Abstract
In pharmaceutical formulations, phospholipids obtained from plant or animal sources and synthetic phospholipids are used. Natural phospholipids are purified from, e.g., soybeans or egg yolk using non-toxic solvent extraction and chromatographic procedures with low consumption of energy and minimum possible waste. Because of the use of validated purification procedures and sourcing of raw materials with consistent quality, the resulting products differing in phosphatidylcholine content possess an excellent batch to batch reproducibility with respect to phospholipid and fatty acid composition. The natural phospholipids are described in pharmacopeias and relevant regulatory guidance documentation of the Food and Drug Administration (FDA) and European Medicines Agency (EMA). Synthetic phospholipids with specific polar head group, fatty acid composition can be manufactured using various synthesis routes. Synthetic phospholipids with the natural stereochemical configuration are preferably synthesized from glycerophosphocholine (GPC), which is obtained from natural phospholipids, using acylation and enzyme catalyzed reactions. Synthetic phospholipids play compared to natural phospholipid (including hydrogenated phospholipids), as derived from the number of drug products containing synthetic phospholipids, a minor role. Only in a few pharmaceutical products synthetic phospholipids are used. Natural phospholipids are used in oral, dermal, and parenteral products including liposomes. Natural phospholipids instead of synthetic phospholipids should be selected as phospholipid excipients for formulation development, whenever possible, because natural phospholipids are derived from renewable sources and produced with more ecologically friendly processes and are available in larger scale at relatively low costs compared to synthetic phospholipids.
Practical applications: For selection of phospholipid excipients for pharmaceutical formulations, natural phospholipids are preferred compared to synthetic phospholipids because they are available at large scale with reproducible quality at lower costs of goods. They are well accepted by regulatory authorities and are produced using less chemicals and solvents at higher yields. In order to avoid scale up problems during pharmaceutical development and production, natural phospholipid excipients instead of synthetic phospholipids should be selected whenever possible.
Keywords: Emulsifier, Lecithin, Liposomes, Natural phospholipids, Phosphatidylcholine, Solubilizer, Synthetic phospholipids
1 Introduction
Phospholipids are well-established excipients for pharmaceutical applications. They are used in many types of formulations, like fat emulsions, mixed micelles, suspensions, and liposomal preparations for any administration route. They are natural compounds and effective alternatives to synthetic, unnatural emulsifiers, like polysorbates, polyoxyethylene castor oil derivatives, and sucrose fatty acid esters.
All lipids that contain phosphorus are called phospholipids. Phospholipids are surface-active, amphiphilic molecules, which comprise a polar head group and a lipophilic tail. Because of this amphiphilic character they are used as emulsifier, wetting agent, solubilizer, and liposome former. The phospholipid molecule comprises a glycerol backbone, which is esterified in positions 1 and 2 with fatty acids and in position 3 with phosphate. The systematic designation of, e.g., phosphatidic acid (PA) is 1,2-diacyl-sn-glycero-3-phosphate (where sn means stereospecific numbering). The sn-2 carbon atom of glycerophospholipids is a chiral center, and natural phospholipids occur in enantiomerically pure form. In typical membrane phospholipids, the phosphate group is further esterified with an additional alcohol, for instance in phosphatidylcholine (PC) with choline, in phosphatidylethanolamine (PE) with ethanolamine, and in phosphatidylglycerol (PG) with glycerol. Depending upon the structure of the polar region and pH of the medium, PE and PC are zwitterionic and have a neutral charge at pH values of about 7, whereas, e.g., PG is negatively charged. The most common phospholipid is PC, and PC is the main component of lecithin. Lecithin is described, e.g., in the United States Pharmacopoeia (USP) as a “complex mixture of acetone-insoluble phosphatides, which consists chiefly of PC, PE, phosphatidylserine, and phosphatidylinositol, combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% of acetone-insoluble matter.” Normally, lecithin grades containing more than 80% PC do not comply anymore with the phamacopoeial definition and are called arbitrarily PC, whereas grades containing less than 80% PC can be arbitrarily called lecithin. However, there is still some confusion on the term “lecithin,” which was originally assigned to the pure PC, but is still used in medicinal literature and documents of health authorities (US-FDA, C-FDA, BfArm). Today “lecithin” has become the trade name for the mixture of phosphatides as typically used in the food and cosmetic industry 1.
Phospholipids are structural and functional components of all cell membranes and therefore endogenous substances. The phospholipid content of membranes and the distribution of fatty acid residues vary within a cell and from one cell type to another 2. Until now, the biological function of membrane asymmetry and the distribution of different types of phospholipids in organs are not completely understood, but the interaction of the manifold of phospholipids and further membrane components seem to fulfil important roles in signal-transduction cascades. To illustrate the importance of phospholipids for the physiological functioning of the body, besides the functional role of phospholipids in cell membranes, phospholipids, mainly PC, have digestion/metabolic functions in bile (as monoacyl-phospholipids) to solubilize cholesterol and fatty components in food and lipophilic drug substances 3, as lipoprotein components for transport of fat between gut and liver, as source for acetylcholine (in case of PC) and as source of (essential) fatty acids and energy 4. In addition, in lung surfactant a specific phospholipid dipalmitoylphosphatidylcholine occurs 4. Phosphatidylserine is a component of the lipid–calcium–phosphate complex for deposition during bone formation 5, regulation of apoptosis (programmed cell death) 6 and blood coagulation 7.
Natural phospholipid excipients are defined as phospholipids isolated from natural sources like, e.g., soybean, rapeseed, and sunflower seed. Natural phospholipids may be further converted to saturated phospholipids by means of hydrogenation or further treated with enzymes to, e.g., remove partially fatty acids (phospholipase A2) or to convert a polar head group (phospholipase D). The saturated phospholipids are considered as natural phospholipids because the resulting saturated lipids are also occurring in nature (i.e., natural identical). For instance, saturated PC can be found in rat tissue at rather high levels in the lung, spleen, brain, and kidney (36, 22, 20, and 16% of total PC, respectively) 8. The additional hydrogenation step represents a minor chemical modification compared to the overall production process of natural phospholipids. Also, enzymatically modified natural (saturated as well as unsaturated) phospholipids are considered as natural phospholipids because the resulting phospholipids occur as well in nature and the enzymatic process used to generate the phospholipids is also natural. Typical for all these natural phospholipids is that the eventual fatty acid and polar head group composition is determined by the (phospholipid and fatty acid) composition of the starting natural material and applied isolation procedures. In contrast, synthetic phospholipid excipients are phospholipids where defined specific molecular species of polar head groups or fatty acids are introduced by means of a tailor made chemical synthesis process.
In this review, the production, use, and properties of natural phospholipids and synthetic phospholipids excipients are compared from the industrial pharmaceutical technical development perspective. The synthetic phospholipids discussed are synthetic analogs to natural alternatives. Chemically modified phospholipids, like, e.g., N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoylphosphatidylethanolamine sodium salt (MPEG-DSPE), which do not occur in nature, are out of the scope of this review.
This comparison offers a rational to select the preferred phospholipid class for pharmaceutical formulation development purposes.
2 Natural phospholipids
Natural phospholipids can be obtained from vegetable sources like, e.g., soybeans, rape (canola) seed, wheat germ, sunflower, and flax seed, and animal material, like egg yolk, milk, or krill. These raw materials are world-wide produced at very large scale. The world-wide harvest of soybeans was in 2013, 280 million tons and the annual world-wide production of soybean oil is about 42 million tons 9. During 2002, 53.4 million tons of table eggs were produced. This represents approximately 17 million tons egg yolk 10. Although only a part of these huge amounts is used for the production of natural phospholipid excipients, it is clear that there is adequate supply for this purpose.
The phospholipid and fatty acid profiles of the lecithin are dependent on the raw material sources. The profiles for de-oiled soybean-, sunflower seed-, and rapeseed lecithin are provided as example (Tables 1 and 2). For an example of the phospholipid and fatty acid composition of a lecithin derived from animal source (egg lecithin), reference is made to the corresponding section below.
Table 1.
Phospholipid composition of vegetable de-oiled lecithins, as derived from corresponding product specifications (%) 11
| Phospholipid | Lecithin | ||
|---|---|---|---|
| Soybean | Sunflower seed | Rapeseed | |
| PC | 20–22 | 20–26 | 23–31 |
| PE | 16–22 | 4–10 | 9–15 |
| PI | 13–16 | 15–19 | 15–18 |
| PA | 5–10 | 2–5 | 5–10 |
| LPC | <3 | <3 | <3 |
Table 2.
Fatty acid composition of typical batches of vegetable de-oiled lecithins (area %) 11
| Fatty acid | Lecithin | ||
|---|---|---|---|
| Soybean | Sunflower seed | Rapeseed | |
| C14:0 | 0.1 | 0.1 | 0.1 |
| C16:0 | 21 | 16 | 10 |
| C18:0 | 4.7 | 5.3 | 0.8 |
| C18:1 | 9.9 | 21 | 49 |
| C18:2 | 57 | 54 | 31 |
| C18:3 | 5.0 | 0.2 | 4.4 |
| C20:0 | 0.1 | 0.3 | 0.1 |
| C22:0 | 0.4 | 1.5 | 0.1 |
In all cases, PC is the main phospholipid component. In order to convert these raw materials into high quality pharmaceutical excipients, meeting pharmacopeial and regulatory requirements for parenteral and other specific formulations, extraction and chromatography procedures are applied.
2.1 Soybean lecithin
To illustrate the production process of natural phospholipid excipients starting from plant oil, the production process of soybean lecithin is given as example. As a first step crude soybean lecithin is isolated by degumming of the crude soybean oil as obtained by extraction from soybeans (Fig. 1) 12.
Figure 1.
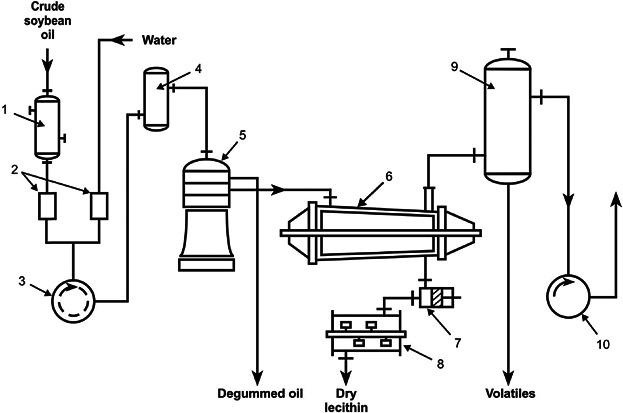
Flow sheet of a lecithin producing unit. Crude soybean oil is heated in the preheater, (1) to 80°C, mixed with 2% water in the proportion control unit, (2) and intensively agitated in (3). The mixture goes to a dwelling container, (4), and is then centrifuged after a residence time of 2–5 min. The degummed oil flows without further drying to the storage tanks. The lecithin sludge is dried in the thin-film evaporator, (6), at 100°C and 6 kPa (60 mbar) for 1–2 min and is discharged after cooling to 50–60°C in the cooler, (8). (9) and (10) are the condenser and vacuum pump, respectively 12.
The crude soybean lecithin obtained serves as starting material for production at large scale of soybean lecithin fractions with higher PC content. They are obtained in high yields by extraction methods using the non-toxic solvents acetone and ethanol followed by chromatographic purification procedures and appropriate solvent removal methods (Fig. 2A (flow chart) and Fig. 2B (visual aspect of phospholipids)). All solvents can be recycled and reused. By selecting appropriate sequential extraction and chromatography methods several lecithin fractions differing in the PC content (20–80%) up to pure PC (to 98%), and in the ratio of phospholipids to non-polar lipids can be reproducibly achieved. This procedure applies to soybean oil and all vegetable oils used to produce lecithin/PC.
Figure 2.
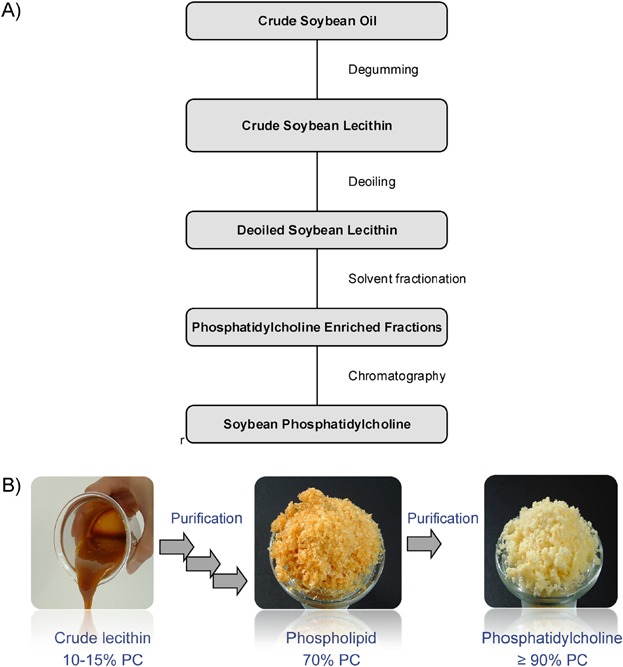
(A) Flow chart of the isolation process steps of soybean phosphatidylcholine from crude soybean oil. (B) Visual aspect of soybean lecithin fractions with varying PC content.
The original fatty acid composition of the starting material is reflected in the fatty acid composition of the purified fractions. The PC content and purity of the various soybean lecithin extraction and chromatography fractions, as measured with normal phase HPLC equipped with an ELSD are demonstrated (Fig. 3). The various phospholipids present in soybean lecithin, N-acyl-PE, PA, PE, PC, PI, and Lyso-PC (LPC) are separated at increasing retention time.
Figure 3.
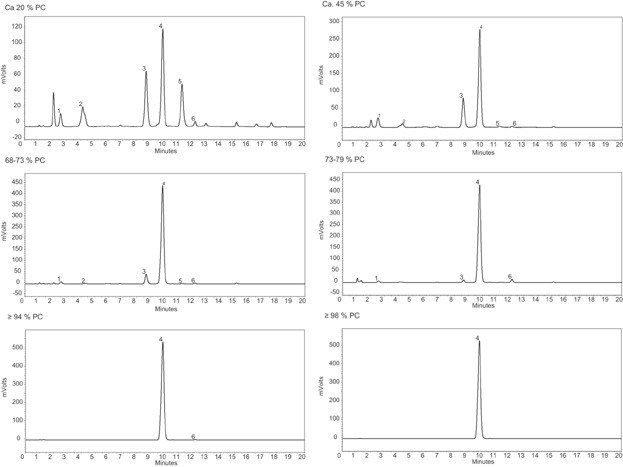
HPLC (normal phase chromatography)-ELSD chromatograms of soybean lecithin extraction and chromatography fractions differing in PC content. Peak numbers reference 1: N-acyl-PE, 2:PA, 3:PE, 4:PC, 5: PI, 6: LPC 11.
As derived from these HPLC chromatograms and additional TLC studies (not shown), the more detailed lipid profile shows dependent on the number of extraction and chromatography steps an increase of the PC content to 98%, and concomitantly a decrease of the other lipid components (Table 3).
Table 3.
Phospholipid composition of the soybean lecithin extraction and chromatography fractions differing in PC content 13
| Component (%w/w) | Fraction | |||||
|---|---|---|---|---|---|---|
| Ca. 20% PC | Ca. 45% PC | 68–73% PC | 73–79% PC | ≥94% PC | ≥98% PC | |
| PC | 22 | 51 | 70 | 79 | 97 | 99 |
| PE | 15 | 18 | 10 | 3.3 | 0 | 0 |
| PI | 15 | 1.7 | 0.7 | 0.2 | 0 | 0 |
| PA | 9.7 | 4.5 | 1.3 | 1.6 | 0 | 0 |
| LPC | 3.2 | 1.9 | 1.4 | 6.1 | 0.4 | 0.1 |
| N-Acyl-PE | 3.2 | 5.0 | 2.3 | 1.7 | 0 | 0 |
The typical fatty acid composition of the soybean lecithin extraction and chromatography fractions differing in PC content is provided in Table 4.
Table 4.
Typical fatty acid composition (area % of total) of soybean lecithin extraction and chromatography fractions differing in PC content 13
| Fatty acid | Fraction | |||||
|---|---|---|---|---|---|---|
| Ca. 20% PC | Ca. 45% PC | 68–73% PC | 73–79% PC | ≥94% PC | ≥98% PC | |
| C14:0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| C16:0 | 21 | 16 | 15 | 15 | 15 | 12 |
| C18:0 | 4.7 | 3.4 | 3.5 | 3.7 | 3.7 | 3.3 |
| C18:1 | 9.9 | 9.5 | 10 | 12 | 12 | 9.7 |
| C18:2 | 57 | 63 | 64 | 63 | 63 | 67 |
| C18:3 | 5.0 | 6.0 | 5.8 | 5.2 | 4.7 | 6.1 |
| C20:0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| C22:0 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
The results in Table 4 indicate that in the fatty acid pattern of the soybean derived lecithin fractions, phospholipids and PCs obtained by extraction and chromatography five fatty acid types account for about 95% of the total fatty acid composition. These are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3). There is a slight variation in the ratio of the fatty acids between the different fractions. The fatty acid composition is influenced by the climate conditions (temperature and humidity) prevailing during the maturation of the soybeans and the genetic origin of the plants.
Control on the quality of the raw material and use of validated purification method guarantees the reproducibility of the composition of the resulting phospholipid excipients. An impression on the inter-batch reproducibility of the quality of the soybean lecithin extraction and chromatography fractions differing in PC content can be obtained from Tables 5A and 5B.
Table 5A.
Inter-batch reproducibility of the composition of soy bean lecithin extraction and chromatography fractions with PC content 20–70% w/w 11
| Component (% w/w) | Fraction | |||||
|---|---|---|---|---|---|---|
| Ca. 20% PC | Ca. 45% PC | 68–73% PC | ||||
| Lot I | Lot II | Lot I | Lot II | Lot I | Lot II | |
| PC | 20–22a) | 20–22a) | 53 | 55 | 71 | 68 |
| PE | 16–22a) | 16–22a) | 14 | 13 | 9.9 | 9.5 |
| LPC | 2.0 | 0.5 | 2.5 | 2.5 | 1.5 | 1.8 |
| Triglycerides | 0.2 | 1.1 | <0.5 | <0.5 | 1.0 | 0.6 |
| Free fatty acids | 0.2 | 0.5 | ≤0.5a) | ≤0.5a) | ≤0.5a) | ≤0.5a) |
| Phosphorus | 3.1 | 3.0 | 3.2 | 3.1 | 3.4 | 3.5 |
| d,L-α-Tocopherol | <0.1 | <0.1 | 0.1 | 0.2 | 0.2 | 0.1 |
| Water | 0.4 | 0.4 | 0.2 | 0.2 | 0.5 | 0.5 |
| Peroxide value | 0.8 | 2.5 | 1.1 | 1.1 | 1.0 | 1.1 |
| Iodine value | n.d.b) | n.d. | n.d. | n.d. | 87 | 87 |
| Acid value | 24 | 23 | 16 | 16 | n.d. | n.d. |
Typical values, not routinely tested.
n.d., not determined.
Table 5B.
Inter-batch reproducibility of the quality of soybean lecithin extraction and chromatography fractions with PC content 76–98% w/w 11
| Component (% w/w) | Fraction | |||||
|---|---|---|---|---|---|---|
| 73–79% PC | ≥94% PC | ≥98% PC | ||||
| Lot I | Lot II | Lot I | Lot II | Lot I | Lot II | |
| PC | 75 | 74 | 96 | 96 | 99 | 98 |
| PE | 3.9 | 3.8 | <LOD | <LOD | <LOD | <LOD |
| LPC | 3.3 | 3.3 | 0.7 | 0.8 | 0.1 | 0.2 |
| Triglycerides | 5.0 | 5.0 | 0.1 | 0.8 | 0.2 | 0.2 |
| Free fatty acids | ≤0.5 | ≤0.5 | <LOD | <LOD | <LOD | <LOD |
| Phosphorus | 3.7 | 3.7 | 3.9 | 3.9 | 3.9 | 4.0 |
| D,L-α-Tocopherol | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Water | 0.5 | 0.5 | 0.5 | 0.5 | 1.2 | 0.8 |
| Peroxide value | 1.2 | 1.2 | 1.7 | 2.0 | 1.7 | 1.3 |
| Iodine value | 90–105 | 90–105 | 104 | 99 | 105 | 103 |
| Acid value | n.d | n.d. | 0.2 | 0.1 | 0.1. | 0.1 |
LOD, limit of detection; n.d., not determined.
The data in the Tables 5A and 5B indicate that the inter-batch reproducibility increases at increasing PC purity. Materials with high PC contents undergo a number of purification steps leading to more constant chemical compositions. For that reason, products with high PC contents are more suited for pharmaceutical applications requiring higher phospholipid purity, like parenteral administration, whereas the lecithin qualities starting at PC 20 are suitable for oral and topical administration.
2.2 Egg lecithin
Egg phospholipids are isolated from egg yolk with similar extraction and chromatography methods as for soybean lecithin. The phospholipid profiles of egg lecithin extraction/chromatography fractions as measured with HPLC, equipped with ELSD shows the presence of PC, PE, SM, LPE, and LPC in the fractions with 64–79% PC, whereas upon further purification, the content of PC increases up to ≥98% and the other lipids are removed with exception of a small amount of SM (Fig. 4 and Table 6A).
Figure 4.
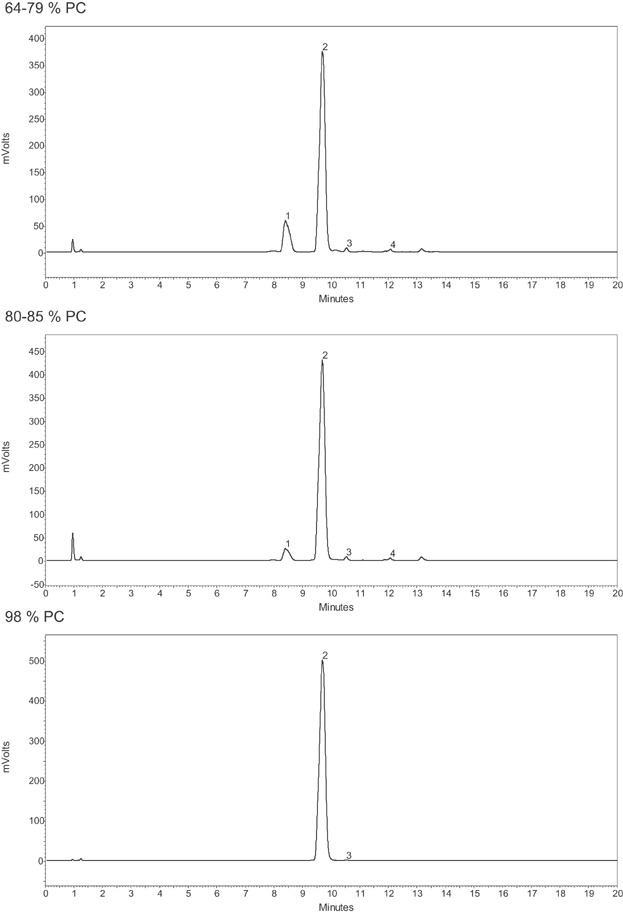
HPLC (normal phase chromatography)-ELSD chromatograms of egg lecithin extraction and chromatography fractions differing in PC content. Peak numbers reference 1: PE, 2: PC, 3: SM, 4: LPC 11.
Table 6A.
Phospholipid composition (% w/w) of the egg lecithin extraction and chromatography fractions differing in PC content 14
| Component | Fraction | ||
|---|---|---|---|
| 64–79% PC | 80–85% PC | ≥98% PC | |
| PC | 72 | 81 | 99 |
| PE | 17 | 8.5 | 0.0 |
| SM | 2.0 | 2.0 | 0.4 |
| LPE | 1.0 | 0.3 | 0.0 |
| LPC | 2.0 | 2.0 | 0.0 |
In the fatty acid pattern of the egg derived phospholipids and PCs, five fatty acid types account for about 92% of the total fatty acid composition (Table 6B).
Table 6B.
Typical fatty acid composition (area % of total) of egg lecithin extraction and chromatography fractions differing in PC content 14
| Fatty acid | Fraction | ||
|---|---|---|---|
| 64–79% PC | 80–85% PC | ≥98% PC | |
| C14:0 | 0.2 | 0.1 | 0.2 |
| C16:0 | 31 | 31 | 34 |
| C18:0 | 15 | 14 | 12 |
| C18:1 | 24 | 28 | 28 |
| C18:2 | 16 | 15 | 16 |
| C20:4 | 5.6 | 4.8 | 3.6 |
| C22:4 | 0.3 | 0.3 | 0.2 |
| C22:5 | 0.2 | 0.2 | 0.1 |
| C22:6 | 2.2 | 1.8 | 1.8 |
These are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and arachidonic acid (C20:4). The presence of the polyunsaturated fatty acids C 20:4 (n-6) and C22:6 (n-3) is typical for egg phospholipids. There is a slight variation in the ratio of the fatty acids between the different fractions. Considering the position of the fatty acid within the phospholipid molecule, egg PC comprises about 40% 1-palmitoyl-2-oleoylphosphatidylcholine.
2.3 Hydrogenated lecithin
Due to the presence of unsaturated fatty acids in natural phospholipids the liquid crystalline to gel phase transition temperature is below 0°C. These phospholipids are at ambient and body temperature in the liquid crystalline state and form upon hydration flexible structures/mesophases, suitable for specific pharmaceutical technological applications. In some formulations, however, when, e.g., more physically stable liposomes with increased stability in blood plasma or when phospholipids with more powder like properties are required, phospholipids with higher phase transition temperatures are preferred 15.
Phospholipids with saturated fatty acids possess these properties and can be obtained by hydrogenation of natural phospholipids with unsaturated fatty acids.
In this process, the unsaturated fatty acids are saturated by hydrogen gas in a catalytic reaction involving metal catalysts (e.g., nickel, palladium, and platinum) bound to porous carrier, typically in a heterogeneous system 16. The progress of the reaction can be followed by monitoring the iodine value 17, which is a measure for the degree of unsaturation of fatty substances. Crude soybean lecithin and pure soy PC have an iodine value of about 90–100. After completion of the reaction, the metal catalyst is completely removed by means of filtration.
Soybean PC predominantly contains the unsaturated acids oleic, linoleic, and linolenic acid, as well as the saturated acids palmitic and stearic acid. The saturated fatty acids are almost exclusively located in position 1 (Table 7) 18.
Table 7.
Fatty acid distribution in soybean phosphatidylcholine (w/w %) 18
| Fatty acid | In 1-position | In 2-position | Total |
|---|---|---|---|
| C16:0 | 24 | 2 | 13 |
| C18:0 | 8 | 1 | 4 |
| C18:1 | 13 | 10 | 11 |
| C18:2 | 50 | 80 | 65 |
| C18:3 | 5 | 7 | 6 |
Thus, hydrogenation of pure soybean PC with a typical iodine value of about 100, results in a mixture of mainly (ca. 85%) 1,2-distearoylphosphatidylcholine (DSPC) and 1-palmitoyl-2-stearoylphosphatidylcholine (PSPC) (ca. 15%).
2.4 Enzyme modified natural phospholipids
Because of the advent of biotechnology, nowadays alternative biochemical synthesis routes via enzyme catalyzed reactions serve as a viable alternative to organic-chemical synthesis steps. In that respect, the use of enzymes for phospholipid modification has moved quickly in recent years, not only in academic research but also in industry 19. The fast growing use of enzymes for polar lipid modification arises from factors such as milder reaction conditions, less environmental pollution, better specificity for improved quality, and higher efficiency of reactions. Different enzymes can be used for different modification purposes to modify/synthesize phospholipids. As illustrated in Fig. 5, a number of phospholipid ester bonds can be modified with appropriate enzymes.
Figure 5.
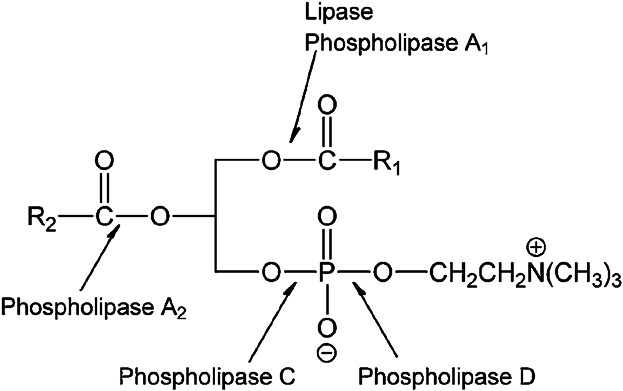
Enzymatic conversion possibilities of phosphatidylcholine 20.
For acyl modifications, the natural enzymes will be phospholipase A1 (EC 3.1.1.32) and A2 (EC 3.1.1.4) (PLA1 and PLA2 respectively). However, lipases (EC 3.1.1.3) in general can also be used for such purposes, in particularly for modification at the sn-1 position. PLA2 in the phospholipase category has specificity for the sn-2 position. Phospholipase D (EC 3.1.4.4) (PLD) is the only potential enzyme for polar group modification. Phospholipase C (EC 3.1.4.3) (PLC) can hydrolyze the bond between the glycerol OH group and the phosphate group but, unfortunately, the enzymes in this category are, at the moment, only active for the hydrolysis reaction.
Examples of enzyme-modified natural phospholipids prepared from natural PC are monoacyl-phosphatidylcholine (lyso PC), soy PE, soy PG, egg PG, and saturated analogs.
3 Synthetic phospholipids
In order to study biophysical/biochemical mechanistic aspects of phospholipids at the molecular level in model-membranes, various synthesis routes for phospholipids were developed to get an access to phospholipids, which are homogeneous with respect to the polar head group and fatty acid composition 19,21–23
In addition to the need for synthetic analogs of natural phospholipids, further synthetic phospholipids were for instance designed to optimize the drug targeting properties of liposomes. Examples are the PEG-ylated phospholipids 24 and the cationic phospholipid 1,2-diacyl-P-O-ethylphosphatidylcholine 25. Also attempts were made to convert by organic chemical means phospholipids into pharmacological active molecules (for instance ether phospholipids 26 or to make phospholipid pro-drugs (example MTP-PE 27). These conversions to pharmacological active moieties are outside the scope of this review.
As a result, during the last decades the general demand for synthetic phospholipids has increased, mainly for use in liposomal formulations. Thus, new synthesis routes to produce phospholipids on a larger scale had to be developed. In general, synthetic phospholipids can be produced using organic chemical synthesis steps and/or enzymatic synthesis steps. In the following, the described various syntheses routes are linked to the starting materials.
3.1 Synthesis starting from mannitol
The classical approach to synthesize diacyl-PC's is starting from d-mannitol, which is acetonated in the presence of anhydrous zinc chloride to give 1,2: 5,6-diisopropylidene-d-mannitol. The bis(acetonide) is treated with lead tetra-acetate to give 1,2-diisopropylidene-sn-3-glyceraldehyde, which is reduced with sodium borohydride or lithium aluminum hydride to (S)-glycerol 1,2-acetonide; the latter is converted to sn-glycerol-3-phosphate and then to phosphoglycerides.
Disadvantages of the classical synthesis method are that the method is lengthy, requiring toxic solvents and chemicals in large excess, and 9 steps to convert mannitol into identical fatty acid chain PC's and 14 steps to mixed fatty acid chain PC's. Also reaction intermediates may be unstable and subject to partial racemization 28,29.
Equally, synthesis methods using acylation of 1-acyl-2-lyso-sn-glycerophosphocholine 30–32 requires the availability of the lyso compound and use of disadvantageous chemical reactions. A simpler method to synthesize symmetric chain gycerophospholipids is a method starting from (R) or (S) glycidyl tosylate 33.The enantiomerically pure glycidyl derivative is, however, expensive, making large scale syntheses of glycerolipids impractical 21.
The method used to synthesize platelet activating factor (PAF = 1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is considered to be the shortest synthesis method of mixed fatty acid chain and identical fatty acid chain phospholipid synthesis 22,34. d-Mannitol (12) is reacting with p-methoxybenzaldehyde under the formation of two 1,3-dioxane rings (13) (Fig. 6).
Figure 6.
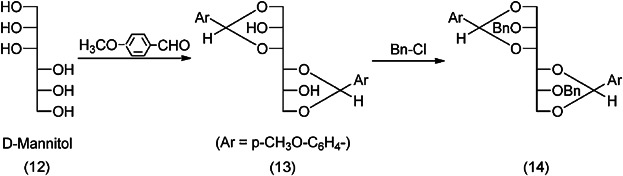
Conversion of d-mannitol to mixed (fatty acid) chain and identical (fatty acid) chain phospholipids (first steps) 22,34.
The free OH-groups are benzylated with benzylchloride (14), and the 1,3-dioxane rings are opened to keep the p-methoxybenzylether specifically only at the primary OH-group (15). The 3,4-diol is split by oxidation and the aldehyde is reduced to (16) (Fig. 7).
Figure 7.
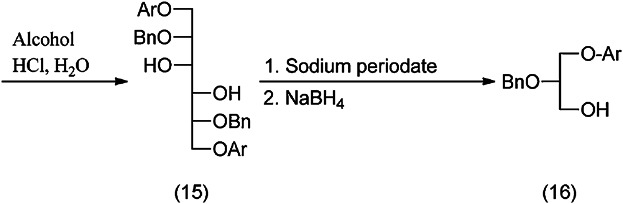
Conversion of d-mannitol to mixed (fatty acid) chain and identical (fatty acid) chain phospholipids (continued).
Since the differentiation of the three OH-groups is given, other phospholipids can also be synthesized starting from (16) by phosphorylation of the free hydroxyl group, followed by selective removal of the protective –Ar and –Bn group and reaction with the selected fatty acids.
Which synthesis route will be selected is, from industrial perspective, dependent on the (commercial) availability of key intermediates or starting materials. In that respect, synthesis of phospholipids may be started with (S)-1,2-isopropylideneglycerol, which is derivatized to 1,2-diacylglycerol, followed by attachment of the polar head group via chemical synthesis 35.
3.2 Synthesis starting from GPC
Another route to produce phospholipids with defined fatty acids is to start such synthesis from glycerophosphocholine (GPC) (sn-glycero-3-phosphocholine: also called: L-alpha glycerylphosphorylcholine, alpha-GPC or choline alfoscerate). This GPC can be synthesized in an enantioselective manner using a biotransformation procedure based on the phosphorylation of glycerol by ATP catalyzed glycerol kinase 36 or, preferably, produced from natural PC. Starting from natural GPC, phospholipids containing two identical acyl moieties can be synthesized with the same sn-glycero-3-configuration as naturally occurring glycerophospholipids in a one step process using activated acyl derivatives, e.g., acylimidazolides or anhydrides 23,37,38.
For the further synthesis from GPC to mixed fatty acid chain phospholipids, organic chemical methods as well as enzymatic procedures can be applied.
When using organic chemical methods GPC is converted to 1-O-trityl GPC, which is then acylated to 1-trityl-2-acyl GPC. In a one-pot reaction, the trityl group is replaced with an acyl group (Fig. 8). For the acylation step of 2 to 3 acyltriazolides can be used instead of fatty acid anhydrides.
Figure 8.
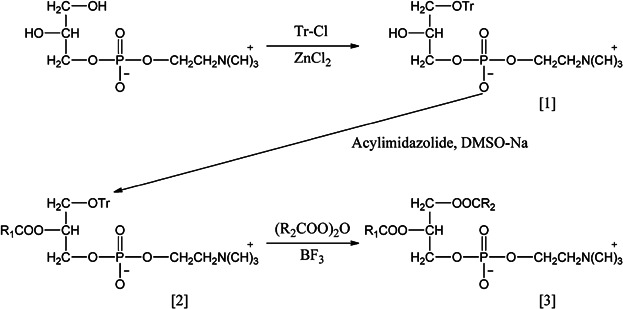
Synthetic preparation of mixed fatty acid chain phosphatidylcholines. Tr = triphenylmethyl 23.
In the presence of borontrifluoride-etherate, as a catalyst, the reaction proceeds within a short time (30 min) at low temperature (around 0°C) and the final product obtained in high yield contains less than 2–3% of positional isomers. Pure mixed fatty acid chain PCs are thus accessible in a three-step procedure, which can be carried out on any scale. This reaction is considerably shorter and therefore less expensive than the total synthesis of the corresponding phospholipids described above.
Alternatively, mixed fatty acid chain phospholipids can be synthesized from (natural or synthetic) GPC using an acylation step followed by cleavage of the fatty acid at the second hydroxyl position by means of an enzymatic modification with phospholipase A2, followed by an acylation of the free hydroxyl group with the desired fatty acid.
In addition, the enzymes (phospholipase D) able to convert the choline head group into another head group like, e.g., glycerol or serine can be used in analogy to the conversion of natural phospholipids (see above), to make, e.g., PG or phosphatidylserine from PC with well-defined mixed fatty acid chains, respectively.
Taking these organic chemical and enzymatic synthesis options into consideration, a process to synthesize mixed fatty acid chain phospholipids, optionally with a conversion of the polar head group, can be designed using natural starting materials and enzymatic procedures (Fig. 9).
Figure 9.
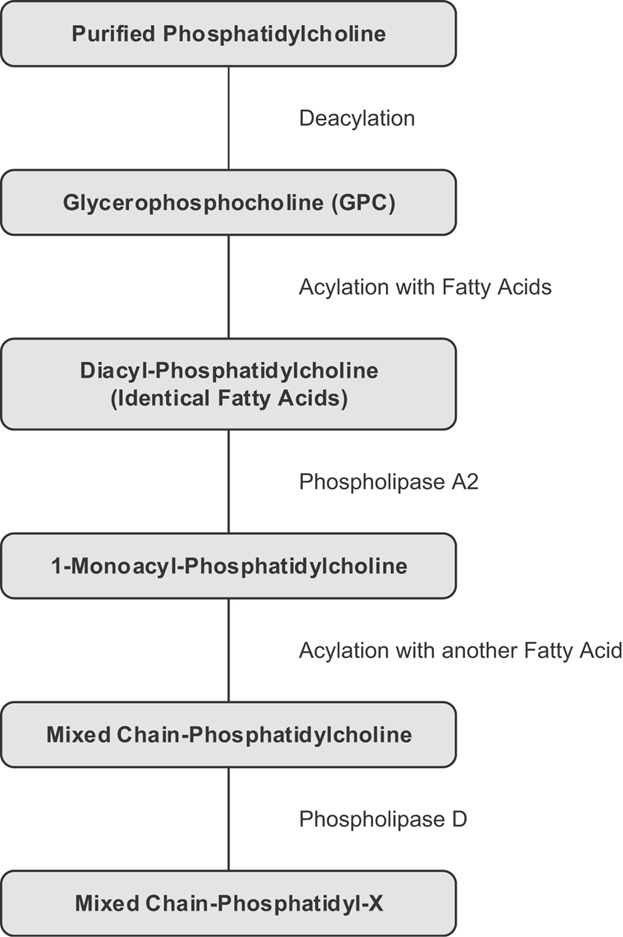
Synthesis of mixed fatty acid chain phospholipids and polar head group modification (X), starting from phosphatidylcholine and using enzymatic procedures.
As a first step, purified natural PC is deacylated to form GPC. Then the GPC is esterified with a selected fatty acid, to yield a diacyl-GPC. The fatty acid at position two of the glycerol backbone can then be selectively removed enzymatically with phospholipase A2 to form 1-monoacyl-phosphatidylcholine. The hydroxyl group at the second position of the monoacyl-phosphatidylcholine can then be re-esterified. Finally, when required, the choline polar head group can be replaced via enzyme catalyzed reaction by another polar head group like glycerol, ethanolamine, or serine to make PG, PE, or phosphatidylserine, respectively.
The number of synthesis steps of this synthesis route is lower compared to the synthesis starting from mannitol or synthesis starting from GPC, prepared by using organic chemical synthesis methods, and requires lower amounts of toxic solvents or chemicals. The yield of this alternative synthesis route is higher compared to the other synthetic procedures, but still hazardous chemicals have to be used.
4 Use of natural phospholipids and synthetic phospholipids in pharmaceutical formulations
Phospholipids are used in pharmaceutical technology as wetting agents, emulsifiers, and builder or components of mesophases like liposomes, micelles, mixed micelles, cubosomes, etc. These functional properties are used in many formulation types, like suspensions, various types of emulsions, mixed micelles, solid dispersions, drug–phospholipid complexes, etc. 39,40. Due to their physiological role, phospholipids possess a very low toxicity profile and can be used for any route of administration.
Scientific academic literature shows that natural as well as synthetic phospholipids are explored for any route of administration. However, for selection of excipients at the academic research level, the costs of goods, large-scale availability, use of green chemistry to manufacture the excipients and registration status/documentation of the used excipients do not play a role. At industrial level, however, these factors play and should play however a significant role in selection of excipients at the beginning of the development process of pharmaceutical products.
It is therefore relevant to assess the occurrence of natural and synthetic phospholipids in the inactive ingredient (excipient) list of the US FDA for approved products (October 24, 2013) 41. In this list, lecithin with CAS No. 8002-43-5 covers a broad range of natural lecithin including soybean lecithin, sunflower seed lecithin, and egg lecithin. When sorting the occurrence of natural and synthetic phospholipids (Table 8), it is clear that synthetic phospholipids are only used for parenteral/injectable administration. In contrast, natural phospholipids are used for any route of administration, including parenteral administration.
Table 8.
Frequency (%) of occurrence of natural and synthetic phospholipids in the US FDA inactive ingredient list of approved drug products dependent on the administration route (status October 24, 2013)
| Excipient | CAS Nr. | Administration route | |||
|---|---|---|---|---|---|
| Oral | Parenteral | Topical | Inhalation | ||
| Natural phospholipids | |||||
| Lecithin | 8002-43-5 | 55 | 10 | 25 | 10 |
| Soybean lecithin | 8030-76-0 | 60 | – | 20 | 20 |
| Egg lecithin | 93685-90-6 | 33 | 66 | – | – |
| Hydrogenated soybean lecithin | 92128-87-5 | 40 | 40 | 20 | |
| Egg PG | 93685906 | 100 | |||
| Synthetic phospholipids | |||||
| DMPC | 18194-24-6 | – | 100 | – | – |
| DOPC | 4235-95-4 | – | 100 | – | – |
| DSPC | 816-94-4 | – | 100 | – | – |
| DPPG | 4537-77-3 | – | 100 | – | – |
| DSPG | 217939974 | – | 100 | – | – |
Since for parenteral administration synthetic as well as natural phospholipids are used and the data provided in Table 10 cover only the products in the USA, the occurrence of natural or synthetic phospholipids in parenteral products in the EU should be considered as well.
The categories of formulations, which are of interest to study the occurrence of either natural or synthetic phospholipids are products used for parenteral administration. These are liposomes, oil-in-water emulsions, mixed micelles for intravenous use and slow release and vaccine vehicles and drug suspensions for intramuscular and subcutaneous administration.
Liposomal products for intravenous administration are formulated with synthetic as well as natural phospholipids (Table 11) 42–52.
Table 9.
Characteristics of intravenous liposomal drug products
| Product | Drug substance | Lipid Excipients | Indication |
|---|---|---|---|
| Abelcet | Amphotericin B | DMPC | Invasive fungal infections |
| DMPG | |||
| AmBisome | Amphotericin B | HSPC | Aspergillus-, Candida- and/or Cryptococcus species infections |
| DSPG | |||
| Cholesterol | Visceral leishmaniasis | ||
| Alpha-tocopherol | |||
| DaunoXome | Daunorubicin citrate | DSPC | Advanced HIV-associated Kaposi's sarcoma |
| Cholesterol | |||
| Doxil/Caelyx | Doxorubicin HCl/sulphate | MPEG-DSPE | AIDS-related Kaposi's sarcoma Ovarian cancer Multiple myeloma |
| HSPC | |||
| Cholesterol | |||
| Marqibo | Vincristine sulphate | Egg SM | Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia |
| Cholesterol | |||
| Mepact | MTP-PE, Mifartumide | POPC | Osteosarcoma |
| DOPS | |||
| Myocet | Doxorubicin HCl/citrate | Egg PC | Metastatic breast cancer |
| Cholesterol | |||
| Visudyne, Verteporfin for injection | Benzoporphyrin | DMPC | Exudative (wet) age-related macular degeneration (AMD) with predominantly classic subfoveal choroidal neovascularisation (CNV) |
| Egg PG | Subfoveal choroidal neovascularisation secondary to pathological myopia. | ||
| Ascorbyl palmitate (Butylated-hydroxytoluene) |
Liposomes can also be used as vehicle for slow release after local parenteral administration (at the surgical site, epidural, or intrathecal) (Table 10) 53–57. In this case, mainly synthetic phospholipids are being used.
Table 10.
Liposomal depot vehicles and their (phospho)lipid composition
| Product | Drug Substance | Lipid Excipients | Indication |
|---|---|---|---|
| Exparel | Bupivacaine | DEPC | Postsurgical analgesia |
| DPPG | |||
| Tricaprylin | |||
| DepoDur | Morphine sulphate | DOPC | Pain treatment prior to surgery or after clamping the umbilical cord during caesarean section |
| DPPG | |||
| Cholesterol | |||
| Tricaprylin | |||
| Triolein | |||
| DepoCyt | Cytarabine | DOPC | Lymphomatous meningitis |
| DPPG | |||
| Cholesterol | |||
| Triolein |
Egg phospholipids are used at a concentration of 1.2% in 10–30% oil-in-water emulsions for parenteral nutrition (e.g., Intralipid and Liposyn II) (Table 11) 58. Derived from a maximal cumulative daily dose of 2.5–3 g triglycerides/kg body weight/day for an adult, maximally 0.30–0.36 g phospholipid/kg body weight/day can be administered 59,60.
Table 11.
Examples of intravenous oil-in-water emulsions using egg phospholipids as emulsifier
| Product | Oil phase |
|---|---|
| Intralipid | Soybean oil |
| Liposyn II | Soybean oil/safflower oil |
| Omegaven | Fish oil |
| ClinOleic | Soybean oil/olive oil |
| Lipofundin | Soybean oil/MCT |
| Structolipid | Interesterified mixture of equimolar amounts of long chain triglycerides (LCT) and medium chain triglycerides (MCT) |
| SMOFlipid | Soybean oil/MCT/olive oil/fish oil |
Oil-in-water emulsions can also be used as carrier for oil soluble drug substances. Also, in this case egg phospholipids are exclusively being used as emulsifier (Table 12) 61–63.
Table 12.
Examples of intravenous drug containing oil-in-water emulsions using egg phospholipids as emulsifier
| Product | Drug substance | Indication |
|---|---|---|
| Diazemuls | Diazepam | Sedative and muscle relaxant |
| Limethason | Dexamethasone palmitate | Rheumatoid arthritis |
| Liple | Alprostadil (prostaglandin E1) | Vasodilator, platelet inhibitor |
| Diprivan/disoprivan | Propofol | Anesthetic |
| Etomidat®-Lipuro | Etomidate | Anesthetic |
| Vitalipid | Vitamin A, D2, E, K1 | Parenteral nutrition |
| Cleviprex | Clevidipine butyrate | Antihypertensive |
In mixed micellar formulations, comprising phospholipids and cholate salts, exclusively soybean phospholipids are being used. These formulations are either used as solubilizer for poorly water soluble compounds, or the soybean PC is used as active principle, through the presence of poly-unsaturated fatty acids for treatment of liver disorders (Table 13) 64.
Table 13.
Examples of intravenous mixed micellar products containing soybean phosphatidylcholine as phospholipid component
| Product | Drug substance/active | Indication |
|---|---|---|
| Valium MM | Diazepam | Anxiolytic |
| Essentiale | PPC | Liver therapy |
| Lipostabil | PPC | Lipid regulation |
| Cernevit | Vitamin blend | Vitamin supplement |
| Konakion MM | Vitamin K | Vitamin supplement |
These products underscore the safe intravenous use of soybean phospholipids. Also the pre-clinical toxicity of mixed micelles comprising soybean PC is well documented. Derived from the soybean PC concentration and the used intravenous dose of the product Essentiale/Lipostabil it can be derived that at least 4 g soybean PC can be administered daily intravenously 65.
Considering the products comprising phospholipids for pulmonary administration, natural as well as synthetic phospholipids are being used (Table 14). Most of these products are used for respiratory distress syndrome, a disease in infants characterized by an immature lung epithelium 66. For treatment of this deficiency, mixtures of phospholipids and extracts from animal origin comprising phospholipid surfactants, neutral lipids, and hydrophobic surfactant-associated proteins B and C (SP-B and SP-C) are inhaled. In case of the product Tobi Podhaler, DSPC is used as emulsifier during the manufacturing process to generate porous particles 67. The product is used to treat lung infections with Pseudomonas aeruginosa 68.
Table 14.
Pulmonary products comprising phospholipids
| Product | Composition |
|---|---|
| Alveofact | Phospholipid fraction from bovine lung |
| Infasurf | Extract of natural surfactant from calf lungs, which includes phospholipids, neutral lipids, and hydrophobic surfactant-associated proteins B and C (SP-B and SP-C) |
| Survanta | Natural bovine lung extract containing phospholipids, neutral lipids, fatty acids, and surfactant-associated proteins to which colfosceril palmitate (dipalmitoylphosphatidylcholine), palmitic acid, tripalmitin |
| Surfaxin | DPPC, POPG Na, palmitic acid, sinapultide (KL4 peptide, a 21-amino acid hydrophobic synthetic peptide) |
| Curosurf | Extract of natural porcine lung surfactant consisting of polar lipids (mainly phospholipids) and hydrophobic low molecular weight proteins (surfactant associated proteins SP-B and SP-C) |
| Tobi Podhaler | Tobramycin/DSPC/CaCl2 |
5 Discussion
As part of an industrial pharmaceutical development process, excipients for the dosage forms have to be selected taking many criteria into consideration. The large-scale availability and related costs of goods and, additionally, sustainability and environmental aspects of the production process play a major role in such selection process in life science industry. The resulting products should have a reproducible composition/quality (and hence functionality).
Compliance with regulatory requirements, the quality of documentation, production under cGMP, the use of validated quality control methods and toxicity/tolerability/biocompatibility profile are also of crucial importance. These aspects are discussed for natural and synthetic phospholipids.
5.1 Production
Natural phospholipids are purified from, e.g., soybeans and egg yolk using non-toxic solvent and chromatography procedures with low consumption of energy and minimum possible waste. When possible, auxiliary materials are continuously recycled. Because of the use of validated purification procedures and sourcing of raw materials with consistent quality, the resulting products differing in PC content possess an excellent batch to batch reproducibility with respect to phospholipid and fatty acid composition. Soybeans and egg yolk as renewable raw materials are abundantly available on the market. The used extraction procedures and chromatography are amenable to scale-up. This means that natural phospholipids comply with all aspects allowing broad pharmaceutical use.
When starting synthesis from mannitol, synthetic phospholipid intermediates, traces of solvents (other than ICH (International Conference on Harmonisation) class 3) and catalysts used may be present in the final product. Large-scale production of such synthetic lipids requires elaborate synthetic procedures and appropriate chemical synthesis infrastructure to achieve the natural stereochemistry.
The synthesis of phospholipids, which are pure with respect to the polar head group and with high purity fatty acid composition, and of the natural stereochemical configuration, starts preferably from GPC. The desired final purity can only efficiently be obtained by starting the synthesis with a high purity GPC. This GPC is obtained from purified natural PC, which is hydrolyzed to remove the fatty acids. The GPC is then further reacylated and/or treated with specific enzymes to synthesize a specific chemical molecule. This procedure means that synthetic phospholipids are per definition more expensive than natural phospholipids. In addition, due to the additional synthesis steps consequently more solvents and chemicals are needed, more chemical waste is produced, and the overall yield of the final product is considerably decreased. For these reasons, synthetic phospholipids are (irrespective of synthesis route) from sustainability and environmental and ecological point of view clearly inferior compared to natural phospholipids.
5.2 Regulatory and safety aspects
Natural phospholipids are in general well known to regulatory authorities. In addition, their track record as excipient with very high tolerability and biocompatibility is outstanding. From regulatory point of view, the definition of lecithin in the USP is important to note. “Lecithin is a complex mixture of acetone-insoluble phosphatides, which consist chiefly of PC, PE, phosphatidylserine, and phosphatidylinositol, combined with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates as separated from the crude vegetable oil source. It contains not less than 50% of acetone-insoluble matter.” Also in other pharmacopoeias soybean lecithin is described (Table 15).
Table 15.
Description of soybean lecithin in regulatory directives
| Pharmacopeia/directive | Food chemicals codex | 231/2012 EC | NF 31 | CP 2010 | JPE 2004 |
|---|---|---|---|---|---|
| Monograph number | INS: 322 | E 322 | – | – | 106893 |
| Monograph title | Lecithin | Lecithins | Lecithin | Soya lecithin | Soybean lecithin |
| Special characteristics | From soybeans and other plant sources | Animal or vegetable origin; additional specifications for hydrolysed lecithins | Origin from the crude vegetable oil source | Extracted and refined from soybean | From soybean, composed mainly of phospholipid |
| Acetone-insoluble matter | n.l.t. 50.0% | n.l.t. 60.0% | n.l.t. 50.0% | n.l.t. 90.0% | n.l.t. 60% |
| Toluene-insoluble matter | – | n.m.t. 0.3% | – | – | – |
| Hexane-insoluble matter | n.m.t. 0.3% | – | n.m.t. 0.3% | n.m.t. 0.3% | – |
| LPC content/LPE content | – | – | – | n.m.t. 3.5%/n.m.t. 0.5% | |
| Water content or loss on drying | n.m.t. 1.5% | n.m.t. 2.0% | n.m.t. 1.5% | n.m.t. 1.5% | n.m.t. 1.5% |
| Peroxide value | n.m.t. 100 | n.m.t. 10 | n.m.t. 10 | n.m.t. 5 | n.m.t. 10 |
| Acid value | n.m.t. 36 | n.m.t. 35 | n.m.t. 36 | n.m.t. 30 | n.m.t. 40 |
| Iodine value | – | – | – | n.l.t. 75 | – |
n.l.t., not less than; n.m.t., not more than.
Monographs for egg phospholipids have been established in the USP NF31, Chinese Pharmacopeia (2010, Egg lecithin) and in the Japanese Pharmaceutical Excipients (2004, Egg lecithin).
The WHO places no limit on the oral intake of lecithin. Also no limit for the ADI value (acceptable daily intake) for lecithin as a food additive 69 is given. After parenteral administration soybean and egg lecithin (unsaturated and saturated) are well tolerated. The European Commission declares that lecithin is a food additive (E322) “generally permitted for use in foodstuffs” 70,71. Also no ADI value has been fixed for lecithin in Europe; the material may be used “quantum satis” 72. The US FDA assigned the generally recognized as safe (GRAS) affirmation for lecithin 73 and enzyme modified lecithin 74,75. The tolerability of natural phospholipids in formulations is further underscored by the use in innumerous products on the market.
Drug products and related formulation technologies show sometimes a remarkable preference for either soybean lecithin or egg lecithin. Oil-in-water emulsions are nowadays exclusively emulsified with egg phospholipids. Mixed micelles are preferably being used with soybean phospholipids. The preference to use egg phospholipids instead of soybean phospholipids was probably related to findings of studies using emulsions, which were not adequately characterized with respect to particle size and chemical composition. For instance, a toxicological study of 1964 wherein in cotton seed oil emulsions emulsified with soybean lecithin was compared with an emulsion using soybean oil and egg lecithin. It was found that dogs treated with the cottonseed oil/soybean lecithin emulsions show serious side effects whereas the soybean oil/egg lecithin emulsion was well tolerated 76. Since this study was poorly designed (e.g., the control of cotton seed oil/egg lecithin is missing), it is not clear that soybean lecithin is responsible for the observed side effects. In addition, it was known that a toxic component in cotton seed oil, gossypol, was responsible for the observed side effects 77. Other studies also showed some adverse reactions of soybean lecithin stabilized emulsions 78, whereas others claim that further purification of soybean lecithin may circumvent toxic effects 79. Because of these uncertainties, egg lecithin has become the preferred emulsifier for parenteral oil-in-water emulsions. Although for this type of emulsion products egg lecithin is preferred, nowadays it is well accepted that soybean phospholipids are acceptable for parenteral administration, because of the use of soybean lecithin (PC) in injectable mixed micellar products (Table 13). The selection of soybean PC for mixed micelles by Hoffmann La Roche for their mixed micelle injectable product diazepam may be related to the presence on the market of the product Essentiale of Nattermann, which comprises mixed micelles of soybean PC and deoxycholate at the time the selection was made.
Saturated PC, prepared from egg yolk or soybeans by means of hydrogenation (HSPC) is not yet mentioned in pharmacopeias. HSPC is, however, listed in the Inactive Ingredient Guide by the FDA Center for Drug Evaluation and, related to the lipid composition of FDA approved drug products, covered in the Draft Guidance on Doxorubicin Hydrochloride (FDA, February 2010) referring to the Draft Guidance for Industry on Liposome Drug Products 80. In addition, HSPC is covered in a reflection paper on the data requirements for (generic) intravenous liposomal products developed with reference to an innovator liposomal product 81.
The pharmaceutical use of phospholipids isolated from animal sources may sometimes be complicated by the occasional occurrence of animal diseases. Examples are the mad cow disease (bovine spongiform encephalopathy (BSE)/transmissible spongiform encephalopathy (TSE)) or avian influenza virus. Although it is unlikely that these diseases can be transmitted to man, it is general policy that, even in the very rare event that starting material is contaminated, the applied extraction and purification methods should be able to remove or inactivate the infectious virus. The processes have to be validated in viral safety studies 82. The raw materials for egg and soy phospholipids are further tested for contaminants possibly originating from the plant or animal origin, e.g., pesticides, aflatoxins, heavy metals, or antibiotic residues and bioburden to ensure the quality and safety of the starting material to obtain pharmaceutical grade phospholipids 83.
Synthetic phospholipids are not mentioned in pharmacopoeias. Just like hydrogenated natural phospholipids they can be found in the Inactive Ingredient Guide by the FDA Center for Drug Evaluation and in the relevant reference documents of FDA and EMA mentioned above. The quality and safety have to be addressed in the application documentation of the drug product. The tolerability of such lipids after parenteral administration can be derived from the doses of the relevant drug products described above. These doses reflect the therapeutic dose of the formulated drugs. The maximal doses of lipids, which can be administered, without drug substance, will be probably significantly higher.
5.3 Reproducibility/purity/heterogeneity
Although natural phospholipids have a heterogeneous composition (regarding polar head group and fatty acid composition), their over-all quality meets that of synthetic phospholipids. This degree of heterogeneity of natural phospholipids is dependent on the degree of purification. In fact, when the specifications of purified egg PC (≥98%) and synthetic POPC are compared the total content of impurities is nearly the same (Table 16).
Table 16.
Comparison of chemical composition (% w/w) of a purified natural phospholipid (egg PC) and a synthetic phospholipid (POPC) 11
| Test Item | Specifications | |
|---|---|---|
| EPC | POPC | |
| PC | ≥98% | ≥99% |
| L-PC | ≤0.2% | ≤0.5% |
| PE | ≤0.1% | – |
| SM | ≤1.0% | – |
| Triglycerides | ≤0.5% | ≤0.2% |
| Cholesterol | ≤0.5% | – |
| Free fatty acids | ≤0.2% | ≤0.3% |
| Unidentified concomitant components | n.d.a) | ≤0.5% |
| Peroxide value | ≤3 | ≤3 |
not detectable, ≤0.1%.
Table 16 shows clearly that the risk for presence of unknown impurities and impurities, which are not qualified by means of toxicity testing, is not eliminated by selecting synthetic phospholipids.
As shown above (Tables 5A and 5B) the various phospholipid fractions obtained from soybean lecithin (as an example of a natural phospholipid) by means of solvent extraction and chromatography are indeed heterogeneous with respect to polar head group and fatty acid profile. However, due to a control on the composition of the raw materials and use of validated purification methods, it is also demonstrated that the resulting natural phospholipids, irrespective their heterogeneity, appear to be highly defined and reproducible with respect to the polar head group and fatty acid composition.
When taking reproducibility of composition (irrespective degree of heterogeneity) and hence functionality is taken as quality aspect then natural phospholipids meet at least the quality of synthetic phospholipids.
5.4 Use of natural and synthetic phospholipids in market products
It is remarkable to note that synthetic phospholipids are almost exclusively being used for parenteral drug products. Their use is restricted to liposomal products and slow release vehicles. Simultaneously, in this product class natural phospholipids are used as well. In oral and dermal products, synthetic phospholipids are not used.
Possible reasons for selecting these synthetic phospholipids instead of natural phospholipids are manifold: First of all, there may not be a natural alternative. An example of such a lipid, with a very specific function to extend the circulation time of intravenously administered liposomes, is a PEG-ylated phospholipid like MPEG-DSPE, which does not occur in nature.
Most of the liposomal products in question have been developed by small pharmaceutical companies with roots in academic research. The original research interest was mainly in the area of biophysics and membrane biochemistry. These scientific disciplines use well-defined molecular mixtures to study the effect of the components on each other for instance in model membranes. In addition, possibly, the selection of synthetic phospholipids may be related to the unfounded fear for unknown impurities, allergens, etc. present in natural phospholipids. Also, for liposomal dosage intended for active or passive drug targeting, the liposomal phospholipids are considered to make an essential contribution to the targeting properties of the liposomes 80. Therefore, the phospholipids should meet quality criteria comparable with new drug substances. Without the knowledge that natural phospholipids meet these requirements, synthetic phospholipids are superficially selected to match the quality of the synthetic drug substance (Impurities in New Drug Substances (ICH Q3A R2)). For all these reasons, there is some preference for using synthetic phospholipids to formulate liposomes.
At this research stage, only relatively small amounts of phospholipids are needed. Scale-up considerations are most of the time at that stage not sufficiently considered. Since cancer is the indication for which such formulations are developed, and therapy improvement for this disease will be rewarded by health insurances, irrespective the costs, consequently relative expensive excipients are being and can be used. These small amounts of phospholipids are also sufficient in the stage of preclinical efficacy testing in mice and rats. In case indeed an improvement of the therapeutic index of the phospholipid based formulations is observed in preclinical models, then most of the time the same formulations will be tested in Phase I clinical trials.
Unfortunately, such products may experience, at a later development stage, severe scale up problems due to lack of availability of the synthetic phospholipids used. This may give rise to delays in development and decrease of return in investment. Also, since the synthetic phospholipids are expensive, the profit margin on the product may be smaller as compared to the product when developed with natural phospholipids. It is therefore advisable to consider, at a very early pharmaceutical development stage, to use natural phospholipids whenever possible.
Considering the discussion above on suitability of the various phospholipid classes for development of dosage forms and scale up, natural phospholipids including hydrogenated natural phospholipids are, irrespective the intended administration route, preferred compared to synthetic phospholipids. When synthetic phospholipids are selected then the synthesis starting from GPC is preferred to syntheses starting from mannitol or (S)-1,2-isopropylideneglycerol to produce the synthetic phospholipids.
Argumentations that natural phospholipids are disadvantageous, because they are heterogeneous and have a high degree of instability due to multiple unsaturation in the fatty acyl chains and are possibly contaminated by trace amounts of proteins, nucleic acids, and other lipids 84,85 do not apply.
This so-called heterogeneity in composition, which can better be described as “presence of well defined other molecular species besides the main lipid component” should not be interpreted as lack of reproducibility. On the contrary, natural phospholipids can be manufactured highly reproducibly with respect to phospholipid and fatty acid composition and their composition is well defined. The presence of well defined other molecular species besides the main lipid component may be even advantageous, compared to mono-component compounds regarding, e.g., emulsifier and solubilizer properties.
The concerns regarding increased instability of natural phospholipids are eliminated by the demonstrated broad use of this class of phospholipids in many products for any route of administration in marketed products for many years.
Contamination risks by traces of proteins, etc. of natural phospholipids are because of the applied extraction solvents and chromatography procedures virtually eliminated.
This selection/preference sequence is of particular interest in technology areas, like liposomes, wherein all classes can be considered. Table 17 shows exemplary some synthetic phospholipids and their natural alternatives, which are more suitable for scale-up.
Table 17.
Natural phospholipids as alternatives to synthetic phospholipids
| Synthetic phospholipid | Alternative natural phospholipid | Main components of alternative phospholipid |
|---|---|---|
| POPC | E PC | POPC 38%, PLPC 26%, SLPC 13%, SOPC 11% |
| POPG (Na) | E PG (Na) | POPG 38%, PLPG 26%, SLPG 13%, SOPG 11% |
| DSPC | HSPC | DSPC 85%, PSPC 15% |
| DSPG (Na) | HSPG (Na) | DSPG 85%, PSPG 15% |
As illustrated in Table 17, alternative natural phospholipids, which have the same head group purity and a similar fatty acid composition may be considered as alternative to synthetic phospholipids. HSPC is preferred compared to DSPC because it is easier to hydrogenate soybean PC than to produce the lipid synthetically. Likewise, it is easier to manufacture hydrogenated soybean phosphatidylglycerol (HSPG) from hydrogenated soy bean PC followed by phospholipase D treatment to convert HSPC to HSPG, than to produce it synthetically.
Since, the fatty acid composition of the alternative lipids is not exactly the same, the liquid crystalline to gel state phase temperature will not be identical. Production processes at which this may play a role need to be rechecked, when the synthetic phospholipid shall be replaced. As mostly mixtures of phospholipids and other ingredients (e.g., cholesterol, which suppresses the phase transition) are used, this should be negligible.
These details point to the importance of selecting the most suitable phospholipid at the beginning of a dosage form development process. In addition to selection of alternative phospholipids based on similarity of chemical composition, alternative natural phospholipids may be selected when considering their functionality and role in the formulation.
For instance, emulsions are known to be more stable when using mixtures of emulsifiers compared to mono-component emulsifier. Purified egg or soybean phospholipids consist of well-defined mixtures of phospholipids, which possess better emulsifier properties for oil-in-water emulsion (see above). For these reasons, they offer an even better alternative to, e.g., POPC. A further example to demonstrate the beneficial alternative use of natural phospholipids is the demonstration that, as part of the design and selection of the phospholipid composition of Doxil liposomes, HSPC could be used instead of DPPC or DSPC to make plasma stable liposomes 86.
6 Conclusions
Natural phospholipids are derived from renewable sources and produced with more ecologically friendly processes and are available in larger scale at relatively low costs compared to synthetic phospholipids. They comply with all requirements of the regulatory authorities and are safe in use for any administration route and any dosage form. Synthetic phospholipids contain chemically specific, defined polar head groups and fatty acids but are synthesized with various chemicals and solvents. Dependent on the synthesis route selected, they may contain intermediates or by-products and unnatural enantiomers may be formed. Synthetic phospholipids are only available in relatively small amounts at high prices.
In the overall phospholipid excipient market, synthetic phospholipids play, compared to natural phospholipid (including hydrogenated and enzyme modified phospholipids), from number of drug products comprising these phospholipids, a minor role. Their use is restricted to some pharmaceutical products. If the use of synthetic phospholipids is unavoidable, synthetic phospholipids using the synthesis route starting from GPC, obtained from natural PC, should be used. At this way, the natural stereochemical configuration is guaranteed and the extra production steps compared to natural phospholipids can be performed with minimal additional usage of solvents and chemicals.
In order to avoid scale up problems during pharmaceutical development and production, natural phospholipid excipients instead of synthetic phospholipids should be selected for formulations whenever possible.
Acknowledgments
The authors thank Lipoid GmbH, Ludwigshafen, Germany, a world-wide supplier of natural as well as synthetic phospholipids, for their kind support in supplying supportive literature data.
The authors have declared no conflict of interest.
Glossary
- BSE
bovine spongiform encephalopathy
- CP
Chinese pharmacopeia
- DEPC
1,2-dierucoylphosphatidylcholine
- DMPC
1,2-dimyristoylphosphatidylcholine
- DOPC
1,2-dioleoylphosphatidylcholine
- DOPS
1,2-dioleoylphosphatidylserine
- DPPC
1,2-dipalmitoylphosphatidylcholine
- DSPC
1,2-distearoylphosphatidylcholine
- DSPG
1,2-distearoylphosphatidylglycerol
- EC
European commission
- ELSD
evaporative light scattering detector
- EMA
European Medicines Agency
- E PC
egg phosphatidylcholine
- E PG
egg phosphatidylglycerol
- FDA
Food and Drug Administration
- GPC
glycerophosphocholine
- HSPC
hydrogenated soybean phosphatidylcholine
- HSPG
hydrogenated soybean phosphatidylglycerol
- ICH
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- LOD
limit of detection
- JPE
Japanese Pharmaceutical Excipients
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- MCT
medium chain triglycerides
- MPEG-DSPE
N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoylphosphatidylethanolamine sodium salt
- MTP-PE
muramyltripeptide-phosphatidylethanolamine
- NF
National Formulary
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PLPC
1-palmitoyl-2-linoleoylphosphatidylcholine
- PLPG
1-palmitoyl-2-linoleoylphosphatidylglycerol
- POPC
1-palmitoyl-2-oleoylphosphatidylcholine
- POPG
1-palmitoyl-2-oleoylphosphatidylglycerol
- PPC
polyenylphosphatidylcholine
- PSPC
1-palmitoyl-2-stearoylphosphatidylcholine
- PSPG
1-palmitoyl-2-stearoylphosphatidylglycerol
- SLPC
1-stearoyl-2-linoleoylphosphatidylcholine
- SLPG
1-stearoyl-2-linoleoylphosphatidylglycerol
- SM
sphingomyelin
- SOPC
1-stearoyl-2-oleoyl phosphatidylcholine
- SOPG
1-stearoyl-2-oleoyl phosphatidylglycerol
- TSE
transmissible spongiform encephalopathy
- USP
United States Pharmacopeia
References
- 1.Shurtleff W, Aoyagi A. 2007. History of Soy Lecithin http://www.soyinfocenter.com/HSS/lecithin1.php.
- 2.Pepeu G, Vannucchi MG, Di Patre PL. In: Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations [Proceedings of the 5th International Colloquium on Lecithin; Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations, Held April 10–12, 1989, in Cannes, France] Hanin I, Pepeu G, editors. New York [u. a.]: Plenum Press; 1990. pp. 43–50. [Google Scholar]
- 3.Vertzoni M, Markopoulos C, Symillides M, Goumas C, et al. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across Caco-2 cell monolayers. Mol. Pharm. 2012;9:1189–1198. doi: 10.1021/mp200479f. [DOI] [PubMed] [Google Scholar]
- 4.Hanin I, Pepeu G. Phospholipids Biochemical, Pharmaceutical, and Analytical Considerations: [Proceedings of the Fifth International Colloquium on Lecithin; Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations, held April 10–12, 1989, in Cannes, France] New York [u.a.]: Plenum Press; 1990. [Google Scholar]
- 5.Merolli A, Santin M. Role of phosphatidyl-serine in bone repair and its technological exploitation. Molecules. 2009;14:5367–5381. doi: 10.3390/molecules14125367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devitt A, Pierce S, Oldreive C, Shingler WH, Gregory CD. CD14-dependent clearance of apoptotic cells by human macrophages: The role of phosphatidylserine. Cell Death Differ. 2003;10:371–382. doi: 10.1038/sj.cdd.4401168. [DOI] [PubMed] [Google Scholar]
- 7.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003;42:423–438. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 8.Shimojo T, Abe M, Ota M. A method for determination of saturated phosphatidylcholine. J. Lipid Res. 1974;15:525–527. [PubMed] [Google Scholar]
- 9.McFerron W. Bloomberg L.P. 2013 Global soybean production seen bigger than expected by oil world. [Google Scholar]
- 10.Bundschuh R, Heinze C. 2013. pp. 246–274. Agrarmärkte 2013, Landesanstalt für Entwicklung der Landwirtschaft und der ländlichen Räume (LEL), Bayrische Landesanstalt für Landwirtschaft (LfL), Schwäbisch Gmünd.
- 11. Data kindly provided by LIPOID GmbH.
- 12.Wendel A. In: Kirk-Othmer Encyclopedia of Chemical Technology. Kirk RE, Othmer DF, editors. New York, Chichester, Weinheim, Brisbane, Singapur, Toronto: John Wiley & Sons, Inc; 1995. pp. 191–210. [Google Scholar]
- 13.Quinkert R-O, Setzer C. 2010. Soybean Lecithins and Phospholipids for Pharmaceutical Applications, Phospholipid Forschungszentrum e. V./Research Cente.
- 14.2008. Egg Lecithins for Pharmaceutical Application, Phospholipid Forschungszentrum e. V./Research Center.
- 15.Senior J, Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: The effect of the phospholipid and cholesterol components. Life Sci. 1982;30:2123–2136. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 16.Pryde EH. In: Lecithins. Szuhaj BF, List GR, editors. Champaign: American Oil Chemists' Society; 1985. pp. 213–246. [Google Scholar]
- 17.European Pharmacopoeia. (Ph. Eur.) 8. 0: 2. 5. 4 Iodine value (reference 01/2008 20504)
- 18.Lekim D. Soya Lecithin: Nutritional and Clinical Aspects: Proceedings of the First International Colloquium Held in Rome, Italy, Nov. 22, 1980. 1981:21–33. The resorption of lecithin administered orally and its physiologic implications, in: Cairella, M., Lekim, D. (Eds.), Societa Editrice Universo, Rome. [Google Scholar]
- 19.Xu X, Vikbjerg AF, Guo Z, Zhang L, Acharya AK. In: Phospholipid Technology and Applications. Gunstone FD, editor. Bridgewater: Oily Press; 2008. pp. 41–82. [Google Scholar]
- 20.Bornscheuer UT, Fettwissenschaft DGF. Enzymes in Lipid Modification. Weinheim: Wiley-VCH; 2000. [Google Scholar]
- 21.Bittmann R. In: Phospholipids Handbook. Cevc G, editor. New York: Marcel Dekker, Inc; 1993. pp. 141–232. [Google Scholar]
- 22.Ghyczy M. In: Lecithins Sources, Manufacture & Uses. Szuhaj BF, editor. Champaign, IL: The American Oil Chemists' Soc; 1989. pp. 131–144. [Google Scholar]
- 23.Paltauf F, Hermetter A. In: Phospholipids Biochemical, Pharmaceutical, and Analytical Considerations: [Proceedings of the Fifth International Colloquium on Lecithin; Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations, held April 10–12, 1989, in Cannes, France] Pepeu G, editor; Hanin I, editor. New York [u.a.]: Plenum Press; 1990. pp. 1–12. [Google Scholar]
- 24.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald RC, Rakhmanova VA, Choi KL, Rosenzweig HS, Lahiri MK. O-Ethylphosphatidylcholine: A metabolizable cationic phospholipid which is a serum-compatible DNA transfection agent. J. Pharm. Sci. 1999;88:896–904. doi: 10.1021/js990006q. [DOI] [PubMed] [Google Scholar]
- 26.Lux H, Heise N, Klenner T, Hart D, Opperdoes FR. Ether–lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether–lipid analogues in Leishmania. Mol. Biochem. Parasitol. 2000;111:1–14. doi: 10.1016/s0166-6851(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 27.Schumann G, Van Hoogevest P, Fankhauser P, Probst A, et al. Liposomes in the therapy of infectious diseases and cancer: Proceedings of a Ciba-Geigy-Squibb-UCLA Colloquium, held at Lake Tahoe, California, February 16-20, 1988. 1989:191. Comparison of free and liposomal MTP-PE: Pharmacological and toxicological aspects, in:, Lopez-Berestein, G., Fidler, I. J. (Eds.), CIBA-GEIGY Corporation, E.R. Squibb & Sons, University of California, A.R. Liss, New York. [Google Scholar]
- 28.Eibl H. Phospholipids as functional constituents of biomembranes. Angew. Chem. Int. Ed. Engl. 1984;23:257–271. [Google Scholar]
- 29.Ohno M, Fujita K, Nakai H, Kobayashi S, et al. An enantioselective synthesis of platelet-activating factors, their enantiomers, and their analogues from d- and l-tartaric acids. Chem. Pharm. Bull. 1985;33:572–582. doi: 10.1248/cpb.33.572. [DOI] [PubMed] [Google Scholar]
- 30.Ali S, Bittman R. Mixed-chain phosphatidylcholine analogues modified in the choline moiety: Preparation of isomerically pure phospholipids with bulky head groups and one acyl chain twice as long as the other. Chem. Phys. Lipids. 1989;50:11–21. doi: 10.1016/0009-3084(89)90022-4. [DOI] [PubMed] [Google Scholar]
- 31.Mangroo D, Gerber GE. Phospholipid synthesis: Effects of solvents and catalysts on acylation. Chem. Phys. Lipids. 1988;48:99–108. [Google Scholar]
- 32.Mason JT, Broccoli AV, Huang C-H. A method for the synthesis of isomerically pure saturated mixed-chain phosphatidylcholines. Anal. Biochem. 1981;113:96–101. doi: 10.1016/0003-2697(81)90049-x. [DOI] [PubMed] [Google Scholar]
- 33.Virtanen JA, Brotherus JR, Renkonen O, Kates M. Synthesis of monoacid 2,3-diacyl-sn-glycerols via 1,6-ditrityl-d-mannitol. Chem. Phys. Lipids. 1980;27:185–190. [Google Scholar]
- 34.Peters U, Bankova W, Welzel P. Platelet activating factor synthetic studies. Tetrahedron. 1987;43:3803–3816. [Google Scholar]
- 35. Synthetic Phospholipids and Other Synthetic Lipids, Corden Pharma Switzerland LLC.
- 36.Crans DC, Whitesides GM. Glycerol kinase: synthesis of dihydroxyacetone phosphate, sn-glycerol-3-phosphate, and chiral analogs. J. Am. Chem. Soc. 1985;107:7019–7027. [Google Scholar]
- 37.Hermetter A, Paltauf F. A facile procedure for the synthesis of saturated phosphatidylcholines. Chem. Phys. Lipids. 1981;28:111–115. [Google Scholar]
- 38.Warner TG, Benson AA. An improved method for the preparation of unsaturated phosphatidylcholines: Acylation of sn-glycero-3-phosphorylcholine in the presence of sodium methylsulfinylmethide. J. Lipid Res. 1977;18:548–552. [PubMed] [Google Scholar]
- 39.Fricker G, Kromp T, Wendel A, Blume A, et al. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010;27:1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 40.Van Hoogevest P, Liu X, Fahr A, Leigh MLS. Role of phospholipids in the oral and parenteral delivery of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2011;21:5–133. [Google Scholar]
- 41. Inactive, Ingredient Search for Approved Drug Products, U.S. Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/iig/index.Cfm.
- 42. Abelcet, RxList Inc. http://www.rxlist.com/abelcet-drug.htm.
- 43. AmBisome® (amphotericin B) liposome for injection, Astellas Pharma US, Inc. http://www.ambisome.com.
- 44. Daunoxome (Daunorubicin Citrate) – Summary http://DrugLib.com http://www.druglib.com/druginfo/daunoxome/
- 45. Doxil, RxList, Inc. http://www.rxlist.com/doxil-drug.htm.
- 46. Assessment report for MEPACT (EMEA/CHMP/635781/2008), European Medicines Agency 2008.
- 47.Van Hoogevest P, Fankhauser P. Liposomes in the therapy of infectious diseases and cancer: Proceedings of a Ciba-Geigy-Squibb-UCLA Colloquium, held at Lake Tahoe, California, February 16–20, 1988. 1989:453. An industrial liposomal dosage form for muramyltripeptidephosphatidylethanolamine (MTP-PE), in:, Lopez-Berestein, G. Fidler, I. J. (Eds.), CIBA-GEIGY Corporation, E. R. Squibb & Sons, University of California, A. R. Liss, New York. [Google Scholar]
- 48.Martin F. Liposome Drug Products – Product Evolution and Influence of Formulation on Pharmaceutical Properties and Pharmacology, U.S. Food and Drug Administration.
- 49.Myocet 50 mg Pulver, Dispersion und Lösungsmittel für ein Konzentrat zur Herstellung einer Infusionsdispersion TEVA Pharma B.V. 2013. http://www.pharmazie.com/graphic/A/50/0-90350.pdf.
- 50. Visudyne® Verteporfin for Injection, Valeant Pharmaceuticals North America LLC. http://www.visudyne.com/
- 51.Regulon Group. http://www.regulon.com.
- 52.Desai NR, Agha BJ, Kale KM. 2000. US6074666.
- 53.Exparel, RxList, Inc. http://www.rxlist.com/exparel-drug.htm.
- 54.DepoDur, RxList, Inc. http://www.rxlist.com/depodur-drug.htm.
- 55.DepoCyt, RxList, Inc. http://www.rxlist.com/depocyt-drug.htm.
- 56.Gambling D, Hughes T, Martin G, Horton W, et al. A comparison of Depodur™, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth. Analg. 2005;100:1065–1074. doi: 10.1213/01.ANE.0000145009.03574.78. 1010.1213/1001.A NE.0000145009.0000103574.0000145078. [DOI] [PubMed] [Google Scholar]
- 57.Cada DJ, Levien T, Baker DE. Morphine Sulfate Extended-Release Liposome Injection. St. Louis, MO: Thomas Land; 2004. , ETATS-UNIS. [Google Scholar]
- 58.Driscoll D. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006;23:1959–1969. doi: 10.1007/s11095-006-9092-4. [DOI] [PubMed] [Google Scholar]
- 59. Intralipid, http://Drugs.com http://www.drugs.com/pro/intralipid.html.
- 60. Intralipid 10%, RxList, Inc. http://www.rxlist.com/intralipid-10-drug/indications-dosage.htm.
- 61. Diazemuls Emulsion for Injection 5 mg/ml (Diazepam), Actavis Nordic A/S, Actavis Group PTC 2013.
- 62.Shah P, Bhalodia D, Shelat P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2010;1:24–32. [Google Scholar]
- 63.Cada D, Levien T, Baker D. Formulary drug reviews – Clevidipine butyrate injectable emulsion. Hosp. Pharm. 2008;43:903–912. [Google Scholar]
- 64.Hammad MA, Müller BW. Increasing drug solubility by means of bile salt–phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998;46:361–367. doi: 10.1016/s0939-6411(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 65.Gundermann K-J, Schneider E. Information Brochure. 1990 LipoStabil in: Rhône-Poulenc Rorer/Nattermann International GmbH. [Google Scholar]
- 66.Gregory TJ, Steinberg KP, Spragg R, Gadek JE, et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997;155:1309–1315. doi: 10.1164/ajrccm.155.4.9105072. [DOI] [PubMed] [Google Scholar]
- 67.Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J. Aerosol Med. Pulm. Drug Deliv. 2011;24:175–182. doi: 10.1089/jamp.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.VanDevanter DR, Geller DE. Tobramycin administered by the TOBI((R)) Podhaler((R)) for persons with cystic fibrosis: A review. Med. Devices (Auckl) 2011;4:179–188. doi: 10.2147/MDER.S16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.1974. p. 234. WHO Food Additives Series No. 5, World Health Organization.
- 70. Commission Regulation (EU) No 231/2012 of March 9, 2012, laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council European Commission, 2012.
- 71. Annex I of European Parliament and Council Directive No 95/2/EC of February 20, 1995, on food additives other than colours and sweeteners, European Parliament and Council 1995.
- 72. Report from the Commission on Dietary Food Additive Intake in the European Union European Commission, http://ec.europa.eu/food/fs/sfp/addit_flavor/flav15_en.pdf, 2001.
- 73. 21CFR184.1400 revised as of April 1, 2013, U. S. Food and Drug Administration.
- 74. 21CFR184.1063 August 1996, U. S. Food and Drug Administration.
- 75. 21CFR184.1063 revised as of April 1, 2013, U. S. Food and Drug Administration.
- 76.Edgren B, Hallberg D, Hakansson I, Meng HC, Wretlind A. Long-term tolerance study of two fat emulsions for intravenous nutrition in dogs. Am. J. Clin. Nutr. 1964;14:28–236. doi: 10.1093/ajcn/14.1.28. [DOI] [PubMed] [Google Scholar]
- 77.Le VHH, Giordano P, Spletzer J. The mechanism of removal of intravenously injected fat: Its relationship to toxicity. Arch. Surg. 1961;83:311–321. doi: 10.1001/archsurg.1961.01300140153029. [DOI] [PubMed] [Google Scholar]
- 78.Schuberth OWA. Intravenous Infusion of Fat Emulsions, Phosphatides and Emulsifying Agents: Clinical and Experimental Studies. 1961 (tr.: P.A. Norstedt), Stockh. [Google Scholar]
- 79.Mueller JF, Iacono J. In: Fette in der Medizin. Henning N, Berg G, editors. München: las Verlag; 1967. pp. 7–10. [Google Scholar]
- 80.2002. Draft Guidance for Industry: Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER),
- 81.2013. Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed With Reference to an Innovator Liposomal Product (EMA/CHMP/806058/2009/Rev. 02), European Medicines Agency,
- 82. European Pharmacopoeia 7.0 general text 5.1.7 Viral Safety.
- 83. Commission Regulation (EC) No 1881/2006 of December 19, 2006, setting maximum levels for certain contaminants in foodstuffs, European Commission 2006.
- 84.Walts AE, Schena DG, Fox EM, Davis JT, Mischke MR. Applications of biocatalysts in the synthesis of phospholipids. Chim. Oggi. 1991;9:29–33. [Google Scholar]
- 85.Walts AE, Schena DG, Fox EM, Davis JT, Mischke MR. In: Chirality in industry: the commercial manufacture and applications of optically active compounds. Collins AN, Sheldrake GN, Crosby J, editors. Chichester [u.a.]: Wiley; 1992. pp. 223–235. [Google Scholar]
- 86.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta (BBA) – Biomembr. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]


