Abstract
Objective
The n-3 polyunsaturated fatty acids (PUFA) are dietary components derived from fish oil with beneficial cardiovascular effects that may relate in part to anti-inflammatory properties. Peripheral artery disease (PAD) is characterized by a marked pro-inflammatory state. We hypothesized that the n-3 PUFA content of red blood cells (omega-3 index) would be correlated with biomarkers of inflammation and vascular function in a PAD cohort.
Methods
This was a cross-sectional study of subjects who presented to an outpatient vascular surgery clinic for evaluation of PAD. We used linear regression to evaluate the independent association between the omega-3 index, inflammatory biomarkers [C-reactive protein (CRP), intercellular adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), and tumor-necrosis-factor-α (TNF-α)] and endothelial function (brachial artery flow mediated dilation [FMD]).
Results
64 subjects (61 claudicants and 3 with critical limb ischemia) were recruited for the study. The mean CRP level was 5.0 ± 5.0 mg/L and the mean omega-3 index was 5.0% ± 1.8%. In an unadjusted model, the omega-3 index was negatively associated with CRP (38% increase in CRP for one standard deviation decrease in the omega-3 index; P=.007) which remained significant after adjustment for age, body-mass index, smoking, the ABI and HDL (33%; P=.04). There was also evidence for independent associations between the omega-3 index and IL-6 (P=.001). There were no significant associations between the omega-3 index and vascular function tests.
Conclusions
In a cohort of patients with PAD, the omega-3 index was inversely associated with biomarkers of inflammation even after adjustment for covariates including the ABI. Because patients with PAD have a high inflammatory burden, further studies should be conducted to determine if manipulation of omega-3 index via dietary changes or fish oil supplementation could improve inflammation and symptoms in these patients.
INTRODUCTION
In a primary care setting, nearly one-third of patients aged 70 and older will suffer from peripheral artery disease (PAD)1 that can significantly impact quality of life and longevity. Despite the available medical therapies, patients with PAD continue to have a higher risk of cardiovascular events compared to patients with coronary artery disease (CAD).2,3 Several studies have demonstrated that C-reactive protein (CRP) and other inflammatory markers, including interleukin-6 (IL-6) and intercellular-adhesion-molecule-1 (ICAM-1), are elevated in patients with PAD.4–6 Inflammation increases the risk of progression to PAD and of its severity,7–10 and more importantly, is a predictor of increased mortality in patients with PAD.11–13
A close relationship exists between inflammation, vascular function, and nutrition. Impaired flow-mediated dilatation (FMD) of the brachial artery independently predicts cardiovascular events in patients undergoing vascular surgery,14,15 emphasizing the relationship between inflammatory markers, thrombosis, and endothelial function.16 Emerging evidence suggests that n-3 polyunsaturated fatty acids (PUFA) can influence both the development and resolution of inflammation,17–19 and can improve endothelial function.20 The omega-3 index—a measure of the red blood cell (RBC) content of the two major long-chain n-3 FAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), expressed as a percentage of total RBC FAs—is a valid marker of tissue n-3 PUFA content and an independent, graded risk factor for death from CAD21. In patients with stable CAD, the omega-3 index has been inversely associated with inflammation as measured with CRP.22 It is important to understand whether this relationship exists in patients with PAD as well, as inflammation appears to be more severe in these patients and correlates with poorer outcomes7–10,23. The goal of this study was to determine if the omega-3 index is associated with circulating markers of inflammation and endothelial function in patients with PAD. A better understanding of this relationship could help guide future treatment of these patients (i.e., nutritional recommendations or supplementation) in an effort to lower inflammation and improve their vascular function.
METHODS
Study Population and protocol
This study was approved by the Committee on Human Research at the University of California, San Francisco (UCSF) and all patients provided informed consent. The study included patients referred to the outpatient vascular surgery clinic of the San Francisco Veterans Affairs Medical Center (SF VAMC) for evaluation of symptomatic PAD. PAD diagnosis was based on current guidelines of an index ABI <0.9 on the affected limb(s) at presentation with the presence of claudication or critical limb ischemia (CLI). We excluded patients with incompressible arteries or an ABI>1.424,25. Claudication was diagnosed based on fatigue, discomfort, or pain that occurs in specific limb muscle groups during effort due to exercise-induced ischemia26. CLI was defined as limb pain that occurs at rest or impending limb loss that is caused by severe compromise of blood flow to the affected extremity26. Both patients with mild-moderate (ABI of 0.41–0.90) and severe PAD (<0.40) were included in the study26. Additional study exclusion criteria included: significant renal disease (eGFR <60ml/min), hepatic disease, or inflammatory disease, concurrent severe infections, acute illness or other major surgery within 30 days of evaluation, or taking immunosuppressive medications. Those meeting the study criteria were invited to participate and informed consent was obtained. Demographic data (including age, race, gender, hip and waist circumference, body mass index, prior supplement use, exercise frequency), cardiovascular history (e.g., CAD, cerebrovascular disease, previous procedures), risk factors (hypertension, diabetes, hyperlipidemia, cigarette smoking), medications, depressive symptoms (using validated nine-item Patient Health Questionnaire - PHQ-9)27, and pertinent cardiovascular examination findings were recorded. Co-morbidities reported in this study (CAD, cerebrovascular disease, hypertension, diabetes and hyperlipidemia) were based on diagnoses previously made by treating physicians and entered in the electronic medical record system of the SF VAMC. A total of 80 consecutive patients meeting study criteria were screened and approached to participate in the study (2011–2012). Sixteen declined to participate for reasons including distance from the hospital and time commitment.
Measurements
n-Fatty Acid Measurements
To measure the omega-3 index, ten milliliters of whole venous blood were collected in a fasting state in an EDTA tube, and centrifuged at 2800 rpm for 10 minutes at 4°C within 30 minutes of collection. Packed RBCs were stored at −80°C until assayed for the omega-3 index according to the HS-Omega-3 index® methodology.28,29 Briefly, fatty acid methyl esters are generated by acid transesterification with boron trifluoride and analyzed by capillary gas chromatography using a GC2010 Gas Chromatograph (Shimadzu Corporation, Columbia, MD) equipped with a SP2560, 100-m column (Supelco, Bellefonte, PA). Fatty acids were identified by comparison with a standard mixture of fatty acids characteristic of RBCs and reported as a percentage of total identified FA after response factor correction. The typical coefficient of variation for the HS-Omega-3 Index (EPA+DHA) using this procedure is 3%. The average omega-3 index in the US population is 4.5%, with values ranging from 2.7% in the lowest 5th percentile to 8.8% in the highest 95th percentile30.
Inflammatory Markers
Inflammatory markers studied included CRP, IL-6, sICAM-1, and tumor-necrosis-factor-α (TNF-α). Selection of these inflammatory biomarkers was based on demonstrated evidence by our group that PUFA alter IL-6, ICAM-1 and TNF-α gene expression in endothelial cells31 and work by Farzaneh-Far et al that circulating CRP and IL-6 are associated with n-3 PUFA in patients with CAD22. Ten milliliters of whole venous blood were collected in a fasting state in a tiger-top tube, clotted for a minimum of 30 minutes at room temperature, and centrifuged at 2800 rpm for 10 minutes at 4°C. Serum was stored at −80°C until assayed for IL-6, sICAM-1, and TNF-α per standard kit protocol (R&D Systems Inc., Minneapolis, MN). The typical coefficients of variation for IL-6, sICAM-1, and TNF-α are 7.4%, 4.6%, and 5.4%, respectively. The lower limits of detection are 0.04pg/ml, 0.1ng/ml, and 0.11pg/ml, respectively. Plasma was assayed for CRP the same day as collection by the SF VAMC lab per standard methodology (Beckman Coulter Analyzer, Miami, FL). The coefficient of variation for CRP using this procedure is 5.1%. Normal range values for CRP are as follow: levels less than 1 mg/L are considered “low,” levels from 1 to 3 mg/L are considered “average,” and levels greater than 3 mg/L are considered “high” and are associated with an increased risk of cardiovascular events32,33. The normal value for IL-6 is less than 7 pg/mL.
Ankle-Brachial Index
The ankle-brachial index (ABI) was measured using current guidelines and standards24,34. Systolic blood pressures of the brachial, posterior tibial and dorsalis pedis arteries were measured bilaterally. For each lower extremity, the highest systolic pressure of the two pedal pulses was divided by the highest systolic pressure of the two brachial arteries.
Renal, Lipid, and Metabolic Measurements
Blood samples were collected in a fasting state for measurement of creatinine (Cr), estimated glomerular filtration rate (eGFR-calculated using the abbreviated MDRD formula based on age, gender, race, and serum creatinine level35), albumin, total cholesterol, triglycerides, low-density lipoprotein, high-density lipoprotein, and hemoglobin A1C (Hgb A1C) if patients were diabetic. Plasma was assayed for these analytes on the same day as collection by the SF VAMC lab per standard methodology (Beckman Coulter Analyzer). Serum was isolated at the same time points for homocysteine and assayed the same day as collection by the SF VAMC lab per standard methodology (Abbott Diagnostics Architect i1000 Analyzer, Lake Forest, IL).
Vascular Reactivity of Brachial Arteries
Flow-mediated vasodilation was performed according to current guidelines and standards36,37 as already described by our group38 and other investigators39. Subjects were asked to fast (≥ 8 hours) and be free of nicotine (≥ 4 hours). A history of recent medications was recorded. Subjects were allowed to rest for ten minutes in a supine position in a darkened room with temperature maintained at 23°C. Each subject’s arm was extended onto a movement-constraining pillow with the palmar aspect oriented anteriorly. A 5 cm tourniquet blood pressure cuff was placed on the upper arm distal to the insertion of the deltoid. Prior to cuff inflation, the baseline vessel diameter and blood-flow velocity were recorded for 60 seconds using EKG-gated image capture software (Brachial Imager, Medical Imaging Applications LLC, Coralville, IA). The blood pressure cuff was then inflated to 50 mm Hg above the subject’s systolic blood pressure and maintained for a period of 5 minutes. Blood-flow velocity was assessed for a period of 30 seconds post-cuff release using the methods described above and B-mode images for 3 minutes post-cuff release.
Analysis of the images was performed using continuous edge-detection software (Brachial Analyzer, Medical Imaging Applications LLC, Coralville, IA). Hyperemia diameter was calculated using a pre-determined time window (55–65 seconds post-cuff release). FMD% was calculated as [(60s Hyperemia diameter-Avg Baseline diameter)/Avg Baseline diameter]*100. The vasoreactivity index was calculated by normalizing FMD to brachial stimulus ratio, with brachial stimulus ratio corresponding to hyperemia flow divided by baseline flow. Time-averaged velocity measurements were obtained using the peak-velocity method. Velocity of the hyperemia stimulus was calculated as the mean velocity of the first four heart beats following cuff-release. Both mean velocity and the velocity time integral were recorded.
Statistical Analysis
For descriptive purposes, we categorized participants by tertiles of the omega-3 index. Differences in characteristics among these groups were compared using ANOVA for continuous variables and Fisher’s exact test for dichotomous variables. We then used linear models to estimate the relationship between decreases in the omega-3 index, rescaled by its standard deviation (SD), and the four inflammatory markers, all log-transformed to meet normality assumptions. After back-transformation using the equation 100*[eβ−1], the resulting regression coefficients are interpretable as percentage changes in the marker for each SD decrease in the omega-3 index. Covariates were retained in the model if omitting them changed the adjusted coefficient for the omega-3 index by more than 5%. The same approach was also used to estimate the association of the omega-3 index with flow-mediated dilation. In addition, we used the Kruskal-Wallis test to evaluate differences in CRP levels across the three omega-3 index categories proposed by Harris et al. (defined as <4%, 4%–8%, >8%, corresponding to high, intermediate, and low CAD risk, respectively21). Finally, having previously shown that patients with PAD and elevated CRP values have adverse cardiovascular and re-vascularization outcomes23,40, we used both unadjusted and fully adjusted logistic models to estimate the association of the omega-3 index with an elevated CRP (>5 mg/dL). Statistical analyses were performed using Stata/SE 12 (StataCorp, College Station, TX).
RESULTS
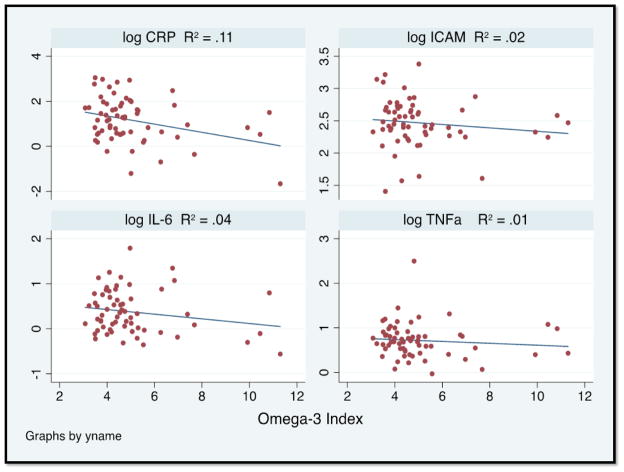
A total of 64 patients that were referred for PAD evaluation at the vascular surgery clinic of the SF VAMC were recruited for the study (61 claudicants and 3 critical limb ischemia-CLI). The mean CRP level was 5.0 ± 5.0 mg/L (median 3.3 mg/L) and the mean omega-3 index was 5.0% ± 1.8% (median 4.5%). One patient had missing inflammatory marker values and was excluded from further analysis. The baseline characteristics of patients with PAD are summarized in Table 1 by omega-3 index tertiles. The patients in the lowest tertile were more likely to be younger, have a history of smoking, and have a higher score on the PHQ-9 (greater depression burden). They also had higher CRP and glucose values and a trend towards higher levels of homocysteine (Table 2). Scatter plots of log-CRP, log-IL6, log-ICAM, and log-TNF-α against the omega-3 index are shown in Figure 1. CRP was inversely correlated with the omega-3 index. There was no statistical difference across tertiles of omega-3 index in brachial artery FMD and ABI measurements (Table 3).
TABLE 1.
The baseline characteristics of the PAD patients categorized by tertiles of the omega-3 index.
| Characteristics | Tertile I 3.7 ± 0.3 (n=21) |
Tertile II 4.5 ± 0.3 (n=21) |
Tertile III 6.8 ± 2.0 (n=21) |
P-value |
|---|---|---|---|---|
| Age, Mean ± SD- | 64 ± 8 | 68 ± 9 | 70 ± 8 | .05 |
| Male Sex (%) | 21 (100) | 21 (100) | 21 (100) | N/A |
| Caucasian | 15 (71) | 16 (76) | 15 (71) | 1.0 |
| Rutherford Classification | 1.9 ± 0.8 | 2.4 ± 0.9 | 2.1 ± 0.9 | .23 |
|
Comorbidities
| ||||
| Hypertension | 19 (90) | 18 (86) | 21 (100) | .35 |
| Hyperlipidemia | 18 (86) | 18 (86) | 19 (90) | 1.0 |
| History of CAD | 6 (29) | 6 (29) | 10 (48) | .37 |
| Diabetes Mellitus | 5 (24) | 6 (29) | 9 (43) | .49 |
| History of previous lower extremity bypass | 4 (19) | 5 (24) | 1 (5) | .31 |
| History of previous lower extremity percutaneous procedure | 7 (33) | 5 (24) | 7 (33) | .83 |
| Current and past history of smoking | 21 (100) | 21 (100) | 17 (81) | .03 |
|
Medications
| ||||
| Aspirin | 14 (67) | 11 (55) | 13 (62) | .76 |
| Ace-inhibitor | 7 (33) | 7 (35) | 10 (48) | .65 |
| β-Blocker | 13 (62) | 7 (35) | 14 (67) | .10 |
| Statin | 19 (90) | 16 (80) | 19 (90) | .57 |
| Anthropometric measures and behavioral health | ||||
|
| ||||
| Body-Mass index (BMI) | 25 ± 4 | 28 ± 4 | 28 ± 5 | .08 |
| Waist-hip ratio | 0.98 ± 0.04 | 1.02 ± 0.07 | 1.00 ± 0.04 | .20 |
| PHQ-9 | 11 ± 7 | 2 ± 3 | 5 ± 7 | .03 |
Values as “mean +/− SD” or “n (%)”. The omega-3 index is the sum of EPA+DHA in RBC membranes.
TABLE 2.
Laboratory values and biomarkers of the population categorized by tertiles of the omega-3 index.
| General Laboratory Values | Tertile I 3.7 ± 0.3 (n=21) |
Tertile II 4.5 ± 0.3 (n=21) |
Tertile III 6.8 ± 2.0 (n=21) |
P-value |
|---|---|---|---|---|
| Cholesterol (mg/dl) | 162 ± 36 | 174 ± 39 | 145 ± 41 | .06 |
| LDL (mg/dl) | 89 ± 29 | 98 ± 38 | 75 ± 36 | .11 |
| HDL (mg/dL) | 40 ± 9 | 43 ± 17 | 47 ± 15 | .31 |
| Triglycerides (mg/dL) | 171 ± 119 | 174 ± 94 | 118 ± 73 | .12 |
| Serum creatinine (mg/dL) | 1.0 ± 0.3 | 1.2 ± 0.4 | 1.0 ± 0.2 | .19 |
| eGFR (mL/min) | 84 ± 29 | 70 ± 27 | 78 ± 23 | .29 |
| Homocysteine | 15 ± 7 | 14 ± 4 | 12 ± 3 | .08 |
| Glucose | 123 ± 83 | 81 ± 53 | 72 ± 32 | .05 |
|
Inflammation
| ||||
| logCRP (mg/L) | 1.4 ± 0.9 | 1.4 ± 0.8 | 0.6 ± 1.1 | .01 |
| logIL-6 (pg/ml) | 0.4 ± 0.4 | 0.5 ± 0.5 | 0.2 ± 0.5 | .14 |
| logICAM | 2.5 ± 0.4 | 2.5 ± 0.3 | 2.4 ± 0.4 | .42 |
| logTNF-α (pg/ml) | 0.8 ± 0.3 | 0.7 ± 0.5 | 0.6 ± 0.4 | .39 |
Values as “mean +/− SD” or “n (%)”. The omega-3 index is the sum of EPA+DHA in RBC membranes.
Figure 1.
Correlations between the omega-3 index and log-CRP (a), log-IL-6 (b), log-ICAM-1 (c), log-TNF-α (d). The omega-3 index is the sum of EPA+DHA in RBC membranes.
TABLE 3.
Non-invasive vascular measurements categorized by tertiles of the omega-3 index.
| Flow-Mediated Brachial Artery Vasodilation | Tertile I 3.7 ± 0.3 (n=21) |
Tertile II 4.5 ± 0.3 (n=21) |
Tertile III 6.8 ± 2.0 (n=21) |
P-value |
|---|---|---|---|---|
| Brachial FMD (%) | 6 ± 5 | 8 ± 3 | 8 ± 5 | .46 |
| Brachial Stimulus Ratio | 4 ± 2 | 5 ± 2 | 6 ± 3 | .13 |
| Vasoreactivity index | 1.5 ± 1.4 | 2.2 ± 1.9 | 1.6 ± 1.2 | .37 |
| Brachial Artery Baseline Diameter (cm) | 0.39 ± .06 | 0.37 ± .07 | 0.38 ± .05 | .66 |
| Brachial Artery Reactive Hyperemia Diameter (cm) | 0.41 ± 0.06 | 0.40 ± 0.07 | 0.40 ± 0.05 | .80 |
| Brachial Artery Baseline Velocity (m/sec) | 0.17 ± 0.06 | 0.15 ± 0.08 | 0.13 ± 0.04 | .26 |
| Brachial Artery Reactive Hyperemia Velocity (m/sec) | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.7 ± 0.2 | .26 |
| Brachial Artery Baseline Flow (ml/min) | 119 ± 50 | 100 ± 67 | 87 ± 33 | .18 |
| Brachial Artery Reactive Hyperemia Flow (ml/min) | 556 ± 261 | 454 ± 208 | 548 ± 254 | .38 |
| Brachial Artery Baseline Shear Stress (dynes/cm2) | 12 ± 4 | 11 ± 7 | 10 ± 4 | .41 |
| Brachial Artery Reactive Hyperemia Shear Stress (dynes/cm2) | 51 ± 25 | 41 ± 16 | 48 ± 16 | .33 |
| Blood Pressure and Ankle-Brachial Indices | ||||
|
| ||||
| Systolic Blood Pressure (mm Hg) | 137 ± 20 | 132 ± 17 | 138 ± 21 | .53 |
| Diastolic Blood Pressure (mmHg) | 76 ± 12 | 77 ± 7 | 76 ± 7 | .97 |
| Index ABI | 0.65 ± 0.25 | 0.76 ± 0.11 | 0.67 ± 0.15 | .12 |
| Mean ABI | 0.75 ± 0.22 | 0.86 ± 0.11 | 0.74 ± 0.15 | .05 |
Values as “mean +/− SD” or “n (%)”. The omega-3 index is the sum of EPA+DHA in RBC membranes.
In an unadjusted model, the omega-3 index was significantly associated with CRP (38% increase in CRP for a 1.8% decrease in the omega-3 index; P=0.007) (Table 4). After adjustment for age, BMI, smoking, index ABI and HDL, we found an independent inverse association between the omega-3 index with CRP (33%; P=0.04). We also found evidence for independent associations between the omega-3 index and IL-6 (22% increase in IL-6 for a 1.8% decrease in the omega-3 index; P=0.001). There was no significant correlation found between the omega-3 index and brachial FMD (unadjusted: 0.26; adjusted: 0.79). There was also no evidence of an interaction between the omega-3 index and CRP on this endpoint.
Table 4.
Percent change in inflammatory markers by per standard deviation (1.8%) decrease in the omega-3 index.
| Inflammatory markera | Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| % Increase | 95% CI | P-value | % Increase | 95% CI | P-value | |
|
| ||||||
| CRP | 38 | 9, 75 | .007 | 33 | 1, 75 | .04 |
| IL6 | 10 | −3, 24 | .13 | 22 | 8, 38 | .001 |
| ICAM-1 | 5 | −4,15 | .31 | 1 | −8, 10 | .87 |
| TNF-α | 4 | −6, 14 | .45 | 2 | −9, 15 | .71 |
Inflammatory markers were log-transformed.
Adjustment variables include age, BMI, smoking, index ABI and HDL (CRP); age, BMI, smoking index ABI and eGFR (IL-6); age, BMI, smoking, index ABI, race, history of CAD and LDL (ICAM-1); and age, BMI, smoking, index ABI, systolic blood pressure, beta blockers, homocysteine and creatinine (TNF-α). The omega-3 index is the sum of EPA+DHA in RBC membranes.
It is known that patients with elevated CRP values have adverse cardiovascular and re-vascularization outcomes23,40. Each standard deviation decrease (1.8%) in the omega-3 index was associated with having an increased likelihood of elevated CRP using a cut-off of 5mg/L, although this did not reach significance (unadjusted odds ratio 2.0; 95% CI 0.9, 4.5; P=.10; adjusted odds ratio 8.6, 95% CI 0.7, 105, P=0.09).
DISCUSSION
This report is the first to examine the relationship between the omega-3 index and systemic inflammation and endothelial function in a PAD cohort. In a cross-sectional cohort of patients presenting for evaluation of PAD in an outpatient vascular surgery clinic, we found a significant inverse association between the omega-3 index and inflammation as measured with CRP and IL-6. Our findings suggest that diet could play an important role in the inflammatory profile of patients with PAD based on their circulating pro-inflammatory biomarkers levels. Although a dietary intervention was not tested in this study, it is possible that manipulation of the omega-3 index, which can be accomplished by increasing consumption of fish or supplementation with fish oil41,42 could have beneficial effects in this patient population. Trials are warranted to test this hypothesis.
Despite attempts at treatment with available medical therapies, patients with PAD continue to have a higher risk of cardiovascular events compared to patients with CAD alone.2,3 Inflammation is known to have a significant association with disease burden in patients with PAD. Inflammation in general, and CRP in particular, has been correlated with impaired endothelial function in vivo and in vitro43,44, and inflammatory markers, including CRP, are known to be elevated in patients with PAD.4–6 Furthermore, in a recent paper by Owens et al, CRP was found to be a strong predictor of mortality in patients with PAD23. Other authors have also reported increased mortality in PAD patients with higher inflammatory biomarker levels.11,45 In our study, CRP and IL-6 were significantly associated with the omega-3 index, but TNF-α and ICAM-1 were not. The failure to demonstrate a significant association between TNF-α and ICAM-1 and the omega-3 index may be related to the relative predictive value of individual biomarkers, as was demonstrated in the Edinburgh Artery Study.10
In the present study, we could not demonstrate a significant association between the omega-3 index and endothelial function. We believe that this could be due to either the absence of an association, the relatively small number of patients in the study, or the fact that the endothelial function in our cohort was so low as to not be affected by this variable. Although our results did not demonstrate an association between omega-3 index and markers of endothelial function using FMD, previous authors have detected a significant correlation in non-PAD cohorts. In one study of young, healthy smokers, Siasos et al. found that FMD values had significantly improved after oral treatment with 2gm/day of n-3 PUFA for several months.20 The presumed mechanism of action is that n-3 FA may decrease the elevated oxidative stress caused by smoking and thereby improve endothelial function. N-3 PUFA supplementation could lead to recovery of endothelial synthesis of nitrous oxide and PGI2, as well as vascular smooth muscle cell sensitivity to nitrous oxide. These mechanisms are especially relevant to the cohort evaluated in our study, 96% (n=67) of whom were current or past smokers and who, as a result, have pro-inflammatory profiles. The results in smokers reported by Siasos et al.20 indicate that supplementation could, in theory, lead to benefits in patients with PAD and should be examined prospectively.
The heightened inflammatory state in PAD is not specifically targeted by existing guideline-directed pharmacotherapies. For example, the American Heart Association/American College of Cardiology guidelines published in 2006 recommend an LDL goal of <100mg/dL for all patients with PAD.46 Feringa and colleagues demonstrated that in patients with PAD, higher doses of statins and lower LDL cholesterol levels are both independently associated with improved outcomes,47 but the effects of cholesterol-lowering per se on inflammatory status is unclear. In the present study, 80–90% of the patients were taking statins and mean total cholesterol and LDL levels were within guidelines, but inflammatory markers were still elevated. The relationship that we found between the omega-3 index and CRP in patients with PAD suggests several avenues for future research: therapy aimed at lowering inflammatory biomarkers (e.g., CRP) could be explored, as well as more aggressive statin therapy in all patients with PAD using a goal LDL<70 mg/dL as recommended by the Third Report of Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults guidelines.48 As noted earlier, based on previous studies demonstrating that fish or fish oil consumption have the potential to increase the omega-3 index41,42, nutritional supplementation with n-3 PUFA or diet modification could possibly lead to an improvement in the levels of circulating inflammatory biomarkers in the PAD population.
It is also important to note that although cholesterol levels were not significantly different by tertiles of omega-3 index in our study, some authors have demonstrated that triglyceride levels are negatively associated and a history of hypercholesterolemia is positively associated with the omega-3 index40. Further studies are needed to better understand this relationship between cholesterol levels and omega-3 index, as well as the impact of statins on the omega-3 index levels.
Several lines of evidence support the beneficial effect of n-3 PUFA on inflammation and atherosclerotic burden. We have recently demonstrated that supplementation with n-3 PUFA in-vitro leads to decreased adhesion of monocytes to the endothelial monolayer.31 Serhan et al. have also made novel discoveries in the field of inflammation with the “resolution-phase interaction products” (resolvins) pathways.17–19 This new paradigm describes the transition from acute to chronic inflammation as involving the loss of endogenously operative resolution processes. This response aims to re-establish homeostasis through resolution of an acute inflammatory response. Lipid autacoids are at the core of these responses as lipoxins and resolvins are thought to be bioactive products of n-6 and n-3 FAs,17,19 respectively. In support of this observation, plasma levels of the pro-resolving mediator 15-epimeric lipoxin were significantly lower in patients with symptomatic PAD than in healthy volunteers, suggesting a “resolution deficit” in PAD5. Considering this evidence, changes in n-3 PUFA intake will likely influence the balance of pro-inflammatory and pro-resolving mediators, potentially leading to clinical improvement in chronic inflammatory states such as atherosclerosis.
Limitations
The patient population studied was not representative of the wider PAD population as it included only male veterans from SF VAMC. These men were predominantly Caucasian and likely had similar diets. Furthermore, the inflammatory phenotype of the population studied at the SF VAMC may be skewed compared to the typical PAD patient with regards to levels of psychological and psychosocial factors affecting overall stress and inflammation.49 The correlation coefficient values were low and limit any conclusions about the relationships between the various inflammatory mediators and the omega-3 index.
CONCLUSIONS
In a cross-sectional study of patients with PAD, the omega-3 index was inversely associated with plasma levels of CRP and IL-6. There were no significant relations with other inflammatory biomarkers nor with measures of vascular/endothelial function. Because patients with PAD and elevated CRP values are at higher risk for cardiovascular and re-vascularization outcomes, further studies are needed to determine if manipulation of omega-3 index via dietary changes or fish oil supplementation could reduce vascular inflammation and related symptoms.
Acknowledgments
FUNDING SOURCES
We thank the Clinical Research Center of the San Francisco Veterans Affairs Medical Center for their invaluable help with this study. The present work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education, by a Clinical Seed Grant from the Society for Vascular Surgery, and by Award Number KL2RR024130 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
This paper will be presented at the SVS Meeting (PVSS Session) in June 2013 in San Francisco, CA (oral presentation).
CONFLICTS OF INTEREST/DISCLOSURES
WSH is the President of OmegaQuant Analytics, LLC (Sioux Falls, SD) and a Sr. Research Scientist at Health Diagnostic Laboratory (Richmond, VA), both are companies which offer the omega-3 index test.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Cotter G, Cannon CP, McCabe CH, et al. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis In Myocardial Infarction (OPUS-TIMI) 16 study. American heart journal. 2003;145:622–7. doi: 10.1067/mhj.2003.6. [DOI] [PubMed] [Google Scholar]
- 3.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120:1041–7. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho KJ, Spite M, Owens CD, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 177:2116–23. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009;50:1063–70. doi: 10.1016/j.jvs.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. Jama. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005;96:1374–8. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 11.Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriere I, Dupuy AM, Lacroux A, Cristol JP, Delcourt C. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J Am Geriatr Soc. 2008;56:840–6. doi: 10.1111/j.1532-5415.2008.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criqui MH, Ho LA, Denenberg JO, Ridker PM, Wassel CL, McDermott MM. Biomarkers in peripheral arterial disease patients and near- and longer-term mortality. Journal of vascular surgery. 2010;52:85–90. doi: 10.1016/j.jvs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 15.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 16.Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–9. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siasos G, Tousoulis D, Oikonomou E, et al. Effects of omega-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: A cross over study. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.10.081. [DOI] [PubMed] [Google Scholar]
- 21.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 22.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–43. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens CD, Kim JM, Hevelone ND, et al. An integrated biochemical prediction model of all-cause mortality in patients undergoing lower extremity bypass surgery for advanced peripheral artery disease. J Vasc Surg. 2012;56:686–95. doi: 10.1016/j.jvs.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenon SM, Gagnon J, Hsiang Y. Video in clinical medicine. Ankle-brachial index for assessment of peripheral arterial disease. N Engl J Med. 2009;361:e40. doi: 10.1056/NEJMvcm0807012. [DOI] [PubMed] [Google Scholar]
- 25.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–45. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–97. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 98. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire Jama. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 28.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. Jama. 2010;303:250–7. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. The Journal of nutrition. 2012;142:1297–303. doi: 10.3945/jn.112.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids. 2013;88:257–63. doi: 10.1016/j.plefa.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Grenon SM, Aguado-Zuniga J, Hatton JP, Owens CD, Conte MS, Hughes-Fulford M. Effects of fatty acids on endothelial cells: inflammation and monocyte adhesion. J Surg Res. 2012;177:e35–43. doi: 10.1016/j.jss.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 34.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 37.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009;50:1063–70. doi: 10.1016/j.jvs.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. Jama. 2009;301:165–74. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens CD, Kim JM, Hevelone ND, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. Journal of vascular surgery. 2010;51:1152–9. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block RC, Harris WS, Pottala JV. Determinants of Blood Cell Omega-3 Fatty Acid Content. Open Biomark J. 2008;1:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazionis L, Ting E, Itsiopoulos C, Wilson A, Hodge A. The effects of fish or fish oil on the omega-3 index. Nutrition & Dietetics. 2012;60:5–12. [Google Scholar]
- 43.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 44.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbonaviciene G, Frystyk J, Flyvbjerg A, Urbonavicius S, Henneberg EW, Lindholt JS. Markers of inflammation in relation to long-term cardiovascular mortality in patients with lower-extremity peripheral arterial disease. Int J Cardiol. 2012;160:89–94. doi: 10.1016/j.ijcard.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Feringa HH, Karagiannis SE, van Waning VH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–43. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 48.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 49.Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease: insights from the heart and soul study. J Am Heart Assoc. 2012;1:e002667. doi: 10.1161/JAHA.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]