Abstract
Objective:
While there is recent interest in using repeated deep inspiratory breath-holds, or prolonged single breath-holds, to improve radiotherapy delivery, breath-holding has risks. There are no published guidelines for monitoring patient safety, and there is little clinical awareness of the pronounced blood pressure rise and the potential for gradual asphyxia that occur during breath-holding. We describe the blood pressure rise during deep inspiratory breath-holding with air and test whether it can be abolished simply by pre-oxygenation and hypocapnia.
Methods:
We measured blood pressure, oxygen saturation (SpO2) and heart rate in 12 healthy, untrained subjects performing breath-holds.
Results:
Even for deep inspiratory breath-holds with air, the blood pressure rose progressively (e.g. mean systolic pressure rose from 133 ± 5 to 175 ± 8 mmHg at breakpoint, p < 0.005, and in two subjects, it reached 200 mmHg). Pre-oxygenation and hypocapnia prolonged breath-hold duration and prevented the development of asphyxia but failed to abolish the pressure rise. The pressure rise was not a function of breath-hold duration and was not signalled by any fall in heart rate (remaining at resting levels of 72 ± 2 beats per minute).
Conclusion:
Colleagues should be aware of the progressive blood pressure rise during deep inspiratory breath-holding that so far is not easily prevented. In breast cancer patients scheduled for breath-holds, we recommend routine screening for heart, cardiovascular, renal and cerebrovascular disease, routine monitoring of patient blood pressure and SpO2 during breath-holding and requesting patients to stop if systolic pressure rises consistently >180 mmHg and or SpO2 falls <94%.
Advances in knowledge:
There is recent interest in using deep inspiratory breath-holds, or prolonged single breath-holding techniques, to improve radiotherapy delivery. But there appears to be no clinical awareness of the risks to patients from breath-holding. We demonstrate the progressive blood pressure rise during deep inspiratory breath-holds with air, which we show cannot be prevented by the simple expedient of pre-oxygenation and hypocapnia. We propose patient screening and safety guidelines for monitoring both blood pressure and SpO2 during breath-holds and discuss their clinical implications.
There is recent clinical interest in using both deep inspiratory breath-holds and techniques for prolonging breath-holding1 for patients undergoing radiotherapy or imaging,2 with up to four radiology articles per week mentioning breath-holding over the past 3 years. In particular, those with left breast cancer may undergo breath-holding to spare the heart from exposure to radiation. Sequentially repeated breath-holds with air of ca. 22 s are generally used,2 but prolonged single breath-holds of 3–7 min1,2 are now feasible.
Blood pressure is not routinely reported in such clinical studies for radiotherapy or imaging,2,3 and we are not aware of any consideration of its implications for patient safety. There appears to be no clinical awareness of the pronounced blood pressure rise (by 40 mmHg or more) that occurs during breath-holding.1,4–10 In young, otherwise healthy patients, brief periods of such hypertension are well tolerated. Patients requiring radiotherapy and complex imaging are, however, typically from an older age group and frequently have significant coexisting disease. Patients with atheromatous coronary artery plaques, aortic disease or cerebrovascular disease are at particular risk from sudden rises in systemic arterial blood pressure. Myocardial infarction, aortic dissection/rupture and haemorrhagic stroke are all potentially lethal complications in these patients. A systolic blood pressure >180 mmHg is considered a “hypertensive crisis” in current medical practice and is associated with a significant risk of end organ damage.11
Whilst multiple breath-holds and prolonged single breath-holding techniques are not yet widely enough used to generate any literature or reporting of serious adverse effects in patients from breath-holding, it is now prudent to consider safety guidelines formally before adverse advents might occur.
We therefore describe the blood pressure rise during the clinically used technique of breath-holding with air and have sought a simple, non-pharmacological and non-invasive method of preventing this rise. The conventional explanation is that the rise is caused by hypoxia and hypercapnia (i.e. asphyxia) of breath-holding stimulating systemic (peripheral) chemoreceptors.12–14 If this is correct, then the rise should be preventable because breath-holding can be easily prolonged with hyperoxia and/or hypocapnia.15
It might be expected that the pronounced blood pressure rise during breath-holding would be signalled, by a corresponding fall in heart rate (owing to baroreflex operation), which might be easier to measure non-invasively than blood pressure. We resolve the long-standing dispute over what happens to the heart rate during simple breath-holding at rest1,6–9,16–18 by using the latest techniques for instantaneous heart rate analysis to show that the blood pressure rise is not signalled by any corresponding fall in heart rate.
METHODS AND MATERIALS
Experiments were conducted following the Declaration of Helsinki19 and the approval of the local research ethics committee in the Wellcome Trust Clinical Research Facility. 12 normal, healthy subjects (9 males and 3 females) aged 20–22 years, with no previous experience of breath-holding, lay at rest in a semi-recumbent position and were instrumented15,20,21 to measure blood pressure [Finapres 2300 Ohmeda (Finapres Ohmeda, Englewood, CA) finger plethysmograph set at heart level], chest electrocardiogram (lead I) and oxygen saturation (SpO2; Nellcor™ finger pulse oximeter; Nellicor, Boulder, CA). Subjects breathed through a face mask. Partial pressure of carbon dioxide (PCO2) end tidal PCO2 (PetCO2) measurements from in-line capnographs (Hewlett-Packard 78536A; Hewlett Packard, Palo Alto) confirmed that subjects did not voluntarily hyperventilate. They listened to music throughout. Data were sampled at 2 kHz and analysed using a CED1401 data acquisition system (Cambridge Electronics Design, Cambridge, UK). We recorded 4 min of eupnoeic breathing in room air preceding the breath-hold. Subjects did not know when the breath-hold would start and did not surreptitiously hyperventilate.
To mimic the deep inspiratory breath-holds used clinically, subjects inhaled air maximally and held it as long as possible with a relaxed posture. They did not attempt pushing or inhaling against a closed glottis, as was easily confirmed1,22 by observation of their chest and neck and of their blood pressure record. The order of experiments was not the same for every subject.
We also studied prolonged single breath-holds. This was achieved by pre-oxygenating subjects by their breathing 60% oxygen (the maximum percentage of oxygen that may safely be given without increasing the risk of atelectasis3,23) for 4 min. It was additionally achieved by rhythmically mechanically ventilating them with positive pressure in 60% oxygen at 16 breaths per minute and at a tidal volume of approximately 1.2 l(depending on their size) using a face mask connected to a Drager Evita 2 ventilator; Drager, Luebeck, Germany.1,20,21 This induced 15 min of hypocapnia at mean PetCO2 level of 20 ± 0 mmHg, the lowest level of hypocapnia that can be safely induced without causing hypocapnic tetany.3,20,21,24
Each heart beat, blood pressure peak and trough were identified, and continuous lines were plotted of instantaneous heart rate (in beats per minute) peak, trough, peak trough difference (to then derive respiratory sinus arrhythmia measured in milliseconds), systolic, diastolic, mean pressure and SpO2. Instantaneous heart rate analysis allows for the presence of respiratory sinus arrhythmia1,9,15 and enabled us to resample all heart rate data at the same frequency (8 Hz was selected to resolve changes at up to 200 beats per minute) to correctly align data in all subjects at the same time points. To reveal any reflex effects that might accelerate heart rate, from the effects of either the final inflation preceding the breath-hold and/or the breakpoint deflation/inflation immediately following the breath-hold, data were also sampled either from time zero that is the start of the maximal inhalation before the breath-hold (where the breath-hold starts at +3 s) or from time zero that is the start of the breakpoint breath (where the breakpoint starts at −0.5 s). Breath-hold durations were also normalized to 100%.
For statistical analysis using PASW® statistics v. 18 (SPSS Inc., Chicago, IL), the whole of the pre-breath-hold period was compared with time periods as indicated in the figures. We used a paired experimental design with each subject compared against their own experimental. For each subject, their mean values in these periods were compared using repeated measures analysis of variance (ANOVA) using within subjects contrasts (with >2000 points analysed per variable per subject) whose probability (p) values are cited in text and in figures. The ANOVA of the SpO2 data used the non-parametric Friedman test. There were no differences between males and females, and all data were combined. Significance was taken at p < 0.05 for two-tailed tests, and the means for 12 subjects are given as ±standard error of the mean.
RESULTS
Breath-hold durations and gases
Over the 4-min rest period, mean systolic blood pressure was 133 ± 5 mmHg, averaged mean pressure was 91 ± 3 mmHg and diastolic pressure was 70 ± 3 mmHg, mean heart rate was 72 ± 2 beats per minute, mean PetCO2 level was 39 ± 1 mmHg, mean SpO2 was 99 ± 0%, and they did not change throughout this period. As found previously,1,20,21 neither pre-oxygenation nor this with hypocapnia changed the resting blood pressure or heart rate.
The mean breath-holding duration was 1.4 ± 0.2 min with air, 2.8 ± 0.4 min with pre-oxygenation and 5.5 ± 0.5 min with pre-oxygenation and hypocapnia.
Breath-holding with air resulted at breakpoint in a large rise in systolic pressure (see below), slight arterial asphyxia, that is, slight desaturation (mean SpO2 fell significantly to reach a nadir of 96 ± 1%; p < 0.05 vs rest at 15 s post breakpoint) and hypercapnia (mean PetCO2 rose significantly to 50 ± 1 mmHg; p < 0.001 vs rest).
Pre-oxygenation prevented the desaturation of breath-holding (mean SpO2 at breakpoint was 100%, non-significant vs resting levels). Such longer breath-holds caused greater hypercapnia (mean PetCO2 was 53 ± 1 mmHg; p < 0.005 vs both rest and breakpoint with air).
Pre-oxygenation and hypocapnia abolished the asphyxia of breath-holding (at breakpoint mean SpO2 was 98 ± 1% and mean PetCO2 was 43 ± 2 mmHg, both non-significant vs resting levels).
Blood pressure rises progressively during breath-holding, even width pre-oxygenation and hypocapnia
Using the clinically used technique of deep inspiratory breath-holds with air, Figures 1 and 2 show that blood pressure rose progressively in all subjects during breath-holding. Mean systolic pressure at breakpoint for breath-holds with air was 175 ± 8 mmHg, and in two subjects (Figure 1), it reached 200 mmHg at breakpoint.
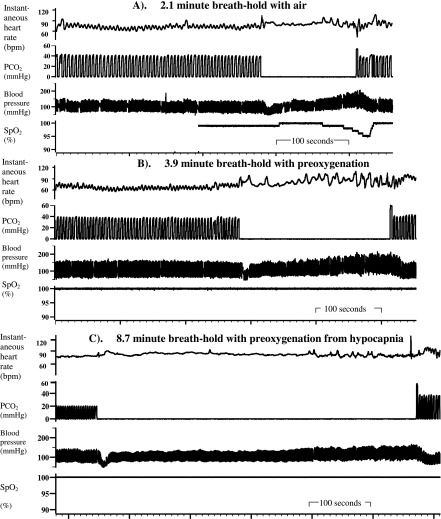
Figure 1.
Blood pressure rises progressively (without heart rate falling) in the subject with the longest breath-hold. Original polygraph record [with the breath-hold indicated by the absence of partial pressure of carbon dioxide (PCO2) fluctuations]. Note the similar pressure rise without heart rate falling for all breath-holds and the slight arterial desaturation following the deep inspiratory breath-hold with air. bpm, beats per minute; SpO2, oxygen saturation.
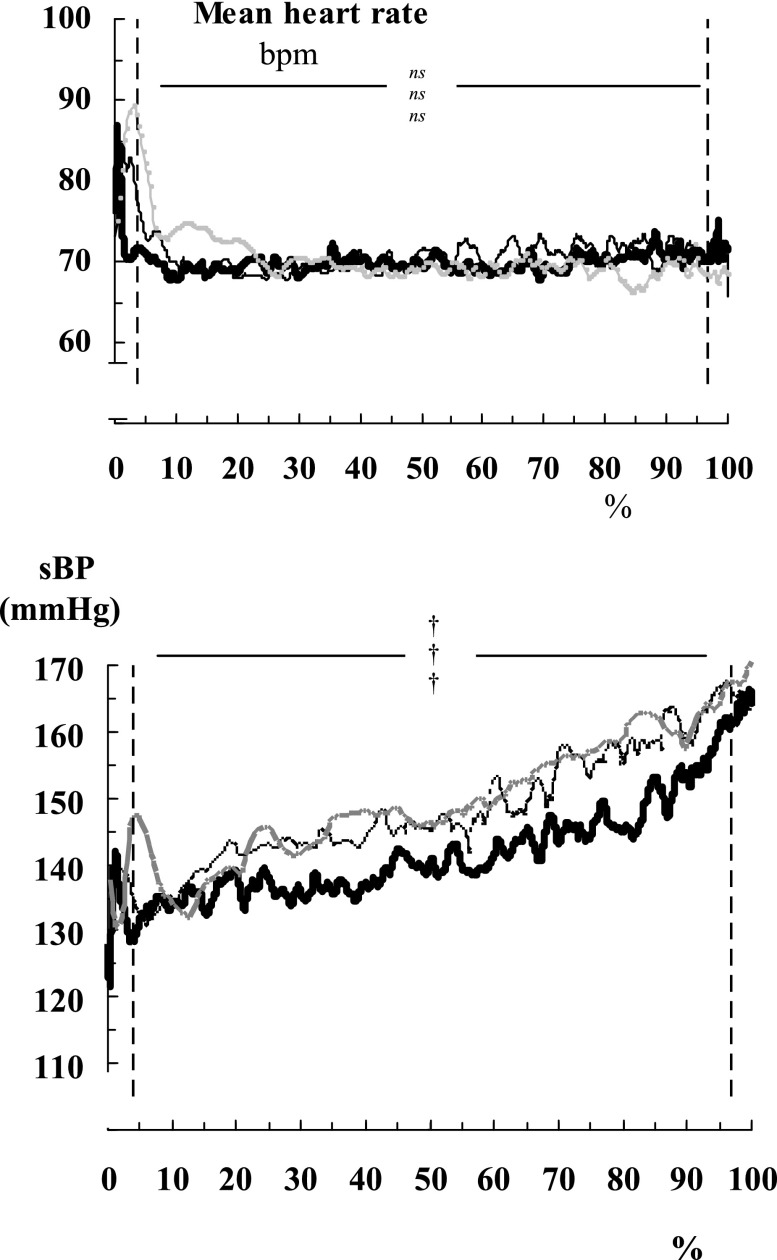
Figure 2.
Averaged systolic blood pressure (sBP) rises progressively (without averaged instantaneous heart rate falling) in 12 subjects. Breath-hold duration is normalized to 100% for each subject, with grey lines indicating breath-holds with air, thin lines indicating breath-holds with 60% oxygen and thick lines indicating breath-holds with 60% oxygen and hypocapnia. Vertical dashed lines indicate the start and end of the breath-hold. nsp > 0.05; †p < 0.005 vs the mean value over the 4 min of pre-breath-hold, with upper symbol indicating breath-holds with air; middle indicating breath-holds with 60% oxygen; lower indicating 60% oxygen and hypocapnia. bpm, beats per minute.
Figure 2 shows that the rise in blood pressure during breath-holding is not caused by asphyxia, because the starting and ending pressures, and hence the overall rises, are not significantly different between breath-holds from air, from pre-oxygenation or from pre-oxygenation and hypocapnia.
It was not the case that subjects with the longest breath-holds had the highest pressure rises. The size of the blood pressure rise was not obviously related simply to breath-hold duration. (The r2 values between duration and the pressure rise were only 0.48, 0.06 and 0.20, while those between duration and the peak pressure at breakpoint were only 0.23, 0.19 and 0.02 for each breath-hold category.)
The blood pressure rise is not signalled by a fall in heart rate during breath-holding
Figures 1 and 2 show that the blood pressure rise is not signalled by a fall in heart rate during breath-holding; that is, it does not cause heart rate to fall. No heart rate fall is detectable even in the subject with the longest breath-hold (approximately 9 min), yet we could successfully detect other changes in the heart rate when expected (e.g. the inflation preceding the breath-hold with air causes a significant increase in mean heart rate, reaching 92 ± 3 beats per minute in the 2nd second, and that the 1st exhalation/inflation causes a significant increase in heart rate, reaching 84 ± 3 beats per minute in the 5th second).
DISCUSSION
Pre-oxygenation and hypocapnia fail to abolish the progressive blood pressure rise during breath-holding
Deep inspiratory breath-holds with air are currently used clinically, and we show the substantial blood pressure rise that occurs, about which there is no warning in current radiotherapy literature.
Our subjects did try hard to breath-hold as long as possible, as shown by the fact that their breath-hold durations and breakpoint PetCO2 levels are as good or better than in previous studies,15,25 that arterial desauration occurred and by how high PetCO2 rose. Neither is the blood pressure rise caused by subjects straining against a closed glottis (the Valsalva manoeuvre) during breath-holding, because we confirmed that none did this.
We are the first to show that this blood pressure rise cannot be prevented by pre-oxygenation and hypocapnia. Thus, the conventional explanation, that it is caused by asphyxia stimulating peripheral chemoreceptors,13,14 is incorrect. Instead, the pressure rise without heart rate falling may be caused by a muscle metaboloreflex originating in the holding muscle, the diaphragm.15 It is disappointing that the pressure rise cannot be prevented by this simple, non-invasive expedient. We do not recommend pharmacological manipulation of autonomic function nor sedation during breath-holding to prevent it, not only because of the further complications of additional medication but also because the involuntary breakpoint mechanism15 is itself a safety feature and should not be suppressed.
Clinical importance for patient safety in radiotherapy of the blood pressure rise during breath-holding
Although, recently, there is widespread clinical interest in using multiple deep inspiratory breath-holds, or prolonged single breath-holds to improve imaging and radiotherapy,1,2 we understand that patients' blood pressure is neither recorded nor reported routinely (see Roth et al2 and the record 14-min breath-hold by Klocke and Rahn3). Moreover, the ability of standard non-invasive blood pressure monitors placed on the arm to reveal the precise pressure rise is limited. This is because, since most breath-holds currently used during radiotherapy and imaging are so short (approximately 22 s duration2), there is only enough time for approximately two measurements. We do not advocate arterial cannulation. The non-invasive devices that continuously estimate blood pressure for each heart beat (such as Finapres) do, however, appear to provide the best indication so far of the time course of the pressure rise.
We also show that routine and non-invasive monitoring of the patient's heart rate cannot warn of this blood pressure rise. Thus, we show that heart rate does not change during breath-holding with air (resolving the long standing dispute18 about what happens to heart rate during breath-holding). Our conclusion is confirmed by the many studies showing that cardiac output does not fall below normal resting levels of approximately 6 l per minute6,8,10 during breath-holding and neither can venous return be impeded sufficiently to alter heart rate. Our more detailed analysis also reveals how previous, simpler heart rate analyses might find either no heart rate change or rises or falls.1,6–10,13,14,16,26–30
Although presently most deep inspiratory breath-holds for radiotherapy and imaging are of only approximately 22 s duration,2 we counsel caution for three reasons. First, we do not yet know what happens to blood pressure in such patients, because no previous studies measured it nor report the patient's maximum breath-hold duration. Since we do not know how near these patients were to their breakpoint, we cannot even estimate from our data how high their blood pressure might rise. Secondly, such studies often ask patients to breath-hold repeatedly31 and the cumulative effects of multiple breath-holds on blood pressure have yet to be measured. Thirdly, if patients already have hypertension, the additional effects of breath-holding on blood pressure might be problematic.
We believe that the primary clinical requirement is simply a greater awareness of the implications of the progressive blood pressure rise for patient safety during breath-holding for radiotherapy. Although pressure does become acutely high, it returns to resting levels approximately 15 s after the breakpoint. Such transient rises in blood pressure are likely to be well tolerated by most patients, but those with pre-existing coronary artery disease, cerebrovascular disease, including cerebral artery aneurysms, aortic disease and long-standing hypertensive end organ damage are all at significant risk of injury from blood pressure rises of this magnitude.
Not only does pressure rise, but breath-holds without pre-oxygenation also cause arterial oxygen desaturation. While moderate desaturation to no lower than 90% is very unlikely to produce any longstanding effects, patients with coexisting ischaemic coronary artery disease or cerebrovascular disease are at risk from permanent damage from episodes of hypoxia. The exact level at which arterial hypoxaemia becomes dangerous varies from patient to patient and is not well understood. With non-invasive monitoring (the finger pulse oximeter), the full extent of this is only apparent at 15 s after the breakpoint (some of this delay being a consequence of the averaging used by pulse oximeters that delays their response time to rapidly changing arterial saturations). Thus, even if a breath-hold is terminated once SpO2 reaches 94%, saturation continues to fall at an increasing rate as normal breathing continues, before eventually stabilizing and rising again. Taking the simple step of monitoring any fall in arterial saturation during breath-holding is, however, prudent. Moreover, we demonstrate that pre-oxygenation with 60% oxygen will prevent any desaturation during even the longest breath-holds.
In conclusion, we show that a clinically significant blood pressure rise occurs during deep inspiratory breath-holds. This rise cannot be prevented by pre-oxygenation and hypocapnia. For breast cancer patients scheduled for deep inspiratory breath-holds, and particularly for prolonged breath-holds, we therefore recommend routine screening for heart, cardiovascular, renal and cerebrovascular disease. We also recommend routine blood pressure and SpO2 monitoring before breath-holds and greater awareness of the possible pressure rises during prolonged breath-holding in such patients, particularly, those with cardiovascular and/or cerebrovascular comorbidity. We also recommend adoption of safety limits, where all patients are requested to stop breath-holding if systolic pressure consistently exceeds 180 mmHg and/or SpO2 falls <94%.
CONFLICTS OF INTEREST
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
FUNDING
This article was supported by Queen Elizabeth Hospital Birmingham Charity.
Acknowledgments
ACKNOWLEDGMENTS
We are grateful for the support from the Queen Elizabeth Hospital Birmingham Charity. We are grateful to Cambridge Electronic Design Ltd, to Dr David McIntyre and to Peter Nightingale for their help with programming and statistical analysis and to Dr Una Martin and the staff at the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility.
REFERENCES
- 1.Cooper HE, Parkes MJ, Clutton-Brock TH. CO2-dependent components of sinus arrhythmia from the start of breath-holding in Man. Am J Physiol 2003; 285: H841–8. [DOI] [PubMed] [Google Scholar]
- 2.Roth J, Engenhart-Cabillic R, Eberhardt L, Timmesfeld N, Strassmann G. Preoxygenated hyperventilated hypocapnic apnea-induced radiation (PHAIR) in breast cancer patients. Radiother Oncol 2011; 100: 231–5. doi: 10.1016/j.radonc.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 3.Klocke FJ, Rahn H. Breath holding after breathing oxygen. J Appl Physiol 1959; 14: 689–93. [DOI] [PubMed] [Google Scholar]
- 4.Muxworthy JF, II. Breath holding studies: the cardio-vascular response. USAF technical report 6528. Dayton, OH: Wright-Patterson Air Force Base; 1951. pp. 457–60. [Google Scholar]
- 5.Hong SK, Moore TO, Seto G, Park HK, Hiatt WR, Bernauer EM. Lung volumes and apneic bradycardia in divers. J Appl Physiol 1970; 29: 172–6. [DOI] [PubMed] [Google Scholar]
- 6.Pingitore A, Gemignani A, Menicucci D, Di Bella G, De Marchi D, Passera M, et al. Cardiovascular response to acute hypoxemia induced by prolonged breath-holding in air. Am J Physiol Heart Circ Physiol 2007; 294: H449–55. [DOI] [PubMed] [Google Scholar]
- 7.Heusser K, Dzamonja G, Tank J, Palada I, Valic Z, Bakovic D, et al. Cardiovascular regulation during apnea in elite divers. Hypertension 2009; 53: 719–24. doi: 10.1161/HYPERTENSIONAHA.108.127530 [DOI] [PubMed] [Google Scholar]
- 8.Heusser K, Dzamonja G, Breskovic T, Steinback CD, Diedrich A, Tank J, et al. Sympathetic and cardiovascular responses to glossopharyngeal insufflation in trained apnea divers. J Appl Physiol 2010; 109: 1728–35. doi: 10.1152/japplphysiol.00522.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YC. Breath-hold diving in terrestrial mammals. Exerc Sport Sci Rev 1982; 10: 270–307. [PubMed] [Google Scholar]
- 10.Hong SK, Lin YC, Lally DA, Yim BJ, Kominami N, Hong PW, et al. Alveolar gas exchanges and cardiovascular functions during breath holding with air. J Appl Physiol 1971; 30: 540–7. [DOI] [PubMed] [Google Scholar]
- 11.Varon J. The diagnosis and treatment of hypertensive crises. Postgrad Med 2009; 121: 5–13. doi: 10.3810/pgm.2009.01.1950 [DOI] [PubMed] [Google Scholar]
- 12.De Burgh Daly M. Interactions between respiration and circulation. In: Fishman AP, ed. Handbook of physiology, section 3, the respiratory system II, the control of breathing. New York, NY: American Physiological Society; 1986. pp. 529–94. [Google Scholar]
- 13.Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol 1993; 74: 2969–75. [DOI] [PubMed] [Google Scholar]
- 14.Gross PM, Whipp BJ, Davidson JT, Koyal SN, Wasserman K. Role of the carotid bodies in the heart rate response to breath holding in man. J Appl Physiol 1976; 41: 336–40. [DOI] [PubMed] [Google Scholar]
- 15.Parkes MJ. Breath-holding and its breakpoint. Exp Physiol 2006; 91: 1–15. [DOI] [PubMed] [Google Scholar]
- 16.Jay O, Christensen JP, White MD. Human face-only immersion in cold water reduces maximal apnoeic times and stimulates ventilation. Exp Physiol 2007; 92: 197–206. [DOI] [PubMed] [Google Scholar]
- 17.Parkes MJ. The limits of breath holding. Sci Am 2012; 306: 76–9. [DOI] [PubMed] [Google Scholar]
- 18.Parkes MJ. Do humans really prolong breath-hold duration by lowering heart rate to reduce metabolic rate? Physiol News 2012; 88: 33–6. [Google Scholar]
- 19.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol 2002; 283: R281–3. [DOI] [PubMed] [Google Scholar]
- 20.Cooper HE, Clutton-Brock TH, Parkes MJ. Contribution of the respiratory rhythm to sinus arrhythmia in normal unanesthetized subjects during mechanical hyperventilation with positive pressure. Am J Physiol 2004; 286: H402–11. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford JJ, Clutton-Brock TH, Parkes MJ. Hypocapnia reduces the T wave of the electrocardiogram in normal human subjects. Am J Physiol 2005; 289: R148–55. [DOI] [PubMed] [Google Scholar]
- 22.Valentinuzzi ME, Geddes LA. The central component of the respiratory heart-rate response. Cardiovasc Res Cent Bull 1974; 12: 87–103. [PubMed] [Google Scholar]
- 23.Agostoni E. Diaphragm activity during breath holding: factors related to its onset. J Appl Physiol 1963; 18: 30–6. [DOI] [PubMed] [Google Scholar]
- 24.Macefield VG, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Brain 1991; 114: 527–40. [DOI] [PubMed] [Google Scholar]
- 25.Ferris EB, Engel GL, Stevens CD, Webb J. Voluntary breath-holding III. The relation of the maximum time of breath holding to the oxygen and carbon dioxide tensions of arterial blood, with a note on its clinical and physiological significance. J Clin Invest 1946; 25: 734–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider EC. Observations on holding the breath. Am J Physiol 1930; 94: 464–70. [Google Scholar]
- 27.Muxworthy JF. 1. Breath holding studies: relationship to lung volume. USAF technical report 6528. Dayton, OH: Wright-Patterson Air Force Base; 1951. pp. 452–6. [Google Scholar]
- 28.Heistad DD, Wheeler RC. Simulated diving during hypoxia in man. J Appl Physiol 1970; 28: 653–6. [DOI] [PubMed] [Google Scholar]
- 29.Whayne TF, Jr, Smith NT, Eger EI, 2nd, Stoelting RK, Whitcher CE. Reflex cardiovascular responses to simulated diving. Angiology 1972; 23: 500–8. [DOI] [PubMed] [Google Scholar]
- 30.Andersson JP, Liner MH, Fredsted A, Schagatay EK. Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J Appl Physiol 2004; 96: 1005–10. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett FR, Colgan RM, Carr K, Donovan EM, McNair HA, Locke I, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol 2013; 108: 242–7. doi: 10.1016/j.radonc.2013.04.021 [DOI] [PubMed] [Google Scholar]