Abstract
Objective:
We investigated the effects of conventional and hypofractionation protocols by modelling tumour control probability (TCP) and tumour recurrence time, and examined their impact on second cancer risks. The main objectives of this study include the following: (a) incorporate tumour recurrence time and second cancer risks into the TCP framework and analyse the effects of variable doses and (b) investigate an efficient protocol to reduce the risk of a secondary malignancy while maximizing disease-free survival and tumour control.
Methods:
A generalized mathematical formalism was developed that incorporated recurrence and second cancer risk models into the TCP dynamics.
Results:
Our results suggest that TCP and relapse time are almost identical for conventional and hypofractionated regimens; however, second cancer risks resulting from hypofractionation were reduced by 22% when compared with the second cancer risk associated with a conventional protocol. The hypofractionated regimen appears to be sensitive to dose escalation and the corresponding impact on tumour recurrence time and reduction in second cancer risks. The reduction in second cancer risks is approximately 20% when the dose is increased from 60 to 72 Gy in a hypofractionated protocol.
Conclusion:
Our results suggest that hypofractionation may be a more efficient regimen in the context of TCP, relapse time and second cancer risks. Overall, our study demonstrates the importance of including a second cancer risk model in designing an efficient radiation regimen.
Advances in knowledge:
The impact of various fractionation protocols on TCP and relapse in conjunction with second cancer risks is an important clinical question that is as yet unexplored
Clinically, it is observed that over half of all cancer patients undergo radiotherapy over the course of their treatment, either as a primary treatment modality or in an adjuvant or a neoadjuvant context. In current radiotherapy treatments, tumours are often irradiated with a heterogeneous dose distribution throughout the treatment volume. The probability that all cancerous cells are removed from the system immediately post treatment is known as tumour control probability (TCP).1 The design and complexity of any treatment regimen in terms of improving therapeutic efficacy can be deduced (to some extent) from TCP values. Although a given heterogeneous dose distribution to the target volume locally controls the disease to a large extent (for a given radiation regimen), there are still shortcomings associated with the currently used radiation protocol. Of these, side effects are of great importance and can be classified based on the time to clinical presentation, with shorter-acting side effects arising from irritation of the skin or mucosa, or irradiation of tissues with sensitive adjacent structures. Late toxicities of radiation are known to manifest after a period of 10–15 years, and one of the major late toxicities is the appearance of a secondary malignancy. Moreover, owing to the gains made in cancer care and patient management, there has been a marked increase in the number of survivors of childhood cancers, or cancers at young ages, and these patients are therefore at increased risk for the delayed consequences of radiotherapy. Several clinical studies have reported tumour recurrence or relapse within a span of 5 years2 and late toxicities in the form of secondary malignancies within 5–20 years3–7 post irradiation. These clinical investigations have been carried out on several types of tumours across a variety of treatment regimens and have also indicated that tumour relapse is a leading cause of death along with radiation-induced second cancers.
Several pre-treatment factors such as age at diagnosis, gender and stage of tumour may impact tumour relapse. Relapse probability and time may vary depending on the treatment modality. In our work, for simplicity, we consider the effect of single treatment modality, namely, radiotherapy on TCP along with analysis of time to relapse. Also, escalating the dose to the target volume will eliminate tumours8–10 and may have an impact on the relapse time (either very long ideally or an increase in the recurrence time) depending on the radiation protocol. Moreover, dose escalation may elevate radiation-induced second cancer risks, but the degree to which it increases these risks remains to be determined.
Clinically, it is widely believed that radiotherapy-induced cancer risks are owing to scattered doses and radiation leakage from the linear accelerator, as well as irradiation to the healthy tissues adjacent to the target volume. Several systematic clinical investigations have indicated that radiation therapy is a significant causative factor of second cancers. Numerous case–control and cohort–control studies have also suggested that there is an increased risk of secondary malignancies with young cancer survivors, for example, survivors of Hodgkin's lymphoma (HL).11–13 Therefore, it is of paramount importance to reduce radiation doses to healthy tissue, while at the same time improve dose conformity to the target volume. The critical organs that get irradiated can be located in-beam (known as serial organs) or out-beam (also known as parallel organs) to the radiation beams and occasionally also receive the same integral dose. It is therefore critical to minimize radiation dosage and schedule radiation doses such that there is minimal impairment of delivery to the critical organs around the primary treatment volume, a probable increase in the relapse time (or with a long relapse time ideally), but also with the goal of reducing the risk of a secondary malignancy. It should be noted that in this work, we concentrated on modelling late complications due to radiotherapy, that is, radiation-induced second cancers only and not on normal tissue complication probability (NTCP) modelling.
The main objective of this work is to develop a generalized mathematical framework that can incorporate relapse dynamics into a TCP model in conjunction with a second cancer risk model. This was carried out to understand the effects of dose escalation on recurrence and second cancer risks. Our minimal model also proposes an efficient paradigm in terms of regimen that may provide insights to be confirmed by future clinical investigations.
METHODS AND MATERIALS
Mathematical model of tumour control probability and recurrence
The linear quadratic (LQ) model is a radiobiological formalism that is used to provide quantitative insights into the evaluation of various clinically relevant treatment protocols. The LQ model describes the fraction of cells that survived owing to administration of a uniform dose D, where D can be an acute dosage or a fractionated dose delivered in several fractions. The survival fraction of cells after radiotherapy is given by:
| (1) |
where D is the total dose and K denotes the number of fractions. In the above equation, α (1/Gy) is the cellular radiosensitivity parameter, which denotes the direct action of lethal cell killing, and β (1/Gy2) represents two-track action where damage from two different radiation tracks interact to inactivate the cell via a double-strand break in the DNA.14–16 The ratio α/β depends on the type of tissue that is being considered. For tissues with early effects, the value of α/β is high, and the linear value is of more importance, and there is therefore a reduced dependence on fractionation. For tissues with late effects, the value of α/β is low, and the quadratic term is more important, so that fractionation also becomes more relevant.
The TCP is a clinical indicator used to estimate the elimination probability of cancerous cells in a tumour and also to compare various fractionation regimens that are used in current clinical practice. In this work, the average number of cells in the tumour volume at any given time is modelled deterministically, and because the true number of cells is a stochastic quantity, we may approximate the probability of tumour control as the Poisson probability that there are zero clonogens remaining in the system, with a given average number of cells. That is, the TCP value is given by Equation (2) as can also be found in O'Rourke et al:1
| (2) |
where N0 is the total number of clonogens in the system and S is the survival fraction of cells after a given acute dosage D.
Clinically, it is not always possible to eliminate all the cancerous cells in the system. There is generally a non-zero probability that a small number of cancerous cells are not eliminated (by either surviving the effects of irradiation or escaping the irradiation itself). These remaining cancerous cells clonally expand post irradiation, to cause a relapse or recurrence of the primary tumour after a period of time. Although several growth functions such as exponential, Gompertz etc. can be chosen, for simplicity, we chose a logistic growth function for recurrence. We define a recurrence of the primary tumour to have occurred after a critical number of cells, 106, has been reached and model the growth of the remaining cancerous cells after the tumour has been irradiated (by a logistic growth function), with the same exponential growth parameter as in the pre-treatment phase. For simplicity (although this can be generalized), we assumed that the critical threshold of cancerous cells diagnosed at relapse is of the order of 106. Then, we determined the time point at which the tumour population level reaches a threshold of 99.9% of 106 (note that the population only approaches 106 asymptotically, since this is the limit of the logistic growth in our framework). We then regard this as the time post treatment to recurrence:
| (3) |
where dnr/dt denote rate of change of the remaining cancerous cells (post irradiation) with time and N denotes carrying capacity of the tissue. Note that nr is the quantity of cancerous cells remaining at the end of the treatment. The output of the TCP model determines the initial condition for the relapse dynamics framework.
Mathematical framework for second cancer risks
Several models have been developed in the literature to estimate radiation-induced second cancer risks, and some of them include, but are not limited to, Lindsay et al,17 Sachs and Brenner,18 Manem et al19 and Timlin et al.20 Also, several types of risks (for instance, relative risk, absolute risk, life time risk) are used to estimate radiation-induced carcinogenic risk. In this work, we used excess relative risk (ERR) similar to the work by Sachs and Brenner.18 A commonly used biologically motivated mathematical model to estimate second cancer risks is the initiation–inactivation–proliferation (IIP) model.17–19 The organ-specific second cancer incidence owing to ionizing radiation is proportional to the yield of pre-malignant (PM) cells at the end of the treatment. Therefore, the functional form used to obtain the ERR estimate is given as the product of the yield of PM cells at the end of the treatment and a proportionality factor as:
| (4) |
where F(D) is the number of radiation-induced initiated PM cells and G(a, e, g) is a constant that depends on the age at exposure a, time since exposure e and gender g. This is standard practice in the modelling of cancer risks as a result of ionizing radiation. From Equation (4), it is evident that the number of PM cells given by F(D) = M is dependent only on the radiation protocol and is independent of patient age. The factor G(a,e,g) = B is independent of the radiation dose but is dependent on time. Since the incidence of second cancers is a multistep carcinogenic process post irradiation, the term B accounts for all these slower biological processes. The IIP formalism gives the evolution of normal and PM cells M during and also at the end of radiation treatment. The proportionality factor B is a constant that denotes time since exposure and is obtained by transferring the risks from the atomic bomb cohort to the corresponding cancer cohort. The underlying rationale behind this risk estimate is that the radiation-induced second cancer risks will not change the slope of the dose–response relationship post irradiation.
We adopted the mathematical framework discussed by Sachs and Brenner.18 There are three biological mechanisms associated with this framework: initiation, inactivation and proliferation components. The proliferation mechanism is assumed to occur during the course of the treatment (i.e. during and in-between fractionation), until the cells reach a steady state number N post irradiation (as a result of tissue homoeostasis). Throughout this work, radiation-induced mutated stem cells are also referred to as mutated or PM cells. In this framework, we track the number of normal n(t) and PM m(t) cells during and at the end of treatment at a given time . As mentioned earlier, the ERR is defined as the product of a proportionality factor (B) with the yield of PM cells (M) at the end of treatment, given by Equation (4).
Denoted by K and T, the number of fractions to be administered and the time between any two fractions (which is assumed constant), respectively, and the average dose per fraction is assumed to be d for the whole organ. Then, the total dose is given by D = Kd.
We let n−, m−, n+ and m+ denote the number of normal and PM cells just before a dose fraction and immediately after the dose fraction, respectively. Suppose k = 1,2,⋯K, then the surviving fraction of cells owing to dose d is given by Equation (1).
The fraction of normal stem cells that are not initiated as PM cells in a given fraction is given by:
| (5) |
where γ (1/Gy) is the mutation-induction parameter.
The number of normal and PM cells that survive the kth dose fraction18 are:
| (6) |
where SPn− denotes the surviving fraction of normal cells that are not made pre-malignant owing a dose d Gy. Owing to homoeostatic regulation of tissue, we assume the repopulation mechanism of normal and PM cells during and after radiation follows a logistic growth model, where λ and rλ are the repopulation rates of normal and PM cells, respectively. Thus, the repopulation mechanism for surviving normal and radiation-initiated PM cells is given by:
| (7) |
The number of PM cells after the last fraction has been administered and until the normal cells cease to repopulate is given by:
| (8) |
Solving the above set of discrete equations with initial conditions n−(1) = N and m−(1) = 0, we obtain the yield of PM cells at the end of treatment and until the repopulation of normal cells reaches its steady state. The above discrete model in terms of its continuous analogue and the equivalence (between them) is discussed by Manem et al:19
| (9) |
where the first term in the above coupled equations denote the repopulation mechanism, the second term denotes the action of cell kill due to dose rate (which is defined as the dose delivered in t minutes) and the third term denotes the radiation-induced initiation due to the dose rate. fτ(t) is a step function that represents the fractionated dose d delivered in K fractions. dn/dt and dm/dt denote rate of change of normal and pre-cancerous cells with time. The function f(t−iT) denotes the step function, representing the dose D delivered in K fractions. In the above equations, all three terms are active during irradiation time, and only the first term is active during fractionation, and after the last dose till the normal cells reach a steady state. It should be noted that the change in the yield of PM cells due to the quadratic term in the discrete version (i.e. in the survival fraction of cells) is minimal (at the order of 10−3 magnitude). Therefore, the quadratic term for the survival fraction of cells is not considered in either the discrete or the continuous versions. However, in this article, we use the continuous analogue given by the set of Equation (9).
We incorporated the TCP model with relapse dynamics of the tumour and estimated the corresponding second cancer risks for various fractionation protocols. Clinically, it is important to eliminate the cancerous cells completely (or reduce them to a possible minimum), such that either the recurrence is zero or the relapse time is increased, which is carried out by escalating the dose. Thus, radiation in this case is considered to be a double-edged sword, which increases local control of disease and increases relapse time (assuming some cancer cells still remain in the system) but at the cost of an increase in the second cancer risks.
Incorporating TCP with relapse dynamics, in conjunction with a second cancer risk model may provide insights into the design of efficient protocols that do not impair treatment to the primary tumour.
RESULTS
Without dose escalation: tumour control probability, recurrence calculations
We first present our investigations on TCP, recurrence and second cancer risks individually. This has been carried out in order to explore an efficient protocol (between traditional and hypofractionation regimens) that can increase TCP and recurrence time.
Owing to the absence of any large body of clinical data to validate our mathematical framework, we resorted to sensitivity analysis of various parameters to address relevant questions related to TCP, relapse and second cancer risks. All the biological parameters were varied within reasonable ranges (that resemble various organs of interest). We also evaluated radiation-induced second cancer risks for conventional and hypofractionation schemes used for the TCP and recurrence model. This was carried out in order to determine the risks associated with each of these individual protocols.
Since it is very important to control primary tumour and to increase relapse time (or ideally to have a relapse time longer than the remaining lifetime of the individual), the TCP can be increased only when dose escalation is carried out for a fixed number of fractions or when there is an increase in the total number of fractions. Later in this section, we present our results related to dose escalation with TCP maximized and recurrence time increased, as well as the corresponding change in second cancer risks. This will help us identify the protocols that can be administered in order to minimize the occurrence of secondary malignancies. Table 1 summarizes the protocols used in our work (all radiation therapies were given at one fraction per day, 5 days per week, with a 2-day break for the weekend).
Table 1.
Summary of fractionation protocols
| Protocol | Fractionation scheme |
|---|---|
| Conventional | 30 fractions with 2 Gy per fraction |
| Hypofraction | 20 fractions with 3 Gy per fraction |
Table 2 summarizes the parameters that were used in our mathematical model.
Table 2.
Summary of parameters
| Parameters (units) | Interpretation |
|---|---|
| N0 | Initial number of tumour cells |
| α (1/Gy) | LQ model parameter |
| β (1/Gy−2) | LQ model parameter |
| tdou (days) | Doubling time of tumour cells |
| λ1 (1/day) | Proliferation rate of tumour cells during treatment |
| λ2 (1/day) | Proliferation rate of tumour cells post-treatment phase |
| λ (1/day) | Proliferation rate of normal cells (in healthy tissue) |
| R | Proliferation rate of pre-malignant cells |
| γ (1/Gy) | Mutation induced by radiation |
LQ, linear quadratic.
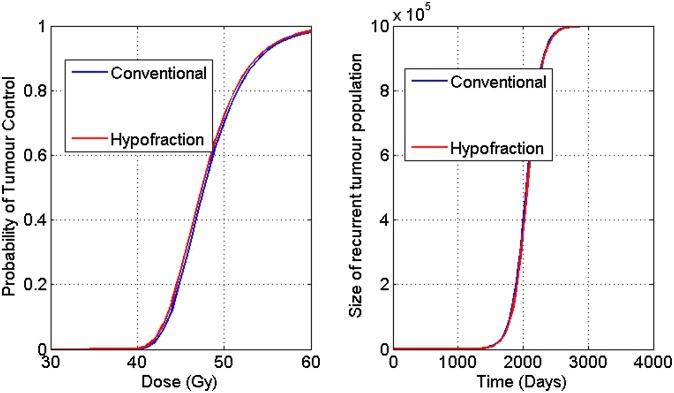
Figure 1 displays TCP and recurrence curves for conventional and hypofractionation protocols.
Figure 1.
Tumour control probability (TCP) vs dose (left panel) and recurrence vs dose (right panel) for various fractionation regimens for a sample parameter set.
The biological range for α (1/Gy) is taken from Kehwar.21 In Table 3, the results of the sensitivity analysis for the radiobiological parameter α (1/Gy) are presented. This investigation was carried out fixing other parameters β (Gy−2) and the growth rate parameters λ1, λ2 (1/day) in the TCP and recurrence framework. TCP and recurrence are reported for each regimen, and we note that TCP reported is that at the completion of the treatment schedule.
Table 3.
Sensitivity analysis for α
| α radiobiological parameter | Conventional regimen |
Hypofractionation regimen |
||
|---|---|---|---|---|
| TCP (%) | Recurrence (days) | TCP (%) | Recurrence (days) | |
| 0.10 | 0 | 1741 | 0 | 1896 |
| 0.15 | 0 | 2095 | 2.26 | 2230 |
| 0.20 | 50.27 | 2445 | 83.25 | 2561 |
| 0.25 | 96.73 | 2789 | 99.11 | 2922 |
| 0.30 | 99.84 | 3122 | 99.95 | 3262 |
| 0.35 | 99.99 | 3477 | 99.99 | 3610 |
TCP, tumour control probability.
All other model parameters are maintained at the following values as α is varied: β = 0.02 Gy−2, tdou = 80 d, .
In Table 3, the big jump in TCP at a cut-off value of α > 0.15 (1/Gy) may be explained entirely by the dynamics of the TCP curve, in the sense that indeed the jump is owing to the assumption of Poisson statistics. This may also be observed from the shape of the TCP curve generally, in which, past a certain dose, a very rapid and large increase in TCP was observed, and since modifying α merely changes the efficacy of a dose of radiation, one would expect to observe similar behaviour past a given cut-off point. From Table 3, it is clear that hypofractionation yields better results for TCP and recurrence than that of conventional protocol, especially for smaller values of α.
In Table 4, the results of a similar analysis carried out by fixing α, and the growth rate parameters λ1, λ2 (1/day) and varying the LQ model parameter β are presented.
Table 4.
Sensitivity analysis for β
| β radiobiological parameter | Conventional regimen |
Hypofractionation regimen |
||
|---|---|---|---|---|
| TCP (%) | Recurrence (days) | TCP (%) | Recurrence (days) | |
| 0 | 98.21 | 2841 | 98.41 | 2878 |
| 0.005 | 99.01 | 2934 | 99.35 | 2973 |
| 0.010 | 99.46 | 2968 | 99.73 | 3052 |
| 0.015 | 99.70 | 3070 | 99.89 | 3164 |
| 0.020 | 99.83 | 3122 | 99.95 | 3262 |
| 0.025 | 99.91 | 3204 | 99.98 | 3397 |
TCP, tumour control probability.
All other model parameters are maintained at the following values as β is varied: α = 0.3 Gy−1, tdou = 80 d, .
As can be seen in Table 4, varying the values of β also gives consistent results that the hypofractionation scheme is an efficient regimen compared with the traditional protocol (in that the recurrence times are longer, which is more desirable), and the TCP at the completion of treatment is higher, across the values of β studied. Moreover, we note that the effect of the β parameter was not as great as the effect of the α parameter, as shown in Table 3, as all TCP values are relatively consistent and close to unity.
A similar analysis was carried out by fixing the LQ parameters α, and the growth rate parameters λ2 (1/day) and varying λ1 (1/day), as in Table 5, or fixing λ1 and varying λ2 (1/day), as shown in Table 6. We recall that the parameter λ1 represents the growth rate of the cancer cells during radiotherapy treatment and the parameter λ2 represents the growth rate of the cancer cells in the period following the completion of radiotherapy.
Table 5.
Sensitivity analysis for λ1
| λ1 growth parameter | Conventional regimen |
Hypofractionation regimen |
||
|---|---|---|---|---|
| TCP (%) | Recurrence (days) | TCP (%) | Recurrence (days) | |
| 0.005 | 99.86 | 3146 | 99.96 | 3304 |
| 0.006 | 99.85 | 3151 | 99.95 | 3298 |
| 0.007 | 99.84 | 3150 | 99.95 | 3288 |
| 0.008 | 99.84 | 3139 | 99.95 | 3274 |
| 0.009 | 99.83 | 3110 | 99.95 | 3255 |
| 0.010 | 99.83 | 3109 | 99.95 | 3258 |
TCP, tumour control probability.
All other model parameters are maintained at the following values as λ1 is varied: α = 0.3 Gy−1, β = 0.02 Gy−2, .
Table 6.
Sensitivity analysis for λ2
| λ2 growth parameter | Conventional regimen |
Hypofractionation regimen |
||
|---|---|---|---|---|
| TCP (%) | Recurrence (days) | TCP (%) | Recurrence (days) | |
| 0.008 | 99.83 | 3382 | 99.95 | 3530 |
| 0.009 | 99.83 | 3006 | 99.95 | 3140 |
| 0.010 | 99.83 | 2705 | 99.95 | 2826 |
| 0.011 | 99.83 | 2459 | 99.95 | 2569 |
| 0.012 | 99.83 | 2254 | 99.95 | 2355 |
TCP, tumour control probability.
All other model parameters are maintained at the following values as λ2 is varied: α = 0.3 Gy−1, β = 0.02 Gy−2, tdou = 80 d.
The results of Tables 5 and 6 show that the effect of the parameter λ1 as it varies, upon TCP and recurrence time is small, and therefore the outcome measures studied are relatively insensitive to this parameter. In contrast to this, we observe that the effect of varying the parameter λ2, on the recurrence time is indeed far more significant, and thus controlling the rate of cell proliferation post treatment for the residual cells has a much greater effect on tumour recurrence time than does the rate of cell proliferation during radiotherapy. Interestingly, this points to the observation that controlling tumour growth rate during radiotherapy is not as efficacious as increasing survival or reducing recurrence time as controlling the tumour growth rate post radiotherapy. We also note that TCP is entirely insensitive to the parameter λ2, as is entirely expected, as this is the post-treatment growth rate, and therefore will not have any effect on the outcome of the treatment at the instant of treatment completion, since λ1 was held constant over these simulations.
Tables 3–6 provide an in-depth analysis of TCP and recurrence calculations. It is clear that overall from these results, the hypofractionation regimen studied is an efficient protocol as compared with the conventional protocol. Moreover, it may be noted that the parameters α and λ2 emerge as important contributors to TCP and recurrence time, respectively, whereas these measures are relatively insensitive to other parameters. Additionally, based on the sensitivity of TCP to the parameter α, we note that in a parametrically heterogeneous population, if small α is represented among the parameter sets, TCP, as observed at the population level, will appear diluted. This represents a shortcoming of using a Poisson model for the TCP with fixed parameters, as there is unmodelled parametric uncertainty that remains unaccounted for, but is characterized by this sensitivity analysis. The variation of various growth and tissue parameters in the biological range, relevant to radiobiology, does not change the conclusion that hypofractionation schemes appear to be theoretically the better option in terms of locally controlling the disease as well as increasing the disease-free survival time of the patient.
Second cancer risks
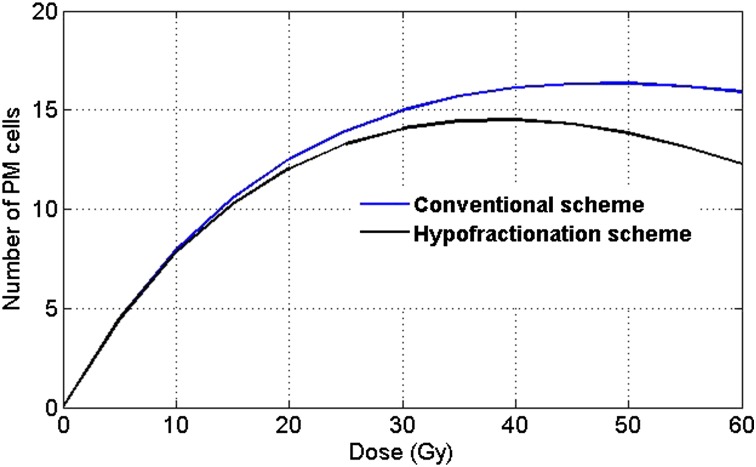
In this article, we consider the critical structure to be one of the irradiated organs in the vicinity of the target volume. As discussed earlier, we assumed that second cancer ERR is proportional to the number of PM cells as in Equation (4), where the value of B is organ specific.18,19 Figure 2 displays a plot between dose and the corresponding number of PM cells, comparing conventional and hypofractionation protocols in the context of second cancer risks for a sample parameter set. The numbers of PM cells are calculated under the assumption that the healthy tissue (also known as the organ of interest) receives 100% of the dose received by the tumour.
Figure 2.
Number of pre-malignant (PM) cells vs dose for various fractionation regimens—conventional and hypofractionation schemes. Parameters: λ = 0.4 (1/day), r = 0.76, γ = 10−6(1/Gy), N = 106, α = 0.18 (1/Gy).
We had varied the above parameters within a biological range (results not shown here) and found the hypofractionation scheme to be a better option in the context of second cancer risks. Based on our calculations, hypofractionation emerges as a more theoretically optimal protocol for the reduction of second cancer risks, which is clinically consistent with the results reported by Schneider et al22 (in the treatment of sarcoma and carcinoma). Owing to the high dose per fraction, the cell kill mechanism dominates the proliferation term in the mathematical model, leading to a reduction in the risk of second cancer compared with the other protocols. For large doses, the cell kill mechanism dominates mutation induction, which causes a decline in second cancer risks. Therefore, in the context of second cancer risk reduction, we observed that hypofractionation is a theoretically more efficient regimen than the other protocols, notably even conventional radiotherapy. Although there are several ongoing discussions on the effect of fractionation on second cancer risks, these theoretical risk predictions cannot be generalized for every critical structure or tissue type.
For a fixed total dose of 60 Gy, Table 7 presents the values of TCP, recurrence time and the corresponding number of PM cells (which is proportional to second cancer risks, ERR) associated with a specific protocol.
Table 7.
Summary of tumour control probability (TCP), recurrence and second cancer risks for a fixed total dose of 60 Gy, with α = 0.3 Gy−1, β = 0 Gy−2, tdou = 80 d,
| Protocol | TCP (%) | Recurrence time (days) | Number of pre-malignant cells |
|---|---|---|---|
| Conventional | 98.21 | 2841 | 15.90 |
| Hypofraction | 98.41 | 2878 | 12.26 |
From Table 7, we noticed that TCP and recurrence time are almost the same for conventional and hypofractionated regimens; and second cancer risks induced by hypofractionation are reduced by 22% in comparison with that of the conventional protocol. Thus, from our analysis, we concluded that hypofractionation appears to be a stronger regimen in terms of improvement in TCP, recurrence time and reduction of second cancer risks (compared with the conventional protocol).
EFFECT OF DOSE ESCALATION
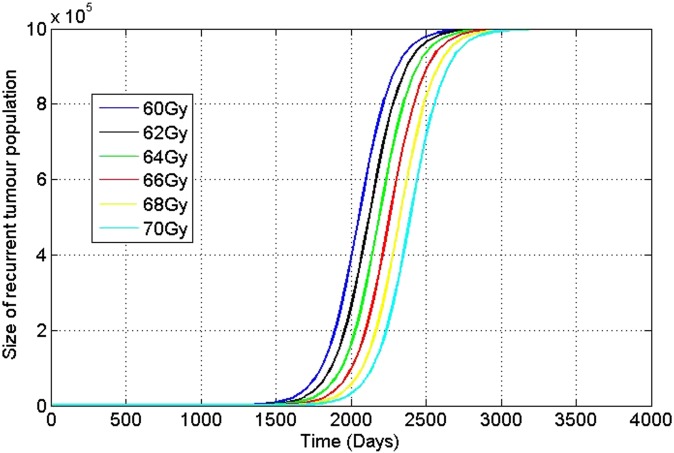
Dose escalation is necessary in order to increase primary tumour control and decrease the recurrence rate (i.e. having a zero relapse time ideally or increasing the relapse time). Increasing the dose applied during treatment, for instance, adding days of treatment (i.e. elongating the treatment window) by 4–5 days, changes TCP, and this in turn changes the average number of cancer cells that remain in the tissue. The effect of these changes on the residual number of cancer cells then changes the recurrence time for cancer, and a “sensitivity” of the recurrence time, to dose applied, can be calculated. Therefore, dose escalation will have a predominant influence on not only TCP but also on the relapse time. For instance, Figure 3 presents plots of how the recurrence dynamics are modified by dose changes in the irradiation protocol (Tables 8 and 9).
Figure 3.
Effect of dose escalation on recurrence time for conventional protocol.
Table 8.
Effect of dose escalation using conventional protocol
| Dose (Gy) | Tumour control probability (%) | Recurrence time (days) | Number of pre-malignant cells |
|---|---|---|---|
| 60 | 98.21 | 2841 | 15.90 |
| 62 | 99.00 | 2930 | 15.75 |
| 64 | 99.45 | 2977 | 15.58 |
| 66 | 99.69 | 3069 | 15.38 |
| 68 | 99.83 | 3107 | 15.17 |
| 70 | 99.91 | 3206 | 14.93 |
Table 9.
Effect of dose escalation using hypofractionated protocol
| Dose (Gy) | Tumour control probability (%) | Recurrence time (days) | Number of pre-malignant cells |
|---|---|---|---|
| 60 | 98.41 | 2878 | 12.26 |
| 63 | 99.34 | 2958 | 11.67 |
| 66 | 99.73 | 3053 | 11.04 |
| 69 | 99.89 | 3171 | 10.38 |
| 72 | 99.96 | 3258 | 9.71 |
The set of curves in Figure 3 represents the variations in dose modification for the conventional protocol.
The following tables (Tables 8 and 9) present an analysis for dose escalation administered using conventional and hypofractionated protocols in current clinical practice, with respect to TCP, recurrence time and second cancer risks. This analysis was carried out for the specific set of parameters: λ1= (1/day), λ2= (1/day), λ1= λ2= ln (2)/80, α = 0.25 (1/Gy), α/β = 4 (Gy) for a slow growing tumour; and λ = 0.4 (1/day), r = 0.76, γ = 10−6(1/Gy), N = 106, α = 0.18 (1/Gy) for an irradiated healthy organ.
The dose escalation and its effects on TCP, recurrence time and second cancer risk behaviour for the two dosing regimens display an interesting behaviour. In all of the above table (Tables 8 and 9), the dose is escalated according to the definitions of the corresponding fractionation protocols. For a conventional protocol, when the dose is escalated by 12 Gy (i.e. from 60 to 72 Gy), TCP is increased by only 1%, recurrence time by 365 days and second cancer risks are reduced by 6%. In the case of the hypofractionated protocol, when the dose is escalated by a similar amount, we observe that recurrence time increases by 380 days, and there is a corresponding reduction in second cancer risks of 20.8%. This suggests that, clinically, while the effects of dose escalation in the hypofractionated and conventional protocols studied provide similar benefits in terms of disease-free survival time increase, the reduction in second cancer risks is much greater in the hypofractionated protocol, providing another rationale for the continued study of this treatment paradigm.
Clinical study—Hodgkin's lymphoma
In this section, we apply our mathematical formalism to a clinical case to evaluate TCP, recurrence and secondary cancer risks. Although recent advancements in radiation therapy have substantially improved the survival rate of patients with Hodgkin's lymphoma, second cancers are a concern for most cancer survivors. We try to compare conventional and hypofractionation regimens to evaluate TCP, recurrence time and the corresponding second cancer risks in HL disease. We assume that the patient diagnosed with HL disease is being treated with radiotherapy only. The primary tumour associated with HL disease is of the lymph nodes in the patient and the irradiated organs are the breast, lung and thyroid tissues. Although patient-specific second cancer risks are important for all organs, for simplicity, we evaluate second cancer risks (ERR) associated only with the breast tissue.
One should note that for a tumour of size N0 ≈ 109, with a proliferative rate in the range of 0.005–0.010 (1/day), the value of TCP will be almost close to zero. In order to obtain a TCP value close to one, the proliferative capacity of tumour cells must be chosen very close to zero. Thus, our mathematical framework can be only applied to small and intermediate tumour sizes, which may arise owing to micrometastases. Hence, TCP and relapse dynamics are associated with primary tumours as well as micrometastatic tumours, and second cancer risks are associated with the neighbouring organs around the primary target volume and those located in the vicinity of the metastatic tumour. Alternatively, not all tumour cells may be in the proliferating stage. In fact, according to the cancer stem cell hypothesis, only a (typically) small population of cancer cells has the potential to divide and maintain the tumour. If we assume that only 1% of cells are tumour-initiating cells, then there will be about 107 proliferating cells and thus the value of TCP will not be close to zero.
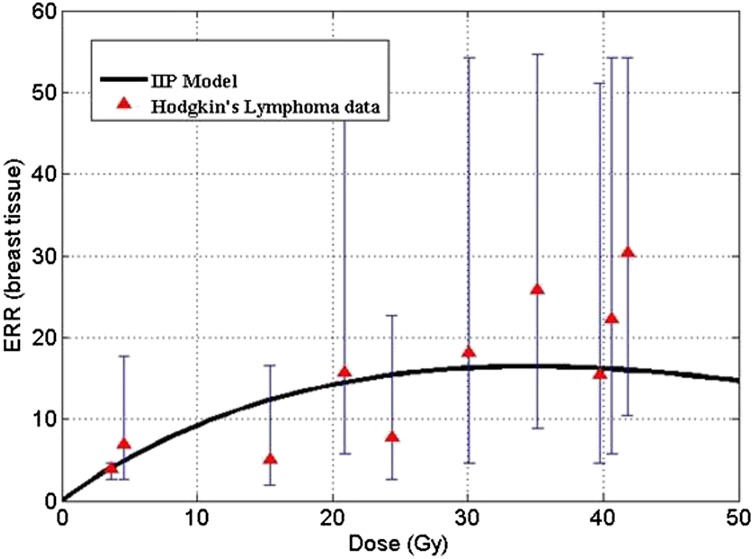
The IIP model developed by Sachs and Brenner18 was validated on epidemiological data of secondary breast cancer risks, which is displayed in Figure 4. The model fit was carried out using the relative proliferation potential of PM cells r to be equal to 0.76.18 Solid lines in the plot denote theoretical risk prediction by IIP formalism. Data points are taken from clinical studies of secondary breast cancers in survivors of HL. The model fits well with the current clinical data under the assumption that the growth advantage is conferred on radiation-initiated PM cells during the recovery period post irradiation.
Figure 4.
Excess relative risk (ERR) for breast tissue vs dose (conventional fractionation regimens). Parameters: λ = 0.4 (1/day), r = 0.76, γ = 10−6 (1/Gy), N = 106, α = 0.18 (1/Gy). IIP, initiation–inactivation–proliferation. Reproduced from Sachs and Brenner18 copyright (2005) National Academy of Sciences, USA.
Tables 10 and 11 present an analysis for dose escalation administered using conventional and hypofractionated regimens to treat HL disease. This investigation was carried out with respect to TCP, recurrence time and second cancer risks. This analysis is carried out for a specific set of parameters: λ1= (1/day), λ2= (1/day), λ1= λ2= ln (2)/80, α = 0.25 (1/Gy) for primary tumour; and α/β = 10 (Gy) (since lymph nodes are fast growing tumours), λ = 0.4 (1/day), r = 0.76, γ = 10−6 (1/Gy), N = 106, α = 0.18 (1/Gy) for irradiated healthy breast tissue and B = 1.2 (proportionality factor for breast tissue taken from Sachs and Brenner18).
Table 10.
Effect of dose escalation using conventional protocol
| Dose (Gy) | Tumour control probability (%) | Recurrence time (days) | Excess relative risk—breast |
|---|---|---|---|
| 60 | 98.19 | 2843 | 19.08 |
| 62 | 98.98 | 2927 | 18.90 |
| 64 | 99.43 | 2979 | 18.69 |
| 66 | 99.69 | 3067 | 18.45 |
| 68 | 99.83 | 3109 | 18.20 |
| 70 | 99.90 | 3206 | 17.91 |
Table 11.
Effect of dose escalation using hypofractionation protocol
| Dose (Gy) | Tumour control probability (%) | Recurrence time (days) | Excess relative risk—breast |
|---|---|---|---|
| 60 | 99.64 | 3038 | 14.71 |
| 63 | 99.86 | 3144 | 14.00 |
| 66 | 99.94 | 3272 | 13.24 |
| 69 | 99.98 | 3345 | 12.45 |
| 72 | 99.99 | 3477 | 11.65 |
The effect of dose escalation on hypofractionation scheme was very effective when compared with conventional protocol, because the increase in recurrence times was seen to be greater for hypofractionated protocol, along with a reduction in second cancer risks. Our analysis therefore indicates that the hypofractionation scheme is a better regimen during dose escalation and has a very significant effect on recurrence time and second cancer risks. Therefore, increasing the overall treatment time (by dose escalation) may theoretically increase the survival time of patients by approximately 1 year or more and may also concomitantly reduce second cancer risks. This analysis therefore may provide a theoretical justification for further clinical trials comparing efficacy and second cancer toxicities between conventional and hypofractionated radiotherapy protocols.
DISCUSSION
One of the most common therapeutic anticancer modalities is radiotherapy (alone or in combination with chemotherapy and/or surgery). However, there are several short- and long-term effects associated with radiation therapy. Short-term toxicities include relapse of the primary tumour, and long-term complications occur as a result of secondary malignancies in one of the proximal organs of the target volume. Several clinical and epidemiological studies have shown that patients may face short-term relapse (within 5 years of treatment) or long-term relapse (5–10 years) post irradiation.23 All these studies provide mounting evidence that tumour relapse and secondary malignancies are two of the main causes of death in cancer survivors. Although there is a probability of late relapse in some patients (which occurs within 5–10 years) and/or a second malignancy within 5–20 years post irradiation, clinicians have yet to determine which of these predominates in practice. Specifically, an example of a tumour relapse 20 years following the initial treatment may occur in HL, as reported by Marková et al;23 while, at the same time, it is well known that the manifestation of secondary solid tumours also occurs within 15–20 years post irradiation for HL survivors. Therefore, as mentioned earlier, it is vital to explore possible fractionation regimens that can maximize local control of the disease, increase disease-free survival (i.e. increase in relapse/recurrence time) and reduce second cancer risks. Recent clinical studies have reported that hypofractionation appears to be a better option to treat carcinoma of the prostate24 and breast cancer in elderly patients.25 More recently, Schneider et al22 found that hypofractionated therapy reduces second cancer risks for carcinoma and sarcoma. In this article, we have developed a generalized mathematical framework that incorporates recurrence and second cancer risk models into the TCP dynamics.
In this study, we first investigated the effect of various protocols on TCP, recurrence time and the corresponding second cancer risks. We observed that tumour control and time to recurrence are identical for both conventional and hypofractionated regimens; however, second cancer risks induced by hypofractionation were reduced by 22% compared with the conventional protocol. The radiobiological benefit from hypofractionation is higher than other protocols, including the conventional regimen. Therefore, from our analysis, we conclude that hypofractionation would theoretically be a more optimal option (taking into consideration TCP, recurrence time and second cancer risks), as compared with a conventional protocol.
Secondly, we mathematically explored the effect of dose escalation on TCP, recurrence time and second cancer risks. This was studied in order to determine whether there is a protocol that can increase disease-free survival time, while simultaneously reducing second cancer risks. Escalating doses beyond the standard treatment protocol of 60 Gy, while lengthening the patient's treatment time changes the recurrence time by 300–400 days, approximately, which can represent a significant treatment gain from a clinical perspective. Moreover, escalating the dose in this regime of parameter values has the interesting effect of reducing the second cancer risks as well. In fact, in the higher dose region, we see a reduction of second cancer risks owing to a large amount of cell killing compared with mutation induction. Based on all of these facts, it would appear that for larger doses, hypofractionation is a better protocol to administer, since second cancer risks have a smaller relative increase, while at the same time, the increase in recurrence time is the largest when compared with other protocols. Moreover, with the parameters used, a very high probability of tumour control was obtained. Additionally, looking at the reduction in second cancer risks for this treatment regimen, as the dose was escalated, shows that for this protocol, the reduction in risk when increasing the dose was one of the largest, by approximately 20%. Thus, in terms of sensitivity, it can be seen that a hypofractionated protocol is not only the most sensitive to dose escalation in terms of recurrence time but also the most sensitive to dose escalation in terms of second cancer risk reduction. Finally, hypofractionated therapy, which is administered in shorter duration for the overall treatment might as a result lead to economic savings in several other resources, and may allow a greater number of patients to benefit from radiotherapy over a given time period. It is on this basis that, within this parameter space and for these models, the hypofractionated protocol is proposed as an efficient alternative therapeutic paradigm. It is the hope that this theoretical framework can be used to provide rational guidance for future clinical trials testing these hypotheses.
As a future study, this work will be extended to include patient-specific features into the model with the corresponding dose–volume histogram (DVH) data in order to obtain individualized estimates of TCP, relapse and second cancer risk. We would also like to compare several fractionation protocols used in current clinical practice for treating various types of cohorts and tumours. To validate our mathematical framework clinically, one can use the DVH data and evaluate TCP, relapse and second cancer risk for patients with HL. The estimation of second cancer risk based on DVH data has been previously carried out by Hodgson et al.26 This work can be extended, upon the availability of clinical data for primary tumours such as the differential DVHs and the relapse data for patients with HL, to validate our modelling results. Additionally, we would also like to extend our model to include tissue recovery and repair owing to radiation therapy. Another future direction that we would like to explore is to understand the dependency of α/β values on radiation quality features of the beam (such as linear energy transfer) and microenvironment. We would like to extend this work to incorporate the above-mentioned features into our model to evaluate TCP, relapse and second cancer risks for various fractionation protocols.
FUNDING
This article is supported by NSERC-CIHR.
Acknowledgments
ACKNOWLEDGMENTS
M Kohandel and S Sivaloganathan are supported by an NSERC/CIHR Collaborative Health Research grant.
REFERENCES
- 1.O'Rourke SF, McAneney H, Hillen T. Linear quadratic and tumor control probability modelling in external beam radiotherapy. J Math Biol 2009; 58: 799–817. doi: 10.1007/s00285-008-0222-y [DOI] [PubMed] [Google Scholar]
- 2.Gaudio F, Giordano A, Pavone V, Perrone T, Curci P, Pastore D, et al. Outcome of very late relapse in patients with Hodgkin's lymphomas. Adv Hematol 2011: 707542. doi: 10.1155/2011/707542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry-Amar M, Somers R. Survival outcome after Hodgkin's disease: a report from the International Data Base on Hodgkin's Disease. Semin Oncol 1990; 17: 758–68. [PubMed] [Google Scholar]
- 4.Cosset J, Henry-Amar M, Meerwaldt J. Long-term toxicity of early stage of Hodgkin’s disease therapy: the EORTC experience. EORTC Lymphoma Cooperative Group. Ann Oncol 1991; 2(Suppl. 2): 77–82. [DOI] [PubMed] [Google Scholar]
- 5.Mauch PM, Kalish LA, Marcus KC, Shulman LN, Krill E, Tarbell NJ, et al. Long-term survival in Hodgkin's disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am 1995; 1: 33–42. [PubMed] [Google Scholar]
- 6.Donaldson SS, Hancock SL, Hoppe RT. The Janeway lecture: Hodgkin's disease-finding the balance between cure and late effects. Cancer J Sci Am 1999; 5: 325–33. [PubMed] [Google Scholar]
- 7.Lee CK, Aeppli D, Nierengarten ME. The need for long-term surveillance for patients treated with curative radiotherapy for Hodgkin’s disease: University of Minnesota experience. Int J Radiat Oncol Biol Phys 2000; 48: 169–79. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. [DOI] [PubMed] [Google Scholar]
- 9.Dasu A, Toma-Dasu I, Karlsson M. The effects of hypoxia on the theoretical modelling of tumor control probability. Acta Oncol 2005; 44: 563–71. [DOI] [PubMed] [Google Scholar]
- 10.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the hypoxic fraction in determining tumor response to fractionated radiotherapy. Radiat Res 1997; 147: 541–50. [PubMed] [Google Scholar]
- 11.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003; 21: 3431–9. [DOI] [PubMed] [Google Scholar]
- 12.Hill DA, Gilbert E, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, et al. Breast cancer risk following radiotherapy for Hodgkin lymphoma: modification by other risk factors. Blood 2005; 106: 3358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, et al. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat Res 2003; 159: 161–73. [DOI] [PubMed] [Google Scholar]
- 14.Chadwick KH, Leenhouts HP. Molecular theory of radiation biology. New York, NY: Springer-Verlag; 1981. [Google Scholar]
- 15.Joiner M, van der Kogel AJ. Basic clinical radiobiology. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- 16.Hall EJ, Amato JG. Radiobiology for the radiologist. New York, NY: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 17.Lindsay KA, Wheldon EG, Deehan C, Wheldon TE. Radiation carcinogenesis modelling for risk of treatment-related second tumors following radiotherapy. Br J Radiol 2001; 74: 529–36. [DOI] [PubMed] [Google Scholar]
- 18.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci 2005; 102: 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manem VSK, Kohandel M, Hodgson DC, Sivaloganathan S. Mathematical modeling of second cancer risks induced by combined modality treatments. Math Med Biol 2014; in press.
- 20.Timlin C, Houston M, Jones B. Malignant induction probability maps for radiotherapy using X-ray and proton beams. Br J Radiol 2011; 84 (Spec Lss 1): S70–8. doi: 10.1259/bjr/70190973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehwar TS. Analytical approach to estimate normal tissue complication probability using best fit of normal tissue tolerance doses into the NTCP equation of the linear quadratic model. J Cancer Res Ther 2005; 1: 168–79. [DOI] [PubMed] [Google Scholar]
- 22.Schneider U, Besserer J, Mack A. Hypofractionated radiotherapy has the potential for second cancer reduction. Theor Biol Med Model. 2010; 7: 4. doi: 10.1186/1742-4682-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marková J, Maaloufová J, Stríteská J, Feltl D, Kozák T. Late recurrence of Hodgkin's disease 20 years after initial diagnosis. Personal observation of two cases and a literature review. [In Czech.] Vnitr Lek 2002; 48: 157–60. [PubMed] [Google Scholar]
- 24.Livsey JE, Cowan RA, Wylie JP, Swindell R, Read G, Khoo VS, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate: five-year outcome analysis. Int J Radiat Oncol Biol Phys 2003; 57: 1254–9. [DOI] [PubMed] [Google Scholar]
- 25.Ortholan C, Hannoun-Lévi JM, Ferrero JM, Largillier R, Courdi A. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys 2005; 61: 154–62. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson DC, Koh ES, Tran TH, Heydarian M, Tsang R, Pintilie M, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer 2007; 110: 2576–86. [DOI] [PubMed] [Google Scholar]