of the CaSR life cycle according to the bound agonist/antagonist that up- or downregulate CaSR availability and consequently its signaling intensity occurring in the NAHAs. Breitwieser [107] designated this recently identified mechanism as “agonist-driven insertional signaling (ADIS)” and our just mentioned findings support her suggestion that ADIS is likely to have therapeutic relevance. In addition, together with the lysosomes the proteasome helps reduce the availability of the total CaSR and the intracellular Aβ42 accrual in the NAHAs since its 20S chymotrypsin-like activity is remarkably though transiently increased by calcilytic NPS 2143 in the presence of exogenous Aβs [5].
CONCLUSIONS
Our view that the CaSRs of human astrocyte-neuron teams play specific and relevant roles in the spreading and progression of the AD neuropathology is supported not only by their just mentioned ability to form specific complexes with Aβ oligomers [6], but also by the opposite effects of CaSR’s highly selective synthetic allosteric agonists or antagonists [5,6,38]. Therefore, the suggestion of using allosteric CaSR antagonists (calcilytics) like NPS 2143 or similarly acting agents to hinder or at least remarkably slow the otherwise inexorable progression of AD stems from the experimental results gained through the use of cultured NAHAs and human cortical HCN-1A neurons [5,6,38,41]. Given the destructive effects of the disease on cortical neurons, it would be advisable to try CaSR antagonists, just like any other anti-AD candidate drug, on MCI or early post-MCI cases to assess their real therapeutic potential.
ACKNOWLEDGEMENTS
This work was partially supported by the Italian Ministry for University and Research.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the drafting of the manuscript and to the drawing of the figures. All the authors read and approved the final manuscript.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
LIST OF ABBREVIATIONS
- aa
= amino acid
- Aβ42
= amyloid-βx-42
- Aβs
= amyloid-β peptides
- AD
= Alzheimer’s disease
- ALS
= amyotrophic lateral sclerosis
- CaSR
= Ca2+-sensing receptor
- ECD
= extracellular domain
- hp
= hyperphosphorylated
- ICD
= intracellular domain
- MCI
= mild cognitive impairment
- MIP
= Maximum Intensity Projection
- NAHA
= normal adult human astrocyte
- NMDAR
= N-methyl-D-aspartate receptor
- α-7-nAChR
= α-7-nicotinic acetylcholine receptor
- NO
= nitric oxide
- ROS
= reactive oxygen species
- TMD
= transmembrane domain
- VEGF
= vascular endothelial growth factor
- VFT
= Venus fly-trap
REFERENCES
- 1.Dal Prà I, Chiarini A, Gui L, Chakravarthy B, Pacchiana R, Gardenal E, Whitfield J F, Armato U. Do astrocytes collaborate with neurons in spreading the “infectious” Aß and Tau drivers of Alzheimer’s diseaseκ. Neuroscientist. 2014 doi: 10.1177/1073858414529828. [DOI] [PubMed] [Google Scholar]
- 2.Armato U, Bonafini C, Chakravarthy B, Pacchiana R, Chiarini A, Whitfield J F, Dal Prà I. The calcium-sensing receptor A novel Alzheimer's disease crucial targetκ. J. Neurol. Sci. 2012;322:137–140. doi: 10.1016/j.jns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Chiarini A, Dal Prà I, Marconi M, Chakravarthy B, Whitfield J F, Armato U. Calcium-sensing receptor (CaSR) in human brain's pathophysiology roles in late-onset Alzheimer's disease (LOAD). Curr. Pharm. Biotechnol. 2009;10:317–326. doi: 10.2174/138920109787847501. [DOI] [PubMed] [Google Scholar]
- 4.Dal Prà I, Chiarini A, Pacchiana R, Chakravarthy B, Whitfield J F, Armato U. Emerging concepts of how ß-amyloid proteins and pro-inflammatory cytokines might collaborate to produce an 'Alzheimer brain'. Mol. Med. Rep. 2008;1(2):173–178. [PubMed] [Google Scholar]
- 5.Armato U, Chiarini A, Chakravarthy B, Chioffi F, Pacchiana R, Colarusso E, Whitfield J F, Dal Prà I. Calcium-sensing receptor antagonist (calcilytic) NPS 2143 specifically blocks the increased secretion of endogenous Aß42 prompted by exogenous fibrillary or soluble Aß25-35 in human cortical astrocytes and neurons-Therapeutic relevance to Alzheimer's disease. Biochim. Biophys. Acta. 2013;1832:1634–1652. doi: 10.1016/j.bbadis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Dal Prà I, Armato U, Chioffi F, Pacchiana R, Whitfield J F, Chakravarthy B, Gui L, Chiarini A. The Aß peptides-activated calcium-sensing receptor stimulates the production and secretion of vascular endothelial growth factor-A by normoxic adult human cortical astrocytes. Neuromolecular Med. 2014 doi: 10.1007/s12017-014-8315-9. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe D J. Alzheimer's disease. Cold Spring Harb. Perspect. Biol. 2011;3(7):a004457. doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Thal D R, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer's disease beginκ. Curr. Opin. Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 10.Attems J, Thal D R, Jellinger K A. The relationship between subcortical Tau pathology and Alzheimer's disease. Biochem. Soc. Trans. 2012;40:711–715. doi: 10.1042/BST20120034. [DOI] [PubMed] [Google Scholar]
- 11.Pluta R. Unresolved questions concerning etiology of Alzheimer's disease hypometabolism. Nutrition. 2011;27(1):1–2. doi: 10.1016/j.nut.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Masters C L, Selkoe D J. Biochemistry of amyloid ß-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ittner L M, Götz J. Amyloid-ß and Tau-a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 14.Klein W L. Synaptotoxic amyloid-ß oligomers A molecular basis for the cause, diagnosis, and treatment of Alzheimer’s diseaseκ. J. Alzheimer s Dis. 2013;33(Suppl 1 ):S49–65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- 15.Ward S M, Himmelstein D S, Lancia J K, Binder L I. Tau oligomers and Tau toxicity in neurodegenerative disease. Biochem. Soc. Trans. 2012;40:667–671. doi: 10.1042/BST20120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bero A W, Yan P, Roh J H, Cirrito J R, Stewart F R, Raichle M E, Lee J M, Holtzman D M. Neuronal activity regulates the regional vulnerability to amyloid-ß deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araque A, Navarrete M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 19.Halassa M M, Haydon P G. Integrated brain circuits astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagele R G, Wegiel J, Venkataraman V, Imaki H, Wang K C, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol. Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Dal Prà I, Whitfileld J F, Pacchiana R, Bonafini C, Talacchi A, Chakravarthy B, Armato U, Chiarini A. The amyloid-ß(42) proxy, amyloid-ß(25-35): induces normal human cerebral astrocytes to produce amyloid-ß(42). J. Alzheimers Dis. 2011;24(2):335–347. doi: 10.3233/JAD-2011-101626. [DOI] [PubMed] [Google Scholar]
- 22.Verkhratsky A, Butt A, editors. JohnWiley &Sons Chichester: 2007. Glial Neurobiology A textbook. [Google Scholar]
- 23.Kettenmann H, Ransom B R, editors. 3rd Edition. New York: Oxford University Press; 2013. Neuroglia. [Google Scholar]
- 24.Tsai H H, Li H, Fuentealba L C, Molofsky A V, Taveira-Marques R, Zhuang H, Tenney A, Murnen A T, Fancy S P, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson W D, Rowitch D H. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushong E A, Martone M E, Jones Y Z, Ellisman M H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113(1):221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 27.Chvátal A, Anderová M, Kirchhoff F. Three-dimensional confocal morphometry-A new approach for studying dynamic changes in cell morphology in brain slices. J. Anat. 2007;210(6):671–683. doi: 10.1111/j.1469-7580.2007.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halassa M M, Fellin T, Takano H, Dong J H, Haydon P G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007;27(24):6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens B. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals. 2008;16(4):278–288. doi: 10.1159/000123038. [DOI] [PubMed] [Google Scholar]
- 30.Molofsky A V, Krencik R, Ullian E M, Tsai H H, Deneen B, Richardson W D, Barres B A, Rowitch D H. Astrocytes and disease a neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molofsky A V, Kelley K W, Tsai H H, Redmond S A, Chang S M, Madireddy L, Chan J R, Baranzini S E, Ullian E M, Rowitch D H. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509(7499):189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antanitus D S. A theory of cortical neuron-astrocyte interaction. Neuroscientist. 1998;4:154–159. [Google Scholar]
- 33.Lovick T A, Brown L A, Key B J. Neuronal activity-related coupling in cortical arterioles involvement of astrocyte-derived factors. Exp. Physiol. 2005;90(1):131–140. doi: 10.1113/expphysiol.2004.028811. [DOI] [PubMed] [Google Scholar]
- 34.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar M W, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt A E, Kang Y J, Tu S, Molokanova E, McKercher S R, Hires S A, Sason H, Stouffer D G, Buczynski M W, Solomon J P, Michael S, Powers E T, Kelly J W, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland E A, Zhang D, Nakanishi N, Chen H S, Wolosker H, Wang Y, Parsons L H, Ambasudhan R, Masliah E, Heinemann S F, Piña-Crespo J C, Lipton S A. Aß induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. U S A. 2013;110(27):E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Re D B, Le Verche V, Yu C, Amoroso M W, Politi K A, Phani S, Ikiz B, Hoffmann L, Koolen M, Nagata T, Papadimitriou D, Nagy P, Mitsumoto H, Kariya S, Wichterle H, Henderson CE, Przedborski S. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–1008. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirooznia S K, Dawson V L, Dawson T M. Motor neuron death in ALS: programmed by astrocytesκ. Neuron. 2014;81:961–963. doi: 10.1016/j.neuron.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raeber A J, Race R E, Brandner S, Priola S A, Sailer A, Bessen R A, Mucke L, Manson J, Aguzzi A, Oldstone M B, Weissmann C, Chesebro B. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 1997;16(20):6057–6065. doi: 10.1093/emboj/16.20.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiarini A, Whitfield J F, Bonafini C, Chakravarthy B, Armato U, Dal Prà I. Amyloid-ß(25-35): an amyloid-ß(1-42) surrogate, and proinflammatory cytokines stimulate VEGF-A secretion by cultured, early passage, normoxic adult human cerebral astrocytes. J. Alzheimers Dis. 2010;21(3):915–926. doi: 10.3233/JAD-2010-100471. [DOI] [PubMed] [Google Scholar]
- 39.Meyer E P, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jantaratnotal N, Ryu J K, Schwab C, McGeer P L, McLarnon JG. Comparison of vascular perturbations in an Aß-injected animal model and in AD brain. Int. J. Alzheimers Dis. 2011;2011:918280. doi: 10.4061/2011/918280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dal Prà I, Chiarini A, Nemeth EF, Armato U, Whitfield J F. Roles of Ca2+ and the Ca2+-sensing receptor (CaSR) in the expression of inducible NOS (nitric oxide synthase)-2 and its BH4 (tetrahydrobiopterin)-dependent activation in cytokine-stimulated adult human astrocytes. J. Cell. Biochem. 2005;96(2):428–438. doi: 10.1002/jcb.20511. [DOI] [PubMed] [Google Scholar]
- 42.Bräuner-Osborne H, Wellendorph P, Jensen A A. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr. Drug Targets. 2007;8(1):169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 43.Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Zhou Y, Yang W, Butters R, Lee H W, Li S, Castiblanco A, Brown E M, ang J J. Identification and dissection of Ca(2+)-binding sites in the extracellular domain of Ca(2+)-sensing receptor. J. Biol. Chem. 2007;282(26):19000–19010. doi: 10.1074/jbc.M701096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrett J E, Capuano I V, Hammerland LG, Hung B C, Brown E M, Hebert S C, Nemeth E F, Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J. Biol. Chem. 1995;270(21):12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 46.Hu J, Spiegel A M. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J. Cell. Mol. Med. 2007;11(5):908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, Reyes-Cruz G, Chen W, Jacobson K A, Spiegel A M. Identification of acidic residues in the extracellular loops of the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ and a positive allosteric modulator. J. Biol. Chem. 2002;277(48):46622–46631. doi: 10.1074/jbc.M207100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conigrave A D, Ward D T. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27(3):315–331. doi: 10.1016/j.beem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Brown E M, Fuleihan G El-H, Chen C J, Kifor O. A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3',5'-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology. 1990;127(3):1064–1071. doi: 10.1210/endo-127-3-1064. [DOI] [PubMed] [Google Scholar]
- 50.Brown E M, Katz C, Butters R, Kifor O. Polyarginine, polylysine, and protamine mimic the effects of high extracellular calcium concentrations on dispersed bovine parathyroid cells. J. Bone Miner. Res. 1991;6(11):1217–1225. doi: 10.1002/jbmr.5650061112. [DOI] [PubMed] [Google Scholar]
- 51.Chakravarti B, Chattopadhyay N, Brown E M. Signaling through the extracellular calcium-sensing receptor (CaSR). Adv. Exp. Med. Biol. 2012;740:103–142. doi: 10.1007/978-94-007-2888-2_5. [DOI] [PubMed] [Google Scholar]
- 52.Hebert S C, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium. 2004;35(3):239–247. doi: 10.1016/j.ceca.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Brennan S C, Davies T S, Schepelmann M, Riccardi D. Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: control of taste modulation and intestinal hormone secretion. Br. J. Nutr. 2014;Jan 2:1–7. doi: 10.1017/S0007114513002250. [DOI] [PubMed] [Google Scholar]
- 54.Ye C, Ho-Pao C L, Kanazirska M, Quinn S, Rogers K, Seidman C E, eidman J G, Brown E M, Vassilev P M. Amyloid-beta proteins activate Ca(2+)-permeable channels through calcium-sensing receptors. J. Neurosci. Res. 1997;47:547–554. doi: 10.1002/(sici)1097-4547(19970301)47:5<547::aid-jnr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 55.Conley Y P, Mukherjee A, Kammerer C, DeKosky S T, Kamboh M I, Finegold D N, Ferrell R E. Evidence supporting a role for the calcium-sensing receptor in Alzheimer disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2009;150B(5):703–709. doi: 10.1002/ajmg.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinn S J, Bai M, Brown E M. pH Sensing by the calcium-sensing receptor. J. Biol. Chem. 2004;279(36):37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 57.Quinn S J, Kifor O, Trivedi S, Diaz R, Vassilev P, Brown E. Sodium and ionic strength sensing by the calcium receptor. J. Biol. Chem. 1998;273(31):19579–19586. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- 58.Canaff L, Hendy G N. Human calcium-sensing receptor gene.Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1 5-dihydroxyvitamin D. J. Biol. Chem. 2002; 277(33):30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 59.Canaff L, Hendy G N. Calcium-sensing receptor gene transcription is up-regulated by the proinflammatory cytokine, interleukin-1beta.Role of the NF-kappaB pathway and kappaB elements. J. Biol. Chem. 2005;280(14):14177–14188. doi: 10.1074/jbc.M408587200. [DOI] [PubMed] [Google Scholar]
- 60.Canaff L, Zhou X, Hendy G N. The proinflammatory cytokine, interleukin-6 up-regulates calcium-sensing receptor gene transcription via Stat1/3 and Sp1/3. J. Biol. Chem. 2008;283(20):13586–13600. doi: 10.1074/jbc.M708087200. [DOI] [PubMed] [Google Scholar]
- 61.Breitwieser G E. The calcium sensing receptor life cycle: trafficking, cell surface expression, and degradation. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27(3):303–313. doi: 10.1016/j.beem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Chang W, Pratt S, Chen T H, Nemeth E, Huang Z, Shoback D. Coupling of calcium receptors to inositol phosphate and cyclic AMP generation in mammalian cells and Xenopus laevis oocytes and immunodetection of receptor protein by region-specific antipeptide antisera. J. Bone Miner. Res. 1998;13(4):570–580. doi: 10.1359/jbmr.1998.13.4.570. [DOI] [PubMed] [Google Scholar]
- 63.Avlani V A, Ma W, Mun H C, Leach K, Delbridge L, Christopoulos A, Conigrave A D. Calcium-sensing receptor-dependent activation of CREB phosphorylation in HEK293 cells and human parathyroid cells. Am. J. Physiol. Endocrinol. Metab. 2013;304(10):E1097–E1104. doi: 10.1152/ajpendo.00054.2013. [DOI] [PubMed] [Google Scholar]
- 64.Lazarus S, Pretorius CJ, Khafagi F, Campion KL, Brennan SC, Conigrave AD, Brown EM, Ward DT. A novel mutation of the primary protein kinase C phosphorylation site in the calcium-sensing receptor causes autosomal dominant hypocalcemia. Eur. J. Endocrinol. 2011;164(3):429–35. doi: 10.1530/EJE-10-0907. [DOI] [PubMed] [Google Scholar]
- 65.Chiarini A, Dal Prà I, Gottardo R, Bortolotti F, Whitfield J F, Armato U. BH(4) (tetrahydrobiopterin)-dependent activation, but not the expression, of inducible NOS (nitric oxide synthase)-2 in proinflammatory cytokine-stimulated, cultured normal human astrocytes is mediated by MEK-ERK kinases. J. Cell. Biochem. 2005;94(4):731–743. doi: 10.1002/jcb.20334. [DOI] [PubMed] [Google Scholar]
- 66.Nemeth E F. Calcimimetic and calcilytic drugs: just for parathyroid cellsκ. Cell Calcium. 2004;35(3):283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 67.Nemeth E F. Allosteric modulators of the extracellular calcium receptor. Drug Discov. Today Technol. 2013;10(2):e277–e284. doi: 10.1016/j.ddtec.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Miedlich S U, Gama L, Seuwen K, Wolf R M, Breitwieser G E. Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J. Biol. Chem. 2004;279(8):7254–7263. doi: 10.1074/jbc.M307191200. [DOI] [PubMed] [Google Scholar]
- 69.Petrel C, Kessler A, Dauban P, Dodd R H, Rognan D, Ruat M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J. Biol. Chem. 2004;279(18):18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- 70.Steddon S J, Cunningham J. Calcimimetics and calcilytics-fooling the calcium receptor. Lancet. 2005;365(9478):2237–2239. doi: 10.1016/S0140-6736(05)66782-7. [DOI] [PubMed] [Google Scholar]
- 71.Letz S, Rus R, Haag C, Dörr H G, Schnabel D, Möhlig M, Schulze E, Frank-Raue K, Raue F, Mayr B, Schöfl C. Novel activating mutations of the calcium-sensing receptor: the calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors. J. Clin. Endocrinol. Metab. 2010;95(10):E229–E233. doi: 10.1210/jc.2010-0651. [DOI] [PubMed] [Google Scholar]
- 72.White E, McKenna J, Cavanaugh A, Breitwieser G E. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol. Endocrinol. 2009;23(7):1115–1123. doi: 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park S Y, Mun H C, Eom Y S, Baek H L, Jung T S, Kim C H, Hong S, Lee S. Identification and characterization of D410E, a novel mutation in the loop 3 domain of CASR, in autosomal dominant hypocalcemia and a therapeutic approach using a novel calcilytic, AXT914. Clin. Endocrinol. (Oxf): 2013;78(5):687–693. doi: 10.1111/cen.12056. [DOI] [PubMed] [Google Scholar]
- 74.Davey A E, Leach K, Valant C, Conigrave A D, Sexton P M, Christopoulos A. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153(3):1232–1241. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 75.Leach K, Sexton P M, Christopoulos A, Conigrave A D. Engendering biased signalling from the calcium-sensing receptor for the pharmacotherapy of diverse disorders. Br. J. Pharmacol. 2014;171(5):1142–1155. doi: 10.1111/bph.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sherwood C C, Stimpson C D, Raghanti M A, Wildman D E, Uddin M, Grossman L I, Goodman M, Redmond J C, Bonar C J, Erwin J M, Hof P R. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. USA. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberheim N A, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29(10):547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Oberheim N A, Takano T, Han X, He W, Lin J H, Wang F, Xu Q, Wyatt J D, Pilcher W, Ojemann J G, Ransom B R, Goldman S A, Nedergaard M. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29(10):3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oberheim N A, Goldman S A, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verkhratsky A, Rodríguez J J, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013;8(2):149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heneka M T, Rodríguez J J, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010;63(1-2):189–211. doi: 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Ruat M, Traiffort E. Roles of the calcium sensing receptor in the central nervous system. Best Pract. Res.Clin. Endocrinol. Metab. 2013;27(3):429–442. doi: 10.1016/j.beem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Verkhratsky A, Olabarria M, Noristani H N, Yeh C Y, Rodriguez J J. Astrocytes in Alzheimer's disease. Neurotherapeutics. 2010;7(4):399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva A J, Takano T, Goldman S A, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894(1):21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 86.Bacskai BJ, Kajdasz S T, McLellan M E, Games D, Seubert P, Schenk D, Hyman B T. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J. Neurosci. 2002;22(18):7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales K R, Paul S M. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 88.Pihlaja R, Koistinaho J, Malm T, Sikkilä H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia. 2008;56(2):154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 89.Wyss-Coray T, Loike JD, Brionne T C, Lu E, Anankov R, Yan F, Silverstein S C, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9(4):453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 90.Heneka M T, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflam. 2005;2(1):22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo J R. Alzheimer's disease beta-secretase BACE1 is not a neurospecific enzyme. J. Neurochem. 2005;92(2):226–234. doi: 10.1111/j.1471-4159.2004.02857.x. [DOI] [PubMed] [Google Scholar]
- 92.Perez J L, Carrero I, Gonzalo P, Arevalo-Serrano J, Sanz-Anquela J M, Ortega J, Rodriguez M, Gonzalo-Ruiz A. Soluble oligomeric forms of beta-amyloid (Abeta) peptide stimulate Abeta production via astrogliosis in the rat brain. Exp. Neurol. 2010;223(2):410–421. doi: 10.1016/j.expneurol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 93.Overmyer M, Helisalmi S, Soininen H, Laakso M, Riekkinen P Sr, Alafuzoff I. Astrogliosis and the ApoE genotype.an immunohistochemical study of postmortem human brain tissue. Dement Geriatr Cogn Disord. 1999;10(4):252–257. doi: 10.1159/000017128. [DOI] [PubMed] [Google Scholar]
- 94.Nordberg A. Astroglia and microglia imaging markers in the progression of Alzheimer’s disease.13th Intl. Geneva/Springfield Symposium on Advances in Alzheimer Therapy. 2014;Abstract Book:65. [Google Scholar]
- 95.Kuchibhotla K V, Lattarulo C R, Hyman B T, Bacskai B J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323(5918):1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abramov A Y, Canevari L, Duchen M R. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J. Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bezprozvanny I, Mattson M P. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattson M P, Chan S L. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003;34(4-5):385–397. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 99.Berridge M J. Calcium regulation of neural rhythms, memory and Alzheimer's disease. J. Physiol. 2014;592(Pt 2):281–293. doi: 10.1113/jphysiol.2013.257527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yano S, Brown E M, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35(3):257–264. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Chattopadhyay N, Espinosa-Jeffrey A, Tfelt-Hansen J, Yano S, Bandyopadhyay S, Brown E M, de Vellis J. Calcium receptor expression and function in oligodendrocyte commitment and lineage progression: potential impact on reduced myelin basic protein in CaR-null mice. J. Neurosci. Res. 2008;86(10):2159–2167. doi: 10.1002/jnr.21662. [DOI] [PubMed] [Google Scholar]
- 102.Chattopadhyay N, Evliyaoglu C, Heese O, Carroll R, Sanders J, Black P, Brown E M. Regulation of secretion of PTHrP by Ca(2+)-sensing receptor in human astrocytes, astrocytomas, and meningiomas. Am. J. Physiol. Cell Physiol. 2000;279(3):C691–C699. doi: 10.1152/ajpcell.2000.279.3.C691. [DOI] [PubMed] [Google Scholar]
- 103.Bandyopadhyay S, Tfelt-Hansen J, Chattopadhyay N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J. Neurosci. Res. 2010;88(10):2073–2082. doi: 10.1002/jnr.22391. [DOI] [PubMed] [Google Scholar]
- 104.Ward B K, Magno A L, Walsh J P, Ratajczak T. The role of the calcium-sensing receptor in human disease. Clin. Biochem. 2012;45(12):943–953. doi: 10.1016/j.clinbiochem.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 105.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius K-J, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson L G, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 106.Kaminsky Y G, Marlatt M W, Smith M A, Kosenko E A. Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: evidence for Abeta(25-35). Exp. Neurol. 2010;221(1):26–37. doi: 10.1016/j.expneurol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Breitwieser G E. The intimate link between calcium sensing receptor trafficking and signaling: Implications for disorders of calcium homeostasis. Mol. Endocrinol. 2012;26:1482–1495. doi: 10.1210/me.2011-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiarini A, Dal Prà I, Menapace L, Pacchiana R, Whitfield J F, Armato U. Soluble amyloid beta-peptide and myelin basic protein strongly stimulate, alone and in synergism with combined proinflammatory cytokines, the expression of functional nitric oxide synthase-2 in normal adult human astrocytes. Int. J. Mol. Med. 2005;16(5):801–807. [PubMed] [Google Scholar]