Abstract
Background
The effort to detect lung cancer in ever-earlier stages leads to the identification of an increasing number of patients without preoperative histological diagnosis. The aim of this study is to determine the prevalence and characteristics of benign lesions excised in the context of lung cancer surgery.
Methods
We retrospectively analyzed data from 125 surgical procedures. We compared the preoperative clinical or cyto-histological diagnosis with the surgical-pathologic diagnosis in order to identify the percentage of benign lesions excised. Furthermore, other parameters were analyzed, such as age, sex, tumor size, the presence of calcification, and the type of surgery according to subgroup.
Results
Of the 125 patients included in the study, 63 (50.4%) had a preoperative histological diagnosis of malignancy, corresponding to 56 cases (44.8%) of primary lung cancer and 7 cases (5.6%) of metastases. The 62 (49.6%) remaining cases without preoperative histological diagnosis were divided among 50 (40%) solitary pulmonary nodules and 12 (9.6%) pulmonary masses. According to the postoperative pathologic examination, we identified 12 (9.6%) benign lesions excised during lung cancer surgery. There were no statistically significant differences by subgroups with respect to age or sex. We found statistically significant evidence regarding the size and wedge resection as the surgical technique of choice for this type of benign lesion.
Conclusion
Our study obtained results similar to those published by other groups regarding the resection of benign lesions in lung cancer surgery. This percentage could be a quality management index of indeterminate lung lesions.
Keywords: Lung neoplasms, Lung surgery, Quality control, Solitary pulmonary nodule
INTRODUCTION
Lung cancer is the most common cause of cancer-related death annually in the world [1]. Therefore, it is important to establish an early diagnosis in order to improve long-term survival. Nowadays, we have a wide range of imaging tests and diagnostic techniques that allow the study of the anatomical features of a tumor, its nature, or the presence of distant metastases.
Despite all these advances, we cannot always obtain a pre-operative cyto-histological diagnosis. Sometimes, tests such as bronchoscopy or transthoracic fine-needle aspiration biopsy (FNAB) have been inconclusive or not possible to perform. In these cases, the next step is diagnostic surgery.
On the other hand, the increasing use of radiological imaging techniques, as low-dose computer tomography (CT) scanning in lung cancer screening or positron emission tomography (PET), is causing an increasing incidental detection of pulmonary lesions suspicious for malignancy [2–4]. Thus, it is increasingly common to find indeterminate pulmonary lesions, which later turn out to be due to a benign disease and in which the treatment of choice would not have been surgery.
The aim of this study is to determine the incidence and characteristics of benign lesions removed in the context of lung cancer surgery.
METHODS
1) Study design
We retrospectively evaluated 125 consecutive surgical procedures from April 2010 to December 2011. We compared the preoperative clinical or cyto-histological diagnosis with the surgical-pathologic diagnosis. Further, we calculated the number and the percentage of benign lesions removed in the context of lung cancer surgery.
Patients were divided into four groups according to the preoperative diagnosis: (1) patients with an indeterminate solitary pulmonary nodule (lesion<3 cm); (2) patients with an indeterminate lung mass (lesion>3 cm); (3) patients with an established diagnosis of lung cancer; and (4) patients with a diagnosis of pulmonary metastases. Subsequently, we analyzed the postoperative histopathology of each group. Moreover, other parameters were analyzed, such as age, sex, tumor size, the presence of calcification, and the type of surgery according subgroup.
2) Statistical analysis
First, we developed a database using Microsoft Access 2007 for Windows. We collected all the necessary information on patients who underwent pulmonary resection surgery for suspected or confirmed neoplastic disease, such as demographic data, preoperative diagnosis, surgical procedure, or postoperative pathological analysis.
All statistical analyses were conducted with SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Qualitative variables were expressed as absolutes frequencies and percentages, and numerical variables as means±standard deviations. Discrete variables were compared using the chi-square test or Fisher’s exact test. Quantitative variables were analyzed using the Student t-test. Significance was set at p<0.05.
3) Management of patients presenting pulmonary nodule
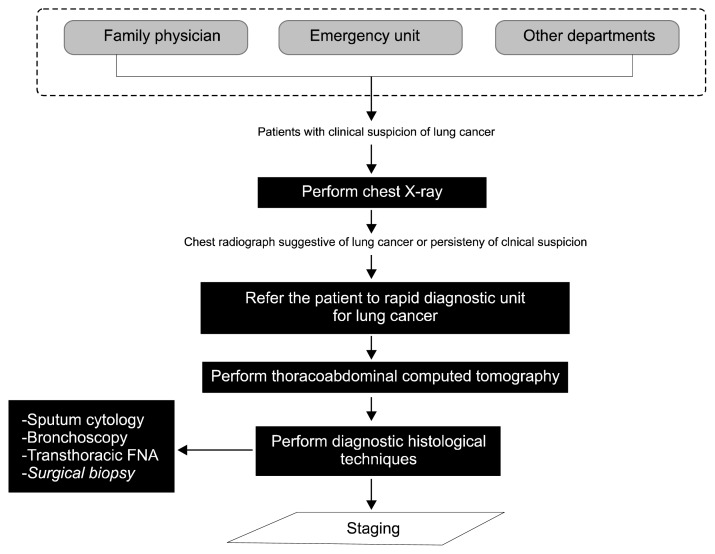
Every patient with a chest radiograph suggestive of lung cancer was referred to the Rapid Diagnostic Unit for Lung Cancer (RDULC) within a maximum of seven days. Thereafter, the patient began the diagnostic process shown in Fig. 1. The management of indeterminate pulmonary lesions was performed according to the classic Evidence-Based Clinical Practice Guidelines [5]. Unfortunately, PET scanning was not available at our hospital at the time of this study.
Fig. 1.
Diagnostic process. Diagnostic algorithm in patients with indeterminate pulmonary lesions. FNA, fine-needle aspiration.
In all cases, a multidisciplinary lung cancer committee consisting of pneumologists, oncologists, radiologists, thoracic surgeons, and pathologists assessed the indications for surgery. Preoperative cardiopulmonary evaluation was performed in all cases. The nutritional status was also studied. All patients underwent posterolateral thoracotomies.
4) Informed consent
Given the retrospective nature of data collection, informed consent was not obtained from the patients, although the Clinical Research Ethics Committee of Vigo University Clinical Hospital approved this study.
RESULTS
A total of 125 patients were retrospectively included in the study. The average age was 63.41±11.11 years with a range of 30.46 to 80.73 years. Eighty-eight of these patients were men versus 37 women. We performed a subgroup analysis for age. We did not find a statistical significance (p=0.319) between Patients with benign lesions (60.36±12.74 years) and the group with a postoperative diagnosis of malignancy (63.73±10.93 years).
With respect to the gender variable, 11 (12.5%) of the 88 men who made up the series underwent surgery for benign lesions, and the remaining 77 (87.5%) male patients had a postoperative diagnosis of malignancy. Thirty-seven cases were women, of which 1 (2.7%) belonged to the group of surgery for benign lesions, and 36 (97.3%) belonged to the group with a postoperative diagnosis of malignancy. There was no statistically significant difference (p=0.108) with respect to gender between these two groups.
When we analyzed the two previous variables, age and gender, together, we observed that the average age of male patients (63.74±10.75 years) was slightly higher than that of the female patients (62.62±12.04 years). This distinction was not statistically significant (p=0.611).
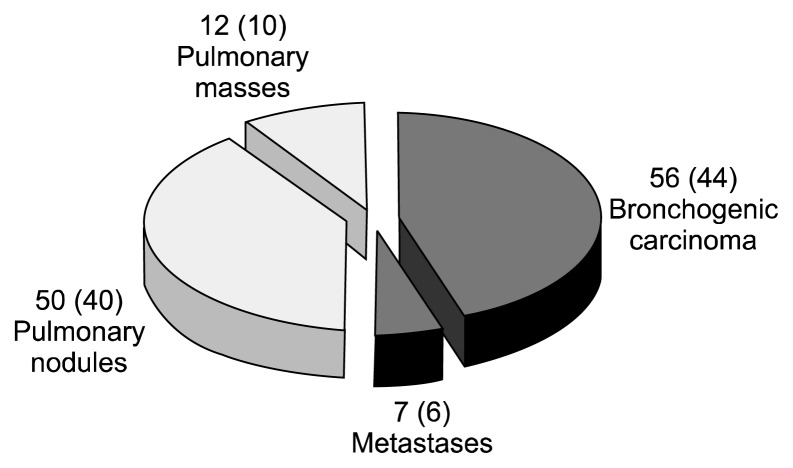
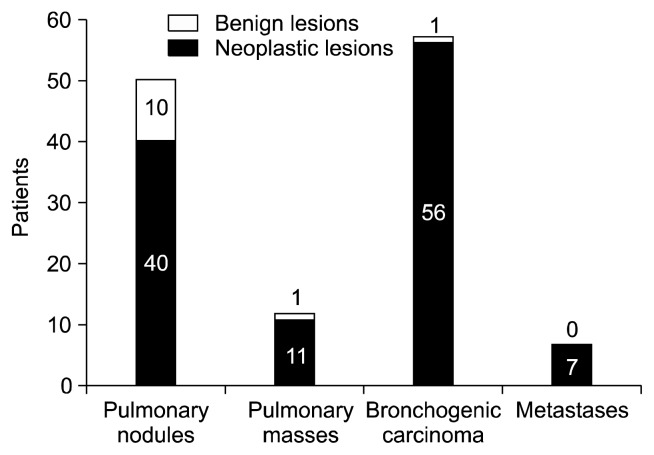
In terms of the diagnosis, of the 125 patients included in the study, 63 (50.4%) had a preoperative histological diagnosis of malignancy, corresponding to 56 (44.8%) primary lung tumors and 7 (5.6%) metastases. CT-guided transthoracic FNAB was diagnostic in 34 cases and bronchoscopy was diagnostic in 29 cases. The remaining 62 (49.6%) cases without a preoperative histological diagnosis were divided among 50 (40%) solitary pulmonary nodules and 12 (9.6%) pulmonary masses (Fig. 2). According to the postoperative pathologic examination, we identified 12 (9.6%) benign lesions excised. Of these, 10 (20%) cases were in the group of 50 solitary pulmonary nodules, 1 case was in a group of 12 undiagnosed lung masses, and 1 case was in the group of 56 patients with a preoperative diagnosis of primary lung cancer (false positive fine-needle aspiration biopsy interpretation). No unnecessary procedures were identified in the group of pulmonary metastases (Fig. 3). Diagnoses of these benign lesions were inflammatory nodules (8 cases, 66.7%), pneumonia (1 case, 8.3%), anthracotic nodule (1 case, 8.3%), fibrosis (1 case, 8.3%), and pulmonary amyloidosis (1 case, 8.3%) (Table 1).
Fig. 2.
Preoperative diagnosis. Values are presented as number (%).
Fig. 3.
Postoperative diagnosis.
Table 1.
Benign lesions
| Diagnosis | Value |
|---|---|
| Inflammatory nodule | 8 (66.7) |
| Pneumonia/pneumonitis | 1 (8.3) |
| Anthracotic nodule | 1 (8.3) |
| Fibrosis | 1 (8.3) |
| Amiloidosis | 1 (8.3) |
Values are presented as number (%).
With respect to the tumor size, we observed that the maximum mean diameter of benign lesions (17 mm) was smaller than the maximum mean diameter of neoplastic lesions (30.38 mm). By analyzing this variable with the Student t-test, we obtained statistical significance (p=0.045). Similarly, calcifications in benign lesions (16.7%) were more frequent than in malignant lesions (6.2%) (Table 2).
Table 2.
Variables and results
| Variables | Benign lesions (n=12) | Neoplastic lesions (n=113) | p-value |
|---|---|---|---|
| Age (yr) | 60.36±12.74 | 63.73±10.93 | 0.319 |
| Sex | 0.108 | ||
| Male | 11 | 77 | |
| Female | 1 | 36 | |
| Tumoral size (mm) | 17±3.66 (σ 13.43) | 30.38±4.73 (σ 22.41) | 0.045 |
| Nodule | 10 (83) | 74 (65.5) | 0.33 |
| Mass | 2 (17) | 39 (34.5) | |
| Location | |||
| Upper lobes/middle lobes | 6 (50) | 56 (49.5) | |
| Lower lobes | 6 (50) | 54 (47.8) | |
| Hilum | 0 | 3 (2.7) | |
| Calcifications | 2 (16.7) | 7 (6.2) | 0.21 |
Values are presented as mean±standard deviation, number, or number (%).
As for the treatment and surgical technique for benign lesions, a wedge resection was performed in 8 cases; enucleation in 1 case; lobectomy in 2 cases; and pneumonectomy in 1 case due to intraoperative complications. In all cases without a preoperative diagnosis, an intraoperative analysis was essential for choosing the appropriate surgical technique. In our analysis we found statistical significance (p=0.025), and wedge resection was considered the surgical technique of choice in all benign lung lesions whenever possible.
DISCUSSION
Cancer is the second leading cause of death (23.1%) in the world, after heart disease, according to the National Centre for Health Statistics [6]. Lung cancer accounts for more deaths than any other type of cancer in both men and women. An estimated 159,480 deaths, accounting for about 27% of all cancer deaths, were expected to occur in 2013 in the United States [1]. A study published [7] in 2009 showed an upward trend in mortality but with a decrease in the growth of the percentage; thus, it is expected that in the coming decades, there will be a stabilization and even a subsequent decline in the incidence of the disease.
Diagnostic tools for the early detection of lung cancer are becoming increasingly common. Nonetheless, most cases are diagnosed at advanced stages and curative treatment is not possible. The creation of a RDULC can improve the prognosis of the disease by favoring early detection. In Vigo University Clinical Hospital, all patients with clinical or radiological suspicion of lung cancer should be referred to a specialist before seven days in order to start the diagnostic process.
Current strategies to distinguish between benign and malignant nodules include close radiological follow-up, tissue biopsy, and surgical resection. As surgeons, we must find a careful balance between attempting to identify and resect early-stage lung cancer and minimizing the rate of resection of benign pulmonary lesions.
A CT scan can provide clues as to whether a solitary pulmonary nodule is benign or malignant. CT scans with dynamic contrast enhancement have been proven to be highly sensitive but non-specific for identifying malignant nodules. In our review, the CT scan showed that benign lesions had a smaller tumor size and an increased presence of calcifications.
Another test that can be useful is a PET scan. A meta-analysis published in 2001 by Gould et al. [8] established the sensitivity and the specificity of PET to be around 96.8% and 77.8%, respectively. This implies that a PET scan can lead to false positive results. Increased uptake of fluorodeoxyglucose is not due exclusively to malignant tumors. Several chronic infectious or inflammatory processes, such as histoplasmosis, tuberculosis, or silicosis, and some benign tumors such as hamartomas, can give false positive images, which can be misleading [9].
Complementing these imaging tests, we have other tools that sometimes let us obtain a preoperative cytological or histological diagnosis. Using flexible bronchoscopy, we can obtain samples through bronchoalveolar lavage, bronchoaspiration, bronchial biopsy, or thoracoscopic fine-needle aspiration. The diagnostic yield of CT-guided transthoracic FNAB confirming malignancy in peripheral 2-cm nodules is 80% to 90%, which decreases to 50% to 70% in nodules smaller than 2 cm or located near the pulmonary hilum [10]. However, FNAB has its limitations in the study of benign lung disease. This was reflected in a study [11] in which 38 of the 140 patients who had undergone lung resection for benign pulmonary nodules had preoperative FNAB. Twenty-nine of the 38 cases (79%) of these biopsies were considered non-diagnostic. Four cases were false positive for neoplastic disease, and only 5 of the 38 cases yielded a benign diagnosis. On the other hand, thoracoscopic fine-needle aspiration biopsy could be a good alternative for a centrally located solitary pulmonary nodule suspected of being lung cancer [12].
The ideal situation would be one in which, once the diagnosis process is complete, we could obtain a histological diagnosis. Discerning between benign and malignant pulmonary lesions is an important factor in surgical decision-making. However, despite all the diagnostic advances made so far, this is not always possible. Sometimes, we have to perform surgery to obtain a diagnosis. The aim of this study is to determine the prevalence and characteristics of benign lesions, for which we had no preoperative diagnosis, that were excised during lung cancer surgery. Although surgery is sometimes a necessary procedure simply for diagnosis, it should be the last resort in a thorough diagnostic process because surgery is an invasive procedure for the patient.
On the basis of a thorough review of medical literature, we observed that the percentage of benign tumors resected in lung cancer surgery has been decreasing over time. This is partly due to the availability of new diagnostic techniques and the formation of multidisciplinary committees on lung cancer. Toomes et al. [13] collected the data of 955 cases that underwent surgery between 1970 and 1980, finding 51% of the benign nodules resected. In 2000, Rubins et al. [14] published a series of reports on 540 patients operated between 1981 and 1994; they identified 21% of the benign nodules resected. In a recent series of cases [15,16], as compiled by Smith from 1995 to 2002, the values were much lower—around 9%, similar to those obtained in our study (9.6%). Therefore, it is expected that over time, these figures will decrease further. We consider this percentage of benign lesions excised to be a quality management index of indeterminate lung lesions. A higher percentage than that reported by different groups in the scientific literature could mean a mishandling of indeterminate lung lesions. Although it is impossible to completely eliminate resections of benign lesions, it is important to exhaust every option to make these resections as uncommon as possible without compromising the early diagnosis and resection of actual lung cancers.
With respect to the histology of benign lung lesions, several diagnoses are possible. The most common findings correspond to infectious processes, inflammatory or cicatricial nodules, or amyloidosis. Further, although parenchymal hamartomas are slow-growing and usually asymptomatic tumors, we decided not to include them in this study. We consider these tumors to have a neoplastic pathology, although most of the time, they do not exhibit malignant behavior.
With respect to surgical treatment, indeterminate pulmonary nodules should be treated by wedge resection whenever possible. The surgical resection should then be extended if in-traoperative cyto-histological analysis shows malignancy. At the time of this study, all our patients underwent surgery through a thoracotomy. We initiated the video-assisted thoracic surgery (VATS) program one year later. Kuo et al. [17] affirmed that VATS has led to an increase in our overall benign lesion resection rate, which can be explained by the increased number of VATS wedge resections that are being performed. Nevertheless, we believe that currently, VATS is a better option in these cases. VATS has been shown to be associated with decreased morbidity and mortality compared with traditional thoracotomy approaches in multiple randomized controlled trials [18].
The limitations of our study include a retrospective design with a small sample size, which decreases the power to detect differences between patients who present benign lesions and patients with a malignant disease. Furthermore, we did not have the essential diagnostic tool of PET at the time of this study.
In conclusion, the study of an indeterminate pulmonary nodule is a major challenge for physicians. The aim of our study was to determine the prevalence and characteristics of benign lesions removed in the context of lung cancer surgery. Our results (9.6% of lung resections) were very similar to those of the most current studies published recently. We conclude that this implies the effective management of an indeterminate pulmonary nodule. An analysis of different variables revealed that the group of patients undergoing surgery for benign lesions had a lower average age, those for lung lesions had a higher percentage of calcifications, and the tumor size was smaller than in the case of neoplastic disease. Furthermore, wedge resection is the surgical technique of choice for pulmonary benign tumors in order to preserve the lung parenchyma.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.American Cancer Society. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer. 2000;89(11 Suppl):2474–82. doi: 10.1002/1097-0142(20001201)89:11+<2474::aid-cncr26>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Jett JR. Limitations of screening for lung cancer with low-dose spiral computed tomography. Clin Cancer Res. 2005;11(13 Pt 2):4988s–4992s. doi: 10.1158/1078-0432.CCR-05-9000. [DOI] [PubMed] [Google Scholar]
- 4.Diederich S, Wormanns D. Impact of low-dose CT on lung cancer screening. Lung Cancer. 2004;45(Suppl 2):S13–9. doi: 10.1016/j.lungcan.2004.07.997. [DOI] [PubMed] [Google Scholar]
- 5.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Escuin JS. Lung cancer in Spain: current epidemiology, survival, and treatment. Arch Bronconeumol. 2009;45:341–8. doi: 10.1016/j.arbres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 9.Yu CH, Wang T, Sun YE, Yao SL, Tian JH, Yin DY. Fluorine-18 fluorodeoxyglucose uptake in patients with benign pulmonary nodules. Zhonghua Wai Ke Za Zhi. 2006;44:90–2. [PubMed] [Google Scholar]
- 10.Cummings SR, Lillington GA, Richard RJ. Managing solitary pulmonary nodules: the choice of strategy is a “close call”. Am Rev Respir Dis. 1986;134:453–60. doi: 10.1164/arrd.1986.134.3.453. [DOI] [PubMed] [Google Scholar]
- 11.Layfield LJ, Coogan A, Johnston WW, Patz EF. Transthoracic fine needle aspiration biopsy. Sensitivity in relation to guidance technique and lesion size and location. Acta Cytol. 1996;40:687–90. doi: 10.1159/000333940. [DOI] [PubMed] [Google Scholar]
- 12.Sung HK, Kim HK, Choi YH. Thoracoscopic needle aspiration biopsy for a centrally located solitary pulmonary nodule. Korean J Thorac Cardiovasc Surg. 2013;46:316–8. doi: 10.5090/kjtcs.2013.46.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toomes H, Delphendahl A, Manke HG, Vogt-Moykopf I. The coin lesion of the lung: a review of 955 resected coin lesions. Cancer. 1983;51:534–7. doi: 10.1002/1097-0142(19830201)51:3<534::aid-cncr2820510328>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Rubins JB, Ewing SL, Leroy S, Humphrey EW, Morrison V. Temporal trends in survival after surgical resection of localized non-small cell lung cancer. Lung Cancer. 2000;28:21–7. doi: 10.1016/s0169-5002(99)00116-6. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Battafarano RJ, Meyers BF, Zoole JB, Cooper JD, Patterson GA. Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann Thorac Surg. 2006;81:1824–8. doi: 10.1016/j.athoracsur.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Lucena J, Coronel P, Orellana Y. Benign pulmonary nodules excised for suspicion of malignancy. Experience in 140 patients. Rev Chilena de Cirugia. 2009;61:27–32. [Google Scholar]
- 17.Kuo E, Bharat A, Bontumasi N, et al. Impact of video-assisted thoracoscopic surgery on benign resections for solitary pulmonary nodules. Ann Thorac Surg. 2012;93:266–72. doi: 10.1016/j.athoracsur.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Sedrakyan A, van der Meulen J, Lewsey J, Treasure T. Video assisted thoracic surgery for treatment of pneumothorax and lung resections: systematic review of randomised clinical trials. BMJ. 2004;329:1008. doi: 10.1136/bmj.38243.440486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]