Abstract
Among patients with coronary artery disease (CAD), those with peripheral artery disease (PAD) have a greater vulnerability to cardiovascular (CV) events than those with CAD alone. In a prospective cohort study of patients with CAD, we evaluated potential mechanisms that might explain the adverse CV outcomes associated with PAD. We performed a prospective cohort study of 1018 patients with stable CAD who were recruited from 2000–2002. Incident symptomatic PAD events were adjudicated during a follow-up period of 7.2 ± 2.6 years. We used Cox proportional hazards models to evaluate the association between incident symptomatic PAD events and subsequent risk of CV events or death. Models were adjusted for demographics, traditional risk factors, inflammation, insulin resistance and health behaviors. Among the 1018 patients, 50 patients who did not report a history of PAD at baseline suffered incident symptomatic PAD events during the follow-up period. Those patients had a higher risk of subsequent CV events and death compared to those who did not develop PAD. After adjustment for traditional risk factors, symptomatic PAD events remained associated with a 70% increased risk of subsequent CV events [adjusted HR 1.7, 95% CI 1.0, 2.9, p=0.04] and a 80% increased risk of death [adjusted HR 1.8, 95% CI 1.2–2.7, p=0.006]. Inflammatory biomarkers were the strongest risk factor contributing to the excess risk. In a contemporary cohort of patients with CAD, incident symptomatic PAD events were associated with an increased risk for subsequent CV events. The increased vulnerability to CV events was partially explained by shared CV risk factors and inflammation.
INTRODUCTION
Cardiovascular (CV) disease is the leading cause of death in the world. Detection and treatment of risk factors for CV events is critical to improving health and longevity. Peripheral artery disease (PAD) is common in patients with coronary artery disease (CAD), with a prevalence of 22–42%1–3. Among patients with CAD, those with comorbid PAD have worse CV outcomes than patients with CAD alone4,5. Whether the increased risk of CV events associated with PAD is explained by shared risk factors, such as hypertension or dyslipidemia, is unknown. Other causal factors that have been postulated include lack of physical activity, impaired endothelial function, depressed cardiac function (ejection fraction) and inflammation6–10. Identification of these causal factors and greater attention to their treatment might decrease the excess morbidity associated with PAD among patients with CAD. Therefore, we followed a prospective cohort of patients with CAD to determine how PAD increases risk for adverse CV outcomes and to identify factors involved in this excess risk. A better understanding of the factors leading to worse outcomes in patients with PAD can help identify high-risk subgroups of patients and aid in the development of targeted interventions to reduce morbidity and mortality.
METHODS
Study Population
We evaluated 1018 patients with CAD who were recruited for The Heart and Soul Study in 2000–02. The study was originally designed to determine how psychological disorders lead to CV events in outpatients with stable CAD. Detailed methods have been previously described11. Participants were recruited from two Departments of Veterans Affairs (VA) medical centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System), one university medical center (University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate in the study if they met at least one of the following conditions: a history of myocardial infarction (MI), angiographic evidence of at least 50% stenosis in 1 or more coronary vessels, previous evidence of exercise-induced ischemia using treadmill or nuclear testing, or a history of coronary revascularization.
All participants completed a baseline examination that included an interview, physical examination including blood pressure measurement by sphygmomanometer, fasting venous blood sample collection, a standardized medical history questionnaire, echocardiography, and exercise treadmill testing. Participants were followed for 7.2 ± 2.6 years (mean +/− SD). Of the 1024 participants who completed the baseline examination, 1018 (>99%) had follow-up information on PAD events. The protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent for participation in the study.
Predictor: Incident Symptomatic Peripheral Artery Disease
Participants were followed by telephone annually to inquire about PAD events. For any reported event, all medical records were collected and reviewed by two independent physician adjudicators, with review by a third physician to resolve any disagreements. A total of 67 patients suffered symptomatic events during the study. Fifty of these events occurred in patients who did not report a history of PAD at baseline and were considered “incident” events our primary analyses. Symptomatic PAD events were defined as meeting one or more of the following criteria, with the majority of events being a combination of 3 or more of the following criteria: obstruction or ulcerated plaque (>50% of diameter or >75% of cross-sectional area) at or below the internal iliac arteries (n=40), revascularization, angioplasty, or thrombolysis for PAD (n=40), exertional leg pain relieved by rest (n=23) and/or a final diagnosis of PAD by a physician (n=56). All diagnoses based on exertional leg pain relieved by rest were coupled with a final diagnosis of PAD by a physician.
Outcome: Cardiovascular Events
Following the baseline examination, we conducted annual follow-up interviews with participants (or their proxies) by telephone asking specifically about hospitalization for “heart trouble.” For any reported event, medical records, electrocardiograms, death certificates, and coroner’s reports were retrieved and reviewed by 2 independent blinded adjudicators. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary. The primary outcome included a composite of events - stroke, transient ischemic attack (TIA), congestive heart failure (CHF), myocardial infarction (MI), coronary revascularization and death. Secondary outcomes included those events assessed individually. CHF was defined as hospitalization for a clinical syndrome involving at least 2 of the following: orthopnea, third heart sound, pulmonary rales, paroxysmal nocturnal dyspnea, elevated jugular venous pressure, and cardiomegaly or pulmonary edema on chest radiography. These signs and symptoms must have represented a clear change from the usual clinical status12. Stroke was defined as a new neurologic deficit not known to be secondary to brain trauma, tumor, infection, or another cause13,14. TIA was defined as a focal neurologic deficit (in the absence of head trauma) lasting more than 30 seconds and no longer than 24 hours, with rapid evolution of the symptoms to the maximal level of deficit in less than 5 minutes and with subsequent complete resolution13,14. Nonfatal MI was defined based on the presence of symptoms, electrocardiographic changes, and cardiac enzymes using standard criteria15,16. Deaths were confirmed by review of death certificates and coroner’s reports.
Patient Characteristics
Age, sex, race, education level, and medical history including history of diabetes mellitus were determined by self-report questionnaire. A history of PAD at baseline was determined by self-report of prior diagnosis by a physician or a nurse, and patients reporting PAD at baseline were excluded from our primary analyses. Height and weight were measured by a standardized protocol, with body mass index calculated as weight in kilograms divided by height in meters squared. Participants were instructed to bring their medication bottles to their appointment, and study personnel recorded all current medications. Medications were categorized using Epocrates Rx (Epocrates Inc, San Mateo, California).
Cardiac Disease Severity and Risk Factors
Low and high-density lipoprotein cholesterol levels were measured from fasting venous blood samples. Participants completed an exercise treadmill test according to a standard Bruce protocol17. Those who could not continue the standard Bruce protocol were switched to slower settings and encouraged to exercise for as long as possible. Exercise capacity was calculated as the total number of metabolic equivalent tasks achieved. Resting and stress echocardiograms were performed using an ultrasound system (Acuson Sequoia; Acuson Siemens, Mountain View, California) with a 3.5-MHz transducer. Before exercise, standard 2-dimensional parasternal long- and short axis and apical 2- and 4-chamber views were obtained and planimetered using a computerized digitization system to determine end diastolic and end systolic left ventricular volume and to calculate left ventricular ejection fraction.
Potential Biological Mediators
Levels of high-sensitivity C-reactive protein (hsCRP), IL-6, TNF-α and fibrinogen were determined from fasting plasma or serum samples. High-sensitivity C-reactive protein (CRP) levels were measured using the Integra assay (Roche, Indianapolis, Indiana) in the first 229 participants and (owing to a change at the laboratory) the Extended Range assay (Beckman Coulter Ireland Inc, Galway, Ireland) in the remaining samples. The lowest detectable CRP measurements with these assays were 0.025 mg/dl and 0.20 mg/dl respectively. Results from the two assays were highly correlated (r = 0.99 in a sample of 185 participants)18. IL-6 was measured by using the Millipore Milliplex Map kit (Millipore, Billerica, Mass.) and TNF-α by using the Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc, St-Charles, MO). Blood levels of two n-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), were measured using capillary gas chromatography as the percentage composition of total fatty acid methyl esters in the red blood cell membranes17. Serum adiponectin, leptin and resistin levels were determined by immunoassay (Linco, Millipore, St. Charles, MO). HOMA-IR was calculated from fasting plasma insulin and serum glucose levels.19
Potential Behavioral Mediators
Smoking and alcohol use were determined by self-report questionnaire. For alcohol use, the AUDIT-C questionnaire was used20. To assess medication adherence, participants were asked, “In the past month, how often did you take your medications as the doctor prescribed?” Possible responses were “all of the time (100%),” “nearly all of the time (90%),” “most of the time (75%),” “about half the time (50%),” or “less than half the time (≤50%).” We defined medication nonadherence as taking prescribed medications 75% of the time or less21. To assess physical activity, participants were asked, “Which of the following statements best describes how physically active you have been during the past month, that is, done activities such as 15 to 20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants chose one of the following six categories: not at all active, a little active (1–2 times per month), fairly active (3–4 times per month), quite active (1–2 times per week), very active (3–4 times per week), or extremely active (≥5 times per week). Participants who reported that they were not at all or a little active were considered physically inactive11. Self-report has been shown to be a reliable, valid, and accurate method of assessing physical activity22–25. In particular, single-response items have demonstrated excellent construct validity24–26.
Statistical Analysis
Differences in baseline characteristics in those who did or did not experience PAD events during the follow-up period were evaluated using t-tests for continuous variables and Chi-square tests for categorical variables. We log-transformed covariates with severely right-skewed distributions, including CRP, other inflammatory markers, and adipocytokines. We used Cox proportional hazards models to assess the association of incident symptomatic PAD events with subsequent CV events, excluding patients who reported a history of PAD at baseline. The variable was treated as a time-dependent covariate, switched on at the time of the incident PAD event. We ran two sets of models for each of the CV endpoints. In the base model, we adjusted for age, sex, and race. The second model also adjusted for all baseline covariates that changed the estimated effect of PAD on the composite CV endpoint by at least 5% using a previously described screening procedure11. Smoking, statin use, diuretic use, and alcohol use were represented as categorical variables. LVEF, IL-6, TNF-α, fibrinogen and HgA1c were entered as continuous variables. For the composite CV endpoint, we ran additional models adding these covariates in groups, categorized as traditional PAD risk factors, measures of cardiac disease severity, biomarkers of inflammation, glycemic control/insulin resistance, and behavioral factors. As in the screening procedure, we summarized the effects of adjustment by the relative change in the coefficient for PAD events with respect to the base model estimate, conventionally known as percent treatment effect (PTE). In Table 3, we present the estimated effect of PAD in models adding these sets of covariates one at a time to the base model. We conducted sensitivity analyses to determine whether alternative categorizations of the PAD exposure group changed our findings, repeating our original analyses using a composite time dependent exposure, defined as a history of self-reported PAD at baseline or incident symptomatic PAD events. Statistical analyses were performed using Stata/SE 12 (StataCorp, College Station, TX).
Table 3.
Association between incident symptomatic PAD events and subsequent CVD events adjusted for groups of risk factors.*
| Cardiovascular outcome event | Model | |||
|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | PTE | |
| Base Model** | 2.7 | 1.6, 4.4 | <0.001 | . |
| Traditional Risk Factors | 2.1 | 1.3, 3.5 | 0.005 | 24.1 |
| Inflammation | 2.0 | 1.2, 3.4 | 0.007 | 28 |
| Insulin Resistance | 2.4 | 1.4, 4.0 | <0.001 | 11.2 |
| Full Model*** | 1.7 | 1.0, 2.9 | 0.04 | 45.1 |
CI, confidence interval; PTE: percent treatment effect.
excluding patients with a baseline history of PAD.
Adjusted for age, sex, and race
Included age, sex, race, and all variables that changed the effect size for incident PAD by >5%: traditional risk factors for PAD (smoking, diuretics), inflammatory markers (IL-6, TNF-a, CRP, fibrinogen) and glycemic control/insulin resistance (hemoglobin A1c).
RESULTS
Baseline characteristics
Patients who had symptomatic PAD events during the follow-up period had more baseline comorbidities than patients with CAD only (Table 1), including hypertension and a history of revascularization. They had a lower treadmill score and were more likely to be on beta blockers, statins, and diuretics. As compared with patients who did not have PAD events during the follow-up period, those with symptomatic PAD events had higher baseline levels of inflammatory biomarkers (IL-6, TNF-alpha, fibrinogen), glycosylated hemoglobin, insulin, glucose, triglycerides, and creatinine.. Smoking was also more common among patients with PAD.
Table 1.
Characteristics of patients with CAD with and without incident PAD events during the follow-up period.
| Incident PAD event (n=50) | No incident PAD event (n=890) | P-value | |
|---|---|---|---|
| Age, Mean (SD), y | 69 ± 10 | 67 ± 11 | 0.19 |
| Male Sex | 45 (90) | 727 (82) | 0.14 |
| Caucasian | 30 (60) | 529 (60) | 0.95 |
|
Comorbidities and Cardiac Disease Severity
| |||
| Hypertension | 43 (86) | 617 (70) | 0.01 |
| Hx of MI | 27 (55) | 468 (53) | 0.77 |
| Coronary Revascularization | 37 (74) | 512 (58) | 0.02 |
| LVEF (%) | 0.60 ± 0.09 | 0.62 ± 0.10 | 0.13 |
| Treadmill Score (METs) | 6 ± 3 | 7 ± 3 | 0.02 |
| Weekly Angina | 6 (12) | 157 (18) | 0.30 |
| History of Stroke | 9 (18) | 121 (14) | 0.39 |
| Diabetes Mellitus | 16 (32) | 218 (25) | 0.24 |
| Systolic Blood Pressure (mm Hg) | 139 ± 27 | 133 ± 21 | 0.10 |
| Diastolic Blood Pressure (mmHg) | 74 ± 12 | 75 ± 11 | 0.47 |
|
Medications
| |||
| Aspirin | 42 (84) | 683 (77) | 0.23 |
| Ace-inhibitor | 32 (64) | 448 (50) | 0.06 |
| B-Blocker | 37 (74) | 511 (57) | 0.02 |
| Statin | 40 (80) | 565 (63) | 0.02 |
| Diuretics | 24 (48) | 248 (28) | 0.002 |
|
PAD Risk Factors
| |||
| Smoking | 41 (82) | 605 (68) | 0.04 |
| Cholesterol (mg/dl) | 175 ± 34 | 178 ± 43 | 0.58 |
| LDL (mg/dl) | 104 ± 28 | 105 ± 34 | 0.86 |
| HDL (mg/dL) | 43 ± 12 | 46 ± 14 | 0.09 |
| Triglycerides (mg/dL) | 176 ± 237 | 137 ± 116 | 0.03 |
| Serum creatinine (mg/dL) | 1.5 ± 1.0 | 1.1 ± 0.6 | 0.0006 |
|
Glycemic Control and Insulin Resistance
|
|||
| Hemoglobin A1c (%) | 6.4 ± 1.4 | 5.9 ± 1.1 | 0.01 |
| Insulin (pg/mL) | 459 ± 461 | 370 ± 470 | 0.20 |
| Glucose (mg/dL) | 128 ± 46 | 119 ± 42 | 0.14 |
| HOMA-IR | 4 ± 4 | 3 ± 4 | 0.16 |
|
Inflammation
| |||
| Log CRP (mg/L) | 0.78 ± 1.2 | 0.67 ± 1.3 | 0.59 |
| Log IL-6 (pg/ml) | 1.3 ± 0.6 | 0.9 ± 0.7 | 0.0002 |
| Log TNF-alpha (pg/ml) | 1.7 ± 0.6 | 1.2 ± 0.9 | 0.0004 |
| Log Fibrinogen (mg/dL) | 6.0 ± 0.2 | 5.9 ± 0.2 | 0.01 |
|
Health Behaviors
| |||
| Physically active | 30 (60) | 568 (64) | 0.56 |
| Medication adherence | 47 (94) | 809 (92) | 0.57 |
| Regular alcohol use | 10 (20) | 261 (30) | 0.15 |
| BMI | 27 ± 5 | 28 ± 5 | 0.17 |
| Waist-hip ratio | 0.97 ± 0.07 | 0.95 ± 0.08 | 0.14 |
| Metabolic syndrome | 37 (76) | 615 (72) | 0.57 |
| Omega-3 Index and Adiponectins | |||
|
| |||
| Omega-3 Index (%) | 0.04 ±0.02 | 0.04 ±0.02 | 0.33 |
| Log Adiponectin | 3.1 ± 0.8 | 3.1 ± 0.8 | 0.52 |
| Log Resistin | 9.2 ± 0.7 | 9.0 ± 0.6 | 0.09 |
| Log Leptin | 8.9 ± 1.2 | 9.0 ± 1.2 | 0.30 |
Values as “mean +/− SD” or “n (%)”
Unadjusted analyses
Among the 50 patients with incident symptomatic PAD events, there were a total of 474 primary composite CV events, corresponding to a rate of 20 CV events per 100 person-years after a symptomatic PAD event versus 8 CV events per 100 person-years among patients who did not have PAD events during follow up (Figure 1). For the secondary endpoints, there were 340 deaths, 118 MIs, 152 CHF events, 58 strokes or TIA and 141 coronary revascularizations. Mortality rates in the two groups were 19 versus 5 per 100 person-years respectively.
FIGURE 1.
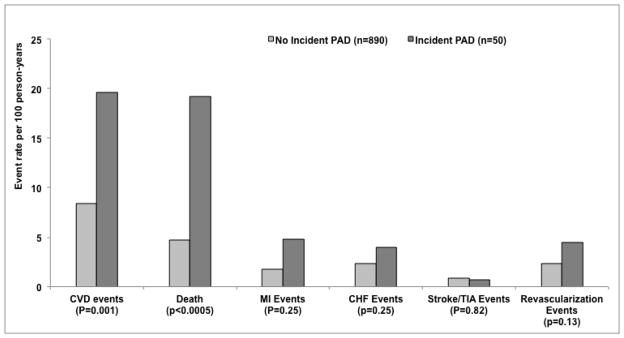
Rates of Subsequent Cardiovascular Events in patients who did or did not develop PAD events.
Effects of potential confounders and mediators
In our screening assessment of potential mediators, the minimally adjusted effect estimate for incident symptomatic PAD on subsequent CV risk was changed by more than 5% after adjustment for IL-6 (18.0%), diuretics (12.1%), HbA1c (11.2%), smoking (10.%), TNF-α (9.9%), fibrinogen (8.5%), CRP (5.1%). Overall, adjusting for inflammatory markers (IL-6, TNF-alpha, CRP and fibrinogen) had the strongest effect on the association between incident symptomatic PAD and subsequent CV events, followed by traditional PAD risk factors (smoking, statins, diuretics) and insulin resistance. Together, these factors together explained 45.1% of the age-adjusted association of incident symptomatic PAD events and future CV events. Factors not meeting the screening criterion included alcohol use (4.2%), HDL (4.1%), history of revascularization (3.6%), LVEF (3.6%), serum creatinine (3.4%) and the remaining CV risk factors and treatments listed in Table 1.
Multivariable-adjusted analyses
After adjusting for age, sex, race and self-reported history of PAD, incident symptomatic PAD was strongly predictive of future CV events, death, MI and the need for revascularization (Table 2). The association between PAD and future CV events remained significant after adjustment for potential mediators including inflammatory biomarkers, glycemic control, co-morbidities and health behaviors. The addition of these groups of mediators to the base model is presented in Table 3, along with the PTE. With regards to secondary outcomes, the association between PAD and death as well as the need for revascularization also remained significant after adjustment for the potential mediators. The association between incident symptomatic PAD and subsequent MI was attenuated after adjustment (Table 2). There was no significant association between incident symptomatic PAD events and subsequent hospitalization for CHF or cerebrovascular events. We found no evidence for modification of the associations between PAD and CV events by age, sex or ethnicity (all p>0.05).
Table 2.
Association between incident symptomatic PAD events and subsequent cardiovascular events in patients with coronary artery disease*
| Cardiovascular outcome event | Base model** | Full model*** | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| Composite endpoint | 2.7 | 1.6, 4.4 | 0.0001 | 1.7 | 1.0, 2.9 | 0.04 |
| Death | 3.2 | 2.2, 4.7 | <0.0001 | 1.8 | 1.2, 2.7 | 0.006 |
| MI | 3.8 | 1.6, 8,7 | 0.002 | 2.1 | 0.8, 5.4 | 0.11 |
| CHF | 2.2 | 0.9, 5.5 | 0.09 | 1.0 | 0.4, 2.5 | 0.996 |
| Stroke/TIA | 0.8 | 0.1, 6.2 | 0.87 | 0.6 | 0.1, 4.3 | 0.60 |
| Revascularization | 3.4 | 1.4, 8.5 | 0.008 | 3.0 | 1.2, 7.7 | 0.02 |
CI, confidence interval.
This is taken from the following number of events (Composite endpoint=474; death=340; MI=118; CHF=152; stroke/TIA=58; coronary revascularization=141).
excluding patients with a baseline history of PAD.
Adjusted for age, sex, and race
Included age, sex, race, and all variables that changed the effect size for incident PAD by >5%: traditional risk factors for PAD (smoking, diuretics), inflammatory markers (IL-6, TNF-a, CRP, fibrinogen) and glycemic control/insulin resistance (hemoglobin A1c).
Alternative definitions of PAD
Sensitivity analysis performed using a combined exposure variable of self-report of PAD at baseline or incident symptomatic PAD events during follow-up did not change our conclusions. Patients with PAD remained at a significantly higher risk for the composite CV endpoint (base model HR 1.6, 95% CI 1.2–2.1, p=0.0003; full model HR 1.4, 95% CI 1.0–1.8, p=0.02; details for other models in Appendix Table 4).
DISCUSSION
In this large cohort of patients with CAD, we found that incident symptomatic PAD was significantly associated with an increased risk of subsequent CV events, death, MI and the need for coronary revascularization. Factors involved in this association included inflammation, glycemic control, cardiac disease severity and traditional PAD risk factors, which explained close to half of the excess risk in cardiovascular morbidity and mortality conferred by an incident symptomatic PAD event. These findings suggest that PAD continues to identify a subgroup at especially high risk for adverse cardiovascular outcomes that might benefit from more intensive secondary prevention.
Previous studies have found that the presence of comorbid PAD is associated with an increased risk of secondary events among patients with CAD27–34. Our study extends these findings by demonstrating that, even beyond the poor prognosis associated with concomitant PAD, hospitalization for incident PAD predicts adverse cardiovascular outcomes.
Different hypotheses have been postulated to explain the association between PAD and adverse CV events. These include 1) worse atherosclerotic burden, 2) an increased inflammatory burden, 3) worse endothelial function (with arterial stiffness that can lead to increased stress on the heart)35 and, 4) loss of benefit of physical activity due to a decrease in ambulation. Hussein and colleagues have suggested that this relationship is due to more extensive and calcified coronary atherosclerosis, constrictive arterial remodeling, and greater disease progression36. Our results support some of these hypotheses by demonstrating that inflammatory markers (IL-6, TNF-α, fibrinogen) explain nearly 20% of the association of PAD and CV morbidity and mortality. Insulin resistance, CV severity, and traditional CV risk factors also played a role. However, given that the association between PAD and adverse CV events remained significant after adjustment for all of these factors, it is possible that other mechanisms, such as endothelial dysfunction or oxidative stress, are also involved in worsening the prognosis of these patients.
Given recent advances in the treatment of CV risk factors and PAD, we might expect that the high morbidity and mortality associated with PAD has declined. In contrast to prior studies37, we found that patients with PAD and CAD were more likely to receive anti-platelet therapy, statins, beta blockers, and ACE-inhibitors than patients with CAD alone. However despite this increased use of protective medications, PAD was still associated with a substantially elevated risk of CV events and death. Our findings suggest that standard medical therapy alone does not fully attenuate the excess risk of PAD.
At the present time, the American Heart Association/American College of Cardiology guidelines published in 2006 recommend a LDL goal of <100mg/dL for all patients with PAD38. Feringa and colleagues demonstrated that in patients with PAD, higher doses of statins and lower LDL cholesterol levels are both independently associated with improved outcomes39. This study, like others, demonstrates that PAD patients have a different pattern of dyslipidemia compared with CAD patients, with lower HDL and higher triglyceride levels. They also have a different inflammatory phenotype. Specific treatment targets for patients with PAD need to be developed based on high quality multi-center trials focused on this population. Our finding that inflammatory biomarkers explained the largest portion of the association of PAD and subsequent CV events suggests several avenues for future research. For example, targeting therapy to lower anti-inflammatory biomarkers, such as CRP, could be explored as well as more aggressive therapy in all patients with PAD using the LDL<70 mg/dL goal recommended by the Third Report of Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults guidelines40
Limitations
Our study findings must be interpreted in light of several limitations. First, the Heart and Soul Study includes mostly urban men with existing heart disease. While this may limit the generalizability of results, this population is important to study given their high risk for development of PAD and for recurrent PAD events. Second, the diagnosis of PAD was not confirmed by ankle-brachial indices. Still, incident symptomatic PAD events were confirmed by careful adjudication of medical records and imaging results. Finally, our power to assess the association of incident symptomatic PAD and subsequent CV events was limited by the relatively small number of patients who developed PAD, which may reduce our ability to look at new mechanisms and novel risk factors.
CONCLUSIONS
Even in the presence of recommended medical therapy for cardiovascular disease and despite taking into account other comorbidities, patients with CAD developing PAD remain at significantly elevated risk for secondary CV events, including death, with inflammation appearing to be a strong risk factor for this excess risk. This suggests that patients with PAD are a highly vulnerable subgroup of patients with CAD who might benefit from more aggressive medical therapy or revascularization. Once a diagnosis of PAD is made, the clinician should remember that these patients are at a particularly high risk of death, MI and need for coronary revascularization and hence should aggressively control known cardiovascular risk factors and be alert to the need for early evaluation of new cardiovascular symptoms.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education. Dr. Cohen was supported by NIH/NHLBI grant K23 HL 094765-01. The Heart and Soul Study was funded by the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), Bethesda, MD, the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, South San Francisco, CA, and the Nancy Kirwan Heart Research Fund, San Francisco, CA. The project described was supported by Award Number KL2RR024130 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
None
References
- 1.Poredos P, Jug B. The prevalence of peripheral arterial disease in high risk subjects and coronary or cerebrovascular patients. Angiology. 2007;58:309–15. doi: 10.1177/0003319707302494. [DOI] [PubMed] [Google Scholar]
- 2.Dieter RS, Tomasson J, Gudjonsson T, et al. Lower extremity peripheral arterial disease in hospitalized patients with coronary artery disease. Vasc Med. 2003;8:233–6. doi: 10.1191/1358863x03vm506ra. [DOI] [PubMed] [Google Scholar]
- 3.Atmer B, Jogestrand T, Laska J, Lund F. Peripheral artery disease in patients with coronary artery disease. Int Angiol. 1995;14:89–93. [PubMed] [Google Scholar]
- 4.Cotter G, Cannon CP, McCabe CH, et al. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis In Myocardial Infarction (OPUS-TIMI) 16 study. Am Heart J. 2003;145:622–7. doi: 10.1067/mhj.2003.6. [DOI] [PubMed] [Google Scholar]
- 5.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 6.Shah AM, Banerjee T, Mukherjee D. Coronary, peripheral and cerebrovascular disease: a complex relationship. Herz. 2008;33:475–80. doi: 10.1007/s00059-008-3152-y. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. Jama. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005;96:1374–8. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 11.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. Jama. 2008;300:2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99:1643–7. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asplund K, Bonita R, Kuulasmaa K, et al. Multinational comparisons of stroke epidemiology. Evaluation of case ascertainment in the WHO MONICA Stroke Study. World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1995;26:355–60. doi: 10.1161/01.str.26.3.355. [DOI] [PubMed] [Google Scholar]
- 14.Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas AM, Schroll M. Stroke incidence, case fatality, and mortality in the WHO MONICA project. World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1995;26:361–7. doi: 10.1161/01.str.26.3.361. [DOI] [PubMed] [Google Scholar]
- 15.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 17.Ali S, Garg SK, Cohen BE, Bhave P, Harris WS, Whooley MA. Association between omega-3 fatty acids and depressive symptoms among patients with established coronary artery disease: data from the Heart and Soul Study. Psychother Psychosom. 2009;78:125–7. doi: 10.1159/000203118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–20. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Dawson DA, Grant BF, Stinson FS. The AUDIT-C: screening for alcohol use disorders and risk drinking in the presence of other psychiatric disorders. Compr Psychiatry. 2005;46:405–16. doi: 10.1016/j.comppsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–86. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 23.Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand J Public Health. 2008;36:52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Jacobs DR, Jr, Leon AS. Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc. 1993;25:92–8. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14:422–8. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 26.Jackson AW, Morrow JR, Jr, Bowles HR, FitzGerald SJ, Blair SN. Construct validity evidence for single-response items to estimate physical activity levels in large sample studies. Res Q Exerc Sport. 2007;78:24–31. doi: 10.1080/02701367.2007.10599400. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 28.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. Jama. 1993;270:487–9. [PubMed] [Google Scholar]
- 30.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. Bmj. 1996;313:1440–4. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 32.Eagle KA, Rihal CS, Foster ED, Mickel MC, Gersh BJ. Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. The Coronary Artery Surgery Study (CASS) Investigators. J Am Coll Cardiol. 1994;23:1091–5. doi: 10.1016/0735-1097(94)90596-7. [DOI] [PubMed] [Google Scholar]
- 33.Jelnes R, Gaardsting O, Hougaard Jensen K, Baekgaard N, Tonnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors. Br Med J (Clin Res Ed) 1986;293:1137–40. doi: 10.1136/bmj.293.6555.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 35.Pasqualini L, Marchesi S, Vaudo G, et al. Association between endothelial dysfunction and major cardiovascular events in peripheral arterial disease. Vasa. 2003;32:139–43. doi: 10.1024/0301-1526.32.3.139. [DOI] [PubMed] [Google Scholar]
- 36.Hussein AA, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57:1220–5. doi: 10.1016/j.jacc.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Welten GM, Schouten O, Hoeks SE, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–96. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Feringa HH, Karagiannis SE, van Waning VH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–43. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


