Abstract
Immunotherapeutic approaches to cancer have shown remarkable promise. A critical barrier to successfully executing such immune-mediated interventions is the selection of safe yet immunogenic targets. As patient deaths have occurred when tumor-associated antigens shared by normal tissue have been targeted by strong cellular immunotherapeutic platforms, route of delivery, target selection and the immune-mediated approach undertaken must work together to maximize efficacy with safety. Selected tumor-specific targets can spare potential toxicity to normal tissue; however, they are far less common than tumor-associated antigens and may not be present on all patients. In the context of immunotherapy for high-grade glioma, 2 of the most prominently studied antigens are the tumor-associated epidermal growth factor receptor and its tumor-specific genetic deletion variant III. In this review, we will summarize the immune-mediated strategies employed against these targets as well as the caveats particular to these approaches.
Keywords: epidermal growth factor receptor, glioma, immunology, immunotherapy
Glioblastoma
Current standard of care (SOC) for glioblastoma multiforme (GBM) consists of resection, radiotherapy, and temozolomide. These therapies are nonspecific, limited in application due to toxicity against normal brain, and ineffective, as overall survival (OS) remains under 15 months. While rarely metastatic, local recurrence after treatment is inevitable, as GBM is characterized by individual, infiltrative, treatment-resistant tumor cells well past the resected tumor margin. Emerging novel therapies must meet 2 key criteria: (i) enhanced tumor specificity to prevent toxicity to surrounding normal brain, and (ii) effective access to, and identification of, single tumor cells embedded in normal tissue to lyse tumor infiltrates and prevent recurrence. Immunotherapy promises an exquisitely precise approach through activating immune responses specifically against cells bearing tumor antigen without damage to the surrounding eloquent cerebral cortex. Furthermore, therapy-induced tumor-reactive lymphocytes are actively mobile biologic agents and possess the potential to harmlessly infiltrate normal brain while seeking and destroying residual tumor. Substantial evidence already suggests that immunotherapeutic intervention can eradicate large, well-established tumors in mice and humans and can destroy immunogenic melanoma metastases residing within the “immunologically privileged” brain.1
Epidermal Growth Factor Receptor and Variant III
While not considered an immunogenic tumor like melanoma, tumor-associated and tumor-specific antigens present on GBM have been identified. Among these potential antigens, epidermal growth factor receptor (EGFR) and the genetic deletion variant III (EGFRvIII) have been targeted by specific therapeutic approaches designed to block signaling or induce antitumor immune responses. In brief, EGFR is a receptor tyrosine kinase that is amplified and overexpressed in 60%–90% of GBM2 with near homogeneous expression upon tumor and expression that is absent to nearly undetectable on normal brain,3 although it has been reported on neural stem cells. While largely undetectable on normal brain, EGFR is present systemically, and an inherent safety risk to targeting this molecule is the potential toxicity to normal EGFR-bearing cells. Patient deaths have occurred when other tumor-associated antigens expressed by normal tissues have been targeted by intensely robust cell-mediated immunotherapeutic approaches.4,5 Therefore, many approaches targeting EGFR amplification on brain tumors have been administered intratumorally behind the blood–brain barrier, although it should be noted that systemic administration of blocking antibodies to EGFR has been well tolerated.6
The EGFRvIII mutation is completely tumor specific, present on approximately one third of GBM,7 present on 67%8 of tumors with amplified EGFR, and expressed on CD133+ brain tumor stem cells. EGFRvIII is produced by the deletion of exons 2–7, resulting in a greatly truncated extracellular domain with a novel glycine residue presented between amino acids 5 and 274.9 Functionally, EGFRvIII is ligand independent and constitutively activated and associated with inducing oncogenic transformation of adjacent cells through paracrine mechanisms10 as well as through the secretion of EGFRvIII positive exosomes.11 However, EGFRvIII is heterogeneously expressed within tumor. While the tumor specificity of targeting EGFRvIII offers exquisite safety, not all tumor cells are EGFRvIII positive, thereby risking the outgrowth of EGFRvIII-negative antigen loss variants under immune-mediated selection pressure.
Inhibition of Signaling
A complex and cooperative oncogenic signaling network between EGFR and EGFRvIII in GBM was recently elucidated and shown to culminate in the phosphorylation of signal transducer and activator of transcription 3.12 Inhibition of this signaling cascade with tyrosine kinase inhibitors has been performed; however, thus far it has proven ineffective in the context of GBM. Signaling from EGFR has also been targeted with the blocking antibodies cetuximab and nimotuzumab. In a phase II study of recurrent GBM, systemic administration of the EGFR-specific monoclonal antibody (mAb) cetuximab resulted in 3 of 55 patients demonstrating a partial response and 17 of 55 patients with stable disease as determined by investigator-assessed stabilization of tumor-associated symptoms.6 During a phase III randomized study of nimotuzumab therapy in adult GBM patients, a trend toward longer overall survival was seen in comparison with standard therapy.13 These modest responses can in part be attributed to limited tumor penetration. As small molecule inhibitors and blocking antibodies must rely on sufficient inhibition of driver mutation pathways, a lack of penetrance or an inability to fully impair signaling compromises efficacy. In contrast to small molecule inhibitors or passive immunotherapeutics, active immunotherapy results in the presence of activated cytotoxic T cells relying solely on antigenic recognition within the target cell population for serial lysis. Thus, engaging the full repertoire of T-cell effector functions resulting from vaccination or adoptive transfer of T cells specifically targeting EGFR and EGFRvIII may be a more successful antitumor approach.
Passive Immunotherapy
As opposed to inhibiting the signaling cascade originating from EGFR and EGFRvIII in GBM, antibodies targeting these molecules can also induce immune-mediated recognition and destruction of tumor by themselves (through antibody-dependent cell-mediated or complement-mediated cytotoxicity) or through conjugation to radionucleotides or toxins to deliver a cytotoxic payload to tumor. While unarmed mAbs to EGFRvIII have not been examined clinically for efficacy, in a murine model of orthotopic EGFRvIII-positive brain tumor, intratumoral EGFRvIII mAb induced immune-mediated cytotoxicity and extended median survival by 286%.14 An EGFRvIII-specific single chain variable fragment (scFv) was also conjugated to recombinant genetically engineered protein derived from the Pseudomonas exotoxin (MR-1). MR-1 binds specifically to EGFRvIII to induce cell death upon endocytosis and significantly increased median survival in a preclinical model of EGFRvIII-positive neoplastic meningitis.15 An immunotoxin with dual specificity against both EGFR and EGFRvIII has also been shown to increase survival in a murine model of EGFR and EGFRvIII-double-positive glioma by 166% versus controls and to possess superior tumor localization in comparison with antibodies targeting EGFR or EGFRvIII alone.2 Intriguingly, a mAb with dual specificity to EGFRvIII and a subset of amplified EGFR demonstrated additive, and sometimes synergistic, antitumor efficacy in a murine model of glioma when used combinatorially with a molecular inhibitor of EGFR signaling.16
Clinically, a phase I study of an EGFRvIII targeted scFv conjugated to a Pseudomonas exotoxin–derived protein delivered intracerebrally by convection enhanced delivery (CED) has been completed in patients with EGFRvIII-positive brain tumors, although results have not yet been published. Clinical studies of EGFR targeted conjugates have also been undertaken, and rhenium-188 labeled nimotuzumab delivered intracavitarily in a phase I trial of recurrent high-grade glioma showed 3 of 11 patients with a partial (n = 1) or complete response (n = 2) for greater than a year.17 Similarly, EGFR was targeted through conjugating its ligand transforming growth factor–α to a Pseudomonas exotoxin–derived chimeric protein to create TP-38. TP-38 was administered to 15 patients with recurrent malignant brain tumors with residual disease by CED directly to the resection cavity, with 2 patients showing radiographic responses and 1 patient with GBM demonstrating a nearly complete response.18 However, imaging from this study revealed that in >80% of the cases, the infusate leaked into the cerebrospinal fluid spaces, thereby not reaching residual tumor. While novel imaging technologies are improving CED, despite the excellent specificity of these passive immunotherapeutics, they still likely have limited penetrance and an inability to reach the infiltrative tumor cells that engender recurrence, even after direct intracerebral administration.
Immunotherapeutic Vaccination
Activated, tumor-specific T cells, however, are not constrained by diffusion and have the capacity to actively survey tissue. Antitumor immunization requires the tumor antigen to be taken up by dendritic cells (DCs) and presented to T cells in the context of the appropriate costimulation. Vaccine-induced activated T cells are able to enter the central nervous system, where CD8+ cytotoxic T lymphocytes (CTLs) can directly lyse tumor cells bearing antigen. More specifically, induced antitumor immunization against EGFRvIII has been demonstrated by both DC and peptide vaccination approaches. In the former, autologous DCs were derived from patients with newly diagnosed GBM and pulsed with a 14-amino-acid peptide encompassing the EGFRvIII peptide conjugated to keyhole limpet hemocyanin (rindopepimut). Three consecutive immunizations were given in the upper thigh with no severe adverse events, and 56% of patients showed an immune response to EGFRvIII after vaccination.19 While survival outcomes were not significantly different than expected, this trial was performed to assess the safety and immunogenicity of rindopepimut. Therefore, EGFRvIII expression on the tumor was not used as an inclusion criterion, and many of the tumors in this small trial were most likely not EGFRvIII positive.
The immunogenicity of rindopepimut as a peptide vaccine was also assessed. In a murine model of orthotopic brain tumor, rindopepimut immunization induced vaccine-specific immunity and extended survival.20 This approach was translated into a multicenter phase II trial in patients with newly diagnosed GBM. Patients immunized with rindopepimut had significantly extended OS in comparison with matched controls (OS of vaccinated patients: 26 mo; OS of matched controls: 15 mo; hazard ratio, 5.3; P = .0013).21 While no patients possessed evidence of EGFRvIII-specific immunity prior to administration of rindopepimut, the development of vaccine-induced immunity correlated with extended survival. Additionally, while patients lacking methylation at the methylguanine methyltransferase (MGMT) promoter have decreased OS and resistance to current SOC, in this phase II trial assessing rindopepimut, vaccination was equivalently efficacious in patients of either methylation status. This suggests that EGFRvIII-targeted vaccination is agnostic to the biological consequences of MGMT methylation that impede current SOC, and perhaps to other biological functions of the cell as well. Furthermore, in a consecutive phase II trial examining the immunogenicity of rindopepimut in the context of standard or dose-intensified temozolomide, OS was 23.6 months (hazard ratio, 0.23; P = .019), and all patients developed EGFRvIII-specific immune responses.22 No patients experienced autoimmunity, and vaccination was well tolerated. As a result of the extended OS seen after rindopepimut, this vaccine is now being tested in an international phase III randomized trial. However, intratumoral EGFRvIII expression is heterogeneous, and the strategy of targeting a single heterogeneous antigen may permit the outgrowth of antigen-negative cells. Analysis of 13 recurrent tumors after rindopepimut treatment showed that 11 no longer expressed EGFRvIII and that 1 of the 2 EGFRvIII-positive recurrent tumors had <1% of cells positive for EGFRvIII.21 A subsequent retrospective analysis of 45 patients with EGFRvIII-positive GBM showed that 15 of 15 evaluated patients who recurred after SOC maintained EGFRvIII, while 16 of 16 evaluated patients who recurred after rindopepimut lost expression of EGFRvIII. These data suggest that EGFRvIII-targeted immunization induces the outgrowth of an EGFRvIII-negative population and points to a mechanism of resistance inherent in targeting a single heterogeneously expressed antigen.
Chimeric Antigen Receptors
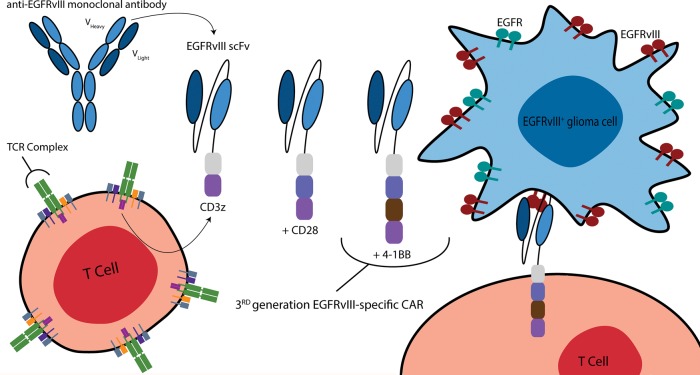
Antitumor vaccination targeting EGFR for the induction of systemic immunity is implausible due to the barrier of central tolerance and localization of the antigen on normal cells. However, genetically engineered T cells specific to EGFR can be generated ex vivo and adoptively transferred intracerebrally to achieve a locoregional active immunotherapeutic approach. As the overexpression of EGFR is homogeneous within tumor, such an approach may lessen the risk of antigen loss variants. Chimeric antigen receptors (CARs) typically combine the variable regions of an antibody with T-cell signaling moieties to confer T-cell activation with the targeting specificity of the parental antibody (Fig. 1), resulting in T-cell activation and target cell lysis. The use of an antibody to redirect T-cell effector function via a CAR confers the potential to recognize any cell surface antigen without presentation through human leukocyte antigen (HLA). The benefits of this engineering of T cell specificity are 3-fold. First, tumors present abnormal HLA molecules or downregulate their expression, which is a major mode of immune evasion. CARs are therefore not prone to the same risks as alternative T-cell therapies that depend on the presence of HLA molecules. Second, the same CAR constructs can be used between patients with tumors expressing the same target antigen (eg, EGFR), since they function independently of HLA restriction. Third, as targeting is antibody based, the specificity of the T cell is not limited by thymic selection, and the CAR-transduced T cell can be redirected against any antigen with an appropriate antibody and thus overcome immune tolerance.
Fig. 1.
Multigeneration EGFRvIII-specific CARs. CARs directed against EGFRvIII are produced by combining the humoral specificity of an EGFRvIII-specific antibody with the intracellular signaling domains of a T-cell receptor (TCR). In general, CARs are composed of the variable heavy and light chains of a mAb fused (via a transmembrane hinge) to CD3ζ. More recently, CAR design has evolved to include additional costimulatory moieties—namely CD28 and/or 4-1BB—to improve OS, proliferation, and antitumor activity. The third-generation EGFRvIII-specific CAR incorporates the CD28, 4-1BB, and CD3ζ signaling constructs. These same CAR designs can be used to target wild-type EGFR.
The CAR molecule has been designed to improve T-cell function. First-generation CARs generally included a single CD3 intracellular domain and successfully redirected cellular cytotoxicity but often possessed limited survival and poor antitumor activity in vivo due to an absence of appropriate costimulation. Second- and third-generation CARs incorporate additional intracellular costimulatory moieties, such as CD28, OX-40, and 4-1BB, to impart improved T-cell proliferation, survival, cytokine secretion, and tumor lysis. Clinical trials utilizing CARs against a variety of antigens and malignancies have demonstrated remarkable therapeutic potential. At MD Anderson Cancer Center, 2 distinct CAR T cells specific to EGFR have been created and tested preclinically. While life-threatening toxicity and death can accompany CARs when targeted against antigens on normal tissue,4,5 it should be noted that the EGFR CAR T cells will be regionally delivered and can also be bioengineered for safety. Indeed, genetic modification of T cells with in vitro–transcribed mRNA results in desired transient CAR expression on T cells and elimination after a few days. This bioengineering approach lends itself to repeated local infusions of transiently expressing EGFR-specific CAR T cells to achieve a therapeutic effect while minimizing systemic toxicity.
Third-generation CARs incorporating the variable region of an EGFRvIII-specific antibody have also been developed.23–26 Due to the tumor specificity of EGFRvIII, these can be administered systemically or intracerebrally and should not lead to direct killing of normal tissues, as seen with CARs targeting ERBB2 and other tumor-associated, but not tumor-specific, antigens.4,5 In a highly infiltrative intracranial xenogeneic model of GBM, EGFRvIII-specific human CAR T cells administered systemically not only trafficked to the brain but localized to invasive tumor deposits to suppress tumor growth and enhance OS.24 EGFRvIII-specific murine CAR T cells were also evaluated against a syngeneic intracranial tumor system in a fully immunocompetent mouse model of established malignant glioma. As the majority of preclinical studies examining CAR efficacy have thus far used immune-deficient xenograft models, the development of a syngeneic system is critical for evaluating CAR function within the immunosuppressive tumor microenvironment and for determining whether CAR therapy can interact with the intact host immune system. Significantly, CAR T cells were shown to be curative at the highest doses following lymphodepletive host conditioning, a common requisite to successful adoptive therapy of T cells.26 Moreover, this study showed that mice that were previously cured of EGFRvIII-positive tumors by CARs were resistant to a secondary tumor challenge with EGFRvIII-negative tumors. This suggests that CAR-induced epitope spreading generated an endogenous host immune response against new tumor antigens. The ability to induce a localized, tumor-specific, de novo host response via tumor-specific CAR therapy would likely eliminate the concern of antigen loss variants and would provide a personalized vaccination effect.27 Supporting this observation is that vaccine-induced epitope spreading was shown to contribute to regression of metastases in a patient with melanoma.28 Clinically, EGFRvIII-specific CARs are now being examined in a phase I/II safety study at the National Cancer Institute for patients with recurrent GBM.25
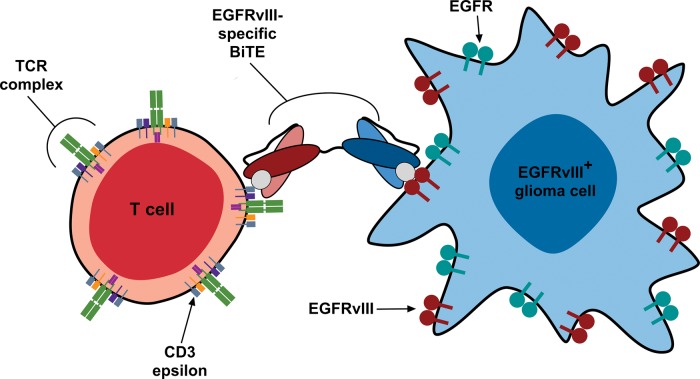
Bispecific T-cell Engagers
Genetically engineered T cell–based therapies, although functionally effective, have limitations because they require viral transduction and heavily trained laboratory personnel to generate individualized patient-specific vaccines. In direct contrast, bispecific antibodies, termed bispecific T-cell engagers (BiTEs), are monomeric proteins composed of 2 antibody-derived scFvs translated in tandem and are relatively easy to manufacture. These constructs consist of an effector-binding arm specific for T-cell CD3 epsilon and an opposing target-binding arm specific for an antigen that is expressed on the surface of tumor cells (Fig. 2). Bispecific antibody constructs are safe and effective because they create a molecular tether resulting in highly localized, HLA-independent, specific T-cell activation with concomitant lysis of tumor cells expressing the BiTE target antigen. In a phase I clinical study, 7 of 7 patients with non-Hodgkin lymphoma treated with a CD19-targeted BiTE at doses as low as 0.06 mg/m2/day for a 1-month continuous infusion period demonstrated objective tumor regression in addition to clearance of tumor from the liver, bone marrow, and blood.29 Using an EGFRvIII scFv, our group at Duke University has recently developed an EGFRvIII-targeted BiTE to redirect T cells against EGFRvIII-expressing glioma. In addition to its tumor specificity, EGFRvIII is an excellent target for BiTE therapy, as its minimal extracellular domain places the antigenic epitope close to the cellular surface, which enhances BiTE-induced cytotoxicity. In preclinical studies, this construct induced T helper (Th)1–type cytokine secretion and polyclonal T-cell proliferation, exclusively in the presence of effector T cells and EGFRvIII-positive glioma.30 Target specificity was further demonstrated through in vitro cytotoxicity assays in which the construct induced significant lysis of EGFRvIII-positive but not EGFRvIII-negative glioma. In vivo, 5 daily doses of the EGFRvIII-targeted BiTE resulted in complete cures in orthotopic tumor-bearing murine models, and even treatment of late-stage disease significantly extended survival (P < .01). Translation of this therapeutic as a safe and effective treatment for patients with EGFRvIII-positive glioma is under way at Duke University.
Fig. 2.
EGFRvIII-specific BiTEs. The EGFRvIII-specific BiTE produces an immunologic synapse between EGFRvIII-positive glioma cells and effector T cells. Synapse formation results in the upregulation of T-cell activation markers, elaboration of Th-1 cytokines, T-cell proliferation, and target cell–specific lysis. Note that the absence of cross-reactivity with the wild-type EGFR ensures a tumor cell–specific immune response. TCR, T-cell receptor.
Future Directions
Immunotherapeutic intervention targeting EGFR and EGFRvIII has already shown promise, most aptly demonstrated by the successful translation of the rindopepimut vaccine into an international phase III trial. Future discovery of additional shared tumor-specific mutations on GBM tumors may permit rindopepimut to be combined into a multi-epitope vaccination to maximize safety while preventing the outgrowth of antigen loss variants. However, it is well established that even platforms that generate robust antitumor responses are still subject to the immunosuppressive milieu of the tumor microenvironment, which can render CTLs into an exhausted and ineffective state.27 Antitumor immunity must be able to persist and function at the tumor in order to engender tumor cell lysis. The discovery and antibody-mediated blocking of immune checkpoint inhibitors such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have been able to potentiate such antitumor immunity and have demonstrated remarkable clinical antitumor efficacy.27 Combinatorial use of these antibodies or immune agonists with immunotherapeutic vaccination to potentiate immune responses and impair immunosuppression is an area of active research. Importantly, an ongoing, international, randomized registration trial of CTLA-4 (ipilimumab) and PD-1 (nivolumab) blockade in patients with recurrent GBM will provide critical insight into the use of these agents in GBM. The development of truly robust immune-mediated approaches against tumor-associated and tumor-specific targets like EGFR and EGFRvIII may permit the type of localized epitope spread that will reveal novel patient-specific tumor mutations and engender de novo immunity to these epitopes. While autoimmune toxicity remains a valid concern, inducing such a localized, multi-epitope, antitumor immune response may be required to eradicate diffuse and heterogeneous tumors like GBM and prevent the specter of recurrence.
Funding
This work was supported by grants NIH R01-NS085412-01 (J.H.S.), NIH R01-CA177476-02 (J.H.S.), NIH RO1-CA120813-08 (A.B.H.), NIH R25-NS065731-05 (J.H.S.), NIH P01 CA154291-02 (Darell D. Bigner/J.H.S.), NIH P01 CA154291-02 (Darell D. Bigner), NIH Brain SPORE 5P50 CA127001-06 (Frederick Lang), 2P30 CA016672-39 (Ron DePinho), Pediatric Brain Tumor Foundation (Darell D. Bigner), Pediatric Brain Tumor Foundation (Darell D. Bigner/J.H.S.), Accelerate Brain Cancer Cure, Inc. (J.H.S.), Kinetics Foundation (J.H.S.), Dr Marnie Rose Foundation (A.B.H.), Cynthia and George Mitchell Foundation (A.B.H.), Annias Immunotherapeutics Agrmnt (J.H.S.), The Cure Starts Now (Oren J. Becher).
Conflict of interest statement. Employment or leadership position: None. Consultant or advisory role: J.H.S., Celldex Therapeutics; A.B.H., Celldex Therapeutics and Bristol-Myers Squibb; L.J.C., Targazyme Inc. (formerly American Stem Cells, Inc.), GE Healthcare, Ferring Pharmaceuticals, Inc., and Bristol-Myers Squibb. Company/stock ownership: L.J.C., InCellerate, Inc. Honoraria: J.H.S., Celldex Therapeutics; L.J.C., Miltenyi Biotec. Research funding: J.H.S., Celldex Therapeutics. Expert testimony: None. Licensed patents and royalties: J.H.S. and A.B.H. received funding under the Duke University Faculty Plan from license fees paid to Duke University by Celldex Therapeutics; L.J.C., Sangamo BioSciences.
References
- 1.Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16(19):4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandramohan V, Bao X, Keir ST, et al. Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin Cancer Res. 2013;19(17):4717–4727. doi: 10.1158/1078-0432.CCR-12-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libermann TA, Razon N, Bartal AD, et al. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44(2):753–760. [PubMed] [Google Scholar]
- 4.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 7.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 8.Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–1387. [PubMed] [Google Scholar]
- 9.Wikstrand CJ, Reist CJ, Archer GE, et al. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4(2):148–158. doi: 10.3109/13550289809114515. [DOI] [PubMed] [Google Scholar]
- 10.Inda MM, Bonavia R, Mukasa A, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24(16):1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 12.Fan QW, Cheng CK, Gustafson WC, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–449. doi: 10.1016/j.ccr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westphal M, Bach F. Chicago, IL: J Clin Oncol; 2012. Final results of a randomized phase III trial of nimotuzumab for the treatment of newly diagnosed glioblastoma in addition to standard radiation and chemotherapy with temozolomide versus standard radiation and temoziolamide. ASCO Annual Meeting. Vol 30. [Google Scholar]
- 14.Sampson JH, Crotty LE, Lee S, et al. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97(13):7503–7508. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer GE, Sampson JH, Lorimer IA, et al. Regional treatment of epidermal growth factor receptor vIII-expressing neoplastic meningitis with a single-chain immunotoxin, MR-1. Clin Cancer Res. 1999;5(9):2646–2652. [PubMed] [Google Scholar]
- 16.Johns TG, Luwor RB, Murone C, et al. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc Natl Acad Sci U S A. 2003;100(26):15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casaco A, Lopez G, Garcia I, et al. Phase I single-dose study of intracavitary-administered nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol Ther. 2008;7(3):333–339. doi: 10.4161/cbt.7.3.5414. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson JH, Archer GE, Mitchell DA, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9(11):4247–4254. [PubMed] [Google Scholar]
- 21.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi BD, Suryadevara CM, Gedeon PC, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J Clin Neurosci. 2014;21(1):189–190. doi: 10.1016/j.jocn.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao H, Choi BD, Suryadevara CM, et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9(4):e94281. doi: 10.1371/journal.pone.0094281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan RA, Johnson LA, Davis JL, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23(10):1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972–984. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbiere V, Chapiro J, Stroobant V, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71(4):1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 29.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell–engaging antibody. Science. 2008;321(5891):974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 30.Choi BD, Kuan CT, Cai M, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci U S A. 2013;110(1):270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]