Abstract
OBJECTIVE
Glucagon delivery in closed-loop control of type 1 diabetes is effective in minimizing hypoglycemia. However, high insulin concentration lowers the hyperglycemic effect of glucagon, and small doses of glucagon in this setting are ineffective. There are no studies clearly defining the relationship between insulin levels, subcutaneous glucagon, and blood glucose.
RESEARCH DESIGN AND METHODS
Using a euglycemic clamp technique in 11 subjects with type 1 diabetes, we examined endogenous glucose production (EGP) of glucagon (25, 75, 125, and 175 μg) at three insulin infusion rates (0.016, 0.032, and 0.05 units/kg/h) in a randomized, crossover study. Infused 6,6-dideuterated glucose was measured every 10 min, and EGP was determined using a validated glucoregulatory model. Area under the curve (AUC) for glucose production was the primary outcome, estimated over 60 min.
RESULTS
At low insulin levels, EGP rose proportionately with glucagon dose, from 5 ± 68 to 112 ± 152 mg/kg (P = 0.038 linear trend), whereas at high levels, there was no increase in glucose output (19 ± 53 to 26 ± 38 mg/kg, P = NS). Peak glucagon serum levels and AUC correlated well with dose (r2 = 0.63, P < 0.001), as did insulin levels with insulin infusion rates (r2 = 0.59, P < 0.001).
CONCLUSIONS
EGP increases steeply with glucagon doses between 25 and 175 μg at lower insulin infusion rates. However, high insulin infusion rates prevent these doses of glucagon from significantly increasing glucose output and may reduce glucagon effectiveness in preventing hypoglycemia when used in the artificial pancreas.
Introduction
Dysfunctional Counter-regulation in Diabetes
Beginning soon after the discovery of insulin, the role of the counter-regulatory hormone glucagon has been studied in light of its impact on glycogenolysis and gluconeogenesis (1). Although β-cell dysfunction is known to be the hallmark of type 1 and type 2 diabetes (2,3), α-cell dysfunction in both of these conditions may also contribute to the diabetic condition (4). The occurrence of α-cell dysfunction in diabetes is primarily due to loss of intrinsic β-cell control: insulin's signaling is believed to be necessary for appropriate and timely release of glucagon during hypoglycemia, as well as for appropriate suppression of glucagon release during hyperglycemia (for example, after meals) (5). Conversely, isolated α-cell dysfunction has little effect on glucose regulation since normal β-cell function is sufficient to regulate glucose levels in the otherwise healthy individual (6). However, the administration of subcutaneous insulin, i.e., nonpulsatile, low portal-systemic ratio, does not sufficiently regulate glucagon release in people with diabetes (7). Additionally, subcutaneously delivered insulin has slow absorption and clearance profiles, even for fast-acting analogs (8). In patients who use insulin, hypoglycemia is accompanied by high insulin concentration at the time of low blood glucose, a nonphysiologic situation and a further cause of reduced glucagon effectiveness (7). For these reasons, endogenous glucagon release by the pancreas in people with diabetes cannot be relied upon in cases of imminent hypoglycemia after subcutaneous injections of insulin. Indeed, Lorenzi et al. (9) demonstrated in the 1980s that the glucagon response to hypoglycemia in people with type 1 diabetes was markedly reduced, especially in those with long-standing diabetes.
Glucagon in the Artificial Endocrine Pancreas
With one early exception, most artificial pancreas systems over the last half century have been single hormone delivery with insulin for the control of type 1 diabetes (10–17). A number of control strategies have been used, along with the incorporation of glucoregulatory models (18–20), in the attempt to better match glycemic control and subcutaneous insulin delivery. However, despite the introduction of fast-acting insulin analogs, the delay in absorption and action of subcutaneous insulin remains one of the greatest hurdles to overcome in single-hormone closed-loop systems (21,22). The recently renewed inclusion of glucagon into artificial pancreas systems has led to a reduced risk of hypoglycemia during closed-loop control (23–26). Small (microgram level) doses of glucagon have been shown by several research groups to minimize time spent in the hypoglycemic range (23,27). Yet a percentage of glucagon doses delivered are unsuccessful in preventing hypoglycemia, despite accounting for insulin on board (IOB) (27). Castle et al. (27) found that correcting glucagon dose based on IOB could account for ∼46% of failures within the hour after a glucagon dose is delivered (37% of failures without accounting for IOB vs. 20% of failures with IOB-adjusted dosing) (28). Factors such as sensor inaccuracy, glucagon degradation, glycogen depletion, and varying pharmacokinetic profiles have been offered as additional explanations (29), but the scarcity of data evaluating microgram doses of glucagon normally used in the setting of bihormonal closed-loop control leaves more questions than provides answers.
Quantifying the Glucagon Response
The glycemic response to doses of intravenous glucagon has been studied extensively since its discovery, both as single dose injections or constant infusions (1,30,31). Like insulin, subcutaneously delivered glucagon has a delayed onset of action compared with intravenous delivery. Unlike insulin, however, the physiological effect of subcutaneously delivered glucagon has received little attention until more recently, evident by the scarcity of integrated glucagon absorption and action models (32,33). As a result, this research study was designed with the intent to quantitatively analyze the glucose response to small doses of glucagon delivered subcutaneously at varying steady-state insulin levels. The primary goal was to elucidate the interaction between insulin and glucagon in order to model the response of endogenous glucose production (EGP) at doses used in the artificial pancreas system. A secondary goal was to determine if high levels of insulin indeed can be overcome by increasing the dose of glucagon.
Research Design and Methods
Subject Recruitment
Subjects were recruited from the Legacy Health Services outpatient clinics in Portland, OR, or from prior contact with our laboratory, between December 2011 and January 2013. Subjects were between 21 and 65 years of age with a diagnosis of type 1 diabetes for >12 months. Exclusionary conditions included pregnancy; ongoing cardiovascular, cerebrovascular, renal, or hepatic disease; any uncontrolled chronic medical condition; oral or parenteral corticosteroid use; immunosuppressant therapy; insulin or glucagon allergy; serum insulin antibody titer >100 µU/mL; or total insulin requirement >200 units/day. The research protocol was reviewed and approved by the Legacy Health Services institutional review board, and all subjects provided written informed consent. Sample size was chosen based on an estimated α error of 0.05, a power of 85%, to detect a 20% difference between groups, with an expected SD of 20% about the mean. Adverse events were monitored and reported by the principal investigator and coinvestigators.
Study Materials
Drugs included regular human insulin (Humulin R; Eli Lilly and Company) and octreotide (Sandostatin; Novartis) for intravenous infusion. Glucagon (GlucaGen) was provided by courtesy of Novo Nordisk. Di-deuterated glucose (6,6-2H2-glucose, 98 atom % D isotopic purity) was purchased through Sigma-Aldrich (St. Louis, MO) for stable isotope infusion. Insulin was infused intravenously at a constant rate for the 10 h of each study day, although at a different rate on each day. Octreotide was prepared as 2 mg in 1,000 mL of 0.9% NaCl, supplemented with 1,330 mg of deuterated glucose and delivered at 0.45 mL/kg/h for the first nine studies. However, after the occurrence of gastrointestinal side effects (loose stools or nausea), the octreotide rate was subsequently lowered to 0.25 mL/kg/h with a resultant increase in the deuterated glucose supplementation to 2,337 mg per liter of fluid, in order to maintain the same infusion rate of deuterated glucose. All protocol changes were instituted after obtaining institutional review board approval. Additionally, each liter of 10% dextrose was supplemented with 800 mg of deuterated glucose (0.8% enrichment) in order to minimize dilution effects from infused glucose (34).
Study Procedures
Each subject underwent three studies on three separate days, each study lasting ∼10 h. Subjects arrived between 7:00 and 8:00 a.m. on each day and were admitted to the Legacy Good Samaritan Hospital after having had breakfast at least 2 h before admission and having turned off their insulin pumps after their breakfast bolus. Subjects were fasted throughout the entire study to remove confounding from meals. On one arm, a double stop-cock system was attached. Arterialization of blood flow was accomplished by application of a heating pad to increase blood flow and allow for blood sampling up to every 5 min. On the other arm, insulin, octreotide, and 10% dextrose were infused. Insulin infusion was constant on each day, at one of three randomly assigned rates: 1) a minimum rate of either 0.01 units/kg/h or an average of the subject’s daytime basal rate, whichever was higher (designated “low”); 2) a rate of 0.05 units/kg/h (designated “high”); and 3) midway between the minimum and maximum rates (designated “medium”). These rates were determined at the time of screening. The lowest rate of insulin infusion was not set (for example at 0.01 units/kg/h) but rather was tailored to the subject’s usual basal infusion rate in order to achieve target glucose levels during the low insulin studies. An infusion rate less than their basal requirement would lead to prolonged hyperglycemia.
Insulin and octreotide infusions were begun once the infusion catheter was available in order to help achieve steady state quickly. Ten percent dextrose infusion was controlled by a proportional integral derivative algorithm design, based upon simulation analyses performed by Bequette (35), using a target glucose level of 85 ± 20 mg/dL. A 2-h run-in period was allowed for achievement of infusion steady states prior to the first dose of glucagon. Every 2 h after the run-in period, glucagon was delivered subcutaneously in a pseudo-random order based on blocks of four, in doses of 25, 75, 125, and 175 μg, varying the initial dose while keeping the same order. Each subject received the same glucagon dose order assigned during screening on each study day. Pseudo-randomization of glucagon doses resulted in the first dose as follows: 25 μg during eight studies, 75 μg during seven studies, 125 μg during nine studies, and 175 μg during five studies. Also, glucagon and insulin levels were drawn every 10 min during the first hour after each glucagon dose, and every 20 min during the second hour. From a total of 116 batches of glucagon samples (4 per study from 29 studies), contamination was noted in 17 batches, in which the glucagon levels exceeded the upper limit of the assay. The remaining 99 batches were used for this analysis, 24 after the 25- and 75-μg doses, 26 after the 125-μg dose, and 25 after the 175-μg dose. Glucose levels were checked every 10 min (every 5 min if the previous check was <60 mg/dL) for the duration of each study utilizing the HemoCue Hb 201 DM analyzer (Cypress, CA). Blood was drawn for measurement of di-deuterated glucose levels at the 0-min time point and every 10 min from the 60-min time point onwards.
Laboratory Testing
For analysis by gas chromatography–mass spectrometry, derivatization of glucose in blood samples was accomplished as follows (34):
A total of 20 µL of plasma was placed into a 13 × 100 mm disposable glass test tube, to which 50 µL of water and 0.5 mL of ice-cold ethanol were added.
The mixture was then vortex mixed before centrifugation at 3,000 rpm for 10 min, with transfer of the supernatant, using gel-loading tips, to a 13 × 100 culture tube, and then allowing evaporation to dryness in a centrifugal evaporator (∼1 h).
To the dry vial, 50 µL of MOX reagent (2% methoxyamine-HCl in pyridine; Thermo Fisher Scientific, Ashville, NC) was added, and the vial was capped with a teflon-lined screw cap and then heated for 2 h at 80°C in a dry block heater.
After cooling, 50 µL of bis(trimethylsilyl)trifluoroacetamide (BSTFA) plus 1% trimethylchlorosilane (TCS) (Regis Technologies, Inc., Morton Grove, IL) was added to the vial, which was left to stand overnight at room temperature.
Using a fume hood, excess solvent was allowed to evaporate using dry nitrogen (∼30 min), and finally 100 µL of a 10% mixture of BSTFA + 1% TCS in dry n-decane was added to the vials.
Di-deuterated glucose ratios were determined at Oregon Health & Science University (OHSU) using a Thermo Scientific DSQ II gas chromatography–mass spectrometry system (Ashville, NC) with standards of 1, 2, and 4% di-deuterated glucose formulated for estimation of glucose concentrations. Insulin and glucagon levels in serum were determined by ELISA (Mercodia, Winston-Salem, NC) and radioimmunoassay techniques (Millipore, St. Charles, MI), respectively.
Data and Statistical Analysis
EGP values were obtained via hierarchical Bayes modeling and adopting a two-compartment glucose model, as described by Haidar et al. (36,37). The method was implemented using WinBUGS version 1.4, extended by the WBDiff package version 1.9.4 (MRC Biostatistics Unit, Cambridge, U.K.) (38). Dextrose infusion rates, di-deuterated glucose infusion rates, and total and di-deuterated glucose concentrations were input into the program and analyzed using non–steady-state equations. EGP was baseline corrected for values at the time of glucagon dosing, and area under the curve (AUC) assessment was performed in Microsoft Excel 2010 (Redmond, WA) using the trapezoidal method. Statistical analysis was performed using Stata 12 (College Station, TX), with the sktest function being used to identify normal distributions. Nonnormally distributed and nonparametric data were presented as median with interquartile ranges (IQRs) where necessary. Generalized estimating equations were used for multivariate analysis accounting for clustering around subjects. MATLAB R2013b (Natick, MA) was used to plot dose-response curves.
Results
Baseline Characteristics
Of 17 subjects initially screened, 11 met screening criteria and took part in the study. Table 1 shows the baseline characteristics of these 11 subjects. The mean age was 42 ± 11.5 years with a mean duration of diabetes of 23.9 ±15.5 years, mean BMI of 27.7 ± 6.2 kg/m2, mean basal infusion rate of 1 ± 0.3 units/h, and a mean HbA1c of 7.5 ± 0.9% (58 mmol/mol). Twenty-nine studies were completed in 11 subjects (2 subjects only completed two studies, and 1 subject only completed one study). All three dropouts were voluntary and related to time constraints preventing the individual from returning for the repeat studies. A single pilot study was done to determine study parameters for the glucose infusion algorithm and is not included in the overall analysis.
Table 1.
Baseline characteristics of study subjects
| Value* (n = 11) | IQR (25–75%) | |
|---|---|---|
| Sex (% males) | 54.5 | — |
| Age (years) | 42.0 ± 11.5 | 36.5–46.0 |
| Duration of diabetes (years) | 23.9 ± 15.5 | 11.0–32.5 |
| HbA1c (% [mmol/mol]) | 7.5 ± 0.9 [58] | 7.0–8.2 [53–66] |
| Body weight (kg) | 84.0 ± 19.0 | 69.1–93.2 |
| BMI (kg/m2) | 27.7 ± 6.2 | 23.0–31.1 |
| Basal insulin infusion rate (units/h)† | 1.0 ± 0.3 | 0.8–1.3 |
*For all except sex, values are expressed as mean ± SD.
†Average of daily basal rates.
Insulin and Glucose Infusion Rates
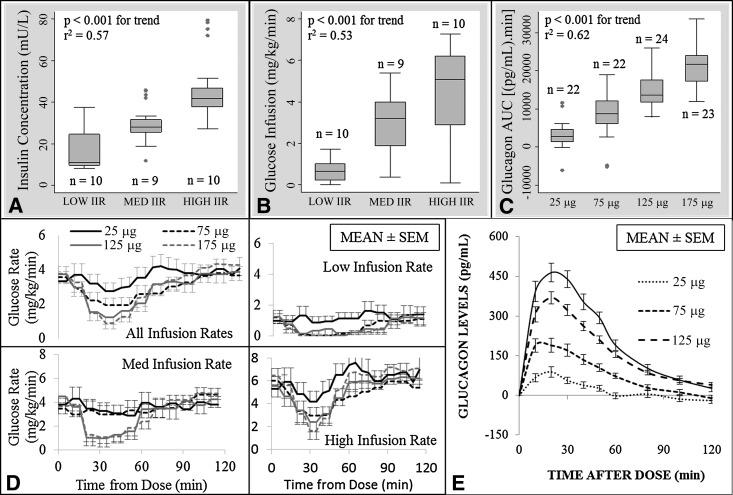
On average, subjects received 0.016 ± 0.006 units/kg/h (median of 0.014) during the low insulin studies (n = 10), 0.032 ± 0.003 units/kg/h (median of 0.03) during the medium insulin studies (n = 9), and 0.05 ± 0.00 units/kg/h (median of 0.05) during the high insulin studies (n = 10). Serum insulin levels were, on average, 17.6 ± 13.0 mU/L (median of 11.0 [IQR 9.7–24.6]) at the low infusion rate, 29.1 ± 8.9 mU/L (median of 28.1 [IQR 25.5–31.5]) at the medium infusion rate, and 46.0 ± 12.5 mU/L (median of 41.7 [IQR 37.5–46.8]) at the high infusion rate (Fig. 1A). Dextrose (D10%) infusion rate increased going from low (mean of 0.7 ± 0.5, median of 0.6 mg/kg/min [IQR 0.2–1]) to medium (mean of 2.9 ± 1.3, median of 3.2 mg/kg/min [IQR 1.9–4]) to high (mean of 4.5 ± 2, median of 5.1 μg/kg/min [IQR 2.9–6.2]) insulin infusion rates (Fig. 1B and D); P < 0.001 for between-group and linear trend analyses. Mean glucose levels during the low insulin studies were higher than those during the medium and high insulin studies (mean glucose after the initial 2-h run-in period: 150.8 ± 68.3, 92.9 ± 21.3, and 88.0 ± 16.0 mg/dL, respectively). The dextrose infusion algorithm used kept subjects within the target range 61% of the study time. Subjects spent, on average, 18 min in the hypoglycemic range (<70 mg/dL), or 3% of the total study time, and only one subject had a single venous glucose reading <50 mg/dL.
Figure 1.
A: Box plot of serum insulin levels (mU/L) at low, medium, and high insulin infusion rates with results of ANOVA. B: Box plot of dextrose (D10%) infusion rate (mg/kg/min) across all studies, by insulin infusion rate group, with results of ANOVA. C: Box plot of glucagon serum level AUC over 60 min, stratified by dose, with results of ANOVA. D: Mean dextrose infusion (mg/kg/min) over time by insulin infusion rate group and glucagon dose: top left, all infusion rates together; top right, bottom left, and bottom right, low, medium, and high insulin infusion rates, respectively. E: Mean incremental change in glucagon serum levels (baseline corrected at time = 0). IIR, insulin infusion rate.
Glucagon Levels
The 60-min AUC and mean incremental change for serum glucagon matched well with glucagon doses used during each study; r2 = 0.63 (Fig. 1C and E). Linear regression identifies a 2,984.6 min ⋅ pg/mL increase in the glucagon AUC over 60 min for each 25-μg increase in the dose (P < 0.001), with a change in the peak serum level of 73.6 pg/mL for each 25-μg increase in the glucagon dose (P < 0.001). Average time to peak glucagon serum concentration was 23.2 ± 13.5 min for the 25-μg dose, 17.1 ± 8.1 min for the 75-μg dose, 19.6 ± 6.1 min for the 125-μg dose, and 20 ± 9.6 min for the 175-μg dose, with no significant difference across the doses.
EGP
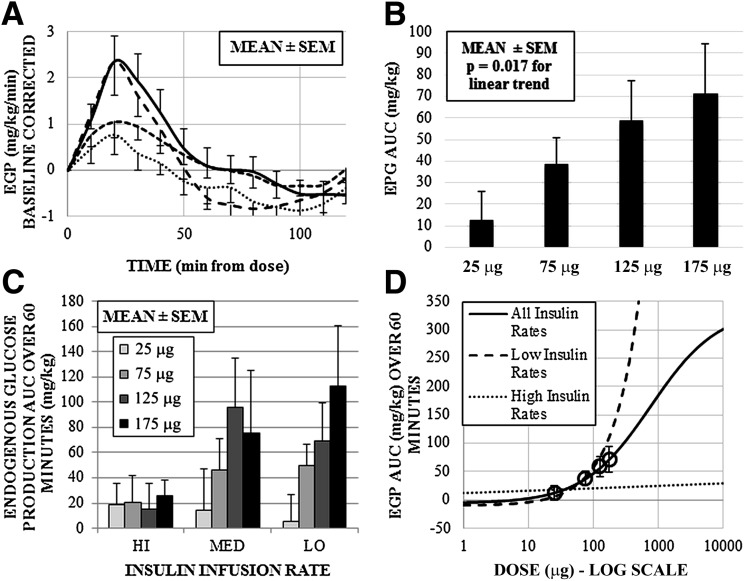
EGP (measured by AUC) for each glucagon dose was calculated and stratified by insulin infusion rate. Two extreme outliers were excluded from the data analysis, as they were beyond the upper third SD level. Figure 2A shows the mean incremental change in EGP (time = 0 used as baseline) across each glucagon dose, and Fig. 2B shows 60-min AUC analysis with P values from linear regression analysis (without accounting for clustering) across glucagon doses. Table 2 and Fig. 2C separate mean EGP by glucagon dose and insulin infusion rate. Trends across the four dose groups were mirrored across the weight-adjusted doses and showed a significant rise in EGP for the low (P = 0.038; slope = 0.632 mg/kg per μg of glucagon) and medium (P = 0.04; slope = 0.59 mg/kg per μg of glucagon) insulin infusion rate experiments. However, as glucagon dose increased within the high insulin infusion rate group, there was no significant elevation in the EGP AUC. Results across all infusion rates show a mean increase in the AUC over 60 min of 20.7 mg/kg, for each 50-μg increase in the glucagon dose. The estimated dose-response curves across all doses, as well as for low and high insulin infusion rates, are plotted in Fig. 2D.
Figure 2.
A: Time profiles of calculated EGP by glucagon dose, baseline corrected for EGP at the time of the dose. B: Mean EGP AUC over 60 min after the dose. C: Three-dimensional graph of mean EGP AUC separated by glucagon dose and insulin infusion rate. D: MATLAB-generated dose-response curve across all doses, and for low and high insulin infusion rate experiments, estimated from study data. Response means for 25-, 75-, 125-, and 175-μg doses indicated by open circles, with 95% CI error bars. Coefficients for parameters of the sigmoid curve with 95% CI: −5.8 (−50 to 272), 336.5 (−3,800 to 3,900), 759.1 (−15,800 to 15,900), and −0.8 (−30.5 to 28.9).
Table 2.
Mean EGP AUC over the first 60 min after each dose
| Insulin infusion rate | Mean EGP AUC (±SD) over 60 min (mg/kg) by glucagon dose |
|||
|---|---|---|---|---|
| 25 μg | 75 μg | 125 μg | 175 μg | |
| Low | 5.05 (±67.9) | 50.04 (±51.5) | 69.29 (±96.3) | 112.8 (±152.5) |
| Medium | 13.96 (±99.7) | 46.6 (±74.3) | 96.31 (±115.4) | 75.62 (±147.5) |
| High | 18.82 (±53.6) | 20.22 (±69.1) | 15.39 (±64.4) | 26.14 (±38.0) |
Adverse Events
There were a total of 42 reported adverse events throughout the study, none of which were severe. Nausea (38%), diarrhea (28%), and headache (21%) were the most frequent occurrences, with episodes of vomiting (10%) and weakness (10%) occurring less frequently. Hyper- and hypoglycemia occurred in 10% of cases as well.
Conclusions
In this study, at the low and medium infusion rates of 0.016 and 0.032 units/kg/h, the EGP response rose proportionately to the glucagon dose, with an average increase in AUC from 5 to 113 mg/kg (P = 0.038) and 14 to 75 mg/kg (P = 0.04). In contrast, during the high insulin infusion rate study, EGP values remained relatively flat (a nonsignificant rise of 18 to 26 mg/mg as glucagon dose increased). This finding suggests that at insulin serum concentrations of >40 mU/L, glucagon doses of 175 μg or lower are largely ineffective at increasing blood glucose levels.
Subcutaneous glucagon has become a useful tool in the bihormonal artificial pancreas, but in some cases, subjects in such studies develop hypoglycemia despite the administration of glucagon. Most cases of hypoglycemia experienced during the management of type 1 diabetes result from a high insulin effect. In the closed-loop setting, administration of glucagon when insulin effect is high would require scaling the dose upwards to compensate for prevailing insulin activity. Most glucoregulatory models currently used can predict insulin serum levels with relative certainty, and this information can then be used to appropriately dose glucagon during online running of an artificial pancreas system. Fear of instability in glucose control, with oscillations in glucose levels between hypoglycemia and hyperglycemia due to competition between insulin and glucagon remains a concern within the artificial pancreas community, but more importantly, the inability to prevent hypoglycemia due to prevailing insulin effect if glucagon is underdosed sets an important precedent for quantifying this relationship.
Glucagon’s primary action is upon increasing hepatic glucose output through glycogenolysis and, to a lesser extent, gluconeogenesis (30). Therefore, the quantification of glucagon action depends upon estimating this effect in vivo. The determination of EGP by tracer methods has evolved over the half century since first introduced (39). Although it remains difficult to accurately estimate EGP in non–steady-state conditions (such as after a dose of glucagon), the use of mathematical models for this estimation has proved useful in elucidating the quantitative relationship between insulin, glucagon, and glucose. Cherrington and colleagues (40) defined the action of intravenously delivered glucagon in the canine model, which is likely translatable to human physiology. Nonetheless, glucagon used in bihormonal systems is delivered via the subcutaneous route. Damiano and colleagues (41), Haidar et al. (33), and Castle et al. (27) have published data analyzing the glycemic response of subcutaneously delivered glucagon in bihormonal closed-loop systems, and Lv et al. (32) from the University of Virginia published an identifiable subcutaneous glucagon absorption model, using data from bihormonal studies by Damiano and colleagues. However, after extensive literature searching, we found no studies in which the effect of subcutaneous glucagon in humans was stratified according to steady-state insulin levels. Therefore, we believe this study, with a stable tracer analysis of glucose output, provides a novel contribution to this field and will be helpful in the modeling of glucagon action in silico. With respect to open-loop therapy, the idea that high IOB can reduce the effect of glucagon on elevating blood glucose would, in theory, be critical in situations of minidosing of glucagon (42–44). We would expect that rescue glucagon therapy, with the approved dose of 1 mg given parenterally, would be less likely to fail even at high IOB, although such large doses were not given in the current study.
Limitations of this study include the following: 1) excessive variability in the estimates of EGP by mathematical modeling of tracer data, likely due to combinations of measurement noise, smoothing effects from the software, and rapid glucose changes during glucagon administration; 2) the study may therefore have been underpowered, based on the larger than expected SD in the EGP data; 3) the use of multiple tracers may have advantages over the use of a single tracer, but a single tracer was chosen for simplicity; 4) the method chosen for determining EGP AUC allowed for negative values and did not take into account a moving baseline prior to calculation, although this method was used in order to ensure standardized estimation across all doses; 5) dosing subjects by microgram per kilogram of fat-free mass would have removed subject weight from the equation, although multivariate analysis did not identify weight as a significant variable at these doses; 6) the continued rise in glucose infusion rates during the medium and high insulin infusion rate studies suggests that the first dose was delivered before steady-state insulin levels were achieved; and 7) the coefficients for the dose-response curve calculated in MATLAB were found to have wide 95% CIs, reducing the strength of the association considerably.
The principal finding in this study was that moderate doses of subcutaneous glucagon were ineffective in stimulating EGP when insulin levels exceeded 40 mU/L. This finding suggests that when insulin levels are elevated, higher doses of glucagon will be required to prevent hypoglycemia, or alternatively, the use of carbohydrate rescue may be necessary. These results also underscore the importance of taking extra precaution to minimize insulin dosing during algorithm design. Although there are several subcutaneous insulin models in use today, the current study provides novel information on the subcutaneous glucagon effect on glucose dynamics during differing steady-state insulin effects. For this reason, these results may be helpful in developing a glucoregulatory model that includes action of both subcutaneously delivered insulin and glucagon.
Article Information
Acknowledgments. The authors thank Dr. Andrea Mari (University of Padova, Padova, Italy) for introduction and guidance into tracer analysis techniques and for the gracious use of his MATLAB software, GLUTRAN. The authors also thank Dr. Wayne Bequette (Rensselaer Polytechnic Institute, New York, NY) for fielding many questions about glucose infusion algorithms and Dr. Mike Lasarev (OHSU) for assistance with methods of statistical analysis. The authors especially thank the nursing and administrative staff of Legacy Good Samaritan Hospital (Portland, OR) for assistance during these studies.
Funding. This study was supported by JDRF, National Institutes of Health grant K23-DK-090133, and Oregon Clinical and Translational Research Institute grant UL1TR000128 from the National Center for Advancing Translational Sciences.
Duality of Interest. J.R.C. has a financial interest in Pacific Diabetes Technologies Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU. W.K.W. has a financial interest in Pacific Diabetes Technologies Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.E.Y., J.R.C., P.A.B., and W.K.W. helped develop and implement the study protocol, performed data analysis, and assisted in the writing and editing of the manuscript. A.H. performed data analysis and provided substantial consulting for interpretation of data. D.L.B. and M.B. provided technical assistance during all studies and assisted in data analysis. J.E.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Data from this study were included in an abstract that was submitted to and presented as a poster at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
References
- 1.Sokal JE. Glucagon—an essential hormone. Am J Med 1966;41:331–341 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langin D. Diabetes, insulin secretion, and the pancreatic beta-cell mitochondrion. N Engl J Med 2001;345:1772–1774 [DOI] [PubMed] [Google Scholar]
- 4.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farhy LS, McCall AL. Models of glucagon secretion, their application to the analysis of the defects in glucagon counterregulation and potential extension to approximate glucagon action. J Diabetes Sci Tech 2010;4:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorel F, Damond N, Chera S, et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 2011;60:2872–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 2012;153:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci 2012;344:136–141 [DOI] [PubMed] [Google Scholar]
- 9.Lorenzi M, Bohannon N, Tsalikian E, Karam JH. Duration of type I diabetes affects glucagon and glucose responses to insulin-induced hypoglycemia. West J Med 1984;141:467–471 [PMC free article] [PubMed] [Google Scholar]
- 10.Albisser AM, Leibel BS, Ewart TG, et al. Clinical control of diabetes by the artificial pancreas. Diabetes 1974;23:397–404 [DOI] [PubMed] [Google Scholar]
- 11.Clemens AH, Chang PH, Myers RW. The development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS). Horm Metab Res 1977;(Suppl. 7):23–33 [PubMed] [Google Scholar]
- 12.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 13.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed]
- 14.Bruttomesso D, Farret A, Costa S, et al. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Tech 2009;3:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care 2010;33:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherr JL, Palau Collazo M, Cengiz E, et al. Safety of nighttime 2-hour suspension of basal insulin in pump-treated type 1 diabetes even in the absence of low glucose. Diabetes Care 2014;37:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toffolo G, Bergman RN, Finegood DT, Bowden CR, Cobelli C. Quantitative estimation of beta cell sensitivity to glucose in the intact organism: a minimal model of insulin kinetics in the dog. Diabetes 1980;29:979–990 [DOI] [PubMed] [Google Scholar]
- 19.Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004;25:905–920 [DOI] [PubMed] [Google Scholar]
- 20.Cobelli C, Man CD, Sparacino G, Magni L, De Nicolao G, Kovatchev BP. Diabetes: models, signals, and control. IEEE Rev Biomed Eng 2009;2:54–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes 2011;60:2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 2011;7:385–395 [DOI] [PubMed] [Google Scholar]
- 23.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther 2014;16:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castle JR, Engle JM, El Youssef J, Massoud RG, Ward WK. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J Diabetes Sci Tech 2010;4:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Youssef J, Castle JR, Branigan DL, et al. A controlled study of the effectiveness of an adaptive closed-loop algorithm to minimize corticosteroid-induced stress hyperglycemia in type 1 diabetes. J Diabetes Sci Tech 2011;5:1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward WK, Castle JR, El Youssef J. Safe glycemic management during closed-loop treatment of type 1 diabetes: the role of glucagon, use of multiple sensors, and compensation for stress hyperglycemia. J Diabetes Sci Tech 2011;5:1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999;48:1198–1214 [DOI] [PubMed] [Google Scholar]
- 31.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF, Jr. Glucagon levels and metabolic effects in fasting man. J Clin Invest 1970;49:2256–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv D, Breton MD, Farhy LS. Pharmacokinetics modeling of exogenous glucagon in type 1 diabetes mellitus patients. Diabetes Technol Ther 2013;15:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haidar A, Duval C, Legault L, Rabasa-Lhoret R. Pharmacokinetics of insulin aspart and glucagon in type 1 diabetes during closed-loop operation. J Diabetes Sci Tech 2013;7:1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magni F, Monti L, Brambilla P, Poma R, Pozza G, Galli Kienle M. Determination of plasma [6,6-2H2]glucose enrichment by a simple and accurate gas chromatographic-mass spectrometric method. J Chromatogr A 1992;573:127–131 [DOI] [PubMed] [Google Scholar]
- 35.Bequette BW. Glucose clamp algorithms and insulin time-action profiles. J Diabetes Sci Tech 2009;3:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haidar A, Potocka E, Boulet B, Umpleby AM, Hovorka R. Estimating postprandial glucose fluxes using hierarchical Bayes modelling. Comput Methods Programs Biomed 2012;108:102–112 [DOI] [PubMed] [Google Scholar]
- 37.Haidar A, Elleri D, Allen JM, et al. Validity of triple- and dual-tracer techniques to estimate glucose appearance. Am J Physiol Endocrinol Metab 2012;302:E1493–E1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS - A Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325–337
- 39.Altszuler N. Remembrance: tracing the glucose tracer dilution technique for measuring glucose turnover. Endocrinology 1992;130:3109–3112 [DOI] [PubMed] [Google Scholar]
- 40.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 2011;13(Suppl. 1):118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Tech 2010;4:1288–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley M, Thomsett MJ, Cotterill AM. Mini-dose glucagon rescue for mild hypoglycaemia in children with type 1 diabetes: the Brisbane experience. J Paediatr Child Health 2006;42:108–111 [DOI] [PubMed] [Google Scholar]
- 43.Hasan KS, Kabbani M. Mini-dose glucagon is effective at diabetes camp. J Pediatr 2004;144:834. [PubMed] [Google Scholar]
- 44.Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care 2001;24:643–645 [DOI] [PubMed] [Google Scholar]