Abstract
OBJECTIVE
The hyperbolic relationship between insulin secretion and sensitivity has been used to assess in vivo β-cell function (i.e., the disposition index). The disposition index emphasizes the importance of taking into account both skeletal muscle and hepatic insulin resistance to depict insulin secretion. However, we propose that adipose tissue insulin resistance also needs to be accounted for when characterizing glucose-stimulated insulin secretion (GSIS) because elevated plasma free fatty acids (FFAs) impair β-cell function.
RESEARCH DESIGN AND METHODS
To characterize the adipose disposition index, we used [1-14C] palmitate infusion to determine basal FFA turnover rate/adipose insulin resistance and an oral glucose tolerance test to characterize the first (i.e., 0–30 min) and second phase (i.e., 60–120 min) of GSIS. We validated a simplified version of the tracer infusion calculation as the product of (1/plasma FFA concentration × plasma insulin concentration) × GSIS in 44 obese insulin-resistant subjects.
RESULTS
The plasma FFA and palmitate tracer infusion calculations of the first- and second-phase disposition index were strongly correlated (r = 0.86, P < 0.000001 and r = 0.89, P < 0.000001, respectively). The first- and second-phase adipose disposition index derived from plasma FFA also was tightly associated with fasting hyperglycemia (r = −0.87, P < 0.00001 and r = −0.89, P < 0.00001, respectively) and 2-h glucose concentrations (r = −0.86, P < 0.00001 and r = −0.90, P < 0.00001).
CONCLUSIONS
Adjusting GSIS for adipose insulin resistance provides an index of β-cell function in obese subjects across the glucose spectrum. Plasma FFA–derived calculations of β-cell function may provide additional insight into the role of adipose tissue in glucose regulation.
Introduction
Prevention of β-cell dysfunction is critical for avoiding the development and progression of type 2 diabetes (1). Assessing in vivo insulin secretion is complex because the release of insulin from the β-cell is influenced by multiple factors including systemic insulin sensitivity, hepatic insulin extraction, plasma free fatty acid (FFA) concentrations, and glucolipid toxicity (2–4). An important aspect of the regulation of insulin secretion in healthy adults with normal glucose tolerance is that a reduction in insulin sensitivity is generally accompanied by a compensatory increase in insulin secretion (5,6). This relationship typically is curvilinear and has been referred to as the disposition index (5).
The disposition index was originally calculated from insulin sensitivity and acute insulin response during the intravenous glucose tolerance test (IVGTT) (5,6). Insulin sensitivity measured with the IVGTT is an aggregate of skeletal muscle, hepatic, and adipose insulin sensitivity. When the disposition index is calculated using the hyperinsulinemic-euglycemic clamp technique with supraphysiological insulin infusion rates (40–80 mU · m−2 · min−1), hepatic glucose production and lipolysis are almost completely suppressed, and 80–90% of glucose is disposed in skeletal muscle (7). Therefore, when using the euglycemic insulin clamp to calculate the disposition index, two physiologically relevant tissues and two physiologically relevant processes, that is, hepatic insulin sensitivity (2) and adipose insulin sensitivity (8), are not taken into account. Adipose tissue should be considered in the interaction of insulin secretion and insulin action because fat tissue secretes factors (e.g., FFAs and adipokines) that contribute to multiorgan insulin resistance (8). These factors may contribute not only to skeletal muscle and hepatic insulin resistance but also to impaired β-cell function in insulin-resistant states, including impaired fasting glucose, impaired glucose tolerance, type 2 diabetes mellitus, and obesity (9,10). Because the fasting plasma FFA concentration is influenced by a feedback loop between adipose tissue and the pancreas (11), a valid estimate of adipose insulin resistance can be obtained from the product of the basal rate of FFA turnover and the fasting insulin concentration (12,13). Relating adipose insulin resistance with glucose-stimulated insulin secretion (GSIS) could yield a novel index of β-cell function. In this study we examine the physiologic relevance of the adipose disposition index as it relates to blood glucose.
Research Design and Methods
Subjects
Forty-four obese individuals (Table 1) who had a stable weight (<2-kg change over the previous 6 months), were sedentary (<90 min/week), and were free of chronic disease participated in the study (14,15). Women were postmenopausal and were not receiving hormone replacement therapy. Subjects who were being treated with insulin or thiazolidinediones were excluded. Subjects were not taking any medications known to influence glucose or insulin metabolism. The diagnosis of type 2 diabetes was based on a standard 75-g oral glucose tolerance test (OGTT): fasting plasma glucose >126 mg/dL and/or 2-h glucose >200 mg/dL. Subjects were in otherwise good health without evidence of renal, hepatic, cardiovascular, or hematological disease as determined by history, physical examination, screening blood chemistries, urinalysis, and electrocardiography. The study protocol was approved by the institutional review board of the University of Texas Health Science Center at San Antonio and the Cleveland Clinic. Subjects gave voluntary written informed consent before their participation.
Table 1.
Demographics, cardiometabolic risk, and basal rate of palmitate appearance, fasting plasma FFA concentration, and adipocyte insulin resistance disposition index details for nondiabetic and type 2 diabetic subjects
| Nondiabetic subjects (n = 11) | Type 2 diabetic subjects (n = 33) | |
|---|---|---|
| Demographics | ||
| Patients (n) | ||
| Male | 3 | 15 |
| Female | 8 | 18 |
| Age (years) | 65.5 ± 1.4 | 57.1 ± 1.5* |
| Weight (kg) | 94.0 ± 5.4 | 86.4 ± 2.6 |
| BMI (kg/m2) | 33.8 ± 2.0 | 30.5 ± 0.7* |
| Fat mass (kg) | 36.3 (30.3, 44.5) | 33.4 (25.9, 40.9) |
| Fat free mass (kg) | 54.5 ± 2.4 | 52.5 ± 1.6 |
| Cardiometabolic outcomes | ||
| HbA1c (%) | — | 8.5 ± 0.2 |
| HbA1c (mmol/mol) | — | 70.1 ± 3.2 |
| Fasting plasma glucose (mg/dL) | 107 ± 3 | 181 ± 8* |
| Fasting plasma insulin (μU/mL) | 17.3 (10.7, 24.4) | 14.3 (10.4, 19.7) |
| 2-h glucose (mg/dL) | 156 ± 7 | 321 ± 12* |
| 2-h insulin (μU/mL) | 105.3 (62.6, 119.3) | 35.6 (24.3, 60.4)* |
| Matsuda Index | 1.9 (1.3, 3.2) | 1.9 (1.5, 2.5) |
| HOMA-IR (glucose × INS) | 4.8 (2.8, 6.2) | 6.6 (4.3, 9.3)* |
| Triglyceride (mg/dL) | 164 ± 16 | 159 ± 11 |
| Cholesterol (mg/dL) | ||
| Total | 192 ± 8 | 180 ± 5 |
| LDL | 129 ± 7 | 112 ± 4 |
| HDL | 38 (33.0, 42.0) | 34 (30.0, 43.0) |
| Adipose outcomes | ||
| Fasting FFA (mEq/L) | 0.69 ± 0.06 | 0.72 ± 0.03 |
| Fasting palmitate Ra (mg/kg FFM/min) | 97.3 (51.4–133.9) | 161.4 (116.1, 237.0)* |
| GSIS | ||
| First phase (0–30 min) | 0.27 (0.18, 0.46) | 0.08 (0.06, 0.22)* |
| Second phase (60–120 min) | 0.57 (0.31, 0.74) | 0.09 (0.06, 0.23)* |
| Adipose IR index | ||
| FFA × INS | 11.4 (6.3, 17.3) | 12.3 (6.3, 14.9) |
| 14C palmitate Ra × INS | 1,595 (650, 3,243) | 2,725 (1,322, 4,324)* |
| First-phase DI adipose plasma† | ||
| 0–30 min | 0.03 (0.01, 0.03) | 0.008 (0.007, 0.01)* |
| 60–120 min | 0.05 (0.04, 0.05) | 0.01 (0.006, 0.01)* |
| Second-phase DI adipose 14C palmitate*† | ||
| 0–30 min | 0.1 (0.1, 0.2) | 0.03 (0.02, 0.04)* |
| 60–120 min | 0.2 (0.2, 0.4) | 0.03 (0.02, 0.05)* |
Normally distributed data are mean ± SEM. Nonnormally distributed data are presented as median (interquartile range). INS, insulin; Ra, rate of appearance. *Different between nondiabetic vs. type 2 diabetic subjects. †Disposition index (DI) was calculated as: GSIS × 1/adipose insulin resistance (plasma FFA and 14C palmitate Ra), where GSIS is INS/glucose area under the curve. DI calculations for 14C palmitate Ra were multiplied here by 1,000 for data presentation of first- and second-phase GSIS.
Body Composition
Height was measured without shoes using a wall-mounted stadiometer, and weight was recorded on a digital platform scale with minimal clothing. Total body fat and fat-free mass were measured by underwater weighing or use of 3H-water radioactivity, as previously described (14,15).
Control Period
Before metabolic testing, subjects were instructed to avoid strenuous physical activity for 24 h. All subjects also were instructed to consume weight-maintenance meals (∼55% carbohydrates, 30% fat, and 15% protein).
Palmitate Turnover
Palmitate turnover, an index of lipolysis, was assessed with an isotopically labeled palmitate infusion. After an overnight fast, a polyethylene catheter was inserted into an antecubital vein for the infusion of [1-14C] palmitate. A second catheter was inserted retrogradely into the distal portion of a vein on the contralateral hand for blood sampling. The hand was kept in a box warmed to ∼60°C for the duration of the study to collect arterialized blood. A prime (2.5 uCi) and continuous (0.1 uCi/min) infusion of 1-14C palmitate was prepared as previously described (14,15) and started at 8 a.m. Briefly, [1-14C] palmitate was bound to human serum albumin and infused for the duration of the study. During the last 30 min of tracer equilibration, plasma samples were obtained at −30, −20, −10, −5, and 0 min for determination of plasma [1-14C] palmitate radioactivity and plasma FFA concentration. At 8 a.m. a bolus of 3.5 uCi of NaH14CO3 was given intravenously. Blood samples were placed on ice and immediately centrifuged and stored at −70°C until subsequent analysis. Adipose insulin resistance index (Adipose-IR) was calculated as the product of the fasting plasma FFA × fasting insulin and compared with the product of basal rate palmitate turnover × fasting plasma insulin, as previously described (12,13).
Insulin Secretion
At 8 a.m. on a different day, following a 10- to 12-h overnight fast, blood samples were collected from a superficial antecubital vein to determine fasting plasma glucose, triglyceride, FFA, total cholesterol, LDL, and HDL concentrations. HbA1c also was measured in patients with type 2 diabetes to further characterize glycemic control. Following collection of baseline samples, subjects orally consumed 75 g of glucose, and blood samples were obtained at 30, 60, 90, and 120 min to determine plasma glucose and insulin concentrations. Whole-body insulin action, which reflects the aggregate of skeletal muscle, hepatic, and adipose insulin sensitivity, was estimated using the Matsuda Index (16). The HOMA of insulin resistance also was calculated as a more reflective surrogate of hepatic insulin resistance (17,18). Glucose and insulin total area under the curve (AUC) during the OGTT were calculated using the trapezoidal method. First- and second-phase GSIS, that is, the insulinogenic index, was calculated during the first 30 min and last 60 min of the OGTT as insulin AUC divided by plasma glucose AUC (i.e., I0–30/G0–30 and I0–60–120/G60–120). The first- and second-phase adipose disposition indices were calculated as follows: I0–30/G0–30 × adipose plasma FFA IR and I60–120/G60–120 × adipose plasma FFA IR, respectively (19).
Biochemical Analysis
Plasma glucose was determined using the glucose oxidase method (YSI 2300 STAT Plus; YSI Life Sciences, Yellow Springs, OH). HbA1c was measured using affinity chromatography (Isolab, Akron, OH). Plasma insulin was measured using a radioimmunoassay (Millipore, Billerica, MA). Plasma triglyceride and cholesterol (total, HDL, and LDL) concentrations were analyzed using enzymatic methods with an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma FFA concentration was measured with a colorimetric assay (Wako Chemicals, Richmond, VA). Basal FFA turnover was determined by dividing the [1-14C] palmitate infusate rate by the plasma [1-14C] palmitate specific activity. Plasma FFA and specific activity were constant during the final 30 min of the tracer infusion.
Determining Hyperbolic Relationships
The hyperbolic relationship between insulin secretion and insulin sensitivity is characterized as insulin secretion = constant/insulin sensitivity. Using log transformation, this equation becomes: log(insulin secretion) = constant – log(insulin sensitivity). When viewed in this context, regression analysis can be applied to OGTT-derived indices of insulin secretion and insulin sensitivity to determine the regression coefficient β. As indicated by Kahn et al. (5), a hyperbolic relationship between a measure of insulin secretion and insulin sensitivity would be represented by β = −1. Thus a hyperbolic relationship can be satisfied if β is approximately equal to –1 and the 95% confidence interval of β excludes 0. If all assumptions with linear regression modeling, y = βx + E (where E is the sampling error associated with the dependent random variable y) are met, such that the variance of sampling error associated with the dependent random variable is constant; if we assume that neither x nor y has measurement error, then orthogonal least squares regression will give a more accurate linear unbiased estimate of β. However, if x or y is associated with random error, larger variances in either parameter will bias the regression coefficient and alter the slope (i.e., β). Further complicating these issues is the view that hyperglycemia amplifies measurement error (20). Because the dependent variable (insulin secretion) and independent variable (insulin sensitivity) are measured with error, use of ordinary least squares regression would yield both a biased coefficient (underestimate of β) and a biased standard error of coefficient estimate (overestimate of β) (5). Thus, as done by Kahn et al. (5), we used perpendicular least squares properly weighted regression, a method that accounts for the error in both independent and dependent variables to accurately predict β (21). We also calculated 95% confidence intervals to establish whether the hyperbolic criteria have been met. We used the bootstrap method to calculate the standard error of the coefficient of estimate of β, as described previously (22,23).
Statistical Analysis
Data were assessed using R (version 2.4.0; The R Foundation, Vienna, Austria). Nonnormally distributed data were log transformed for analysis. Independent two-tailed t tests were used to compare nondiabetic and type 2 diabetic cohorts. Pearson product moment correlation was used to examine the relationship between adipose disposition index and metabolic characteristics. Significance was accepted as P ≤ 0.05. Data are expressed as mean ± SEM or median (interquartile range), as appropriate.
Results
Metabolic Characteristics
Individuals with type 2 diabetes were slightly younger and had a lower BMI compared with nondiabetic subjects (P < 0.05; Table 1). Although whole-body insulin sensitivity was comparable between groups, type 2 diabetic subjects had higher hepatic and adipose insulin resistance compared with nondiabetic subjects (P < 0.05; Table 1). Adipose tissue insulin resistance using the plasma FFA concentration was strongly correlated with the [1-14C] palmitate–derived measures (r = 0.80; P < 0.00001), although this relationship was attenuated when plasma FFA and [1-14C] palmitate were analyzed without adjusting for insulin (r = 0.45; P < 0.003).
Insulin Secretion
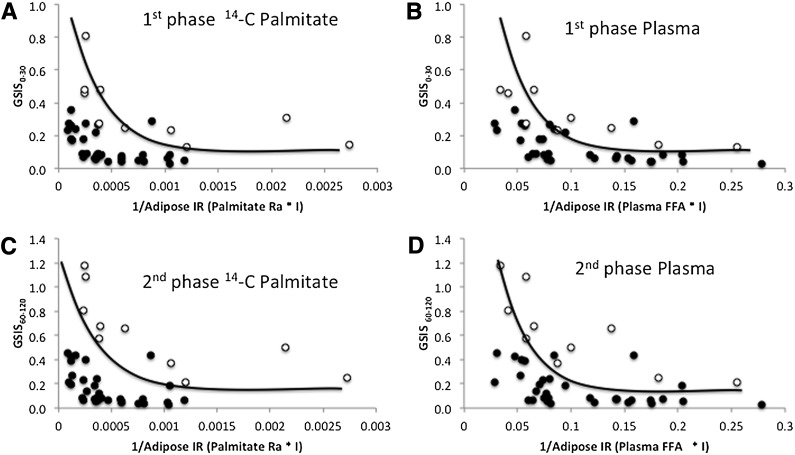
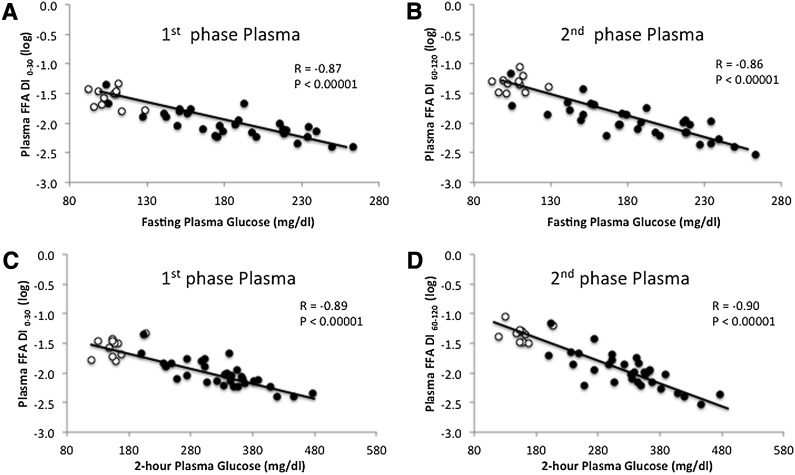
As expected, GSIS and the adipose disposition index were lower in type 2 diabetic compared with nondiabetic subjects (P < 0.05; Table 1). Both [1-14C] palmitate– and plasma FFA–derived adipose disposition indices (i.e., GSIS × adipose tissue insulin resistance) satisfied the hyperbolic relationship whether calculated using the first phase (0–30 min) or second phase (60–120 min) of GSIS in nondiabetic and type 2 diabetic subjects (Fig. 1 and Table 2). Following log transformation there was no difference in the slope of the disposition index between nondiabetic and type 2 diabetic subjects for plasma FFA (first phase: P = 0.73; second phase: P = 0.88) and [1-14C] palmitate (first phase: P = 0.93; second phase: P = 0.98), suggesting that both groups exhibited a similar curvilinear relationship. Importantly, in nondiabetic subjects the curve was shifted up and to the right compared with patients with type 2 diabetes for both first- and second-phase plasma FFA and [1-14C] palmitate calculations (P < 0.001; data not shown). First- and second-phase adipose tissue disposition index using plasma FFA concentration was strongly correlated with the first- and second-phase adipose tissue disposition index derived from [1-14C] palmitate (r = 0.86, P < 0.000001 and r = 0.89, P < 0.000001, respectively). A similar correlation between the plasma FFA–derived and [1-14C] palmitate–derived adipose disposition indices was observed in men (first phase: r = 0.80, P < 0.00001; second phase: r = 0.89, P < 0.000001) and women (first phase: r = 0.88, P < 0.00001; second phase: r = 0.90, P < 0.000001). Both first- and second-phase adipose disposition indices derived from plasma FFA concentrations were closely correlated with fasting (r = −0.87, P < 0.00001 and r = −0.89, P < 0.00001, respectively) and 2-h plasma glucose (OGTT) (r = −0.86, P < 0.00001 and r = −0.90, P < 0.00001; Fig. 2). The first- and second-phase adipose tissue disposition indices using plasma FFA concentration also was significantly correlated with age (r = 0.35, P < 0.03 and r = 0.45, P < 0.004, respectively) and BMI (r = 0.42, P < 0.007 and r = 0.39, P < 0.01, respectively).
Figure 1.
GSIS adjusted for adipose insulin resistance (IR) (adipose tissue disposition index). A: 14C palmitate–calculated adipose disposition index: first phase. B: Plasma FFA–calculated adipose disposition index: first phase. C: 14C palmitate–calculated adipose disposition index: second phase. D: Plasma FFA–calculated adipose disposition index: second phase. Open circles represent nondiabetic subjects. Closed circles represent diabetic subjects.
Table 2.
Estimated regression coefficients (β) and 95% CIs using orthogonal slope for β based on first- and second-phase GSIS and adipose insulin sensitivity
| Insulin secretion | Insulin sensitivity | β | 95% CI |
|---|---|---|---|
| Nondiabetic subjects | |||
| First-phase GSIS (0–30 min) | 1/Plasma FFA × INS | −0.81 | (−1.42, −0.20) |
| 1/Ra palmitate × INS | −0.88 | (−12.81, 11.06) | |
| Second-phase GSIS (60–120 min) | 1/Plasma FFA × INS | −0.85 | (−1.19, −0.50) |
| 1/Ra palmitate × INS | −0.87 | (−1.24, −0.50) | |
| Type 2 diabetic subjects | |||
| First-phase GSIS (0–30 min) | 1/Plasma FFA × INS | −1.47 | (−2.02, −0.92) |
| 1/Ra palmitate × INS | −0.96 | (−1.27, −0.65) | |
| Second-phase GSIS (60–120 min) | 1/Plasma FFA × INS | −1.93 | (−3.13, −0.73) |
| 1/Ra palmitate × INS | −1.21 | (−2.63, 0.21) |
No between-group difference in slope parameters was detected, suggesting diabetic and nondiabetic groups had similar curvilinear relationships between insulin secretion and sensitivity. INS, insulin; Ra, rate of palmitate appearance.
Figure 2.
Correlation between plasma FFA–derived calculation of the adipose tissue disposition index versus fasting and 2-h plasma glucose concentration (OGTT). Open circles represent nondiabetic subjects. Closed circles represent diabetic subjects.
Conclusions
Elevated fasting plasma FFA concentrations and impaired suppression of plasma FFA by insulin plays an important role in not only the development of insulin resistance (8,14,24) but also β-cell dysfunction (9,10). The major finding from this study is that the plasma FFA–derived adipose tissue disposition index, whether calculated using first- or second-phase GSIS, approximates the hyperbolic relationship (Fig. 1) that is purported to maintain normal glucose concentrations and correlates strongly with [1-14C] palmitate kinetic assessments. The variability in slope parameters detected in our study (Table 2) are comparable with those reported using whole-body estimates of insulin resistance (23) and is consistent with work by Ferrannini and Mari (25) describing the utility of the hyperbolic relationship when scaling GSIS to insulin resistance across the glucose tolerance spectrum. Therefore, based on our modest sample size of obese nondiabetic and diabetic subjects, adjusting GSIS for adipose insulin resistance seems to be a reasonable approach to understanding the integrated multitissue metabolic abnormalities associated with β-cell dysfunction in obesity- and diabetes-related glucose intolerance.
To maintain normoglycemia the pancreatic β-cell must compensate for multiorgan insulin resistance with an appropriate increase in insulin secretion. Our data are consistent with the view that the capacity of pancreatic β-cells to secrete insulin for the prevailing degree of insulin resistance declines as glucose intolerance worsens in obese subjects, that is, diabetic subjects have a downward, left-shifted insulin curve (20) (see Fig. 1). However, it should be acknowledged that, in vivo, the compensatory increase in pancreatic insulin secretion in response to insulin resistance may be unable to completely normalize circulating glucose concentrations. In Pima Indians and Caucasians, Stumvoll and colleagues (26) reported that the feedback between insulin resistance on pancreatic β-cell demand involves a signal from circulating glucose, such that when GSIS is divided by insulin resistance to depict the β-cell demand for maintaining blood glucose concentrations (as opposed to multiplying GSIS by insulin resistance), glycemia increases for a given decrease in insulin sensitivity despite normal compensation of insulin secretion (termed glucose allostasis). Thus GSIS compensation for insulin resistance is likely more complex than that provided by a single calculation of a whole-body insulin sensitivity–derived disposition index, and additional models of β-cell response are needed (25). Recent work suggested that alterations in hepatic disposition index may precede changes in peripheral disposition index in response to overfeeding and/or physical inactivity, suggesting that multiple organs influence compensatory insulin secretion (2,27,28). The novel calculation presented herein justifies the use of the adipose disposition index to characterize the contribution of adipose insulin resistance to GSIS in obese nondiabetic and obese diabetic subjects. Future work is needed to examine the role of skeletal muscle, hepatic, and adipose disposition indices following lifestyle modification and/or bariatric surgery to better understand the complexity of human glucose regulation. Ultimately, these findings may provide greater clinical insight for targeted treatment strategies that prevent/reverse type 2 diabetes.
There are important considerations of the current work that should be acknowledged. We recognize that using the plasma C-peptide concentration as a measure of prehepatic insulin secretion provides a more direct measure of GSIS and β-cell function, although work by our laboratory suggested that hepatic insulin extraction does not affect insulin-derived calculations of β-cell function before or after lifestyle modification in a cohort of obese insulin-resistant adults similar to that used in the current study (29). In addition, use of the IVGTT or hyperglycemic clamp may provide a more accurate assessment of insulin secretion compared with OGTT-derived measures (5,6). However, exclusion of the gastrointestinal tract limits the physiologic understanding of in vivo β-cell function. Further, circulating glycerol may represent a better biomarker for lipolysis because plasma FFA can be re-esterified and/or taken up for storage, thereby limiting FFA as a lipolytic indicator. Consistent with this notion, basal [1-14C] palmitate turnover was only modestly associated with plasma FFA in our study (r = 0.45; P < 0.003). Therefore, despite the observation that groups in our study exhibited similar fasting plasma FFA concentrations, the higher basal [1-14C] palmitate turnover in diabetic compared with nondiabetic subjects suggest differences in FFA metabolism (i.e., production vs. storage) were present. It is worth recognizing, however, that FFAs are implicated in the development of β-cell dysfunction, and FFAs are released from adipocytes, which make them a reasonable marker of lipolysis (9,10,27,30). Both fasting and 2-h plasma glucose concentrations were closely associated with adipose disposition index, and part of this finding could be related to estimation of the disposition index including plasma glucose. However, the possibility of autocorrelation is lessened by the fact that first-phase GSIS (0–30 min of insulin divided by glucose) adjusted for adipose insulin resistance (fasting FFA × fasting insulin) correlates strongly with 2-h plasma glucose. This time course difference in glucose and insulin pattern suggests that the adipose disposition index calculation has physiologic relevance in characterizing β-cell function across the glucose tolerance continuum (Fig. 2). The index also correlated with age and body mass, which further confirms that it has physiological relevance.
In conclusion, GSIS is essential for preventing the progression from normal glucose tolerance to type 2 diabetes (1,2,30). Our results suggest that adjusting GSIS for adipose insulin resistance provides a reasonable index of β-cell function in obese nondiabetic and obese diabetic subjects. Because a high-fat diet and lipid infusion in humans impair GSIS (27,31), characterization of in vivo pancreatic β-cell insulin secretion in relation to adipose tissue insulin resistance may provide additional mechanistic insight into the regulation of glucose homeostasis.
Article Information
Acknowledgments. The authors thank the nursing staff for technical assistance and the dedicated research assistants and subjects for their efforts.
Funding. This research was supported by National Institutes of Health grant RO1-AG-12834 (J.P.K.). S.K.M. was supported by National Institutes of Health grant T32-DK-007319. The majority of R.A.D.’s salary is supported by the Veterans Administration Health Care Service.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.K.M. developed the hypothesis for this study and performed statistical analysis. J.P.K., S.R.K., and Y.M. collected and organized data, performed analysis, and edited the manuscript. J.H. performed statistical analysis. R.A.D. and J.P.K. wrote and revised the manuscript. R.A.D. and J.P.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab 2011;96:2354–2366 [DOI] [PubMed] [Google Scholar]
- 2.Faerch K, Brøns C, Alibegovic AC, Vaag A. The disposition index: adjustment for peripheral vs. hepatic insulin sensitivity? J Physiol 2010;588:759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 4.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. β-Cell dysfunction is associated with metabolic syndrome severity in adults. Metab Syndr Relat Disord 2014;12:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 6.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007;30:89–94 [DOI] [PubMed] [Google Scholar]
- 8.Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:121–124 [DOI] [PubMed] [Google Scholar]
- 9.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 10.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- 11.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 13.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon TPJ, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab 2009;297:E552–E559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki Y, Glass L, Triplitt C, et al. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia 2001;44:2210–2219 [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 19.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 20.Krudys KM, Kahn SE, Vicini P. Population approaches to estimate minimal model indexes of insulin sensitivity and glucose effectiveness using full and reduced sampling schedules. Am J Physiol Endocrinol Metab 2006;291:E716–E723 [DOI] [PubMed] [Google Scholar]
- 21.Riggs DS, Guarnieri JA, Addelman S. Fitting straight lines when both variables are subject to error. Life Sci 1978;22:1305–1360 [DOI] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R. Statistical data analysis in the computer age. Science 1991;253:390–395 [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907 [DOI] [PubMed] [Google Scholar]
- 24.Haus JM, Solomon TPJ, Marchetti CM, Edmison JM, González F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 2010;95:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 2004;47:943–956 [DOI] [PubMed] [Google Scholar]
- 26.Stumvoll M, Tataranni PA, Stefan N, Vozarova B, Bogardus C. Glucose allostasis. Diabetes 2003;52:903–909 [DOI] [PubMed] [Google Scholar]
- 27.Brøns C, Jensen CB, Storgaard H, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 2009;587:2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alibegovic AC, Højbjerre L, Sonne MP, et al. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 2009;58:2749–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malin SK, Solomon TPJ, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic β-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab 2013;305:E1248–E1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon TPJ, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010;33:1561–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]