Abstract
Objective:
Treatment planning for charged particle therapy in the thoracic and abdominal regions should take account of range uncertainty due to intrafractional motion. Here, we developed a design tool (4Dtool) for the target volume [field-specific target volume (FTV)], which accounts for this uncertainty using four-dimensional CT (4DCT).
Methods:
Target and normal tissue contours were input manually into a treatment planning system (TPS). These data were transferred to the 4Dtool via the picture archiving and communication system (PACS). Contours at the reference phase were propagated to other phases by deformable image registration. FTV was calculated using 4DCT on the 4Dtool. The TPS displays FTV contours using digital imaging and communications in medicine files imported from the PACS. These treatment parameters on the CT image at the reference phase were then used for dose calculation on the TPS. The tool was tested in single clinical case randomly selected from patients treated at our centre for lung cancer.
Results:
In this clinical case, calculation of dose distribution with the 4Dtool resulted in the successful delivery of carbon-ion beam at the reference phase of 95% of the prescribed dose to the clinical target volume (CTV). Application to the other phases also provided sufficient dose to the CTV.
Conclusion:
The 4Dtool software allows the design of the target volume with consideration to intrafractional range variation and is now in routine clinical use at our institution.
Advances in knowledge:
Our alternative technique represents a practical approach to four-dimensional treatment planning within the current state of charged particle therapy.
Our centre treats >700 patients per year by passive carbon-ion beam irradiation therapy, conducted over 4 days per week. Carbon-ion scanning beam treatment without respiratory gating was successfully implemented in 2011.1,2 To extend treatment anatomical sites, we are now preparing to introduce carbon-ion scanning treatment for the thoracic and abdominal regions. One of our goals is to increase capacity to meet the increasing numbers of patients, while maintaining high treatment accuracy and patient comfort.3–5 For the thoracic and abdominal regions, passive beam treatment of the internal target volume (ITV) is used with respiratory gating, with replacement of density values within the ITV to avoid cold spots within the target.6
Recent progresses in treatment beam irradiation techniques include four-dimensional (4D)-based dose calculation algorithms and image-guided techniques (= three dimensions plus time axis). Nevertheless, most commercial treatment planning systems (TPSs) remain basically three-dimensional (3D) in nature. Charged particle therapy faces the challenge of interfractional geometry changes and intrafractional motion. General sources of intrafractional motion are respiration, pulsation, peristalsis and patient motion. Several commercial software strategies for the management of four dimensions have appeared, such as the design of geometrical-based target volume (= ITV) and deformable image registration (DIR). However, while assessing doses to the target and organs at risk (OARs) requires quantitative information on beam range fluctuations, TPSs do not provide such data. Given that both inter- and intrafractional motion characteristics can differ among individual patients, many radiation oncologists and physicists are likely concerned about judging the acceptability of 3D treatment planning at other respiratory phases. Furthermore, while several solutions to these problems with 4D approaches have been suggested,7–12 most are still at the research level or in use at only a few institutions.
We routinely use TPSs for passive and scanning carbon-ion beam treatment in three dimensions, and we considered it would be more practical to extend the TPS to four dimensions. Here, we developed a graphical user interface (GUI)-based software application, which addresses the improvement for charged particle scanning beam therapy for a moving target and enables the exchange of data with commercial TPSs in routine clinical use.
METHODS AND MATERIALS
Concept of four-dimensional charged particle treatment planning
In essence, the main advantage of particles, their finite range, is also the main challenge in particle radiotherapy (RT) owing to the potential uncertainties it involves. Most treatment procedures for 4D charged particle treatment planning are similar in process to those for 3D planning. In principle, a treatment beam range is chosen to adequately irradiate the moving tumour and spare normal tissues at risk. Capturing intrafractional motion in the thoracic and abdominal regions requires time-resolved 3DCT imaging (4DCT). For this, the target volume and OARs on the 4DCT data at the reference phase (generally peak exhalation) are delineated. These contours are transferred to the other phases using DIR. Treatment planning parameters are then selected, including beam angles, beam spot weight map, gating window, number of rescans, prescribed dose, etc. Target volumes with consideration to intrafractional range variation (described below) are defined using 4DCT imaging.
Dose calculations at respective phases and beam spots are performed with consideration to dose rate, energy change time and respiratory cycle using 4DCT data sets. DIR is then applied to warp the resulting dose distributions back to the reference phase. This allows delivery of the accumulated dose over the motion cycle, with consideration of the interplay effect (full 4D treatment plan).
4Dtool software specification
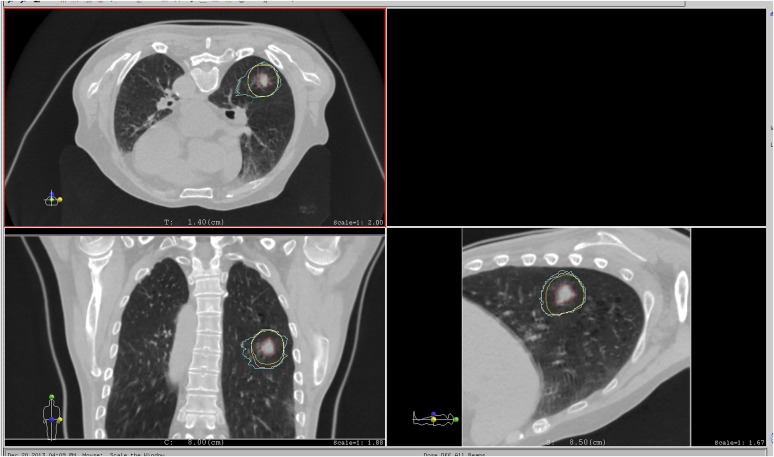
The 4Dtool imports and exports various data inputs in several digital formats, namely digital imaging and communications in medicine (DICOM) CT image, RT-plan and RT-Structure Set from the picture archiving and communication system (PACS). The tool incorporates a GUI with six image panels (axial, sagittal and coronal at reference and respective phases) (Figure 1). It also integrates several visualization functions, including zoom in/out of the whole image area, image overlay (subtraction, checker board, blend with images at reference and respective phases), etc. as well as projected contour data (derived from the RT-Structure Set) and measurements (length, angle, CT number). The DIR function allows the propagation of contour data at the reference phase to other phases. 4Dtool is programmed using C++ program language with Intel® integrated performance library and Intel math kernel library (Intel Corporation, Santa Clara, CA) on Microsoft® Visual Studio 2010 (Microsoft, Redmond, WA) and works under a Windows® 7 environment and is installed on a workstation (Dell™ Precision R5400, 2.66-GHz quad-core central processing unit Intel processor, 8-GB physical memory; Dell, Round Rock, TX).
Figure 1.
Main screen of the 4Dtool software. The upper panel shows CT images at the reference phase. The lower panel shows four-dimensional CT images at respective phases overlaid on the CT image at the reference phase. Red, yellow, white and light blue lines on the upper panel are the gross tumour volume, clinical target volume, internal target volume and field-specific target volume, respectively. Calculating beam angle is set to 20°. Exp, exposure; resp, respiratory. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140233.
Practical four-dimensional treatment planning procedure
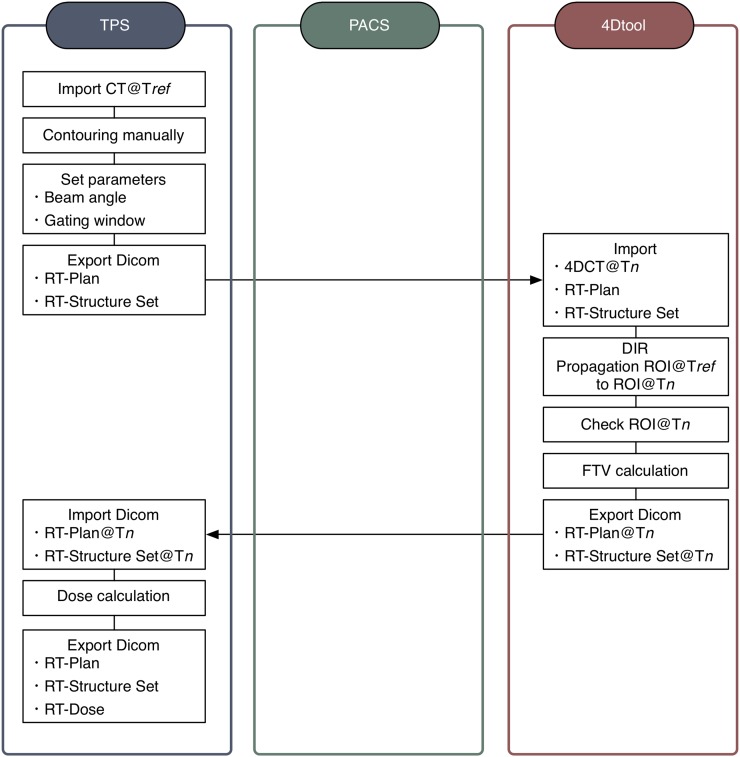
To expand the commercial TPS to four dimensions, the treatment procedure flow for TPS allows an alternative approach to the full 4D treatment planning (Figure 2).
Figure 2.
Overview of the four-dimensional (4D) treatment planning system. DICOM, digital imaging and communications in medicine; DIR, deformable image registration; FTV, field-specific target volume; PACS, picture archiving and communication system; ROI, region of interest; RT, radiotherapy; Tn, time at respective respiratory phase; Tref, time at reference respiratory phase; TPS, treatment planning system.
Import data and input contouring
The first step in treatment planning is to import patient CT data to TPS from PACS. A number of commercial software applications for target contouring at multiple respiratory phases are now available, but most limit the import of CT data for dose calculation to a single respiratory phase only. Gross tumour volume (GTV) and clinical target volume (CTV) are defined by the radiation oncologist, and OARs are segmented. A treatment plan is then designed, typically using beams from several angles. These procedures are generally performed on the TPS.
Propagation of contours
The TPS exports target and OAR structure data and treatment plan information to the 4Dtool software via PACS in DICOM-RT format (RT-Structure Set and RT-Plan, respectively). The 4Dtool imports 4DCT data sets from the PACS. The oncologist and medical physicist are then able to check all data sets from the TPS and PACS by display on the 4Dtool software GUI.
Structure sets (target and OARs) at the reference phase are transferred to other phases using B-spline-based DIR.13 The DIR technique reduces the need for manual contouring at other phases. DIR does not remove registration error completely, however, and the transferred structure sets may not be registered correctly. Since the 4Dtool integrates several contouring functions (manual input in axial, sagittal and coronal sections, auto segmentation, etc.), the radiation oncologist and medical physicist can modify structure sets on the respective phases.
Field-specific target volume calculation
To account for intra- and interfractional motion, we use ITV in photon and charged particle beam therapy. ICRU 62 describes the “geometrical” rather than “radiological pathlength” concept for ITV creation.14 The major difference between charged particle beam therapy and photon beam therapy is that the former considers radiological pathlength variation along respective given rays as a function of time. ITV is therefore unsuitable for particle beam therapy. While ICRU report 78 introduced range incorporation margins added to CTV, however, it is not clearly stated how to use it for intrafractional moving target.15 The maximum intensity volume and average intensity projection approaches have been used in passive particle beam irradiation16,17 and are utilized by a commercial TPS for treatment beam delivery to a moving target. Because these methods may result in expansion of the smeared beam field and density regions, however, and subsequent overdosage to normal tissue regions, their use in calculating intrafractional range variation has not been fully investigated.18 To solve this problem, we integrated the field-specific target volume (FTV) calculation to the 4Dtool software, which is similar to ITV but not identical, and FTV is likely based on an idea stated originally in Graeff et al.9
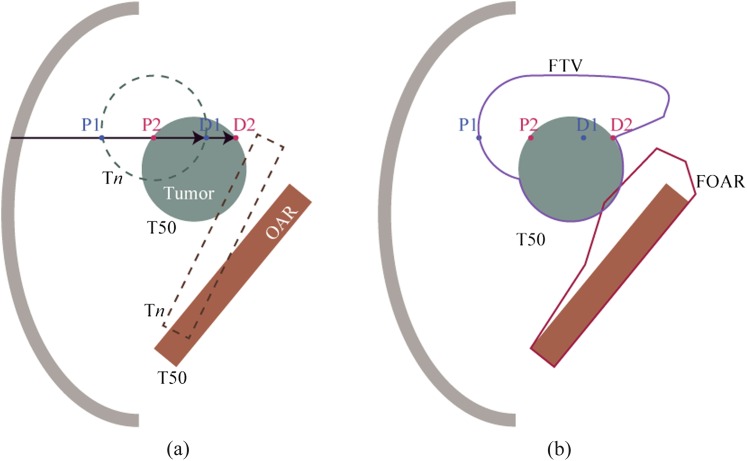
First, the user specifies the gating window phase interval, which is commonly set to a 30% beam on phase (window near exhalation, but can be set to any desired interval). Second, FTV/field-specific organs at risk (FOAR) are designed as follows. Water equivalent pathlength (WEPL) values at the proximal and distal edge of the target at respective phases are calculated (Figure 3a), and maximum and minimum WEPL values at the respective distal and proximal sides are selected. For example, WEPL at points P1, P2, D1 and D2 are on the same ray line at respective phases (Figure 3a). WEPL at points P1 and D2, the minimum and maximum values, respectively, are projected on the CT data at the reference phase (Figure 3b). The FTV is designed by doing this at respective ray lines. FOAR is designed by the same procedures.
Figure 3.
(a) Target and organs-at-risk positions at the reference phase (T50) and Tn. D1 and P1 are distal and target proximal position at Tn, respectively. D2 and P2 are distal and target proximal position at T50, respectively. (b) Field-specific target volume (FTV) and field-specific organs at risk (FOAR) were projected on the CT image at the reference phase (T50). OAR, organ at risk.
4Dtool calculates ITV within the gating window, and it is more helpful in assisting users to understand intrafractional respiratory motion than in displaying the FTV/FOAR because FTV/FOAR shapes are often more complex (zigzags, spikes, etc.) than CTV shapes, particularly in the lung region. Calculated FTV/FOAR and ITV contours are displayed on the 4Dtool software GUI (Figure 1). Users can check that CTV contours during the gating window are included in the ITV and FTV. 4Dtool then exports DICOM files (RT-Plan RT-Structure set) at respective phases to the PACS.
Dose calculation on the treatment planning system
The TPS displays the FTV/FOAR and ITV contours by importing the DICOM files above from the PACS (Figure 4). The user sets treatment parameters on the TPS, such as FTV and FOARs to the treated target and OARs, respectively. Other treatment procedures such as beam weight optimization, prescribed dose set, etc. are the same as in conventional 3D treatment planning. These treatment parameters on the CT image at the reference phase are used to calculate dose distribution on the TPS. Since treatment planning is FTV based, the prescribed dose is given to CTVs at the respective phases within the gating window. Also, the weighting applied to each 4DCT data set was derived from the respiratory signal. Since most commercial 4DCT equally subdivided respiratory cycle, equal weighing is generally used for each 4DCT phase. To assess dose distributions at other phases, the CT image at other phases and the treatment parameters defined at the reference phase are imported into the TPS, and the dose distributions are recalculated.
Figure 4.
Main screen of the treatment planning system. CT images at the reference phase and contours imported from the 4Dtool software are displayed. Red, yellow, white and light blue lines on the upper panel are the gross tumour volume, clinical target volume, internal target volume and field-specific target volume, respectively. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140233.
Clinical example
A single clinical case was studied by randomly selecting a patient treated at our centre for lung cancer. The patient agreed to participate in the study, which was approved by the institutional review board of National Institute of Radiological Sciences, Chiba, Japan.
Treatment planning CT was acquired under free breathing conditions in 4D mode (Aquilion One™ Vision; Toshiba Medical Systems, Otawara, Japan) with monitoring of respiration using a respiratory sensing system. Because this CT scanner is able to obtain approximately 16 cm in the longitudinal direction in a single rotation, the scan region does not include the 4DCT artefacts typically observed in conventional multislice CT.19 Detector collimation of 270.0 × 1.0 mm was selected to avoid the degradation of CT image quality close to the end of the CT scan region owing to insufficient data in the Radon space. The scan revolution time was 0.5 s. The 4DCT data sets were equally subdivided into ten phases (T00, peak inhalation; T50, peak exhalation).
GTV and OARs, including the normal bilateral lung (excluding the defined GTV), spinal cord and heart were manually delineated on the 4DCT data in the reference phase (T50). A CTV was created by adding 10-mm margins to the GTV in all directions. The FTV was designed for a 20° beam angle using the 4DCT data sets. The gating window was set from mid-exhalation (T30) to mid-inhalation (T70). Planning target volume (PTV) was defined by adding a 0-mm-WEPL proximal margin and a 2-mm-WEPL distal margin to the FTV. A set-up margin was not added. The prescribed dose of 12 Gy (RBE) was administered to the PTV via a single beam angle from 20°.
RESULTS
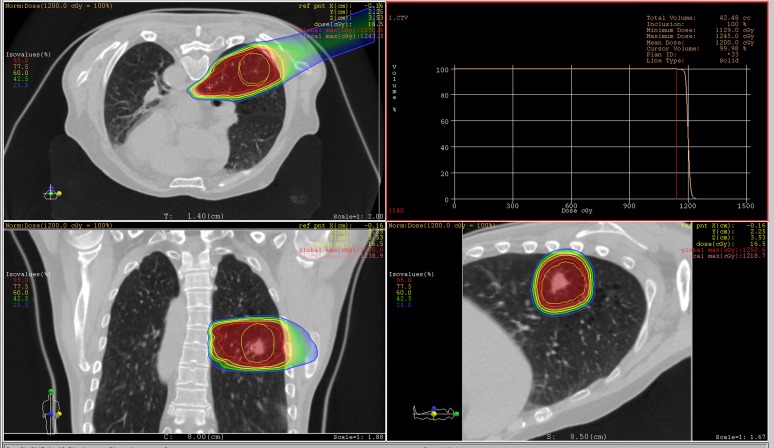
The carbon-ion beam dose distributions at the reference phase (T50) successfully gave sufficient dose to the CTV (Figure 5). Dose for >95% volume irradiation (D95) of the CTV at the reference phase was 100% of the prescribed dose.
Figure 5.
Carbon-ion dose distribution at the reference phase (T50, peak exhalation). The beam angle was set to 20°. Red, yellow and light green lines around the target show the gross tumour volume, clinical target volume and internal target volume (ITV), respectively. Red, orange, yellow, light green and blue lines out of the ITV are the 95%, 77.5%, 60%, 42.5% and 25% isodose lines, respectively. The right upper panel shows dose–volume histogram analysis. max, maximum; norm, normal; ref pnt, reference point. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140233.
To assess dose coverage within the gating window, the treatment planning parameters were applied to the 4DCT at other phases. Figure 6 shows carbon-ion beam dose distributions at mid-inhalation (T70) in the same image sections (axial, sagittal and coronal) as in Figure 5. The tumour position was moved to the inferior side owing to respiration, which resulted in an increased magnitude of beam overshoot around the superior side owing to replacement of the solid tumour density by the lower density of the lung. Despite this, sufficient dose was given to the CTV because of the FTV-based treatment planning. D95 of the CTV at T70 was 99.5%.
Figure 6.
Carbon-ion dose distribution at the reference phase (T70, mid inhalation). The beam angle was set to 20°. The red line around the target shows the clinical target volume. Red, orange, yellow, light green and blue lines out of the internal target volume are the 95%, 77.5%, 60%, 42.5% and 25% isodose lines, respectively. The right upper panel shows dose–volume histogram analysis. GTV, gross tumour volume; max, maximum; norm, normal; ref pnt, reference point. For colour images see online: www.birpublications.org/doi/abs/10.1259/bjr.20140233.
DISCUSSION
To enable treatment planning in charged particle therapy for the thoracic and abdominal regions with consideration to intrafractional respiratory motion, we developed a software application for design of the FTV using 4DCT data sets. This application interactively exchanges data with any commercial TPSs via the PACS and keeps the quality of the commercial TPS (dose calculation accuracy, etc.). This alternative approach to 4D treatment planning showed good dose coverage to a moving target and is now in routine clinical use.
Although treatment planning is more realistic when dose distributions are calculated at respective phases with inclusion of the interplay effect, most commercial TPSs are not able to calculate dose distribution with interplay effect. Our approach to 4D treatment planning with FTV irradiates all beam spots in respective phases; accordingly, the interplay effect does not occur and is not considered. We do this on the basis of our previous studies, which showed that four or more phase-controlled rescans with FTV should substantially improve the accuracy of dose delivery, and it is indeed close to that calculated by our present approach.20–22
In the present study, we introduced an alternative approach to four dimensions. This approach does not calculate 4D accumulated dose distribution using DIR, for the following reason. The most common approach to 4D treatment planning is to calculate the dose in each phase of a 4DCT and then to use DIR to warp the resulting dose distributions back to the reference phase,12,23,24 and carbon-ion beam accumulated dose is considered the non-linear biologically effective dose.25 While, TRiP4D performed different approach for the biological doses by accumulating a particle spectrum.25 To pursue this approach for a 4DCT acquisition with 10 different phases, the dose calculation needs to be performed 10 times. Each resulting 3D dose distribution then needs to be warped back to the reference phase. As described above, when propagated contours from other respiratory phases do not exclude DIR errors completely, the radiation oncologist modifies the contours manually. By contrast, however, oncologists cannot modify dose distributions. In other words, dose calculation with 4D treatment planning relies on DIR, the accuracy of which has been evaluated.26 There are different approaches to DIR, however, and, as Zhang et al27 showed, various algorithms lead to different results with uncertainties of up to 20%. On this basis, the National Institute of Radiological Sciences has yet to accept the calculation of accumulated dose with DIR into clinical routine practice.
However, to assess whether intrafractional motion results in unacceptable irradiation of target and normal tissues, full 4D treatment planning with DIR is needed because dose distribution on respective voxels can be averaged out by motion, especially given larger overshoots present in lung cancer treatment in different phases. Dose assessments for target and OARs in the 4Dtool are therefore limited to evaluation in each respiratory phase only. For target dose assessment, however, if dose assessments to the CTV at respective phases within the gating window reach a clinically acceptable level, such as a D95 of >95%, the accumulated dose to the CTV may also reach an acceptable level when we composed uniform beam field to the target, although here we did not calculate the accumulated dose.
With regard to gating window selection, our clinical example here had a duty cycle of 50% (gating window, T30–T70). A longer treatment time may degrade respiratory pattern reproducibility (amplitude or cycle, etc.), and tumour position at treatment may accordingly differ from that at treatment planning. Moreover, extending treatment time is not comfortable for patients. Although a longer gating window is preferred, it should be defined by considering the tolerance dose to normal tissues. As described above, dose assessment to OARs in our approach did not completely consider intrafractional motion because accumulated dose was not calculated, but rather simply evaluated in respective phases, as also occurs in conventional 3D treatment planning.
CONCLUSION
We developed a software application for the design of the target volume with consideration to intrafractional range variation, and successfully integrated it into routine clinical practice in the National Institute of Radiological Sciences. Although a number of 4D treatment planning techniques have been introduced, we believe our alternative procedure is a practical approach for 4D treatment planning within the current state of practice. However, when DIR accuracy is improved and dose calculation with inclusion of the interplay effect is integrated, full 4D treatment planning will be integrated into routine clinical use. Several commercial TPSs already provide ITV design tools, which use 4DCT data sets. Given the importance of FTV/FOAR design function in charged particle therapy in thoracic and abdominal sites, the upgrading of existing commercially available 3D-based TPSs to 4D capability is now urgently required.
REFERENCES
- 1.Mori S, Shibayama K, Tanimoto K, Kumagai M, Matsuzaki Y, Furukawa T, et al. First clinical experience in carbon ion scanning beam therapy: retrospective analysis of patient positional accuracy. J Radiat Res 2012; 53: 760–8. doi: 10.1093/jrr/rrs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol 2012; 42: 670–85. doi: 10.1093/jjco/hys104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaniwa T, Furukawa T, Nagano A, Sato S, Saotome N, Noda K, et al. Field-size effect of physical doses in carbon-ion scanning using range shifter plates. Med Phys 2009; 36: 2889–97. [DOI] [PubMed] [Google Scholar]
- 4.Inaniwa T, Furukawa T, Tomitani T, Sato S, Noda K, Kanai T. Optimization for fast-scanning irradiation in particle therapy. Med Phys 2007; 34: 3302–11. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa T, Inaniwa T, Sato S, Shirai T, Takei Y, Takeshita E, et al. Performance of the NIRS fast scanning system for heavy-ion radiotherapy. Med Phys 2010; 37: 5672–82. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Asakura H, Komatsu S, Yashiro T, Kumagai M, Kandatsu S, et al. Design of a compensating bolus by use of exhalation CT data for covering residual motion in respiratory-gated charged-particle lung therapy: four-dimensional carbon beam dose calculation. Radiol Phys Technol 2008; 1: 83–8. doi: 10.1007/s12194-007-0012-z [DOI] [PubMed] [Google Scholar]
- 7.Mori S, Chen GT. Quantification and visualization of charged particle range variations. Int J Radiat Oncol Biol Phys 2008; 72: 268–77. doi: 10.1016/j.ijrobp.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Hara R, Yanagi T, Sharp GC, Kumagai M, Asakura H, et al. Four-dimensional measurement of intrafractional respiratory motion of pancreatic tumors using a 256 multi-slice CT scanner. Radiother Oncol 2009; 92: 231–7. doi: 10.1016/j.radonc.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Graeff C, Durante M, Bert C. Motion mitigation in intensity modulated particle therapy by internal target volumes covering range changes. Med Phys 2012; 39: 6004–13. doi: 10.1118/1.4749964 [DOI] [PubMed] [Google Scholar]
- 10.Rietzel E, Bert C. Respiratory motion management in particle therapy. Med Phys 2010; 37: 449–60. [DOI] [PubMed] [Google Scholar]
- 11.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys 2006; 64: 1589–95. [DOI] [PubMed] [Google Scholar]
- 12.Richter D, Schwarzkopf A, Trautmann J, Krämer M, Durante M, Jäkel O, et al. Upgrade and benchmarking of a 4D treatment planning system for scanned ion beam therapy. Med Phys 2013; 40: 051722. doi: 10.1118/1.4800802 [DOI] [PubMed] [Google Scholar]
- 13.Sharp GC, Kandasamy N, Singh H, Folkert M. GPU-based streaming architectures for fast cone-beam CT image reconstruction and demons deformable registration. Phys Med Biol 2007; 52: 5771–83. [DOI] [PubMed] [Google Scholar]
- 14.ICRU-62. Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50). Bethesda, MD: International Commission on Radiation Units and Measurements; 1999. [Google Scholar]
- 15.DeLuca PM; ICRU-72. Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 78). Bethesda, MA: International Commission on Radiation Units and Measurements; 2007. [Google Scholar]
- 16.Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys 2007; 67: 906–14. [DOI] [PubMed] [Google Scholar]
- 17.Rietzel E, Liu AK, Doppke KP, Wolfgang JA, Chen AB, Chen GT, et al. Design of 4D treatment planning target volumes. Int J Radiat Oncol Biol Phys 2006; 66: 287–95. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Wu Z, Folkert MR, Kumagai M, Dobashi S, Sugane T, et al. Practical approaches to four-dimensional heavy-charged-particle lung therapy. Radiol Phys Technol 2010; 3: 23–33. doi: 10.1007/s12194-009-0072-3 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Langner U, Loo BW, Jr, Shen J, Keall PJ. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int J Radiat Oncol Biol Phys 2008; 72: 1250–8. doi: 10.1016/j.ijrobp.2008.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Furukawa T, Inaniwa T, Zenklusen S, Nakao M, Shirai T, et al. Systematic evaluation of four-dimensional hybrid depth scanning for carbon-ion lung therapy. Med Phys 2013; 40: 031720. doi: 10.1118/1.4792295 [DOI] [PubMed] [Google Scholar]
- 21.Mori S, Inaniwa T, Furukawa T, Zenklusen S, Shirai T, Noda K. Effects of a difference in respiratory cycle between treatment planning and irradiation for phase-controlled rescanning and carbon pencil beam scanning. Br J Radiol 2013; 86: 20130163. doi: 10.1259/bjr.20130163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa T, Inaniwa T, Sato S, Shirai T, Mori S, Takeshita E, et al. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 2010; 37: 4874–9. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Zenklusen S, Knopf AC. Current status and future prospects of multi-dimensional image-guided particle therapy. Radiol Phys Technol 2013; 6: 249–72. doi: 10.1007/s12194-013-0199-0 [DOI] [PubMed] [Google Scholar]
- 24.Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol 2011; 56: R113–44. doi: 10.1088/0031-9155/56/16/R01 [DOI] [PubMed] [Google Scholar]
- 25.Kramer M. Treatment planning for heavy-ion radiotherapy: biological optimization of multiple beam ports. J Radiat Res 2001; 42: 39–46. [DOI] [PubMed] [Google Scholar]
- 26.Brock KK. Results of a multi-institution deformable registration accuracy study (MIDRAS). Int J Radiat Oncol Biol Phys 2010; 76: 583–96. doi: 10.1016/j.ijrobp.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Boye D, Tanner C, Lomax AJ, Knopf A. Respiratory liver motion estimation and its effect on scanned proton beam therapy. Phys Med Biol 2012; 57: 1779–95. doi: 10.1088/0031-9155/57/7/1779 [DOI] [PubMed] [Google Scholar]