Abstract
Objective:
To evaluate the usefulness of MR computer-aided detection (CAD) in patients undergoing neoadjuvant chemotherapy for prediction of the pathological complete response of tumours.
Methods:
148 patients with breast cancer (mean age, 47.3 years; range, 29–72 years) who underwent neoadjuvant chemotherapy were included in our study. They underwent MRI before and after neoadjuvant chemotherapy, and we reviewed the pathological result as the gold standard. The computer-generated kinetic features for each lesion were recorded, and the features analysed included “threshold enhancement” at 50% and 100% minimum thresholds; degree of initial peak enhancement; and enhancement profiles comprising lesion percentages of washout, plateau and persistent enhancement. The final pathological size and character of tumours were correlated with post-chemotherapy mammography, ultrasonography and MR CAD findings. Kruskal–Wallis test and intraclass correlation coefficient were used to analyse the findings.
Results:
We divided the 148 patients into complete pathological response and non-complete pathological response groups. A complete pathological response was defined as no histopathological evidence of any residual invasive cancer cells in the breast or axillary lymph nodes. 39 patients showed complete pathological response, and 109 patients showed non-complete pathological response. Between enhancement profiles of MR CAD, plateau proportion of tumours was significantly correlated with the pathological response of tumours (mean proportion of plateau on complete pathological response group was 27%, p = 0.007).
Conclusion:
When plateau proportion of tumours is high, we can predict non-complete pathological response of neoadjuvant chemotherapy.
Advances in knowledge:
MR CAD can be a useful tool for the assessment of response to neoadjuvant chemotherapy and prediction of pathological results.
In the early 1980s, neoadjuvant chemotherapy was introduced to improve outcomes in patients with advanced breast cancer.1 This therapeutic method has been known for its fascinating advantages. Large and advanced breast cancers might be downstaged for conservative surgery rather than mastectomy. It also offers an improved survival rate. Furthermore, tumour response may be assessed in vivo by measuring tumour size. As a result, ineffective chemotherapy can be stopped and patients can avoid unnecessary toxicity.2,3 Previous studies have shown that the size of residual tumours and the response of a tumour to neoadjuvant chemotherapy are related to the recurrence-free survival rate.4–8 It was also exhibited8,9 that neoadjuvant chemotherapy could lead to a pathological complete response (pCR) in up to 30% of patients with breast cancers, and these patients showed a better survival outcome than patients with residual cancers. This result emphasizes that prediction of chemotherapeutic effects before treatment could be critical for a successful cancer treatment.
Pathological correlation with MRI has demonstrated greater sensitivity for evaluating breast cancers than do conventional imaging methods such as mammography and ultrasonography. MRI can predict the size and extent of lesions, including margins, with sensitivity near 100%.10 It also accurately evaluates residual tumour and better determines chemotherapeutic response. This imaging method could be beneficial for evaluation of response to neoadjuvant chemotherapy. As a result, it might enable physicians to optimize treatment regimens both early in the course of chemotherapy and post-operatively and to offer more opportunities for breast conservation.11
However, breast MRI requires more time for image processing and interpretation than do other conventional methods and has demonstrated variable specificity. To overcome these limitations, computer-aided detection (CAD) programs for breast MRI are widely used. These systems have the potential to improve efficiency of breast MRI and reduce the number of false-positive diagnoses.12 CAD may not only improve consistency and detection rate but also provide new methods of analysis that are not available with manual interpretation such as quantitative measurement of kinetic curve thresholds.13 But, a previous study has shown that CAD was less accurate than a radiologist in the assessment of tumour size in patients with breast cancer undergoing neoadjuvant chemotherapy.14 Other research reported that CAD is sufficiently accurate for the assessment of the extent of residual tumours, but the assessment by a radiologist and CAD showed a fair-to-poor agreement for assessment of response to chemotherapy.15 However, controversy remains as to whether CAD is accurate for MRI of patients with breast cancer who were treated with neoadjuvant chemotherapy.
Therefore, the purposes of this study were to retrospectively evaluate whether MRI parameters assessed with CAD are associated with the pCR of tumours, and to evaluate the accuracy of CAD in breast MRI for the assessment of the extent of residual tumours in patients undergoing neoadjuvant chemotherapy for breast cancer.
METHODS AND MATERIALS
Patient selection
Patient selection for this study was approved by the ethics committee of our institution (Asan Medical Center, Seoul, Korea).
Selected patients with histopathologically confirmed breast cancer underwent neoadjuvant chemotherapy from November 2007 to February 2011. Patients who underwent both baseline and follow-up MRI measurements before and after treatment as well as surgery after completing neoadjuvant chemotherapy were included in our study. 151 patients were identified from a retrospective review of our breast MRI database, which involved dynamic contrast-enhanced breast MRI.
In cases of multifocal disease, the largest one was selected as the index tumour. After elimination of 3 females who underwent MR examination after the first cycle of neoadjuvant chemotherapy, 148 lesions of 148 patients (mean age, 47.3 years; range, 29–72 years) were included in the analysis. All patients underwent mammography, ultrasonography and core biopsy with a histological diagnosis of breast cancer. The mean interval between pre-neoadjuvant chemotherapy MR examinations and initiation of neoadjuvant chemotherapy was 10 days (range, 0–33 days), and the mean interval between the initiation of neoadjuvant chemotherapy and post-treatment MR examination was 141 days (range, 59–205 days). The median number of neoadjuvant chemotherapy cycles was seven (range, four to nine cycles). The median interval between post-treatment MR examination and surgery was 17 days (range, 1–74 days). 107 patients (72%) received taxane plus anthracycline regimens, whereas 29 patients (20%) received an anthracycline-based regimen. The remaining 12 patients (8%) received a trastuzumab plus taxane regimen.
MRI protocol
MRI was performed using a 1.5-T MR scanner (MAGNETOM® Avanto; Siemens Medical Solutions, Erlangen, Germany). The body coil was used as the transmitter, and a dedicated four-channel phased-array breast coil (Siemens Medical Solutions) as the receiver. Bilateral breast imaging was performed with the following protocol: an axial short T1 inversion recovery sequence [repetition time (TR)/echo time (TE) = 4400/74 ms; inversion time, 130 ms; 5-mm thickness without an interslice gap; field of view, 340 × 340 mm2; matrix size, 224 × 448 pixels; acquisition time, 134 s], a three dimensional (3D) T1 weighted fast low-angle shot dynamic gradient-echo sequence (TR/TE = 5.0/2.4 ms; flip angle, 10°; 0.9-mm thickness without an interslice gap; 0.9 × 0.9 × 0.9 mm3 isotropic voxel; one unenhanced and five contrast-enhanced acquisitions with a temporal resolution of 60 s) and an intravenous bolus injection of 0.2 ml kg−1 gadoterate meglumine (Dotarem®; Guerbet, Paris, France) administered using a MR-compatible power injector (Spectris; Medrad®, Pittsburgh, PA) with a flow of 1 ml s−1, followed by a 20-ml saline flush. One pre-contrast and five post-contrast dynamic series were obtained at every 60 s after injection of a contrast agent. Post-processing manipulations included subtraction images and maximum-intensity projection images.
Computer-aided detection data collection and analysis
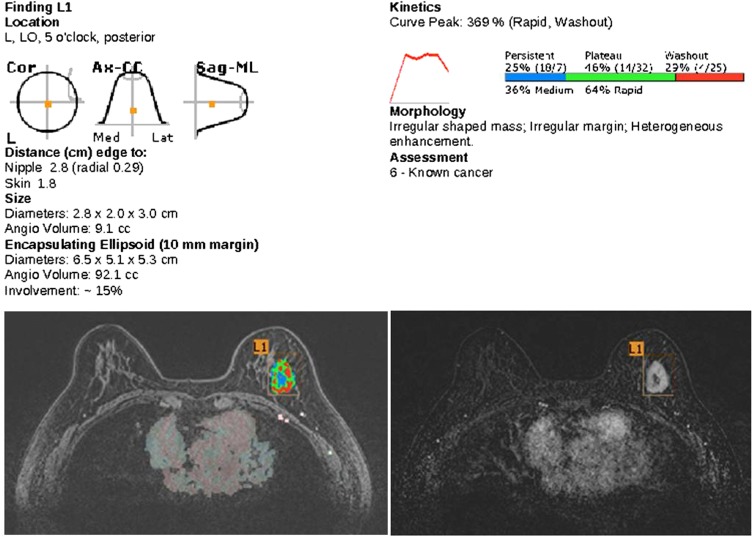
All MRI examinations were subsequently processed by a computer-aided evaluation software (CADstream®; Confirma Inc., Kirkland, WA), a commercially available CAD system. For measurement of MR image parameters, pre-contrast and all post-contrast T1 weighted image series were transferred to a CAD system. The system automatically segmented the tumours into three dimensions and calculated the tumour diameter (maximal size of an enhancing lesion), tumour volume (total enhancing lesion volume), peak enhancement value (highest pixel signal intensity at the first post-contrast series) and proportions of persistent, plateau and washout-enhancing components within a tumour. We took the 50% enhancement threshold level to compare the pre-contrast and first post-contrast series to attain greater sensitivity in the detection of slowly enhancing lesions frequently found in the neoadjuvant chemotherapy setting.16–18 A colour map was generated according to the delayed phase enhancement type after peak enhancement as follows: persistent type, which indicated increased pixel signal intensity of >10% from the first post-contrast series; washout type, which indicated decreased pixel signal intensity at the last post-contrast series of >10% from the first post-contrast series; or plateau type, which indicated increased pixel signal intensity at the last post-contrast series of <10% and decreased intensity of <10% from the first post-contrast series (Figure 1).
Figure 1.
Definition of computer-assisted diagnosis-generated variabls.
The percentages of regions demonstrating different enhancement curves in a given lesion are automatically summarized in an enhancement profile of the lesion by the program (Figure 2).
Figure 2.
Demonstration of the kinetic features of tumour on a report from MR computer-aided detection (CAD). The tumour location, distance from the nipple, size and angio volume are present in the standardized report of the CAD system. It also shows kinetic parameters of the tumour with a colour-coded image. For breast studies, it is only able to show the washout map. Radiologists can manually add information about the morphology of the tumour and the final assessment in the report. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140142.
The CAD-processed MRI examinations were reviewed on a CAD workstation by a breast radiologist. The same radiologists, who had performed a one-dimensional measurement at a picture archiving and communication system workstation, selected the same lesions for CAD measurement. CAD-generated enhancement profiles were recorded, with the percentages of each total enhancement of the lesions allocated to persistent, plateau and washout enhancement types. CAD-generated maximum tumour sizes were recorded. Maximum sizes of residual invasive malignancy at final surgery were obtained from the surgical pathology reports.
Histopathological analysis
Histopathological examinations were performed by board-certified breast pathologists according to the TNM classification. Final tumour diameters were determined on the basis of gross and microscopic evaluation of the surgical specimens. Standardized report templates included the number and size of measurable invasive components and carcinoma in situ components of the tumours. The definition of pCR was the absence of invasive tumour cells in the primary tumour sites (ductal carcinoma in situ may be present).
Statistical analysis
The Kruskal–Wallis test was utilized to analyse the relationship between pCR and kinetic features of MR CAD. Intraclass correlation coefficient (ICC) was used each for evaluation of consensus between MRI, MR CAD and final pathological diameter.
All statistical analyses were performed with a statistical software (SPSS® for Windows v. 12.0; SPSS, Chicago, IL). A p < 0.05 was considered to indicate a statistically significant difference. The Kruskal–Wallis test was used for analysis, and ICC was applied for evaluation of consensus between measures.
RESULTS
Patients and lesions
A total of 148 patients were included in our study. The mean age of the included patients was 47.3 years (range, 29–72 years). The size of the lesions ranged from 1.9 to 13.0 cm on pre-operative MRI. Pre-operative histopathological evaluation after core needle biopsy revealed invasive ductal carcinoma (n = 140, 95%), invasive lobular carcinoma (n = 3, 2%), metaplastic carcinoma (n = 3, 2%), mucinous carcinoma (n = 1, 0.7%) and invasive apocrine carcinoma (n = 1, 0.7%).
Lesion features after neoadjuvant chemotherapy
After neoadjuvant chemotherapy, 75 lesions did not show any abnormal enhancing signal in the non-visible MR CAD. The mean size of lesion on the final pathology was 1.2 cm (range, 0–8.8 cm). The final pathological findings of the no visible MR CAD signal group were residual invasive ductal carcinoma (n = 34, 45%), no residual tumour (n = 18, 24%), ductal carcinoma in situ (n = 17, 23%), invasive lobular carcinoma (n = 3, 4%) and others (n = 3, 4%). Pathological findings of the visible signal group (n = 73) were residual invasive ductal carcinoma (n = 67, 92%), ductal carcinoma in situ (n = 3, 4%), no residual tumour (n = 2, 3%) and metaplastic carcinoma (n = 1, 1%).
While 39 patients exhibited complete pathological response, 109 patients showed non-complete pathological response. Between enhancement profiles of MR CAD, plateau proportion of tumours indicated a significant negative correlation with pCR (mean, 27%; p-value, 0.007; Table 1). Proportion of plateau enhancement on total tumour volume was quite low in the complete pathological response group. The p-value for persistent enhancement was nearly significant (p-value, 0.067; one-tailed p-value, 0.034). Other profiles showed no significant correlation (Figures 3 and 4).
Table 1.
Relationship between enhancement profiles of MR computer-aided detection and pathological result of tumour
| Variable (%) | Total (n = 148) | pCR (n = 39) | Non-pCR (n = 109) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Mean | SD | Mean | SD | p-value | |
| Rapid | 3 | 97 | 50.2770 | 20.31983 | 50.7949 | 21.25556 | 50.0917 | 20.07234 | 0.846 |
| Medium | 0 | 97 | 49.7162 | 20.32727 | 49.2051 | 21.25556 | 49.8991 | 20.08267 | 0.846 |
| Persistent | 4 | 66 | 44.0203 | 18.33493 | 48.7179 | 19.96042 | 42.3394 | 17.50884 | 0.067 |
| Plateau | 3 | 75 | 31.3581 | 10.10423 | 27.4872 | 8.95566 | 32.7431 | 10.16840 | 0.007 |
| Washout | 0 | 40 | 24.6041 | 15.79028 | 23.4718 | 16.87655 | 25.0092 | 15.44434 | 0.445 |
pCR, pathological complete response; SD, standard deviation.
Kruskal–Wallis test (p < 0.05).
Figure 3.
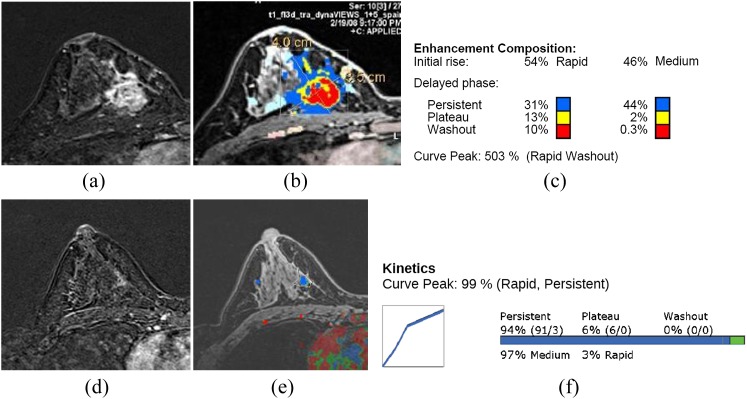
MR computer-aided detection (CAD) images of a 51-year-old patient with invasive ductal carcinoma. Before chemotherapy, there is a well enhancing mass with internal non-enhancing area in the inner portion of the right breast (a). The colour-coded kinetic pattern was overlaid in the MR CAD image (b), delayed plateau enhancement component was 15% of the total tumour angio volume (c). After chemotherapy, the previously noted malignant mass markedly decreased and a subtle enhancing parenchymal lesion remained (d). The kinetic curve showed predominant progressive enhancing pattern on MR CAD (e, f). After a breast conserving operation, there was 1 cm of focal ductal carcinoma in situ on the final pathology. Ax, axial; CC, craniocaudal; Cor, coronal; Lat, lateral; L1, lesion 1; LO, lower outer; Med, medial; ML, mediolateral; Sag, sagittal. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140142.
Figure 4.
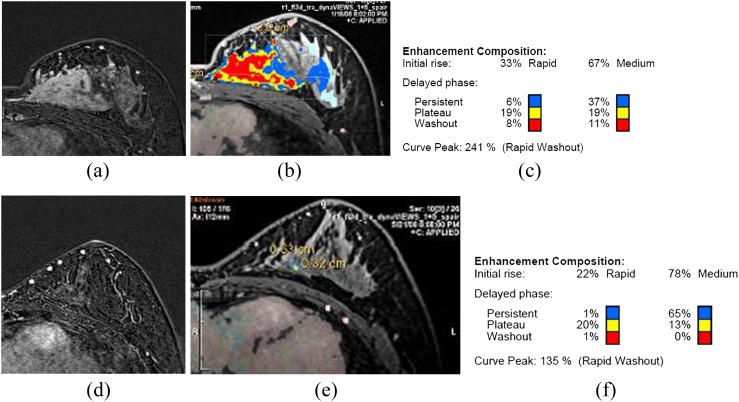
MR computer-aided detection (CAD) images of a 48-year-old patient with invasive ductal carcinoma. Before chemotherapy, there was a large well enhancing mass in the left breast on breast MRI (a). The colour-coded kinetic pattern was overlaid in the MR CAD image (b), plateau enhancement component was 38% of the total tumour angio volume (c). After chemotherapy, the size of the large mass significantly decreased (d), but there was still an enhancing parenchymal lesion on MRI of about 8 cm. The colour-coded kinetic pattern on MR CAD was also significantly improved from the previous study (e, f). There was 10 cm of residual invasive ductal carcinoma on final pathological result after modified radical mastectomy. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140142.
Agreement with pathological findings
On evaluation of consensus to pathological diameter, manual measurement of conventional MRI revealed higher agreement (ICC = 0.6378) than that of automated measurement of MR CAD (ICC = 0.3844) (Figure 5).
Figure 5.
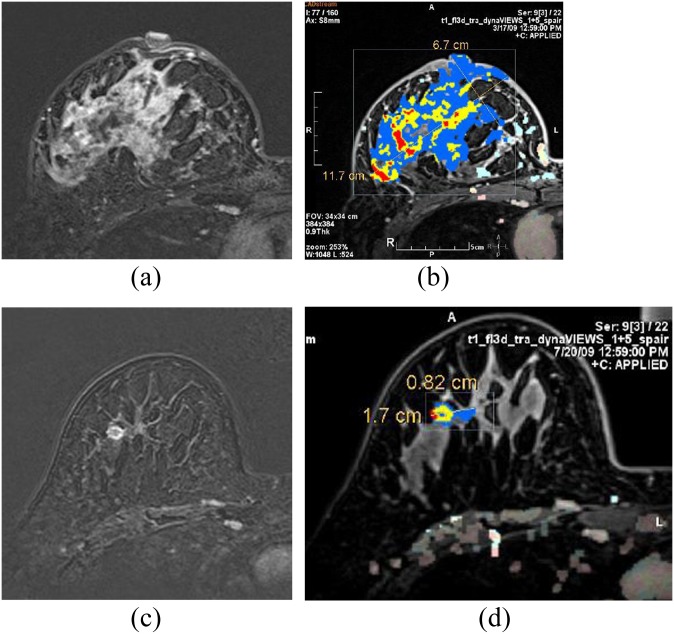
MR computer-aided detection (CAD) images of a 52-year-old invasive ductal carcinoma patient. In breast MRI, there was a well enhancing large mass in the right breast (a). The colour-coded kinetic pattern was overlaid in the MR CAD image with calculated tumour size (b). After chemotherapy, the size of the tumour markedly decreased (c, d). After modified radical mastectomy, there was 2.0 cm of residual invasive ductal carcinoma on the final pathology. FOV, field of view; L, length; W, width. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140142.
DISCUSSION
Neoadjuvant chemotherapy was undertaken with the aim of shrinking tumours in patients who were not candidates for primary surgery and in the hope of allowing greater conservation of the breast.19 In addition, neovascularity associated with cancers may affect the effectiveness of chemotherapeutic agents for the treatment of primary breast cancer as well as potential metastasis. While the tumour vasculature remains intact, prior chemotherapeutic treatment before surgery might improve the delivery of chemotherapeutic agents to the targeted tumour cells, leading to more successful chemotherapy. Owing to these potential advantages of neoadjuvant chemotherapy in the treatment of advanced breast cancer, it has become widely adopted over the past several years.20
All conventional methods for initial assessment of breast cancers, such as mammography and sonography, have been known to be suboptimal in the accurate assessment of response to neoadjuvant chemotherapy.21 Some previous studies have suggested that the lack of concordance may be related to chemotherapy-induced fibrosis.22 It has been accepted that breast MRI with contrast enhancement indicates a high degree of sensitivity ranging from 95% to 100% and a variable specificity ranging from 37% to 97% to detect breast cancers.23–30 Measuring the size of residual tumour and enhancement patterns in predicting response to neoadjuvant chemotherapy with breast MRI has been also investigated in many previous studies.31,32 Chemotherapy-induced fibrosis can be difficult to differentiate from residual disease on conventional imaging methods, but MRI could be a more effective method for differential diagnosis of residual lesion vs chemotherapy-induced fibrosis.
We applied a computer-assisted program capable of semi-automatic assessment of kinetic features in a given lesion. The advantage of this approach lies in the exclusion of interobserver variability, as well as additional information on the enhancement pattern distribution of the whole lesion.
After chemotherapy, >70% of patients (33/46) showed false-negative findings on MR CAD. The potential for falsely negative MRI examinations following neoadjuvant chemotherapy has been demonstrated in multiple previous studies, by Abraham et al11 (1/31 lesions), Rieber et al33 (4/13 lesions), Wasser et al34 (1/20 lesions), Rosen et al35 (1/19 lesions) and Chen et al36 (7/13 lesions). Multiple previous studies have shown a significant reduction in the peak contrast enhancement of tumours following chemotherapy, and a MRI false-negative rate has been attributed to this phenomenon. In case a residual enhancement below threshold could be present, lowering the specified minimum for significant enhancement might reduce the false-negative rate of CAD. However, a false-positive rate could be increased when the threshold is decreased.
Comparing the pre-chemotherapy enhancement profiles of MR CAD and tumour response, a significant correlation between the total volume of delayed plateau enhancement and tumours showing final pathological incomplete response was identified. The proportion of delayed persistent enhancement did not reach statistical significance but showed nearly significant results. We think that this phenomenon might be correlated with angiogenesis of tumours. The reaction of chemotherapeutic agents through vessels could be disturbed owing to poorly expressed or overexpressed neovascular structures. Our findings can be associated with a previous study by Weidner et al.37 Their research that highlighted blood microvessel density as a prognostic factor to breast cancer was initially accepted as a powerful parameter to identify more aggressive phenotypes of breast cancer. However, different opinions have been published on this subject that new vessels developed in tumour settings are not adequately assembled and these fragile conduits have been demonstrated to be collapsed in intratumour masses. Accordingly, the newly formed intratumour blood vessels are faint or not even functional.38 The application of this finding for patients who pre-arranged neoadjuvant chemotherapy may be expected to prevent unnecessary and inconducive chemotherapy. Furthermore, a recent multi-institutional prospective study39 also reported that pCR is more highly predictive of recurrence-free survival for every established receptor subset than overall breast cancers.
Multiple studies have shown that lesion size measured on MRI after neoadjuvant chemotherapy correlates well with residual malignancy at pathology as well as correlation coefficients ranging from 0.70 to 0.98.40–44 In our study, conventional MRI showed the highest agreement compared with other methods and MR CAD, similar to previous studies.
There are some limitations in our study. First, this was a retrospective study with a small sample size and was conducted at a single institute. Second, it was not possible to correlate computer-aided volumetric results with tumour volumes determined by histopathology, as the 3D volumetric results were not available in the clinical situation. Third, performed neoadjuvant chemotherapy regimens were not the same in all of the patients. Fourth, some part of the residual invasive ductal carcinoma component showing no significant enhancement owing to its small size could be excluded in the final MRI measurement.
In conclusion, MR CAD can be a useful tool for the assessment of response to neoadjuvant chemotherapy and prediction of pathological results. While the poor response of neoadjuvant chemotherapy could be expected in cases of a high plateau proportion of tumour, less invasive components of final pathological findings might be suspected when the signal of MR CAD disappeared after neoadjuvant chemotherapy. The prediction of therapeutic response with MR CAD could help determine effectiveness of treatment and avoid ineffective chemotherapy with its associated possible complications. This subsequently offers other treatment choices to physicians and patients.
REFERENCES
- 1.Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, et al. A Phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia 2010; 26: 514–21. doi: 10.3109/02656731003639364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg 2005; 92: 14–23. doi: 10.1002/bjs.4840 [DOI] [PubMed] [Google Scholar]
- 3.Sapunar F, Smith IE. Neoadjuvant chemotherapy for breast cancer. Ann Med 2000; 32: 43–50. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bryant J, Wolmark N, Mamousnas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–85. [DOI] [PubMed] [Google Scholar]
- 5.Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 6.Buchholz TA, Hill BS, Tucker SL, Frye DK, Kuerer HM, Buzdar AU, et al. Factors predictive of outcome in patients with breast cancer refractory to neoadjuvant chemotherapy. Cancer J 2001; 7: 413–20. [PubMed] [Google Scholar]
- 7.Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol 2008; 9: 10–18. doi: 10.3348/kjr.2008.9.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999; 17: 460–9. [DOI] [PubMed] [Google Scholar]
- 9.Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 2008; 26: 814–19. doi: 10.1200/JCO.2007.15.3510 [DOI] [PubMed] [Google Scholar]
- 10.Drew PJ, Chatterjee S, Turnbull LW, Read J, Carleton PJ, Fox JN, et al. Dynamic contrast enhanced magnetic resonance imaging of the breast is superior to triple assessment for the pre-operative detection of multifocal breast cancer. Ann Surg Oncol 1999; 6: 599–603. [DOI] [PubMed] [Google Scholar]
- 11.Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer 1996; 78: 91–100. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD, Peacock S, DeMartini WB, Chen X. A new automated software system to evaluate breast MR examinations: improved specificity without decreased sensitivity. AJR Am J Roentgenol 2006; 187: 51–6. [DOI] [PubMed] [Google Scholar]
- 13.Meeuwis C, Van de Ven SM, Stapper G, Fernandes Gallardo AM, van den Bosch MA, Mali WPM, et al. Computer-aided detection (CAD) for breast MRI: evaluation of efficacy at 3.0 T. Eur Radiol 2010; 20: 522–8. doi: 10.1007/s00330-009-1573-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demartini WB, Lehman CD, Peacock S, Russell MT. Computer aided detection applied to breast MRI: assessment of CAD generated enhancement and tumor sizes in breast cancers before and after neoadjuvant chemotherapy. Acad Radiol 2005; 12: 806–14. [DOI] [PubMed] [Google Scholar]
- 15.Lyou CY, Cho N, Kim SM, Jang M, Park JS, Baek SY, et al. Computer-aided evaluation of breast MRI for the residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy. Korean J Radiol 2011; 12: 34–43. doi: 10.3348/kjr.2011.12.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge SC, Gibbs JE, Lu Y, Essserman LJ, Tripathy D, Wolverton DS, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184: 1774–81. [DOI] [PubMed] [Google Scholar]
- 17.Williams TC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. Breast MR imaging: computer-aided evaluation program for discriminating benign from malignant lesions. Radiology 2007; 244: 94–103. [DOI] [PubMed] [Google Scholar]
- 18.Yi A, Cho N, Im SA, Chang JM, Kim SJ, Moon HG, et al. Survival outcomes of breast cancer patients who receive neoadjuvant chemotherapy: association with dynamic contrast-enhanced MR imaging with computer-aided evaluation. Radiology 2013; 268: 662–72. doi: 10.1148/radiol.13121801 [DOI] [PubMed] [Google Scholar]
- 19.Aapro MS. Neoadjuvant therapy in breast cancer: can we define its role? Oncologist 2001; 6(Suppl. 3): 36–9. [DOI] [PubMed] [Google Scholar]
- 20.Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol 2005; 184: 868–77. [DOI] [PubMed] [Google Scholar]
- 21.Singletary SE, McNeese MD, Hortobagyi GN. Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 1992; 69: 2849–52. [DOI] [PubMed] [Google Scholar]
- 22.Dao TH, Rahmouni A, Campana F, Laurent M, Asselain B, Fourquet A. Tumor recurrence versus fibrosis in the irradiated breast: differentiation with dynamic gadolinium-enhanced MR imaging. Radiology 1993; 187: 751–5. [DOI] [PubMed] [Google Scholar]
- 23.Heywang SH, Wolf A, Pruss E, Hilbertz T, Eiermann W, Permanetter W. MR imaging of the breast with Gd-DTPA: use and limitations. Radiology 1989; 171: 95–103. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA: preliminary observations. Radiology 1989; 170: 681–6. [DOI] [PubMed] [Google Scholar]
- 25.Revel D, Brasch RC, Paajanen H, Rosenau W, Grodd W, Engelstad B, et al. Gd-DTPA contrast enhancement and tissue differentiation in MR imaging of experimental breast carcinoma. Radiology 1986; 158: 319–23. [DOI] [PubMed] [Google Scholar]
- 26.Stack JP, Redmond OM, Codd MB, Dervan PA, Ennis JT. Breast disease: tissue characterization with Gd-DTPA enhancement profiles. Radiology 1990; 174: 491–4. [DOI] [PubMed] [Google Scholar]
- 27.Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic–pathologic correlation. Radiology 1994; 190: 485–93. [DOI] [PubMed] [Google Scholar]
- 28.Gribbestad IS, Nilsen G, Fjosne H, Fougner R, Haugen OA, Petersen SB, et al. Contrast-enhanced magnetic resonance imaging of the breast. Acta Oncol 1992; 31: 833–42. [DOI] [PubMed] [Google Scholar]
- 29.Gilles R, Guinebretière JM, Shapeero LG, Lesnik A, Contesso G, Sarrazin D, et al. Assessment of breast cancer recurrence with contrast-enhanced subtraction MR imaging: preliminary results in 26 patients. Radiology 1993; 188: 473–8. [DOI] [PubMed] [Google Scholar]
- 30.Rubens D, Totterman S, Chacko AK, Kothari K, Logan-Young W, Szumowski J, et al. Gadopentetate dimeglumine–enhanced chemical-shift MR imaging of the breast. AJR Am J Roentgenol 1991; 157: 267–70. [DOI] [PubMed] [Google Scholar]
- 31.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002; 179: 1193–9. [DOI] [PubMed] [Google Scholar]
- 32.Delille JP, Slanetz PJ, Yeh ED, Halpern EF, Kopans DB, Garrido L. Invasive ductal breast carcinoma response to neoadjuvant chemotherapy: noninvasive monitoring with functional MR imaging pilot study. Radiology 2003; 228: 63–9. [DOI] [PubMed] [Google Scholar]
- 33.Rieber A, Zeitler H, Rosenthal H, Görich J, Kreienberg R, Brambs HJ, et al. MRI of breast cancer: influence of chemotherapy on sensitivity. Br J Radiol 1997; 70: 452–8. [DOI] [PubMed] [Google Scholar]
- 34.Wasser K, Klein SK, Fink C, Junkermann H, Sinn HP, Zuna I, et al. Evaluation of neoadjuvant chemotherapeutic response of breast cancer using dynamic MRI with high temporal resolution. Eur Radiol 2003; 13: 80–7. [DOI] [PubMed] [Google Scholar]
- 35.Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol 2003; 181: 1275–82. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Moore MO, Lehman CD, Mankoff DA, Lawton TJ, Peacock S, et al. Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad Radiol 2004; 11: 1115–24. [DOI] [PubMed] [Google Scholar]
- 37.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 38.Duarte M, Longatto FA, Schmitt FC. Angiogenesis, haemostasis and cancer: new paradigms and old concerns. J Bras Patol Med Lab 2007; 43: 441–9. [Google Scholar]
- 39.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012; 30: 3242–9. doi: 10.1200/JCO.2011.39.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung YC, Chen SC, Su MY, See LC, Hsueh S, Chang HK, et al. Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat 2003; 78: 51–8. [DOI] [PubMed] [Google Scholar]
- 41.Martincich L, Montemurro F, Cirillo S, Marra V, De Rosa G, Ponzone R, et al. Role of magnetic resonance imaging in the prediction of tumor response in patients with locally advanced breast cancer receiving neoadjuvant chemo-therapy. [In English and Italian.] Radiol Med 2003; 106: 51–8. [PubMed] [Google Scholar]
- 42.Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs histologic measurement of breast cancer following chemotherapy: comparison with x-ray mammography and palpation. J Magn Reson Imaging 2001; 13: 868–75. [DOI] [PubMed] [Google Scholar]
- 43.Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat 2002; 72: 145–52. [DOI] [PubMed] [Google Scholar]
- 44.Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol 1999; 17: 110–19. [DOI] [PubMed] [Google Scholar]