Abstract
Diabetic retinopathy (DR) is one of the major complications of diabetes causing vision loss and blindness worldwide. DR is widely recognized as a neurodegenerative disease as evidenced from early changes at cellular and molecular levels in the neuronal component of the diabetic retina, which is further supported by various retinal functional tests indicating functional deficits in the retina soon after diabetes progression. Diabetes alters the level of a number of neurodegenerative metabolites, which increases influx through several metabolic pathways which in turn induce an increase in oxidative stress and a decrease in neurotrophic factors, thereby damage retinal neurons. Loss of neurons may implicate in vascular pathology, a clinical signs of DR observed at later stages of the disease. Here, we discuss diabetes-induced potential metabolites known to be detrimental to neuronal damage and their mechanism of action. In addition, we highlight important neurotrophic factors, whose level have been found to be dysregulated in diabetic retina and may damage neurons. Furthermore, we discuss potential drugs and strategies based on targeting diabetes-induced metabolites, metabolic pathways, oxidative stress, and neurotrophins to protect retinal neurons, which may ameliorate vision loss and vascular damage in DR.
Keywords: Metabolites, neurodegeneration, neurotrophic factor, neurons, retina.
INTRODUCTION
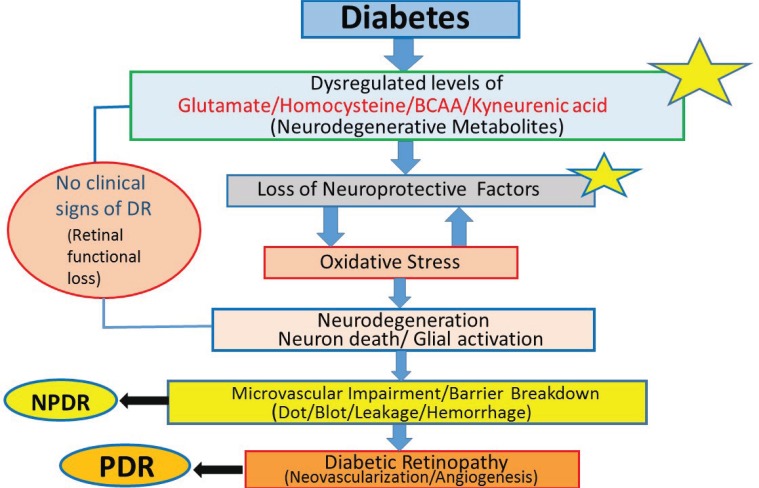
Diabetic retinopathy (DR) is the leading cause of vision loss and blindness in the working-age population worldwide. DR is being recognized as a neurodegenerative disease of the retina as opposed to previously considered solely as a microvascular disease. Numerous studies in diabetic patients showed functional deficits in the neural retinas [1-3]. In addition, a large body of cellular and molecular studies suggest changes in the neural retina before any vascular changes shortly after diabetes [2, 3]. Moreover, various studies reported damage of neurons due to apoptosis in the diabetic retina [3-6]. Glial cells, a vital component of neural retina are found to be activated in diabetes which is another feature of retinal neurodegeneration [5]. Thus, neural retina comprising of both glial and neuronal cells are compromised in diabetes thereby disturbing the homeostasis and interaction between these cells. Diabetes being a metabolic disease, alters levels of a number of metabolites both systemically and locally in the retina of diabetic patients and rodents. Dysregulated metabolites increases flux through a number of metabolic pathways which in turn increases oxidative stress and decreases neurotrophic support as shown in the flow diagram (Fig. 1). These altered factors, may damage neurons early in diabetic retina leading to progression of DR. However, the exact link between the levels of those potential metabolite(s) or factor(s) and their mechanism of neuronal damage at early stages in the disease progression has not been fully understood. In this review article, we discuss mechanisms of neurodegeneration especially due to altered levels of metabolites and neurotrophic factors in the diabetic retina and also highlight a number of potential neuro-protective strategies, drugs and treatments.
Fig. (1).
Depicts the stages and potential factors influencing the progression of diabetic retinopathy. *indicates the key site(s) and factor(s) early in diabetes progression implicating in diabetic retinopathy. Strategies to ameliorate their levels may arrest or prevent the progression of diabetic retinopathy. DR (diabetic retinopathy), NPDR (non-proliferative diabetic retinopathy), PDR (proliferative diabetic retinopathy) and BCAA (branched chain amino acid).
MECHANISM OF NEURODEGENERATION IN THE DIABETIC RETINA: IMPLICATION OF ALTERED METABOLITES IN DIABETES
Hyperglycemia
Among metabolites, hyperglycemia is known to be the major factor which activates several metabolic pathways including increases in flux through polyol, hexosamine, protein kinase C (PKC) pathways and advanced glycation end products (AGEs) which have been nicely summarized in few recent review articles [7, 8]. These activated pathways mediate an increase in oxidative stress by decreasing the level of antioxidant glutathione, leading to tissue damage. These pathways also activate nuclear factor kappa B, a transcription factor which in turn activates a number of genes of inflammatory molecules, cytokines, chemokines and decreases expression and signaling through various growth factors leading to a feedback loop in increasing oxidative stress and severe damage to neurons in the retinal tissue [9, 10]. Diabetes also induce a number of other metabolites and factors including various excitatory amino acids, lower vitamins, nutrients, hormones, and neurotrophic factors which affect several pathways and factors implicated in cellular damage and more specifically neuronal damage in the diabetic retina.
GLUTAMATE
An increased level of glutamate has been reported in the diabetic retina and also in the vitreous of diabetic patients, suggesting a neurotoxic role of glutamate which may damage retinal neurons and especially retinal ganglion cells by excitotoxicity [11, 12]. Increased extracellular level of glutamate in the neuronal tissue activates N-methyl D-Aspartate (NMDA) receptors, depolarizing the neuronal cells which increases the influx of calcium and sodium ions into the cell and in turn generates free radicals and induce apoptosis [14]. Exact reasons for the increase in glutamate level in diabetic retina is not known. However, we found a high level of branched chain amino acids (BCAA) level in the serum and retina of diabetic rat, which may also be responsible for extracellular glutamate levels in the retina [15].
In addition, recently Jiang et al [16] found an increased level of D-serine in the aqueous and vitreous humour of PDR patients. Earlier, the same group reported high level of serine racemase, D-serine and glutamate in the diabetic eye [17]. Since, D-serine acts as an agonist of NDMA receptor, causing excitotoxicity to neurons [18, 19]. It is likely that increased levels of both D-serine and glutamate in diabetic retina might equally implicate in neurodegeneration in diabetic retina. Thus, by lowering extracellular level of glutamate, D-serine and/or inactivating NMDA receptor, excitotoxicty of glutamate/D-serine can be ameliorated [13].
HOMOCYSTEINE AND VITAMINS
Another potential neurodegenerative metabolite is homocysteine whose elevated level has been associated with various neurodegenerative diseases including diabetic retinopathy [20, 21]. Homocysteine is a sulphur containing amino acid formed by demethylation of methionine and the level is reduced by the enzyme methione synthetase in presence of vitamin B12 and folate as cofactors [22, 23]. Earlier, we have reported a reduced expression of the folate transporter and a decreased folate level in the diabetic retina [24]. Thus lower level of folate in diabetic retina may cause an increase in the homocysteine levels. The elevated homocysteine levels has been found to induce apoptosis in retinal ganglion cells (RGC) [25, 26]. Homocysteine has also been shown to activate NMDA receptors, thereby may cause excitotoxicty of RGCs in diabetic retina [27, 28].
KYNURENIC ACID
Kynurenic acid is the product of tryptophan metabolism which is suggested to play an important role in neuro-degeneration. A correlation between decreased levels of kynurenic acid and glutamate excitotoxicty and free radical generation has been found. Kynurenic acid has been found to influence the excitotoxcity of neuronal cells by homocysteine [29]. In kynurenine pathway, 3-hydroxykynurenine and quinolinic acid have neurotoxic effect; however, kynurenic acid is a neuroprotectant [30]. Therefore, kynurenic acid might be a potential neuroprotective agent in diabetic retina.
RENIN ANGIOTENSIN SYSTEM
A large body evidence suggest activated metabolites of renin–angiotensin system (RAS) in diabetic retina plays a significant role in retinal neurodegeneration. Angiotensin II, a component of RAS activates angiotensin type 1 receptor (AT1R) and produces reactive oxygen species, which damages retinal cells and particularly retinal ganglion cell in the diabetic retina [31-33]. Kurihara et al. [32] showed that the increased level of AT1R in diabetic retina resulted in impaired neuronal function and the AT1R blocker telmisartan suppressed the impaired inner retinal function. Recently, we also found a beneficial effect of AT1R blocker, telmisartan towards neuroprotection in the retina of diabetic rats [34]. Thus role of RAS and its therapeutic target may have important role towards neuroprotection in diabetic retinopathy.
OXIDATIVE STRESS
Hyperglycemia in diabetic state activates a number of metabolic pathways including polyol, hexosamine, PKC and AGEs. Increase in flux through these pathways have been shown to enhance the production of reactive oxygen and nitrogen species (ROS/RNS) [7, 35, 36]. Diabetes induced increase in the level of excitatory amino acids in the retina also increase the production of ROS/RNS. Thus, ROS/RNS becomes a central player in damaging cells which in turn increases production of more ROS/RNS activating several metabolic and apoptotic pathways associated with neurodegeneration [37, 38]. Antioxidant defense systems via enzymatic and nonenzymatic pathways counterbalance ROS damage. Important enzymatic antioxidants includes superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase while nonezymatic antioxidants include vitamins A, C and E and glutathione (GSH). However, in diabetic retina, these antioxidant systems are not effective in balancing the levels of oxidants which makes neuronal cells vulnerable to be damaged.
DYSREGULATION OF NEUROTROPHINS IN DIABETIC RETINA
Dysregulation of neurotrophic factors is considered as the major hallmark of neurodegeneration in diabetic retina. Neurotrophins are important for neuronal survival, growth and functional maintenance [39-43]. It is reported that imbalance of these factors cause damage to retinal neurons both in case of proliferative diabetic retinopathy and oxygen-induced retinopathy [44, 45]. Neuronal retina produces a substantial amount of neurotrophic factors. Among these, brain derived neurotrophic factor (BDNF) is produced by retinal neurons and glia which affects cell differentiation, growth and neurotransmission [46-48]. We and others have reported a reduced level of BDNF in the serum and retina of diabetic rodents [48]. Absence or reduction in the level of BDNF and its receptor cause serious alteration in retinal function. Another important neurotrophic factor is nerve growth factor (NGF) whose level is found to be increased in DR patients [49]. NGF levels positively correlated with the stages of DR and other diabetic parameters [39].
Pigment epithelial derived factor (PEDF) plays a significant role in retinal homeostasis since it has both antiangiogenic and neuroprotective properties. PEDF blocks the production of ROS and also prevents glutamate excitotoxicity [50, 51]. Therefore, reduced level of PEDF seems crucial for neurodegeneration in diabetic retina.
Insulin is an important neurotrophic factor for retinal neurons. An increase in neuronal apoptosis and cell death has been observed in insulin deficient diabetic retina [52-54]. It is observed that diabetes impairs the retinal insulin receptor signaling pathway that may initiate the progression of DR [55]. Thus retinal neurons survival depend on insulin and insulin receptor signaling [56].
Erythropoietin (Epo) is another potent neuroprotective factor synthesized in the retina [57, 58]. In addition to neuroprotection, Epo helps in the mobilization of endothelial progenitor cells (EPCs) toward injured retinal sites, thus involves in the neurovascular repair [59, 60]. Therefore, a better understanding of the molecular mechanism and function of neurotrophins in the retina is necessary which may contribute as therapeutic agents in neuroprotection.
NEUROPROTECTION STRATEGIES AND POTENTIAL DRUG TARGETS
One of the primary steps toward prevention or amelioration of neurodegeneration in diabetic retina is targeting dysregulated metabolites and blood pressure control, the root cause of neurodegeneration early in diabetes. The most effective strategy to ameliorate metabolic alterations such as hyperglycemia, hyperlipidemia, increased level of excitatory amino acids, metabolites of RAS which exacerbate diabetic complications including DR is through lifestyle modifications. Numerous reports suggest that modifications in diet and exercise prevent or slow the progression of the disease, thereby ameliorate neuronal damage in DR. In addition, a number of neurorpotective treatment strategies have attracted significant interest towards discovering drugs/agents that could protect retinal neurons, particularly retinal ganglion cells and possibly prevent or protect vision loss.
N-methyl D-aspartate (NMDA) receptor antagonist, MK-801, has been found to be effective in protecting neurons after intraocular injection in diabetic rats [61]. Glutamate receptor antagonist memantine treatment exhibited neuro-protection in diabetic rodents [62]. Pentazocine, a specific sigma receptor-1 ligand protected neurons in diabetic rat retina, suggesting its potential role in neuroprotection [63]. We found that gabapentin (Neurontin) a specific inhibitor of the neuronal, cytosolic isoform of branched chain amino-transferase (BCATc) inhibited the synthesis of glutamate, decreased caspase-3 activity and lowered ROS level in the diabetic retina, suggesting a neuroprotective role of the drug [64]. Another strategy to decrease the excitotoxic level of glutamate might be by increasing the ratio of BCKA/BCAA which may decreases glutamate synthesis and increases the rate of glutamate oxidation in the Muller cell, thereby may protect retinal neurons [13].
Neuroprotective factors such as BDNF, NGF, PEDF, VEGF, Insulin and Epo have been shown to be effective in protecting neurons in experimental diabetic retinopathy. BDNF reduced the damage to ganglion cells under oxidative stress conditions [65, 66]. In addition, BDNF promotes the survival of neurons and plays a key role in the synaptic connections and neurotransmission [67-69]. BDNF also provides a neuroprotective effect by detoxifying the excitotoxic level of glutamate by increasing uptake and the expression of glutamine synthetase in Muller cells under stress conditions [70]. Intraocular injection of BDNF in combination with ciliary neurotrophic factor is found to protect retinal neurons [71].
Intraocular gene transfer of PEDF increased the survival of retinal neurons under ischemic damage [72]. In addition, intravitreal injections of PEDF prevented neuronal loss and vascular damage early in DR [73].
Angiogenic molecule VEGF is also a potential neurotrophic factor in the retina. Endogenous VEGF plays important role in the survival and maintenance of retinal neurons. Inhibition of VEGF in the normal adult retina induced a significant loss of ganglion cells [74]. Li group has demonstrated that VEGF treatment rescued neurons in the retina of mouse models of neurotoxicity [75].
Basic Fibroblast Growth Factor (bFGF) is a neurotrophic factor which plays important role in the survival, maturation and regeneration of both glial cells and neuronal cells [76, 77].
Insulin rescues retinal neurons from cell death in the diabetic rat retina. Intraocular injection of insulin restores insulin receptor activity and Akt signaling prosurvival pathway in diabetic rat retinas [53, 55, 78]. Therefore, insulin delivery locally in the retina may protect neurons in the diabetic retina.
Administration of Epo-peptide either by intravitreal [79] or intraperitoneal injection [80] protected degeneration of retinal neurons in diabetic rats [81]. Epo may help both in protecting neurons as well as repair of vessels, thus making a therapeutic agent to protect neurovascular damage in DR.
ANTIOXIDANTS
Evidence from numerous pharmacological studies suggest that lowering oxidative stress in diabetic retina is an effective way to combat neurodegeneration [31, 82, 83].
Administration of antioxidants showed inhibition of the activation of transcription factor NF-kB, which regulates a number of inflammatory genes. Feeding rats with diet supplemented with antioxidants, including alpha-tocopherol, N-acetyl cysteine, ascorbic acid, and beta-carotene, inhibited the increase in caspase-3 activity and apoptosis of neurons in the diabetic retina. In addition, supplementation of vitamin C and vitamin E increased the activities of enzymes such as glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase. Benfotiamine (vitamin B1), a lipid-soluble thiamine derivative, blocked major hyperglycemia-induced pathways and prevented experimental diabetic retinopathy [84]. A combination of oral benfotiamine and alpha-lipoic acid reduced AGEs and ROS formation in animal studies [85]. The administration of antioxidants in a study of type 2 diabetic patients with non-PDR maintained the antioxidant plasma status levels as measured by oxidative malonyldialdehyde and total antioxidant status [86]. However, the antioxidant therapy could not improve visual acuity. The use of PEDF as a therapeutic option to block pathways that lead to the production of ROS are being extensively studied and remain to be validated for human use [87].
Polyphenolic compounds are known for their strong antioxidant activities. Recently, Sasaki et al 2011 showed the beneficial effect of a polyphenolic compound leutin, towards amelioration of oxidative stress and neurodegeneration in diabetic retina [82]. Previously, a study reported that supplementation of leutin to diabetic rats prevented the impairment of electroretinogram [88]. More recently, we have also found leutin supplementation ameliorated oxidative stress and neurodegeneration in the retina of diabetic rats (unpublished data). (−)-Epigallocatechingallate from green tea has been demonstrated to have neuroprotective properties in the retina [83]. Curcumin, a major component of turmeric is known for its antioxidant activity, has a promising role in preventing a decrease in antioxidant level in diabetic retina [89, 90].
Lipid peroxidation was found to be significantly higher in diabetic retinopathy patients [91]. Clinical studies suggest that lipid-lowering agent fenofibrate reduced the progression of neurodegeneration in patients with DR possibly by reducing apoptosis, oxidative stress and inflammation [92]. Thus, fenofibrate may be a useful neuroprotective agent in diabetic retina. Therefore, antioxidant therapy may be useful as an adjunct treatment in combination with other treatments for the prevention of retinal neurodegeneration.
CONCLUSIONS
Continuous efforts toward better understanding of the mechanism(s) of neurodegeneration especially due to dysregulation of metabolites and neurotrophic factors are required in diabetic retina. Amelioration of dysregulated metabolites and neurotrophic factors may arrest or prevent neurodegneration in diabetic retinopathy. In addition, investigation of the root cause of neurodegeneration early in diabetes would implicate into better treatment or prevention strategy for neurodegeneration. Diabetic patients who develop neurodegeneration early in the disease progression require early treatment utilizing drugs which may protect neurons. Drug delivery into the eye and specifically into the retina is a challenge, however, different mode of efficient drug delivery system are being developed. Topical administration of brimonidine, NGF, PEDF and insulin seems to be effective in experimental animals. Neuroprotective drugs in combination with other treatments might be better option for retinal neuroprotection. Still clinical trials are required for the drugs to protect retinal neurons and also to test the safety and effectiveness of those drugs in diabetes.
ACKNOWLEDGEMENTS
Authors would like to thank funding support from King Abdul Aziz City for Science and Technology (KACST), grant number ARP: 30-23.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Curr. Diab.Rep. 2010;10:380–4. doi: 10.1007/s11892-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 2.Bearse MA , Jr, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest. Ophthalmol. Vis. Sci. 2004;45:296–304. doi: 10.1167/iovs.03-0424. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr. Pharm. Des. 2007;13:2699–712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 4.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy.Penn State Retina Research Group. Diabetes. 1998;47:815–20. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 5.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes.Early onset and effect of insulin. J. Clin. Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–9. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- 7.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Compl. 2012;26(1):56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013;343560 doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010;53: 2656–66. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]
- 11.Diederen RM, La Heij EC, Deutz NE, Kijlstra A, Kessels AG, van Eijk HM, Liem AT, Dieudonné S, Hendrikse F. Increased glutamate levels in the vitreous of patients with retinal detachment. Exp. Eye Res. 2006;83:45–50. doi: 10.1016/j.exer.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Yu XH, Zhang H, Wang YH, Liu LJ, Teng Y, Liu P. Time-dependent reduction of glutamine synthetase in retina of diabetic rats. Exp. Eye Res. 2009;89:967–71. doi: 10.1016/j.exer.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Ola MS, Hosoya K, LaNoue KF. Regulation of glutamate metabolism by hydrocortisone and branched chain keto acids in cultured rat retinal Müller cells (TR-MUL). Neurochem. Int. 2011;59:656–63. doi: 10.1016/j.neuint.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;15:749. doi: 10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gowda K, Zinnanti WJ, LaNoue KF. The influence of diabetes on glutamate metabolism in retinas. J. Neurochem. 2011;117:309–20. doi: 10.1111/j.1471-4159.2011.07206.x. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Du J, He T, Qu J, Song Z, Wu S. Increased D-serine in the aqueous and vitreous humour in patients with proliferative diabetic retinopathy. Clin. Exper. Ophthalmol. 2014 doi: 10.1111/ceo.12329. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Fang J, Wu B, Yin G, Sun L, Qu J, Barger SW, Wu S. Overexpression of serine racemase in retina and overproduction of D-serine in eyes of streptozotocin-induced diabetic retinopathy. J. Neuroinflamm. 2011;8:119. doi: 10.1186/1742-2094-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. 2003;100(11):6789–94. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hama Y, Katsuki H, Tochikawa Y, Suminaka C, Kume T, Akaike A. Contribution of endogenous glycine site NMDA agonists to excitotoxic retinal damage in vivo. Neurosci. Res. 2006;56(3):279–85. doi: 10.1016/j.neures.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Brazionis L, Rowley K , Sr, Itsiopoulos C, Harper CA, O'Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–6. doi: 10.2337/dc07-0632. [DOI] [PubMed] [Google Scholar]
- 21.Lim CP, Loo AV, Khaw KW, Sthaneshwar P, Khang TF, Hassan M, Subrayan V. Plasma aqueous and vitreous homocysteine levels in proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012;96:704–7. doi: 10.1136/bjophthalmol-2011-301044. [DOI] [PubMed] [Google Scholar]
- 22.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem. Soc. Trans. 2007;35:1175–9. doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- 23.Wright AD, Martin N, Dodson PM. Homocysteine, folates, and the eye. Eye (Lond) 2008;22:989–93. doi: 10.1038/sj.eye.6703061. [DOI] [PubMed] [Google Scholar]
- 24.Naggar H, Ola MS, Moore P, Huang W, Bridges CC, Ganapathy V, Smith SB. Downregulation of reduced-folate transporter by glucose in cultured RPE cells and in RPE of diabetic mice. Invest. Ophthalmol. Vis. Sci. 2002;43:556–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Moore P, El-sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith. SB. Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp. Eye Res. 2001;73:45–57. doi: 10.1006/exer.2001.1009. [DOI] [PubMed] [Google Scholar]
- 26.Ganapathy PS, Perry RL, Tawfik A, Smith RM, Perry E, Roon P, Bozard BR, Ha Y, Smith SB. Homocysteine-mediated modulation of mitochondrial dynamics in retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2011;52:5551–8. doi: 10.1167/iovs.11-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganapathy PS, White RE, Ha Y, Bozard BR, McNeil PL, Caldwell RW, Kumar S, Black SM, Smith SB. The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2011;52:5515–24. doi: 10.1167/iovs.10-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U.S. A. 1997;94:5923–8. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chmiel-Perzyκska I, Perzyκski A, Wielosz M, Urbaκska EM. Hyperglycemia enhances the inhibitory effect of mitochondrial toxins and, D.L-homocysteine on the brain production of kynurenic acid. Pharmacol. Rep. 2007;59:268–73. [PubMed] [Google Scholar]
- 30.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–74. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva KC, Rosales MA, Biswas SK, Lopes de Faria JB, Lopes de Faria JM. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382–90. doi: 10.2337/db09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, Tsubota K, Okano H, Oike Y, Ishida S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–8. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38:752–65. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem. Res. 2013;38(8):572–9. doi: 10.1007/s11064-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 35.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species In the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front. Biosci. 2009;14:3974–87. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 38.Lam CS, Benzie IF, Choi SW. Relationships among diabetic retinopathy, antioxidants, and glycemic control. Optom. Vis. Sci. 2011;88:251–56. doi: 10.1097/OPX.0b013e318208494a. [DOI] [PubMed] [Google Scholar]
- 39.Park KS, Kim SS, Kim JC, Kim HC, Im YS, Ahn CW, Lee HK. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am. J. Ophthalmol. 2008;145:432–7. doi: 10.1016/j.ajo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011;10: 209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 42.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 43.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–21. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 44.Suchting S, Bicknell R, Eichmann A. Neuronal clues to vascular guidance. Exp. Cell Res. 2006;312:668–75. doi: 10.1016/j.yexcr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 46.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010;70:304–22. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010;70:271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ola MS, Nawaz MI, El-Asrar A. Reduced levels of brain derived neuro¬trophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol. Neurobiol. 2013;33:359–67. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barhwal K, Hota SK, Prasad D, Singh SB, Ilavazhagan G. Hypoxia-induced deactivation of NGF-mediated ERK1/2 signaling in hippocampal cells Neuroprotection by acetyl-L-carnitine. J. Neurosci. Res. 2008;86:2705–2721. doi: 10.1002/jnr.21722. [DOI] [PubMed] [Google Scholar]
- 50.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye Molecular targets and therapeutic potential. Prog. Retin. Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Shen X, Xie B, Cheng Y, Jiao Q, Zhong Y. Effect of pigment epithelium derived factor on the expression of glutamine synthetase in early phase of experimental diabetic retinopathy. Ocul. Immunol. Inflamm. 2011;19:246–54. doi: 10.3109/09273948.2011.580073. [DOI] [PubMed] [Google Scholar]
- 52.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes.Early onset and effect of insulin. J. Clin. Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 54.Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- 55.Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS, Fort PE, Antonetti DA, Gardner TW. Diabetes reduces basal retinal insulin receptor signaling Reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- 56.Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J. Biol. Chem. 2004;279:37997–38006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- 57.Becerra SP, Amaral J. Erythropoietin--an endogenous retinal survival factor. N.; Engl. J. Med. 2002;347:1968–70. doi: 10.1056/NEJMcibr022629. [DOI] [PubMed] [Google Scholar]
- 58.Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, Xu G, Li W, Xu GT. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest. Ophthalmol. Vis. Sci. 2010;51:35–46. doi: 10.1167/iovs.09-3544. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. 2008;118:526–33. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant MB, Boulton ME, Ljubimov AV. Erythropoietin when liability becomes asset in neurovascular repair. J. Clin. Invest. 2008;118:467–70. doi: 10.1172/JCI34643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber M, Bonaventure N, Sahel JA. Protective role of excitatory amino acid antagonists in experimental retinal ischemia. Graefes. Arch. Clin. Exp. Ophthalmol. 1995;233:360–5. doi: 10.1007/BF00200485. [DOI] [PubMed] [Google Scholar]
- 62.Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest. Ophthalmol. Vis. Sci. 2007;48:5152–5159. doi: 10.1167/iovs.07-0427. [DOI] [PubMed] [Google Scholar]
- 63.Martin PM, Roon P, van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest. Ophthalmol. Vis. Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 64.Ola MS, Xu Y, Barber AJ, Lanove KF. Gabapentin ameliorates apoptosis of the diabetic retina. ARVO meeting abstract. 2007;48:632. [Google Scholar]
- 65.Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest. Ophthalmol. Vis. Sci. 2001;42:966–74. [PubMed] [Google Scholar]
- 66.Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J. Neurosci. 2002;22:7639–49. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinzón-Duarte G, Arango-González B, Guenther E, Kohler K. Effects of brain-derived neurotrophic factor on cell survival, differentiation and patterning of neuronal connections and Müller glia cells in the developing retina. Eur. J. Neurosci. 2004;19:1475–1484. doi: 10.1111/j.1460-9568.2004.03252.x. [DOI] [PubMed] [Google Scholar]
- 68.Hu Y, Cho S, Goldberg JL. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2010;51:1747–1754. doi: 10.1167/iovs.09-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Migallón MC, Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp. Eye Res. 2011;92:260–267. doi: 10.1016/j.exer.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Min D, Xiao-Bo X, Si-Qi X. BDNF regulates GLAST and glutamine synthetase in mouse retinal Müller cells. J. Cell Physiol. 2011 doi: 10.1002/jcp.22762. [DOI] [PubMed] [Google Scholar]
- 71.Azadi S, Johnson LE, Paquet-Durand F, Perez MT, Zhang Y, Ekström PA, van Veen T. CNTF + BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res. 2007;1129:116–129. doi: 10.1016/j.brainres.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 72.Takita H, Yoneya S, Gehlbach PL, Duh EJ, Wei LL, Mori K. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest. Ophthalmol. Vis. Sci. 2003;44:4497–504. doi: 10.1167/iovs.03-0052. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida Y, Yamagishi S, Matsui T, Jinnouchi Y, Fukami K, Imaizumi T, Yamakawa R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab. Res. Rev. 2009;25:678–86. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 74.Nishijima K, Ng Y, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP. Vascular Endothelial Growth Factor-A Is a Survival Factor for Retinal Neurons and a Critical Neuroprotectant during the Adaptive Response to Ischemic Injury. Am. J. Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J. Clin. Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 77.Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol. Psychiatry. 2001;6:285–92. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- 78.Reiter CE, Sandirasegarane L, Wolpert EB, Klinger M, Simpson IA, Barber AJ, Antonetti DA, Kester M, Gardner TW. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am. J. Physiol. Endocrinol. Metab. 2003;285:E763–74. doi: 10.1152/ajpendo.00507.2002. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, Xu G, Lu L, Dai W, Yanoff M, Li W, Xu GT. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol. Vis. Sci. 2008;49(2):732–42. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- 80.Wang Q, Gorbey S, Pfister F, Höger S, Dorn-Beineke A, Krügel K, Berrone E, Wu L, Korff T, Lin J, Busch S, Reichenbach A, Feng Y, Hammes HP. Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell Physiol. Biochem. 2011;27:769–82. doi: 10.1159/000330085. [DOI] [PubMed] [Google Scholar]
- 81.McVicar CM, Hamilton R, Colhoun LM, Gardiner TA, Brines M, Cerami A, Stitt AW. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60:2995–3005. doi: 10.2337/db11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Faria JB, Faria JM. Green tea is neuro-protective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:1325–36. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 84.Balakumar P, Rohilla A, Krishan P. The multifaceted therapeutic potential of benfotiamine. Pharmacol. Res. 2010;61:482–88. doi: 10.1016/j.phrs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid nor malises complication-causing pathways in type 1 diabetes. Diabetologia. 2008;51:1930,–32. doi: 10.1007/s00125-008-1100-2. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M. A 5-year follow-up of antioxidant Supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011;21:637–43. doi: 10.5301/EJO.2010.6212. [DOI] [PubMed] [Google Scholar]
- 87.Yamagishi S, Matsui T. Advanced glycation end products (AGEs): oxidative stress and diabetic retinopathy. Curr. Pharm. Biotechnol. 2011;12:36228. doi: 10.2174/138920111794480534. [DOI] [PubMed] [Google Scholar]
- 88.Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E, Almansa I, Romero FJ, Miranda M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006;41:979–84. doi: 10.1016/j.freeradbiomed.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 89.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab (Lond). 2007;16:4–8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011;27:123–30. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 91.Mancino R, Di Pierro D, Varesi C, Cerulli A, Feraco A, Cedrone C, Pinazo-Duran MD, Coletta M, Nucci C. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol. Vis. 2011;17:1298–1304. [PMC free article] [PubMed] [Google Scholar]
- 92.Wong TY, Simó R, Mitchell P. Fenofibrate - a potential systemic treatment for diabetic retinopathyκ. Am. J. Ophthalmol. 2012;154:6–12. doi: 10.1016/j.ajo.2012.03.013. [DOI] [PubMed] [Google Scholar]