Abstract
Digital breast tomosynthesis (DBT) has gained acceptance as an adjunct to digital mammography in screening. Now that breast density reporting is mandated in several states in the USA, it is increasingly important that the methods of breast density measurement be robust, reliable and consistent. Breast density assessment with DBT needs some consideration since quantitative methods are modelled for two-dimensional (2D) mammography. A review of methods used for breast density assessment with DBT was performed. Existing evidence shows Cumulus has better reproducibility than that of the breast imaging reporting and data system (BI-RADS®) but still suffers from subjective variability; MedDensity is limited by image noise, whilst Volpara and Quantra are robust and consistent. The reported BI-RADs inter-reader breast density agreement (k) ranged from 0.65 to 0.91, with inter-reader correlation (r) ranging from 0.70 to 0.93. The correlation (r) between BI-RADS and Cumulus ranged from 0.54–0.94, whilst that of BI-RADs and MedDensity ranged from 0.48–0.78. The reported agreement (k) between BI-RADs and Volpara is 0.953. Breast density correlation between DBT and 2D mammography ranged from 0.73 to 0.97, with agreement (k) ranging from 0.56 to 0.96. To avoid variability and provide more reliable breast density information for clinicians, automated volumetric methods are preferred.
Breast cancer accounts for approximately 23% of all cancers in females and is the most frequent cause of cancer deaths in females worldwide.1–3 The exact aetiology of the disease is complex, but many risk factors have been documented in the literature amongst which is breast density.4–7 Breast density refers to the proportion of the breast that is composed of fibroglandular tissue. Breasts with high density contain more epithelial and stromal cells and collagen, which are significant for tumorigenesis as well as tissue-specific progenitor cells that are at risk of transformation to cancer cells.8,9 Studies have shown that breast density is a strong, modifiable and measureable risk factor for breast cancer.10–13 Additionally, the masking effect from breast density reduces the performance of screening mammography and limits early detection and treatment of breast cancer.14 Encouragingly, breast density is reducible, and its reduction has been shown to mitigate breast cancer risk.13 Therefore, mammographic breast density measurement can be used for breast cancer risk prediction and personalization of breast cancer prevention and control strategies, such as the selection of females who may require breast density reduction interventions. It may also be used for selection of more appropriate imaging pathways for earlier detection of breast cancer.5,13 Utilization of breast density for these purposes requires robust and consistent methods for its assessment.
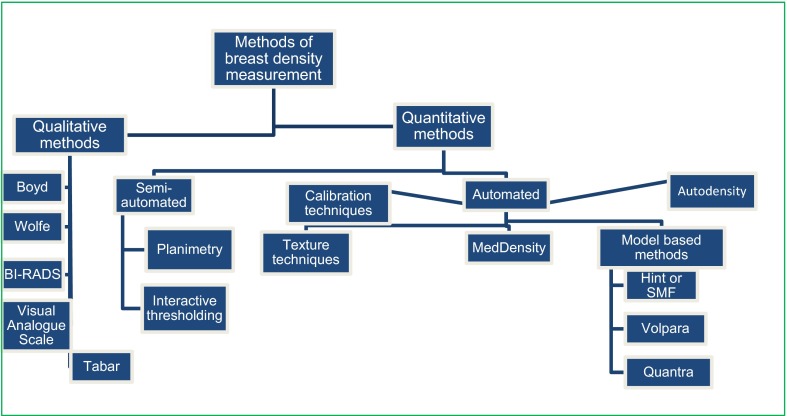
Breast density depicted by the radio-opaque areas on a mammogram can be assessed using qualitative and quantitative (semi-automated and automated) methods.15–17 Qualitative methods assign breast density grades based on visual assessment of the relative proportions of dense tissue, fat and prominence of ducts and include breast imaging reporting and data system (BI-RADS®), visual analogue scale and Wolfe, Tabar and Boyd assessment methods.15,18,19 Semi-automated methods use segmentation and thresholding techniques to quantify the percentage of dense tissue on a mammogram and include planimetry and interactive thresholding methods such as Cumulus and Madena.20,21 Automated methods use mathematical, statistical and physical modelling to calculate breast density; such automated methods include computerized texture-based techniques, calibration approaches and dual X-ray absorptiometry.22–24 Others are automated thresholding approaches, such as Autodensity and MedDensity,25,26 and three physical model-based techniques: standard mammographic form (SMF), Volpara and Quantra.27–29 Irrespective of the method of measurement, breast density has been shown to be a potent risk factor for breast cancer.
Many studies on mammographic breast density measurement are based on film–screen mammography and digital mammography (DM), which produce two-dimensional (2D) images of a three-dimensional (3D) breast. Qualitative methods have been shown to be poorly reproducible with these modalities; they have wide inter-reader agreement with Kappa (k) values ranging from 0.37 to 0.91.26,30 Quantitative methods have better reproducibility with these modalities; however, there are concerns that quantitative area measurement of breast density as percentage mammographic density (PMD) is not representative of the tissue at risk of breast cancer, and that it is more reasonable to measure the volume of only the fibroglandular tissue, which is more related to the dense tissue at risk instead of PMD.16,31 Another concern is that volumetric breast density measurement with 2D mammography is limited owing to the absence of depth information in such mammograms;31 methods estimating mammographic breast density with 2D mammography attempt to take into account variation in breast tissue thickness by modelling; however, with all models, there are assumptions made that may not be necessarily correct for an individual patient.
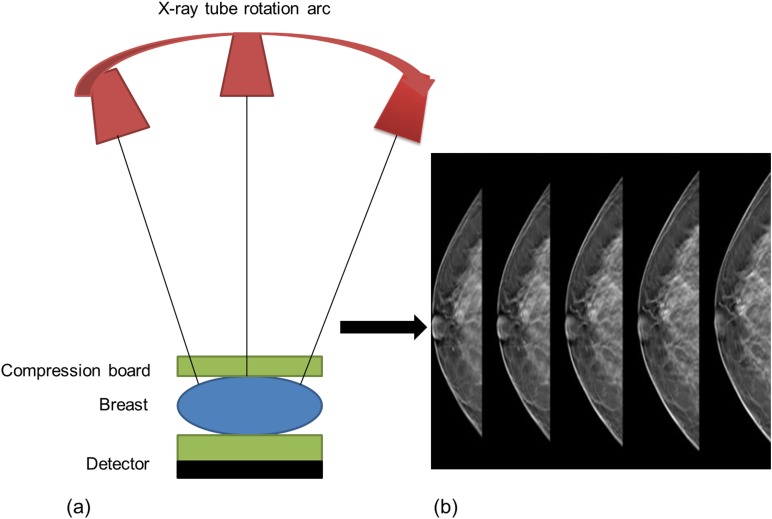
Digital breast tomosynthesis (DBT) has gained acceptance as a tool for imaging of the symptomatic breast and as an adjunct to DM in screening.32,33 Breast density assessment with DBT needs some consideration since quantitative methods are modelled for 2D mammography. DBT is a 3D imaging modality utilizing the concept of conventional tomography but a limited angle of tube movement (11–60°) to acquire depth information from the breast (Figure 1a,b).34 With the removal of anatomical noise (superimposed skin and subcutaneous tissue) in DBT images, quantitatively assessed breast density is expected to be lower than DM. On the other hand, more dense tissue becomes apparent to a subjective reader and qualitatively assessed breast density with DBT is expected to be higher relative to DM. It is therefore important to have a standardized robust, reliable and reproducible assessment method to avoid variability in breast density measurement as this will impact on clinical decision-making for females undergoing breast screening. There are several contending methods (Figure 2), each of which has its own merits; this review briefly examines the links between breast density and breast cancer. It also examines methods that have been used for measurement of mammographic breast density with DBT to ascertain which can be considered the best approach.
Figure 1.
Principles of digital breast tomosynthesis: (a) tube rotations relative to the detector and (b) acquired image slices. Image courtesy of Hologic Inc.; Bedford, MA © 2011. All rights reserved.
Figure 2.
Methods of breast density measurement. BI-RADS®, breast imaging reporting and data systems; SMF, standard mammographic form.
METHODS AND MATERIALS
Search strategy
The preferred reporting items for systematic reviews and meta analysis (PRISMA) search strategy was employed to search for articles in MEDLINE, EMBASE, CINAHL (EbSCOhost), PubMed, SPIE library, Cochrane library, Web of Science and Scopus databases. In order to access more information, we conducted a Google search, and reference lists of published articles were examined to identify additional articles not identified in the database searches. Searches were conducted using the following terms: breast density assessment with digital breast tomosynthesis, breast density and digital breast tomosynthesis, methods for breast density assessment, and breast density and breast cancer risk. The PICOS system was used to evaluate each article for relevance (Table 1).
Table 1.
Criteria for determining study eligibility.
| Characteristics | Criteria |
|---|---|
| Study year | Studies published from January 2000 to March 2014 |
| Study type | 1. Case–control trials |
| 2. Cohort studies | |
| Population | Females of all ages |
| Intervention | 1. Breast density measurement methods |
| Comparator | 1. Robustness, reliability and reproducibility of density measurement methods |
| Outcomes | 1. Mammographic breast density measurement performance |
| 2. Breast cancer risk prediction capability |
Studies were characterized according to the year of publication, study type, population, intervention, comparators and outcomes.
Inclusion criteria
Articles were included if they described breast density and breast density measurement with DBT and were published in English language from January 2000 to March 2014. Articles were qualitatively assessed for study quality and risk of bias to ensure that they fit the inclusion criteria. Articles that did not fulfil these criteria were excluded.
Data synthesis
Data extraction was performed independently and blindly by two reviewers with differences of opinion resolved by discussion. Where a consensus was not reached, articles were excluded. The selection was strongly influenced by the guidelines for assessing study quality and risk of bias.35 Each study was scored high or low by each reviewer; this enabled us to appraise the conduct of such research. Only articles rated high were included in this review.
Results
The search strategy identified 812 articles published from 2000 to March 2014. Out of these, 11 studies fulfilled the inclusion criteria. All studies were rated high and were used to assess the performance of breast density assessment methods with DBT. A total of 842 cases (mammograms) were evaluated by these studies.
Breast density and breast cancer risk
Studies have shown that breasts with >75% dense tissue have a four- to six-fold higher risk of developing breast cancer relative to those with <10% dense tissue.4,5,15 The high risk of breast cancer from breast density has been linked to high epithelial and stromal cells and collagen concentrations in the microenvironment of dense breasts8,36 and increased activity of mitogens and mutagens in dense breast tissue.37,38 Breast cancers evolve from epithelial cells; stromal cells stimulate epithelial cells' proliferation through insulin-like growth factor 1 (IGF-1) and transforming growth factor beta,39 and collagen in the breast microenvironment assists in tumour reorganization.40 Therefore, increased concentration of each of these components increases the risk of carcinogenesis. Similarly, biological interaction among these three components results in stretching and stiffening of each component, initiating processes that lead to cancer.36,40 Additionally, dense tissue contains high concentrations of mitogens, such as IGF-1 and oestrogen,41,42 and mutagens such as cytochrome P450 1A2.37 Therefore, there is increased exposure of proliferating progenitor epithelial and stromal cells in dense breasts to the toxic metabolites of mitogens and mutagens, increasing the probability of their transformation to cancer.43 Thus, it is clear that measuring breast density can give an indication of breast cancer risk, which may allow for earlier adoption of preventive and control measures.
Qualitative methods of mammographic breast density assessment
Qualitative methods involve subjective decision-making and grading of mammographic breast density based on visual perception. Such methods include Wolfe, Tabar and Boyd assessment methods, as well as visual analogue scale and BI-RADS. These methods have potential applicability for breast density assessment with DBT; however, only BI-RADS has been used with DBT to date.26,44
BI-RADS grades breast density into four categories: D1 (almost entirely fatty breast); D2 (breast with scattered areas of fibroglandular tissues); D3 (heterogeneously dense breast); D4 (extremely dense breast). It considers D3 and D4 as high-grade densities and D1 and D2 as low-grade densities.45 BI-RADS has demonstrated strong positive intrareader (k = 0.79–0.86) and inter-reader (k = 0.65–0.91) agreement with DBT;26,44,46,47 reported inter-reader correlation (r) ranged from 0.7 to 0.93, with correlations better for D1 and D4 than for D2 and D3 breast density categories.26,44 BI-RADS has also shown strong positive intrareader (k = 0.79–0.96) and inter-reader (k = 0.79–0.91) breast density agreement between DBT and DM44,46–49 (Table 2). The reproducibility of BI-RADS is generally poor owing to reader subjectivity in breast density assessment.30,45 Poor reproducibility could have different implications on breast cancer risk prediction and choices in screening. To reduce variability and provide an objective measurement of breast density, quantitative approaches were developed for breast density evaluation.
Table 2.
Summaries of studies on breast density measurement with digital breast tomosynthesis (DBT)
| Author | Study method | Quality rating of study35 | Number of participants | Results/comparator | Conclusion/outcomes | |
|---|---|---|---|---|---|---|
| Kontos et al50 | Cumulus (cohort study) | High | 52 females | Correlation between texture features on DBT vs DM, r = 0.73 Correlation of %BD vs parenchymal features 
|
Cumulus is feasible for breast density measurement in DBT, and parenchymal texture features are more related to breast density on DBT than DM | |
| Kontos et al48 | Cumulus (cohort study) | High | 71 females |
Relationship %BD vs parenchymal features
|
Cumulus is reliable for breast density estimation in DBT, and parenchymal texture features are more strongly correlated with percentage mammographic density on DBT than DM | |
| Ren et al51 | Quantra (cohort study) | High | 15 projection phantom images |  |
Quantra is a promising tool for density estimation in DBT but needs further algorithm optimization | |
| Bakic et al20 | Cumulus (cohort study) | High | 40 females | Cumulus intrauser correlation for DBT, r = 0.94 Breast density correlation for DBT vs DM, r = 0.90 |
Cumulus is very reliable for density estimation with DBT, and breast density of DM is strongly correlated with DBT | |
| Bakic et al52 | Cumulus (cohort study) | High | 35 females |
Cumulus estimated density DBT = 28 ± 19%; DM = 36 ± 20% Correlation DBT vs DM, r = 0.76 Agreement DBT vs DM, κ = 0.56 |
Cumulus is suitable for measuring breast density with DBT. Breast density is lower in DBT than in DM | |
| Bakic et al46 | Cumulus (cohort study) | High | 39 females |
Cumulus interuser correlation DBT, ρ = 0.85 DM, ρ = 0.75 Breast density with DBT vs DM ρ = 0.91; k = 0.79 |
Cumulus is suitable for density estimation on DBT, and percentage density estimated from reconstructed and projection images are similar | |
| Tagliafico et al26 |
MedDensity and BI-RADS® (cohort study) | High | 50 females |
MedDensity vs BI-RADS correlation Four-grade scale (D1–4), r = 0.54 Two-grade scale (D1–2 vs D3–4), r = 0.78 BI-RADS inter-reader agreement, k = 0.80 Breast density with DBT vs DM BI-RADS intrareader agreement, k = 0.81–0.86 BI-RADS inter-reader agreement, k = 0.91 |
Moderate breast density correlation between MedDensity and BI-RADS. Density is underestimated on DBT relative to DM | |
| Tagliafico et al47 | Cumulus and BI-RADS (cohort study) |
High | 160 females | Correlation between BI-RADS and Cumulus, r = 0.94 Cumulus density correlation between modalities MRI vs DBT, r = 0.95 DM vs DBT, r = 0.97 BI-RADS agreement for DM and DBT, k = 0.91 |
Cumulus and BI-RADS are reliable methods for estimating breast density from DBT, DM and MRI | |
| Tagliafico et al44 | MedDensity and BI-RADS (cohort study) | High | 160 females |
MedDensity vs BI-RADS correlation Four-grade scale (D1–4), r = 0.48 Two-grade scale (D1–2 vs D3–4), r = 0.76 BI-RADS inter-reader agreement, k = 0.81 Breast density with DBT vs DM BI-RADS intrareader agreement, k = 0.79–0.81 BI-RADS inter-reader agreement, k = 0.89 |
Moderate density correlation between BI-RADS and MedDensity. Breast density is lower on DBT than on DM | |
| Tromans et al53 | Volpara (cohort study) | High | 20 females | Volumetric breast density correlation for DBT vs 2D mammograms, r = 0.903 Agreement between BI-RADS and Volpara, k = 0.953 |
Volpara is robust and a promising substitute for BI-RADS | |
| Regini et al49 | BI-RADS + Quantra for DM (cohort study) | High | 200 mammograms |
Breast density with DBT vs DM BI-RADS intrareader agreement, k = 0.96 |
There is no variation in breast density assessed with DBT and DM |
BI-RADS, breast imaging reporting and data system; DM, digital mammography; k, weighted kappa agreement; κ, quadratic kappa agreement; r, correlation coefficient; ρ, Spearman's correlation.
Quantitative methods of mammographic breast density assessment
Quantitative methods use mathematical, statistical and physical principles to calculate mammographic breast density and are classified into semi-automated and automated methods.
Semi-automated methods
Semi-automated methods use thresholding and segmentation techniques to perform area measurement of mammographic breast density as percentages and include planimetry and interactive thresholding methods such as Cumulus and Madena. Of the available semi-automated methods, only Cumulus has as yet been used for breast density estimation with DBT (Table 2).
Cumulus (University of Toronto, Canada) employs computerized thresholding and segmentation techniques to select grey levels for density assessment mainly from the central DBT image slice. Two grey levels are usually selected; the first of these separates the pixels in the image representing the breast from the background and sums these pixels to provide a measure of breast area (AB). The second threshold outlines the fibroglandular (dense) breast tissue and sums the pixels over this area to calculate the area of dense tissue (AD). The software calculates PMD as the percentage of the dense tissue and the total breast area (PMD = AD/AB × 100).20
Cumulus has demonstrated strong positive intrauser (ρ = 0.88, r = 0.89–0.94) and interuser (ρ = 0.85) correlations as well as intrauser (k = 0.81) and interuser (k = 0.56–0.79) agreement with DBT. The correlation between Cumulus and BI-RADS ranged from 0.54 to 0.94.20,50,52 Cumulus has shown a strong positive correlation between parenchymal texture features and breast density with DBT (r = 0.73);50 such texture features were shown to be more related to mammographic breast density with DBT than with DM. Cumulus estimated mammographic density values are lower on DBT than on DM;20,50,52 however, the software has been shown to overestimate breast density by 3% with DBT relative to DM in another study.20 Reported Cumulus-assessed mammographic breast density correlation between DBT and DM ranged from r = 0.76 to 0.97 and ρ = 0.78 to 0.91.20,47,52 Cumulus is generally thought to be limited by its binomial categorization of pixel into either 100% dense or 100% fat, and area measurement of breast density as percentages ignoring the 3D characteristics of the breast. Additionally, the dependence of the software on user expertise reduces its reproducibility.54,55 Removing the human from the evaluation completely would obviously be the solution to the issue of subjective variability; therefore, automated methods of breast density evaluation have been developed.
Automated methods
Automated methods were developed to allow for objective and consistent breast density assessment of mammograms. Such methods include texture-based techniques, calibration approaches, automated thresholding techniques such as Autodensity and MedDensity and three physics model-based techniques: SMF, Volpara and Quantra. Whilst these methods may have potential for mammographic density estimation with DBT, only three of these have as yet been used with DBT mainly on the central slice and include MedDensity, Volpara and Quantra.26,51–53 MedDensity performs area measurement of breast density as percentages, whereas Volpara and Quantra measure volumetric breast density and grade such densities into BI-RADS categories. Both Volpara and Quantra are extensions of SMF and employ similar principles for volumetric density estimation, but with some differences such as internal calibration as well as thresholds for classifying density into BI-RADS categories.
MedDensity (developed by Guilio Tagliafico, University of Genova, Italy) is a method based on maximum entropy and uses spatial information for automatic thresholding and segmentation of breast into fatty and dense tissue. The software uses the pixel values of the segmented areas to estimate the area of the dense tissue and total breast area. It calculates PMD as the percentage of the area of the dense tissue and the total breast area. Breast density assessed with DBT using this software is moderately positively correlated with BI-RADS breast density measures; reported correlations (r) ranged from 0.48 to 0.78, with correlation better on a two-grade (D1–2 vs D3–4) than a four-grade (D1–4) breast density scale.26,44,56 MedDensity has shown breast density evaluated with DBT to be lower than that for DM by 11.4%, with the level of breast density underestimation with DBT varying according to BI-RADS categories: 16.0%, 11.0%, 3.5% and 18.1% for BI-RADS 1, 2, 3 and 4, respectively.44 Although MedDensity is automated, quantum and anatomical noise in the image limits its thresholding capability and reduces the reliability of the software.46
Volpara (Volpara Solutions, Mãtakina Company, Wellington, New Zealand) is based on a relative physics model estimating the volume of fibroglandular tissue relative to the entire breast. It measures breast density by finding a reference point of entirely fat in each image and then estimating X-ray attenuation relative to that point for all other points in the image.57 The software calculates the volume of dense tissue by integrating the thickness of dense tissue at each pixel level values over the image; it computes the volume of the breast by multiplying the area of the breast by the recorded breast thickness.58 Volpara calculates average percentage volumetric breast density as a percentage of the volume of fibroglandular tissue and the total volume of the breast. Volpara generates BI-RADS breast density categories by classifying estimated densities into four volumetric density grades (VDGs): VDG 1 (<4.5%); VDG 2 (4.5–7.5%), VDG 3 (>7.5–15.5%) and VDG 4 (>15.5%); these VDGs correspond to BI-RADS 1–4, respectively.57,58 Although Volpara is modelled to generate objective BI-RADS scores, it generates relatively lower density values than BI-RADS with DM, but such density values have been shown to be strongly positively correlated with BI-RADS categories.30,53,58 With DBT, Volpara has shown strong positive agreement (k) with BI-RADS (0.953) and a volumetric breast density correlation (r) between DBT and 2D mammography of 0.903.53
Quantra (Hologic Inc., Bedford, MA) uses the physical modelling of mammographic systems as a basis to calculate volumetric breast density.51 It estimates the thickness of the fibroglandular breast tissue above each pixel in the image, and sums these pixel values to quantify the total volume of fibroglandular tissue in the breast. It also examines the whole silhouette of the imaged breast to estimate the total volume of the breast. The percentage volumetric breast density is then calculated as a percentage of the estimated fibroglandular tissue volume and the total breast volume.49,59 The software generates BI-RADS breast density categories by classifying the estimated volumetric breast density into four segments: segment 1 (0–<5.20); segment 2 (5.20–<12.6); segment 3 (12.6–<25.7); and segment 4 (25.7–100.0). These segments correspond to BI-RADS 1–4 density grades, respectively. Quantra has been shown to be an accurate60 and reproducible tool for quantifying breast density with DM.61,62 To date, there has been no clinical trial on the feasibility of Quantra for breast density estimation with DBT; it has, however, been assessed on phantoms using the Quantra results of 2D mammograms as a reference and has shown that breast density based on the central slice is 10% higher in DBT than in DM.51
The limitations of this review include that it was restricted to studies published in English. Additionally, DBT is a relatively new technology and few studies have assessed breast density with it. With the acceptance of DBT as an auxiliary for screening, it is increasingly likely that clinical breast density assessment will be performed with it in the near future. This review has presented the evidence related to the performance of breast density assessment methods used with DBT, and the relevance of DBT for volumetric breast density assessment.
CONCLUSION
DBT images contain depth information useful for volumetric breast density estimation, which is more related to the fibroglandular tissue at risk of breast cancer. With DBT, Cumulus has better reproducibility than BI-RADS but still suffers from subjective variability; MedDensity is limited by image noise, and together with Cumulus do not perform volumetric breast density assessment. Volpara and Quantra calculate volumetric breast density; they are robust, reliable and reproducible and are therefore preferred to other methods. Since BI-RADS is the most common clinical methodology, it may be necessary to calibrate Volpara and Quantra so that their thresholds reflect the BI-RADS categories assigned by expert radiologists. Automation and standardization of breast density measurements across sites may provide clinicians with more reliable breast density information and, therefore, more reliable and consistent selection of choices for breast cancer prevention and control. Without this, variations in breast density measurement will lead to unnecessary differences in clinical decision-making for females undergoing breast screening.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–403. doi: 10.1016/j.ejca.2012.12.02 [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Fernandez LM. Use of statistics to assess the global burden of breast cancer. Breast J 2006; 12(Suppl. 1): S70–80. doi: 10.1111/j.1075-122X.2006.00205.x [DOI] [PubMed] [Google Scholar]
- 4.Boyd N, Martin L, Gunasekara A, Melnichouk O, Maudsley G, Peressotti C, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prev 2009; 18: 1754–62. doi: 10.1158/1055-9965 [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356: 227–36. doi: 10.1158/1055-9965.EPI-09-0107 [DOI] [PubMed] [Google Scholar]
- 6.Baglietto L, Krishnan K, Stone J, Apicella C, Southey MC, English DR, et al. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am J Epidemiol 2013; 179: 475–83. doi: 10.1093/aje/kwt260 [DOI] [PubMed] [Google Scholar]
- 7.Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol 2005; 162: 743–52. [DOI] [PubMed] [Google Scholar]
- 8.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res 2003; 5: R129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh K, Brandt KR, Reynolds C, Scott CG, Pankratz VS, Riehle DL, et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat 2012; 131: 267–75. doi: 10.1007/s10549-011-1727-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habel LA, Capra AM, Achacoso NS, Janga A, Acton L, Puligandla B, et al. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev 2010; 19: 2488–95. doi: 10.1158/1055-9965 [DOI] [PubMed] [Google Scholar]
- 11.Lokate M, Peeters PH, Peelen LM, Haars G, Veldhuis WB, van Gils CH. Mammographic density and breast cancer risk: the role of the fat surrounding the fibroglandular tissue. Breast Cancer Res 2011; 13: R103. doi: 10.1186/bcr3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell G, Antoniou AC, Warren R, Peock S, Brown J, Davies R, et al. Mammographic density and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Res 2006; 66: 1866–72. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011; 103: 744–52. doi: 10.1093/jnci/djr079 [DOI] [PubMed] [Google Scholar]
- 14.Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology 2008; 246: 376–83. doi: 10.1148/radiol.2461070200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy SW, Nagtegaal ID, Astley SM, Gillan MG, McGee MA, Boggis CR, et al. Visually assessed breast density, breast cancer risk and the importance of the craniocaudal view. Breast Cancer Res 2008; 10: R64. doi: 10.1186/bcr2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Highnam R, Jeffreys M, McCormack V, Warren R, Smith GD, Brady M. Comparing measurements of breast density. Phys Med Biol 2007; 52: 5881–95. [DOI] [PubMed] [Google Scholar]
- 17.McCormack VA, Highnam R, Perry N, Silva ID. Comparison of a new and existing method of mammographic density measurement: intramethod reliability and associations with known risk factors. Cancer Epidemiol Biomarkers Prev 2007; 16: 1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Warren R, Warren-Forward H, Forbes JF. Reproducibility of visual assessment on mammographic density. Breast Cancer Res Treat 2008; 108: 121–7. [DOI] [PubMed] [Google Scholar]
- 19.Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast 2005; 14: 269–75. [DOI] [PubMed] [Google Scholar]
- 20.Bakic PR, Kontos D, Zhang C, Yaffe MJ, Maidment ADA. Analysis of percent density estimates from digital breast tomosynthesis projection images—art. no. 651424. Proc SPIE 2007; 651424. doi: 10.1117/12.713855 [DOI]
- 21.Palomares MR, Machia JR, Lehman CD, Daling JR, McTiernan A. Mammographic density correlation with Gail model breast cancer risk estimates and component risk factors. Cancer Epidemiol Biomarkers Prev 2006; 15: 1324–30. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Morimoto Y, Daida Y, Laidevant A, Malkov S, Shepherd JA, et al. Comparison of breast density measured by dual energy X-ray absorptiometry with mammographic density among adult women in Hawaii. Cancer Epidemiol 2011; 35: 188–93. doi: 10.1016/j.canep.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heine JJ, Cao K, Rollison DE. Calibrated measures for breast density estimation. Acad Radiol 2011; 18: 547–55. doi: 10.1016/j.acra.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver A, Lladó X, Pérez E, Pont J, Denton ER, Freixenet J, et al. A statistical approach for breast density segmentation. J Digit Imaging 2010; 23: 527–37. doi: 10.1007/s10278-009-9217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickson C, Arzhaeva Y, Aitken Z, Elgindy T, Buckley M, Li M, et al. AutoDensity: an automated method to measure mammographic breast density that predicts breast cancer risk and screening outcomes. Breast Cancer Res 2013; 15: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tagliafico A, Tagliafico G, Astengo D, Cavagnetto F, Rosasco R, Rescinito G, et al. Mammographic density estimation: one-to-one comparison of digital mammography and digital breast tomosynthesis using fully automated software. Eur Radiol 2012; 22: 1265–70. doi: 10.1007/s00330-012-2380-y [DOI] [PubMed] [Google Scholar]
- 27.Ciatto S, Bernardi D, Calabrese M, Durando M, Gentilini MA, Mariscotti G, et al. A first evaluation of breast radiological density assessment by QUANTRA software as compared to visual classification. Breast 2012; 21: 503–6. doi: 10.1016/j.breast.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 28.Highnam R, Brady M, Yaffe MJ, Karssemeijer N, Harvey J. Robust breast composition measurement—Volpara™. In: Marti J, Oliver A, Freixenet J, Marti R, eds. Digital mammography. Lecture notes in computer science. Vol. 6136. Berlin, Heidelberg: Springer-Verlag; 2010. pp. 342–9. [Google Scholar]
- 29.Skippage P, Wilkinson L, Allen S, Roche N, Dowsett M, A'Hern R. Correlation of age and HRT use with breast density as assessed by Quantra™. Breast J 2013; 19: 79–86. doi: 10.1111/tbj.12046 [DOI] [PubMed] [Google Scholar]
- 30.Gweon HM, Youk JH, Kim JA, Son EJ. Radiologist assessment of breast density by BI-RADS categories versus fully automated volumetric assessment. AJR Am J Roentgenol 2013; 201: 692–7. doi: 10.2214/AJR.12.10197 [DOI] [PubMed] [Google Scholar]
- 31.Alonzo-Proulx O, Packard N, Boone JM, Al-Mayah A, Brock KK, Shen SZ, et al. Validation of a method for measuring the volumetric breast density from digital mammograms. Phys Med Biol 2010; 55: 3027–44. doi: 10.1088/0031-9155/55/11/003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 2013; 266: 104–13. doi: 10.1148/radiol.12120674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. doi: 10.1148/radiol.12121373 [DOI] [PubMed] [Google Scholar]
- 34.Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 2010; 20: 1545–53. doi: 10.1007/s00330-009-1699-5 [DOI] [PubMed] [Google Scholar]
- 35.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 36.Hawes D, Downey S, Pearce CL, Bartow S, Wan P, Pike MC, et al. Dense breast stromal tissue shows greatly increased concentration of breast epithelium but no increase in its proliferative activity. Breast Cancer Res 2006; 8: R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong CC, Tang BK, Rao V, Agarwal S, Martin L, Tritchler D, et al. Cytochrome P450 1A2 (CYP1A2) activity, mammographic density, and oxidative stress: a cross-sectional study. Breast Cancer Res 2004; 6: R338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee E, Van Den Berg D, Hsu C, Ursin G, Koh WP, Yuan JM, et al. Genetic variation in transforming growth factor beta 1 and mammographic density in Singapore Chinese women. Cancer Res 2013; 73: 1876–82. doi: 10.1158/0008-5472.CAN-12-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev 2001; 10: 243–8. [PubMed] [Google Scholar]
- 40.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF)-1, IGF-binding protein 3, and mammographic density. Cancer Res 2000; 60: 3744–8. [PubMed] [Google Scholar]
- 42.van Duijnhoven FJ, Peeters PH, Warren RM, Bingham SA, Uitterlinden AG, van Noord PA, et al. Influence of estrogen receptor alpha and progesterone receptor polymorphisms on the effects of hormone therapy on mammographic density. Cancer Epidemiol Biomarkers Prev 2006; 15: 462–7. [DOI] [PubMed] [Google Scholar]
- 43.Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol 2003; 86: 477–86. [DOI] [PubMed] [Google Scholar]
- 44.Tagliafico AS, Tagliafico G, Cavagnetto F, Calabrese M, Houssami N. Estimation of percentage breast tissue density: comparison between digital mammography (2D full field digital mammography) and digital breast tomosynthesis according to different BI-RADS categories. Br J Radiol 2013; 86: 20130255. doi: 10.1259/bjr.20130255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redondo A, Comas M, Macià F, Ferrer F, Murta-Nascimento C, Maristany MT, et al. Inter- and intraradiologist variability in the BI-RADS assessment and breast density categories for screening mammograms. Br J Radiol 2012; 85: 1465–70. doi: 10.1259/bjr/21256379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakic PR, Carton AK, Kontos D, Zhang C, Troxel AB, Maidment AD. Breast percent density: estimation on digital mammograms and central tomosynthesis projections. Radiology 2009; 252: 40–9. doi: 10.1148/radiol.2521081621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tagliafico A, Tagliafico G, Astengo D, Airaldi S, Calabrese M, Houssami N. Comparative estimation of percentage breast tissue density for digital mammography, digital breast tomosynthesis, and magnetic resonance imaging. Breast Cancer Res Treat 2013; 138: 311–17. doi: 10.1007/s10549-013-2419-z [DOI] [PubMed] [Google Scholar]
- 48.Kontos D, Ikejimba LC, Bakic PR, Troxel AB, Conant EF, Maidment AD. Analysis of parenchymal texture with digital breast tomosynthesis: comparison with digital mammography and implications for cancer risk assessment. Radiology 2011; 261: 80–91. doi: 10.1148/radiol.11100966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Regini E, Mariscotti G, Durando M, Ghione G, Luparia A, Campanino PP, et al. Radiological assessment of breast density by visual classification (BI-RADS) compared to automated volumetric digital software (Quantra): implications for clinical practice. Radiol Med March 2014. Epub ahead of print. doi: 10.1007/511547-014-0390-3 [DOI] [PubMed] [Google Scholar]
- 50.Kontos D, Bakic PR, Carton AK, Troxel AB, Conant EF, Maidment AD. Parenchymal texture analysis in digital breast tomosynthesis for breast cancer risk estimation: a preliminary study. Acad Radiol 2009; 16: 283–98. doi: 10.1016/j.acra.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren BR, Smith A, Jing ZX. Measurement of breast density with digital breast tomosynthesis. Proc SPIE 2012; 83134Q. doi: 10.1117/12.913357 [DOI]
- 52.Bakic PR, Kontos D, Carton A-K, Maidment ADA. Breast percent density estimation from 3D reconstructed digital breast tomosynthesis. Proc SPIE 2008; 691318. doi: 10.1117/12.773890 [DOI]
- 53.Tromans C, Highnam R, Morrish O, Black R, Tucker L, Gilbert FJ. Volumetric breast density estimation on conventional mammography versus digital breast tomosynthesis. [Scientific Exhibit.] 2014; Poster no. C-0363. doi: 10.1594/ecr2014/C-0363 [DOI]
- 54.Diffey J, Berks M, Hufton A, Chung C, Verow R, Morrison J, et al. A stepwedge-based method for measuring breast density: observer variability and comparison with human reading. Proc SPIE 2010; 76220A. doi: 10.1117/12.844265 [DOI]
- 55.Kontos D, Bakic PR, Acciavatti RJ, Conant EF, Maidment ADA. A comparative study of volumetric and area-based breast density estimation in digital mammography: results from a screening population. Proceedings of the Digital Mammography 10th International Workshop, IWOM 2010; 16–18 June 2010; Girona, Spain, 2010. [Google Scholar]
- 56.Tagliafico A, Tagliafico G, Tosto S, Chiesa F, Martinoli C, Derchi LE, et al. Mammographic density estimation: comparison among BI-RADS categories, a semi-automated software and a fully automated one. Breast 2009; 18: 35–40. doi: 10.1016/j.breast.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 57.Gubern-Merida A, Kallenberg M, Platel B, Mann RM, Marti R, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms: a validation study. PLoS One 2014; 9: e85952. doi: 10.1371/journal.pone.0085952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo JM, Ko ES, Han BK, Ko EY, Shin JH, Hahn SY. Automated volumetric breast density estimation: a comparison with visual assessment. Clin Radiol 2013; 68: 690–5. doi: 10.1016/j.crad.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 59.Kontos D, Xing Y, Bakic PR, Conant EF, Maidment ADA. A comparative study of volumetric breast density estimation in digital mammography and magnetic resonance imaging: results from a high-risk population. Proc SPIE 2010; 762409. doi: 10.117/12.845568 [DOI]
- 60.Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D, et al. Agreement of mammographic measures of volumetric breast density to MRI. PLoS One 2013; 8: e81653. doi: 10.1371/journal.pone.0081653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh JM, Fallenberg EM, Diekmann F, Renz DM, Witlandt R, Bick U, et al. Volumetric breast density assessment: reproducibility in serial examinations and comparison with visual assessment. Rofo 2013; 185: 844–8. doi: 10.1055/s-0033-1335981 [DOI] [PubMed] [Google Scholar]
- 62.Engelken F, Singh JM, Fallenberg EM, Bick U, Böttcher J, Renz DM. Volumetric breast composition analysis: reproducibility of breast percent density and fibroglandular tissue volume measurements in serial mammograms. Acta Radiol 2014; 55: 32–8. doi: 10.1177/0284185113492721 [DOI] [PubMed] [Google Scholar]