Abstract
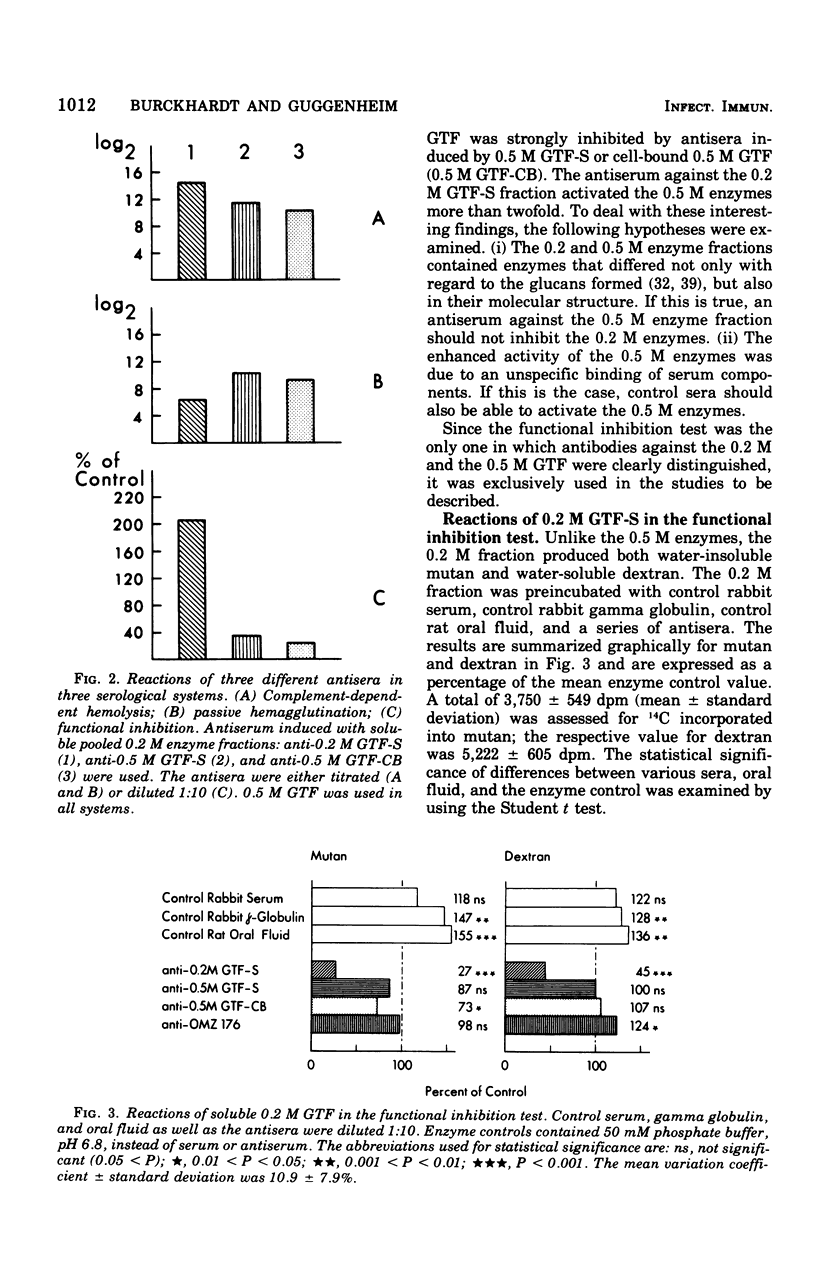
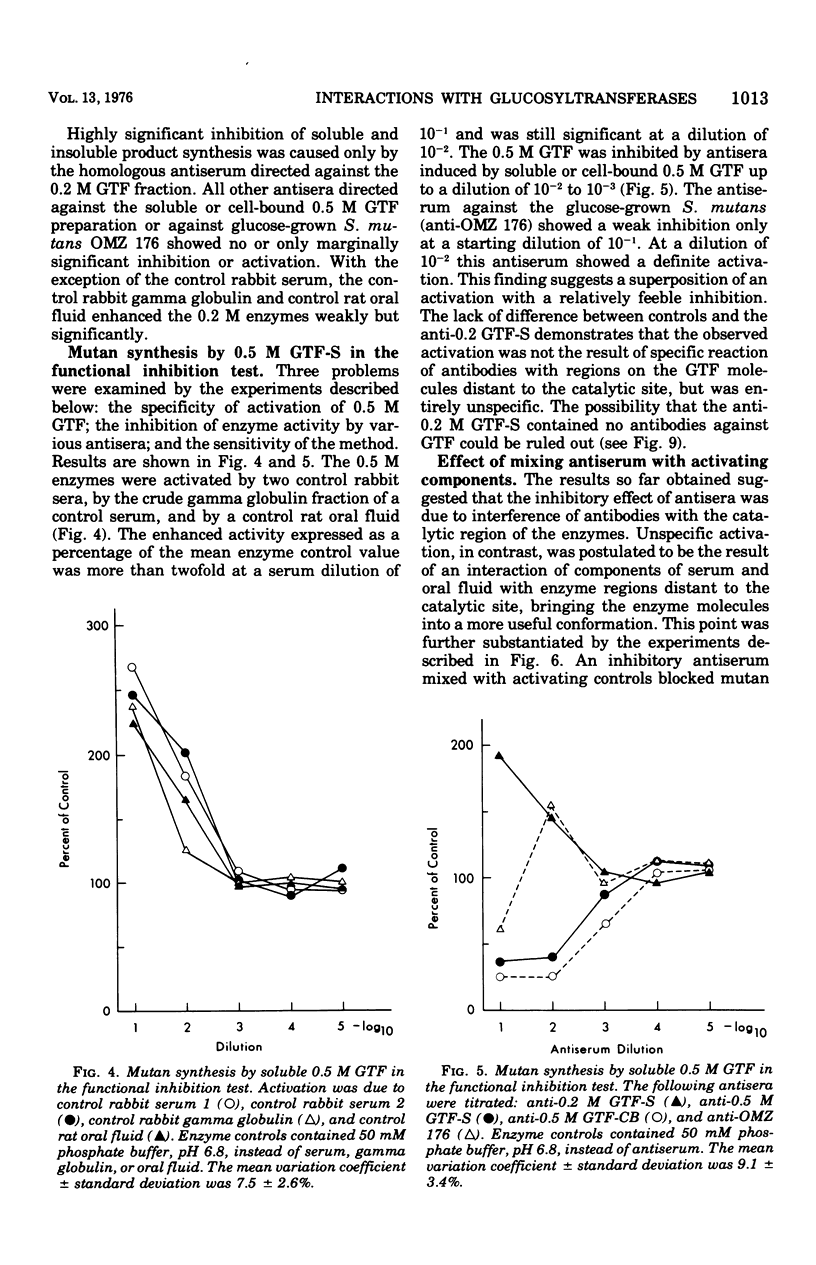
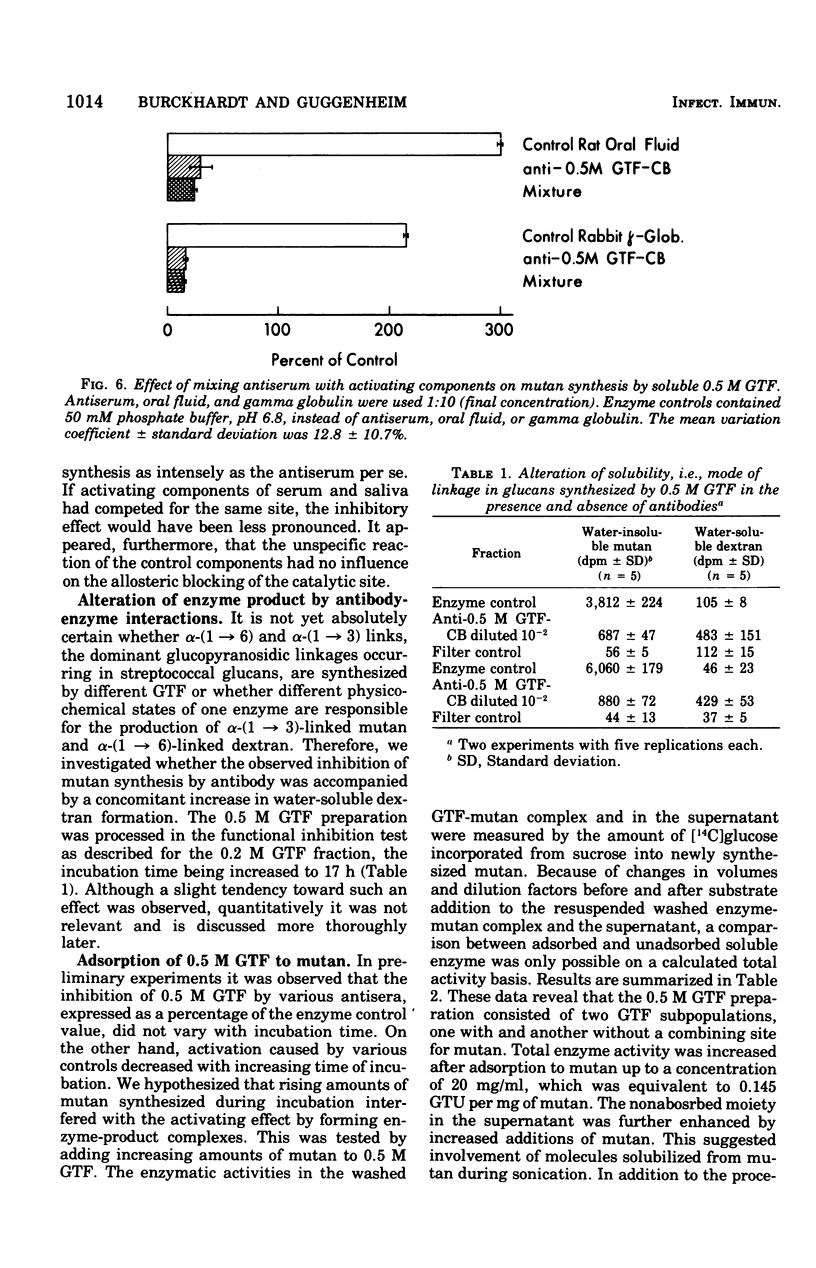
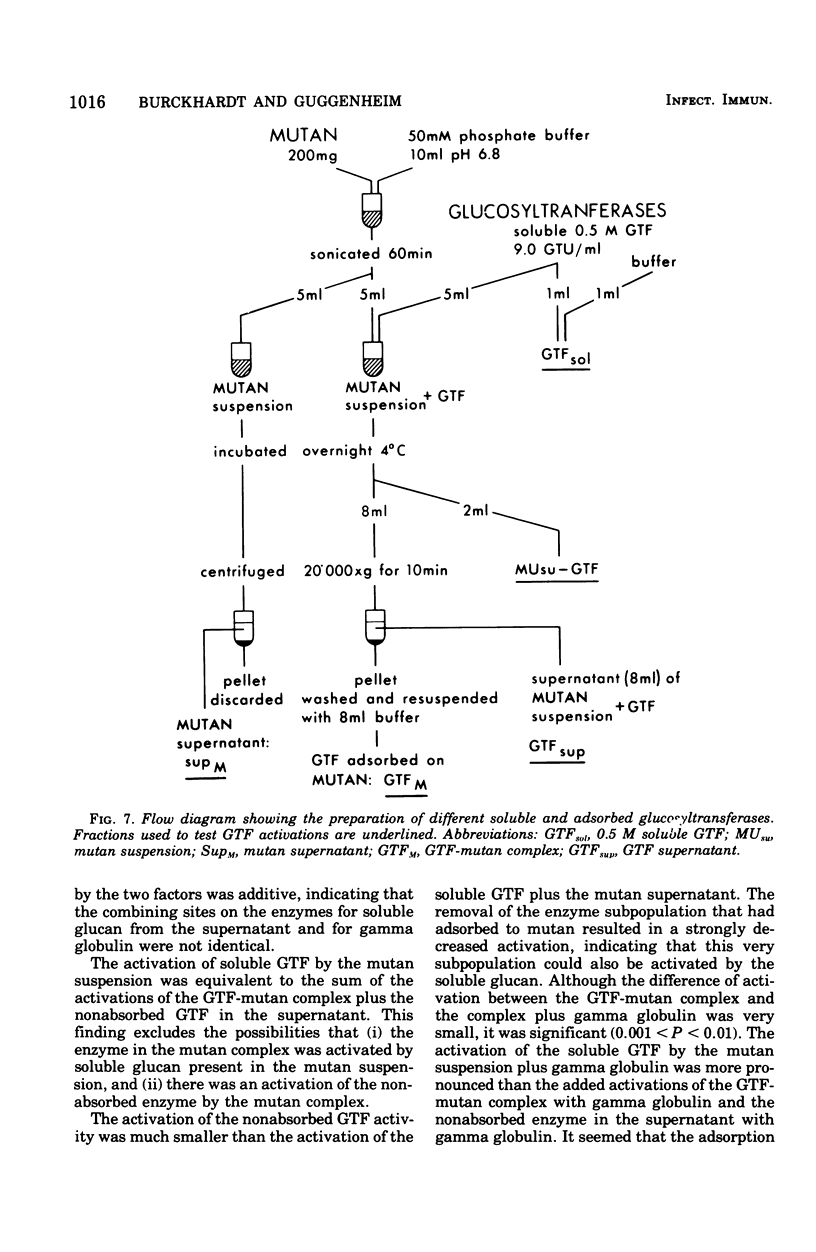
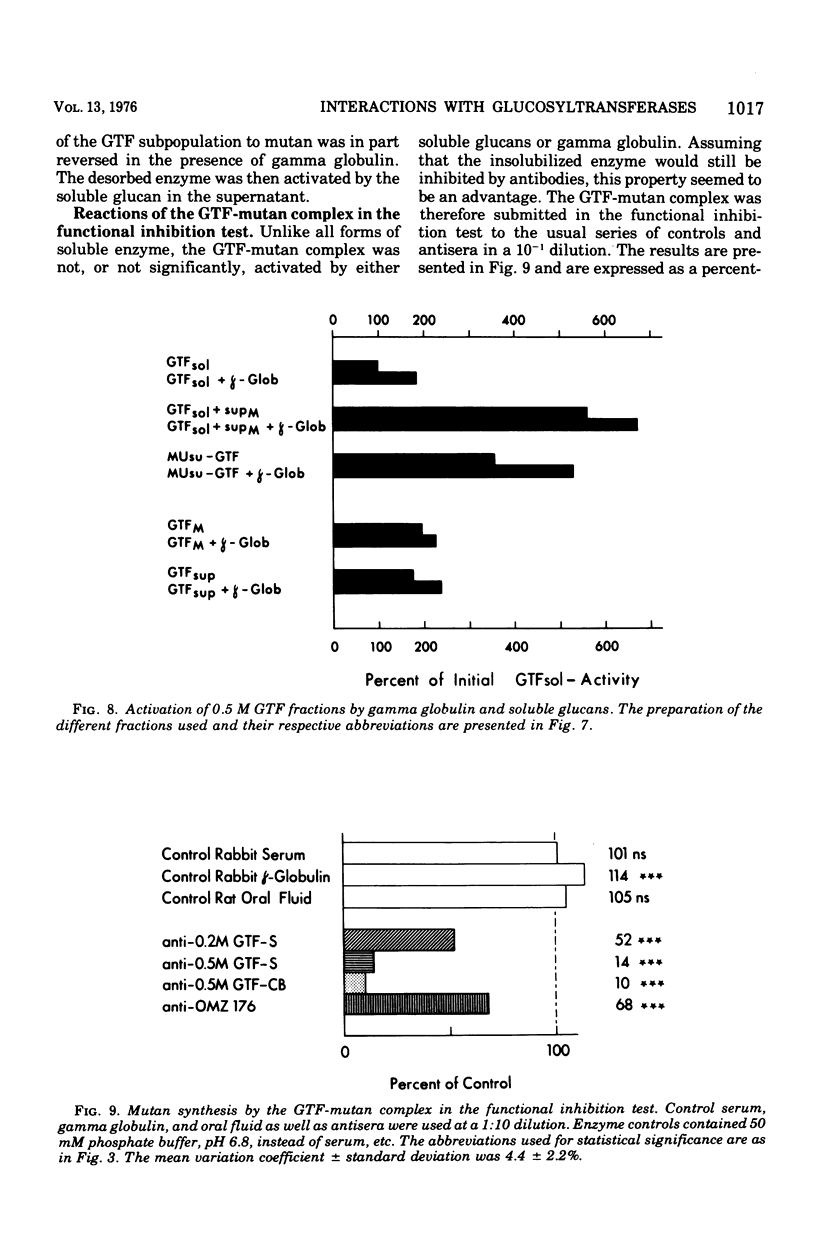
Partially purified glucosyltransferases (GTF) isolated from Streptococcus mutans OMZ 176 and respective rabbit antisera were used to study enzyme-antibody interactions. A comparison between sensitive serological techniques and a functional inhibition test based on a radioenzyme assay demonstrated that the latter test system was the only one that discriminated between different antisera. Positive reactions in high dilutions in the former test systems were explained by the involvement of non-GTF contaminants and/or antibodies against enzyme regions distant to the catalytic site. The minute cross-reactions between two enzyme fractions and the respective antisera in the functional inhibition test indicated that the two immunogens contained mainly GTF that differed in the structure of their catalytic region. Control rabbit sera, rat oral fluid, and insoluble and soluble glucans considerably activated the GTF eluted with a 0.5 M phosphate buffer from hydroxapatite. It is suggested that these enzymes had additional binding sites for macromolecules inherent to rabbit sera and rat oral fluid, respectively, and that the observed increase in enzyme activity was due to a more stable enzyme conformation. Possibly the stimulation of GTF by the soluble glucan fraction was caused by a primer and/or acceptor function; however, this was not the case of the insoluble glucan. A stable complex was formed in the absence of the enzyme substrate, sucrose, the activity of which was not readily enhanced. It is concluded that the GTF of strain OMZ 176 are composed of multiple, multi-reactive molecules that enable these enzymes to act as cross-linking agents.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. The reaction of pentoses with anthrone. Biochem J. 1958 Apr;68(4):669–672. doi: 10.1042/bj0680669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W. H., Cohen B., Cole M. F., Colman G. Immunization against dental caries. Br Dent J. 1975 Jul 15;139(2):45–58. [PubMed] [Google Scholar]
- CINADER B. Antibody to enzymes--a three-component system. Introduction: immunochemistry of enzymes. Ann N Y Acad Sci. 1963 May 8;103:495–548. doi: 10.1111/j.1749-6632.1963.tb53717.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Krasse B. Inhibition of streptococcal dextransucrase by sera of rabbits infected with Streptococcus sanguis. Arch Oral Biol. 1968 Jul;13(7):849–852. doi: 10.1016/0003-9969(68)90109-x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Sundström B. Variations in composition of early dental plaque following ingestion of sucruse and glucose. Odontol Revy. 1968;19(2):161–169. [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Guggenheim B., Lehner T. Antibodies to an extract of Streptococcus mutans, containing glucosyltransferase activity, related to dental caries in man. Arch Oral Biol. 1973 Jun;18(6):657–668. doi: 10.1016/0003-9969(73)90001-0. [DOI] [PubMed] [Google Scholar]
- Challacombe S. J., Lehner T., Guggenheim B. Serum and salivary antibodies to glucosyltransferase in dental caries in man. Nature. 1972 Jul 28;238(5361):219–219. doi: 10.1038/238219a0. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Dewar M. D., Walker G. J. Metabolism of the polysaccharides of human dental plaque. I. Dextranase activity of streptococci, and the extracellular polysaccharides synthesized from sucrose. Caries Res. 1975;9(1):21–35. doi: 10.1159/000260139. [DOI] [PubMed] [Google Scholar]
- Ebert K. H., Schenk G. Mechanisms of biopolymer growth: the formation of dextran and levan. Adv Enzymol Relat Areas Mol Biol. 1968;30:179–221. doi: 10.1002/9780470122754.ch4. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Acceleration of dextransucrase activity of Streptococcus mutans by secretory immunoglobulin A. J Bacteriol. 1974 Jun;118(3):805–809. doi: 10.1128/jb.118.3.805-809.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitzelmann R., Steinmann B., Bally C., Lebherz H. G. Antibody activation of mutant human fructosediphosphate aldolase B in liver extracts of patients with hereditary fructose intolerance. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1270–1277. doi: 10.1016/0006-291x(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B. Extracellular polysaccharides and microbial plaque. Int Dent J. 1970 Dec;20(4):657–678. [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Herzog E., Mühlemann H. R. The cariogenicity of different dietary carbohydrates tested on rats in relative gnotobiosis with a Streptococcus producing extracellular polysaccharide. Helv Odontol Acta. 1966 Oct;10(2):101–113. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hayashi J. A., Shklair I. L., Bahn A. N. Immunization with dextransucrases and glycosidic hydrolases. J Dent Res. 1972 Mar-Apr;51(2):436–442. doi: 10.1177/00220345720510023201. [DOI] [PubMed] [Google Scholar]
- Hotz P., Guggenheim B., Schmid R. Carbohydrates in pooled dental plaque. Caries Res. 1972;6(2):103–121. doi: 10.1159/000259783. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- KOEPSELL H. J., TSUCHIYA H. M. Enzymatic synthesis of dextran. J Bacteriol. 1952 Feb;63(2):293–295. doi: 10.1128/jb.63.2.293-295.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Lehmann F. G. Glutamatdehydrogenase aus menschlicher Leber. V. Aktivierung, Hemmung und allosterische Umfaltung des Enzymmoleküls durch seinen Antikörper. Z Gesamte Exp Med. 1971;155(2):172–186. [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Caldwell J. Immunological and bacteriological basis for vaccination against dental caries in rhesus monkeys. Nature. 1975 Apr 10;254(5500):517–520. doi: 10.1038/254517a0. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee, Michalek S. M., Webb J., Navia J. M., Rahman A. F., Legler D. W. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with Streptococcus mutans. J Immunol. 1975 Jan;114(1 Pt 2):300–305. [PubMed] [Google Scholar]
- McLean R. J., Bosmann H. B. Cell-cell interactions: enhancement of glycosyl transferase ectoenzyme systems during Chlamydomonas gametic contact. Proc Natl Acad Sci U S A. 1975 Jan;72(1):310–313. doi: 10.1073/pnas.72.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Schamschula R. G., Barmes D. E. A study of the streptococcal flora of plaque in caries free and caries active primitive peoples. Aust Dent J. 1970 Oct;15(5):377–382. doi: 10.1111/j.1834-7819.1970.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Simonson L. G. Distribution and frequency of streptococcus mutants in caries-active individuals. J Dent Res. 1972 May-Jun;51(3):882–882. doi: 10.1177/00220345720510034201. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbman M. A., Smith D. J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974 Jun;9(6):1079–1091. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]


