Abstract
Caged compounds are light-sensitive probes that functionally encapsulate biomolecules in an inactive form. Irradiation liberates the trapped molecule, permitting targeted perturbation of a biological process. Uncaging technology and fluorescence microscopy are 'optically orthogonal': the former allows control, and the latter, observation of cellular function. Used in conjunction with other technologies (for example, patch clamp and/or genetics), the light beam becomes a uniquely powerful tool to stimulate a selected biological target in space or time. Here I describe important examples of widely used caged compounds, their design features and synthesis, as well as practical details of how to use them with living cells.
The idea behind the caging technique is that a molecule of interest can be rendered biologically inert (or caged) by chemical modification with a photoremovable protecting group (Fig. 1). Illumination results in a concentration jump of the biologically active molecule that can bind to its cellular receptor, switching on (or off) the targeted process. Virtually every kind of signaling molecule or second messenger, of every size|[mdash]|from protons to proteins|[mdash]|has been caged1.
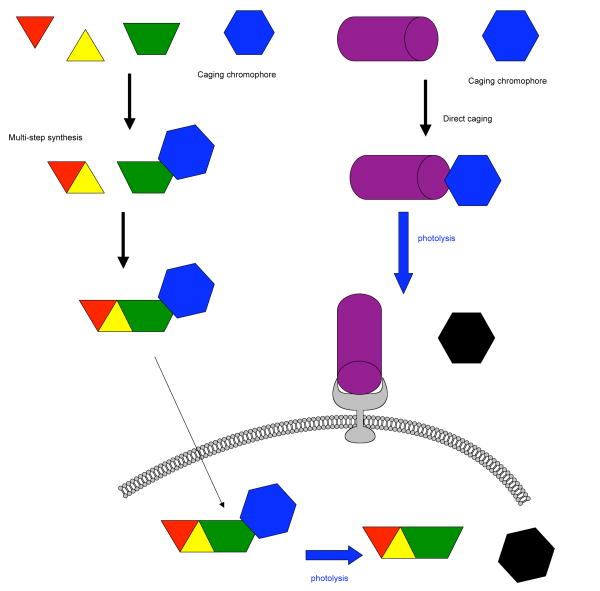
Figure 1. General strategies caged by either multistep or direct caging.
The caging chromophore prevents receptor binding until it is cleaved by light. Second messengers and hormones can be caged by both strategies, but the illustration shows only two examples for simplicity.
Why are caged compounds so useful? A single component of cellular chemistry can control the function of a cell, and such cellular regulation can be temporally or spatially defined, intracellular or extracellular, and amplitude- or frequency-modulated2. Photomanipulation of cellular chemistry using caged compounds provides a uniquely powerful means to interact with such cellular dynamics, as it can touch upon any one of the above dimensions. Thus, since light passes through cell membranes, uncaging can rapidly release a biomolecule in an intracellular compartment. This space is not readily accessible to many second messengers (for example, inositol-1,4,5-trisphosphate (IP3), ATP, Ca2+, cAMP, cGMP) when they are applied to cells externally, as their charge makes them impermeable to the plasma membrane. Furthermore, uniform illumination results in release throughout the cytosol, or the release can be localized by focusing the uncaging beam on one part of a cell. Likewise, extracellular uncaging of neurotransmitters and hormones is tunable, allowing stimulation of many neurons simultaneously or of single synapses|[mdash]|by global or focused illumination, respectively. Light cannot only be directed, but also modulated in time and amplitude. Thus, uncaging can also be used to produce rapid, repetitive release of biomolecules or finely graded changes in the magnitude of stimulation.
Examples of important caged biomolecules or second messengers are calcium3, 4, 5, 6, neurotransmitters7, 8, 9, 10, inositols11, 12, nucleotides13, 14, peptides15, 16, enzymes17, 18, 19, mRNA20 and DNA21. Apart from Ca2+, all these mole-cules are caged by covalent modification of one part of their structure with a photoremovable chromophore. Although chemists create most new caged compounds, many biologists have embraced the use of these powerful tools to answer biological questions. For example, calcium uncaging with molecules like NP-EGTA (Fig. 2) has been widely used to study many Ca2+-controlled processes. In particular, secretory processes in neuronal and non-neuronal cells have been extensively studied with caged calcium22. Rapid uncaging of glutamate using two-photon photolysis is particularly useful for the rational stimulation of visually identified synapses in complex tissue preparations such as acutely isolated brain slices from the hippocampus23 (Fig. 2b). Finally, uncaging of mRNA in vivo in zebrafish is an excellent example of a biological application of uncaging in whole animals20 (Fig. 2c). Before any of these experiments can be performed, however, it is necessary to cage the molecule of interest. The basic methods for the construction of caged compounds are discussed below. Although some methods require complex chemistry, others are simple enough for biologists to do themselves.
Figure 2. Structures and photochemistry of caged compounds.
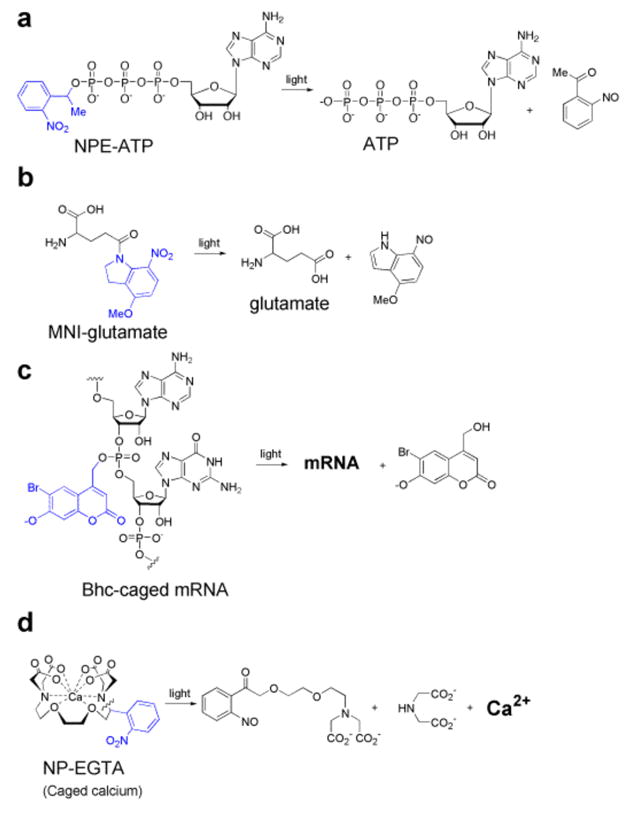
(a) NPE-ATP13 cannot be hydrolyzed before uncaging as covalent attachment of the caging chromophore to the | [gamma] |-phosphate prevents enzymatic access. Photolysis breaks the bond between the benzylic carbon of the chromophore and an oxygen atom, liberating the caged ATP. NPE-ATP has been widely used to control molecular motors31. (b) MNI-Glu does not bind to postsynaptic glutamate-gated ion channels because of modification of the side-chain carboxylate with the caging chromophore10. Uncaging restores the | [gamma]|-carboxylate by donation of an oxygen atom from the nitro group. (c) The translational activity of Bhc-mRNA is latent because of chemical modification of multiple phosphates on the backbone (for simplicity only a single cage is shown). Irradiation initiates a photosolvolysis reaction that releases the caged mRNA20. (d) NP-EGTA cages Ca2+ by high affinity binding5, photolysis breaks the Ca2+ coordination sphere in two, yielding low-affinity fragments that release the bound Ca2+.
Making caged compounds
Caged compounds are made using synthetic organic chemistry. Syntheses are usually multistep, but some caged compounds are made with one-step 'direct' caging (Fig. 1). Multistep syntheses are usually required because most natural products have many functional groups of equivalent reactivity.
As organic chemists have made a huge number of caged compounds, a full description of all of them is beyond the scope of this review (for chemistry reviews, see refs. 1,24). Furthermore, chemical applications of photorelease technology are not covered in this review (for example, Affymetrix production of genechips25, 26). In this section I outline syntheses of the caged compounds that have been widely used by biologists as well as new, conceptually important methods of synthesizing caged macromolecules.
General design guidelines
Caged compounds must be biologically inert before photolysis. This means the probe should be neither an agonist nor antagonist when applied at a useful concentration to the biological preparation. The rate of uncaging of the caged biomolecules needs to be faster than the process being studied, if kinetics is an essential part of the process being studied. The absolute speed requirement will vary depending on the application: neurotransmission is much faster than gene transcription, but both can be regulated by calcium. Finally, the higher the efficiency of uncaging, the easier it is to use a caged compound, but caged compounds with modest uncaging efficiencies (for example, 1-(ortho-nitrophenyl)-ethyl (NPE)-caged ATP and IP3) have been used to great effect in many biological experiments. Lower uncaging efficiencies can, however, be deleterious for cell health in the case of UV light|[ndash]|mediated uncaging, or in the case of two-photon excitation, the rate of uncaging could be too slow to be useful (see Box 1 and Fig. 3 for a description of important optical characteristics of compounds and their effect on uncaging efficiency).
Box 1. Definition of Photochemical Properties of Caged Compounds.
The absorption of light
When we think of light as an electromagnetic wave, the key idea for light absorption by a chromophore is that the oscillating electric field of light interacts with an electron as if it were an oscillating dipole. Effective interaction requires a ‘resonance’ between the light wave and a molecular electronic energy gap (that is, the ground state and an excited state, hence absorption is strictly quantized). Electrons oscillate at about 1015 Hz, corresponding to wavelengths of about 200–700 nm, hence this is called the ‘photochemical range’ of light. If we think of light as a stream of particles, instead of an electromagnetic wave, then a photon becomes a ‘reagent’, and absorption is a bimolecular reaction that occurs by collision of particles. The propensity for this reaction is quantified in the extinction coefficient (ε) of a molecule (units: M–1 cm–1). The larger the ε, the more likely it is that a photon will be absorbed. Two laws relate ε to actual measurement of absorption: (i) the proportion of light absorbed is independent of initial intensity, I0 (Lambert's law), and (ii) transmitted light intensity, It, is proportional to concentration (c) of absorbing molecules (Beer's law). Hence the optical density of a solution is log(I0 / It), and ε= log(I0 / It) × c × l, where l is the optical pathlength.
The use of absorbed light
The quantum yield of reaction (=) is the measure of how many excited state molecules lead to uncaged products (units: moles per Einstein, but normally given as a unitless number).
Two-photon excitation
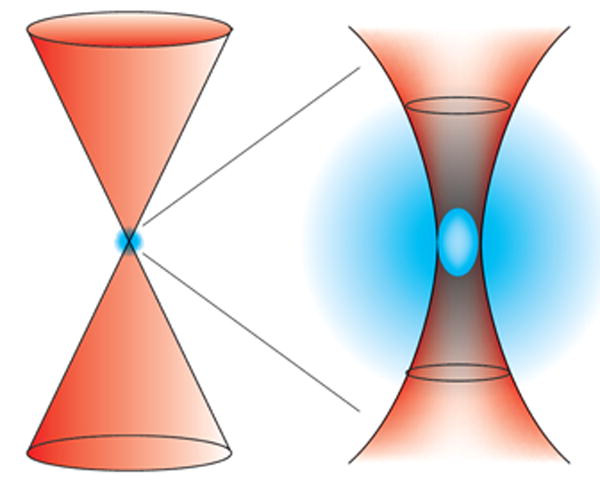
Normally an excited state produced by the absorption of a single photonic quantum, as described above, occurs at a rate of about 1015 s–1. If two photons of half the energy of such a quantum collide with a chromophore within 10–18 s, then ‘virtually simultaneous’ absorption of two photons occurs that varies quadratically with the incident flux density. The probability of two-photon excitation (n) is dependent on several variables97:
(where δ in the two-photon cross section, P the incident power, τ the pulse-width, ƒ the repetition rate of the laser light, λ, the wavelength of excitation light, NA the numerical aperture of the microscope objective, and h, c are constants). One consequence of the nonlinear nature of two-photon is that excitation is dramatically localized the z direction. Thus, when used for uncaging, two-photon excitation may allow focal bursts of release with a volume of much less than 1 fL. This strict three-dimensional confinement is dependent upon rapid uncaging, as the residence time of an excited molecule with the two-photon volume is ∼0.3 ms (ref. 98). Thus, two-photon excitation of a slowly released caged glutamate64 does not allow highly localized released but creates a ‘mist’ of uncaged glutamate (Fig. 4). But if glutamate is released quickly, uncaging can be close to the diffraction limit10,88.
Figure 3. The degree of spatial confinement of two-photon uncaging depends on the rate of substrate release.
Caging small molecules and ions
Caged neurotransmitters
Caged glutamate is the most widely used caged neurotransmitter by biologists, and many syntheses of caged glutamate have been published using different chromophores and/or different caging strategies27. Very few of these caged glutamates actually satisfy all the requirements of neuroscientists for photorelease in complex biological tissue such as acutely isolated brain slices (that is, rapid uncaging, photochemically efficient release, hydrolytic stability, biological inertness and, ideally, two-photon excitation sensitivity).
The |[alpha]|-carboxy-ortho-nitrobenzyl (CNB) protecting group was the first truly successful caging chromophore for neurotransmitters. The four-step synthesis of CNB-Glu9 has proved especially important for neurobiology. Glutamate is released quickly (half-time, 25 |[mu]|s) and efficiently to activate |[alpha]|-amino-5-hydroxy-3-methyl-4-isoxazole propionic acid (AMPA) receptors with their normal time course (that is, a rise of about 0.5 ms). CNB-GABA has also been synthesized28, but it has been reported to be mildly antagonistic29. Unfortunately, CNB-Glu is not sensitive to two-photon uncaging, so 4-methoxy-7-nitroindolinyl (MNI)-Glu has been developed to address this need10. Both caged compounds require careful purification after the final deprotection step, which is identical in both syntheses, because the presence of even the slightest amount of free glutamate can be toxic to acutely isolated brain slices. The synthesis of CNB- and MNI-caged amino acids involves several steps requiring silica gel column chromatography to remove organic side products. This makes the preparation of these compounds much more challenging for biologists to do themselves, but CNB-Glu and MNI-Glu are commercially available (Table 1).
Table 1. Properties of selected commercially available caged compounds.
| Caged compound | Φ | ε(M–1 cm–1) | Φ × ε | Rate (s–1) | Stability |
|---|---|---|---|---|---|
| Calcium chelators | |||||
| DM-nitrophena,b | 0.18 | 4,300 | 774 | 3.8 × 104 | Complete |
| NP-EGTAa | 0.23 | 970 | 194 | 6.8 × 104 | Complete |
| nitr-5b | 0.012 | 5,500 | 66 | 2.5 × 103 | Complete |
| diazo-2a | 0.03 | 22,800 | 1,596 | 2.3 × 103 | Complete |
| Neurotransmitters | |||||
| CNB-Glua | 0.14 | 500 | 70 | 4.8 × 104 | Fair |
| CNB-GABAa | 0.16 | 500 | 70 | 3.6 × 104 | Fair |
| CNB- | 0.8 | 430 | 344 | 1.7 × 104 | Excellent |
| carbamoylcholinea | |||||
| MNI-Gluc | 0.085 | 4,300 | 366 | ∼105 | Excellent |
| Phosphates | |||||
| NPE-IP3a,b | 0.65 | 430 | 280 | 225 and 280 | Excellent |
| NPE-cAMPb | 0.51 | 430 | 219 | 200 | Fair |
| DMNPE-cAMPa | 0.05 | 5,000 | 250 | 300 | Poor |
| NPE-cADPribosea | 0.11 | 430 | 271 | 18 | Excellent |
| NPE-ATP-a,b | 0.63 | 430 | 271 | 90 | Excellent |
| DMNPE-ATP a | 0.07 | 5,000 | 350 | 18 | Fair |
| Fluorophores | |||||
| bis-CMNB-fluoresceina | ND | 2,000 | ND | ND | Complete |
| DMNB-HPTS a | ND | ∼5,000 | ND | ND | Complete |
From Invitrogen (Molecular Probes).
From Calbiochem.
From Tocris. ε, extinction coefficient; Φ, quantum yield. ND, not determined.
Caged nucleotides, nucleosides and inositols
Caged ATP13 and cAMP30 were the first caged compounds to be synthesized and uncaged in living cells. Caged ATP was made by coupling NPE-caged phosphate to ADP and required three synthetic steps, whereas caged cAMP was caged directly using hyper-reactive diazo chemistry that allows selective caging of phosphates. The diazo approach has proved very useful for caging phosphates in a wide variety of molecules. ATP and IP3 have both been caged in one step using diazo chemistry12, 14. There have also been many studies published using NPE-caged ATP and IP3 by biologists who make these caged compounds31, 32, 33. There are two advantages to biologists in making their own caged ATP or IP3: a large quantity can be made a reasonable price, and purity can be guaranteed.
Although NPE-caged compounds have proven extremely useful for biologists, they are not perfect caged compounds. Improvements in one or more of their properties (solubility, stability, rate of uncaging and two-photon cross-section) have been reported, however. For example, NPE-cAMP is not very soluble at pH 7, so a highly soluble version of this compound has been synthesized in nine synthetic steps34, which has the added benefit of also being more hydrolytically stable. The rate of uncaging of NPE-ATP is a little slow for some studies (rate is 83 s|[minus]|1), so many faster-uncaging caged ATP probes have been synthesized. Two interesting biological studies have been published showing that the rate of photolysis of NPE-ATP can be rate-limiting35, 36. NPE-IP3 has a low two-photon cross-section (|[sim]| 0.001 GM), so a caged IP3 with larger cross-section (0.035 GM) has been synthesized in seven synthetic steps, permitting localized two-photon uncaging of IP3 in living cells37. Finally, membrane-permeant ester derivatives of caged cAMP and IP3 probes have been made using multistep syntheses that permit loading of these second messengers into intact cells38, 39, 40.
Caged calcium
The inorganic cation calcium cannot form covalent bonds to caging groups the way organic molecules can, so a new caging strategy has been developed. Photolabile derivatives of known high-affinity calcium chelators (BAPTA, EDTA and EGTA) have been synthesized. These molecules decrease their affinity for calcium upon irradiation, thus uncaging some of the bound calcium (Fig. 1d). Ideally the Ca2+ cage should bind as much Ca2+ as possible, but as the dissociation constant is always finite, there must always be some free Ca2+ and free (unloaded) cage. This situation is quite different from that of other caged molecules. Thus, in the case of caged Ca2+, two additional properties define efficiency of caging, the affinity for Ca2+ before and after photolysis (Table 2). The higher the affinity of the chelator, the more it can be loaded with Ca2+ before [Ca2+]free reaches an activating level, and the lower to photoproduct affinity, the more Ca2+ is released by photolysis (Table 2). To produce net release of bound Ca2+, all of the unloaded cage must be photolyzed, else it acts as a 'calcium sink' that will re-complex the photoreleased calcium (reviewed in ref. 41).
Table 2. Properties of calcium cages.
| Ca2+ Kd (nMa) | Product Kd (mM) | ΔKd | Φ | ε (M–1 cm–1) | Φ × ε | Rate calcium release (s–1) | 2PCS (GM) | |
|---|---|---|---|---|---|---|---|---|
| NDBF-EGTA | 100 | 2.0 | 20,000 | 0.7 | 18,400 | 12,880 | 20,000 | 0.6 |
| DMNPE-4 | 48 | 2.0 | 42,000 | 0.09 | 5,120 | 461 | 45,000 | 0.01 |
| NP-EGTA | 80 | 1.0 | 12,500 | 0.23 | 975 | 224 | 68,000 | 0.001 |
| DM-nitrophen | 5 | 3.0 | 60,0000 | 0.18 | 4,300 | 774 | 38,000 | 0.01 |
| nitr-5 | 145 | 0.0063 | 54 | 0.012 | 5,500 | 66 | 2,500b | NDc |
| azid-1 | 230 | 0.12 | 520 | 1.0 | 33,000 | 33,000 | 500 | 1 |
Measured at pH 7.2.
Given is the rate of appearance of organic photo product; Ca2* is probably released at the same rate.
This value has not been measured, but is probably 0.01 by analogy with DM-nitrophen. ε, extinction coefficient; Φ, quantum yield. 2PCS, two-photon cross-section. ND, not determined.
The general strategy of calcium uncaging has been applied in two different ways. One based on photochemical modification of the buffering capacity of BAPTA derivatives (nitr-5, (ref. 3) or azid-1 (ref. 42)), and the other based on photochemical scission of the backbone of either EDTA (dimethoxy (DM)-nitrophen4) or EGTA (nitrophenyl (NP)-EGTA5). The compounds 1-(4,5-dimethoxy-2-nitrophenyl)-EGTA (DMNPE)-4 (ref. 6), NDBF-EGTA43. DM-nitrophen, NP-EGTA and nitr-5 are all commercially available, and therefore have been used in hundreds of biological experiments41, 44, 45. The properties of all these calcium cages are summarized in Table 2.
Caging macromolecules
A variety of caged peptides and enzymes have been made. The former are usually small, inhibitory peptides that can be used to disrupt an important intracellular protein-protein interaction. The latter are macromolecular counterparts of standard caged compounds, in which the catalytic function of the enzyme is blocked by the caging chromophore. Such syntheses are often more challenging than those discussed above, as peptides seem to be inherently unstable. But the development and use of caged peptides is important because they provide another means of controlling the fate of a cell that is conceptually distinct from the uncaging of small molecules, especially given that many cellular processes are not regulated by essential cofactors but simply by protein-protein interactions. An alternative to such direct protein caging is to cage the translational or transcriptional machinery that produces such gene products. Both strategies have been extensively reviewed46, so I outline only the basic concepts involved in caging macromolecules below.
Caging peptides and proteins
Proteins and peptides have been caged with commercially available reagents that covalently modify specific amino acid residues. G-actin was the first protein to be caged using this ‘shotgun’ approach17. Proteins such as PKA and cofilin have also been caged using a similar strategy19, 47, 48. There are several specific points to consider when planning the synthesis of a caged enzyme: (i) use of caged proteins is complicated by issues of residual activity of the caged protein (it is very difficult to have 100% caging, so a few percent residual activity may give rise to ambiguous results), (ii) recovery of activity after uncaging is problematic, especially when ‘shotgun’ caging is used as multiple sites must be uncaged for activation, and (iii) caged proteins must be microinjected into cells46.
Two approaches to the synthesis of caged proteins using modern molecular techniques have been developed. The first uses unnatural amino acid mutagenesis. T4 lysozyme was caged as proof-of-principle of this idea18. Caged ion channels and phosphoproteins have been synthesized using this method, and such syntheses require the combination of a dazzling array of technical skills (reviewed in ref. 49). The second approach uses expressed protein ligation (reviewed in ref. 50) to assemble a caged semi-synthetic protein. For example, Smad2 is regulated by dephosphorylation of two C-terminal serine residues, and so a caged version of the protein was synthesized by solid phase synthesis of the caged phosphoserine portion of the enzyme, which was then coupled to recombinant Smad2-MH2 domain to yield a caged protein51.
The basic strategy for synthesizing caged peptides was delineated with the development of caged MLKII inhibitors. Using solid-phase peptide synthesis, a caged tyrosine was incorporated into a small inhibitory peptide15. Other peptides have been caged using the same approach16, 52, 53, 54.
Peptides and enzymes are normally caged by some site-specific crucial modification with a standard small caging chromophore such as that first used with ATP. But it is often hard to know the position of a single crucial residue, or it is difficult to construct such uniquely caged compounds. An alternative means of disrupting protein-protein interactions is with shear bulk. An amyloidogeic human prion protein fragment was caged by adding a polycationic peptide to the N terminus via a photocleavable cross-linker55. This constitutes a conceptually novel addition to the arsenal of caging strategies that could be adapted for many other macromolecules for which precise structure-function relationships are not known or perhaps for which important sites are hard to modify selectively with small blocking groups. A similar strategy has been applied, using cross-linked antibodies56.
Caged mRNA and DNA
Turning on one gene in space-time is especially attractive for delineation of protein function in whole organisms, and this is where uncaging technology has found important applications. Genetic function can be controlled by caging large mRNA or DNA fragments.
The simplest way to cage mRNA has also proven to be the most useful biologically. Direct, multi-site caging of mRNA20, 57, 58, 59 or DNA21 with a reactive diazo coumarin chromophore, similar to the original caged cAMP synthesis30, (Bhc; Fig. 2c) efficiently inactivates the molecules.
The techniques for caging essentially any biomolecule or second messenger have now been developed, so that protons60, inorganic cations, gases61, small organic molecules and macromolecules46, 62 can be caged. But given the complexity of the syntheses of most caged compounds, commercial availability constrains their use for most laboratories. As caged peptides and proteins are quite unstable, companies cannot keep large amounts in stock. This means that most biologists are restricted to the few caged compounds that are commercially available as a result of high demand (Table 1) or they must collaborate with academic laboratories that synthesize caged compounds.
Using caged compounds
This section aims to give biologists an idea how to think about the practicalities of using caged compounds and provides some biological examples illustrating the basic principles of caged compounds.
Photochemical guidelines
Chemical synthesis of a biologically inert caged compound is only the first step in developing a useful caged probe. There are many other chemical and photochemical properties that must be understood for the cages to be designed and used effectively (Box 1).
Chemical considerations
Organic synthesis is typically performed in nonaqueous solvents, but all caged compounds are used with living cells. So the final step of the synthesis usually renders the caged compound water-soluble. This essential property is often much more difficult to achieve than it sounds, for two reasons: (i) adding a hydrophobic caging chromophore to a moderately soluble natural product often makes it sparingly soluble at physiological pH, and (ii) for practical purposes, one often requires a high concentration of the caged compound as a stock solution. Some substrates are so highly soluble (for example, EGTA, IP3) that adding the caging chromophore to these molecules makes little difference to their solubility, whereas others can be profoundly affected by caging (for example, serotonin, cAMP, GABA, DAG, sphingosine-1-phosphate). This problem is often ignored in the literature, but can be solved by adding additional (usually negative) charge to the caging chromophore, which unfortunately complicates the synthesis considerably34.
Aqueous stability is also a vital property of caged compounds. Some chemical bonds are sensitive to aqueous hydrolysis (most importantly esters and to a lesser extent amides), whereas others are not (ethers, amines and carbamates). Poor aqueous stability results in the spontaneous hydrolytic release of the caged compound and has bedeviled the development of a good caged glutamate (ref. 27 and Table 1). Caging the amine either directly or via the carbamate is the obvious solution to this crucial problem63, but these caged glutamates are uncaged too slowly to be useful27. Caging glutamate as an amide has provided a recent solution to this important dilemma10, 64.
The rate of uncaging is an important property of most caged compounds. The actual rate of uncaging has been reported for only a few caged bioactive molecules. The fundamental problem with such rate determinations is that there are few indicators for biomolecules that respond with useful temporal speed. Thus whilst indicators for glutamate, IP3, cAMP, cGMP and ATP do exist, only the kinetics of the ATP indicator have been characterized and therefore used to quantify the rate of uncaging14. Good indicators for cations (for example, Ca2+) exist, and have been used to characterize some caged calcium compounds. Chemists attempted to solve this difficult situation by studying the photochemistry of the caging chromophores. But this approach has turned out to be much more complicated than expected (reviewed in ref. 24). The primary photochemical reaction of ortho-nitrobenzyl, the most widely used and only generically applicable type of caging chromophore, is wellstudied and involves a photochromic aci-nitro intermediate65. It was thought that since the appearance of ATP from NPE-caged ATP was concomitant with the decay of its aci-nitro intermediate14, that the decay of this species could be used as an indicator of the release of other caged compounds (for example, IP3 (ref. 12) and glutamate9). Rigorous study of the kinetics of model ortho-nitrobenzyl (ortho-NB)|[ndash]|caged compounds (caged ethers) has however shown this is not always the case66. The situation is further complicated by a report that 4,5-dimethoxy-2-nitrobenzyl (DMNB)-caged ethers uncage much faster than simple ortho-NB|[ndash]|caged ethers67. It seems for ortho-NB|[ndash]|caged phosphates and carboxylates, aci-nitro decay is rate-limiting, whereas for ortho-NB|[ndash]|caged ethers it is not, and for DMNB-caged ethers the situation is unclear. These data suggest that for nitroaromatic caged compounds the rate of appearance of each caged effector from photolysis of caged compounds must be determined.
The reaction mechanisms of two other caging chromophores have been thoroughly studied: coumarin-caged phosphates and para-hydrophenacetyl|[ndash]|caged acids (reviewed in ref. 24). Both uncage via photosolvolysis68, which is a rapid process (rates >106 s|[minus]|1), but it only works for certain functional groups, so unfortunately such chemistry is of limited applicability. Additionally, there are no commercially available molecules caged with these chromophores.
The efficiency of use of the incident uncaging light, the product of the extinction coefficient and quantum yield of reaction, expressed as |[epsi]| |[times]| |[phgr]|, is an important property of caged compounds and may constrain feasible applications of any caged compound. The size of the |[epsi]| can also constrain application of a caged compound. For practical purposes, optical densities of the cage in a cell should be less than 20%, else inhomogeneous uncaging will result from inner filtering effects across the cell during rapid photolysis. For example, if the concentration of the cage is 2 mM, then the extinction coefficient at 350 nm of the cage must be less than 10 mM|[minus]|1 cm|[minus]|1 for a 100 | [mu]|m pathlength. In chromaffin cells of about 20 |[mu]|m diameter, the concentration of DM-nitrophen often used is 10 mM (refs. 69, 70, 71, 72). This solution has an optical density of about 0.09. If the same concentration of azid-1 (ref. 42) was used, then the optical density would rise to 0.66. Rapid illumination in such a situation would produce a graded photolysis of the cage and an uneven release of Ca2+ across the cell because of the inner filtering effect by azid-1 as a result of its strong absorption. Thus, for most onephoton uncaging experiments, moderate extinction coefficients (500|[ndash]|5,000 M|[minus]|1 cm|[minus]|1) can be advantageous. But smaller cells or experiments that require low concentrations of substrate to be uncaged or have a slow time frame can tolerate caging chromophores with large extinction coefficients20, 59, 73.
The compatibility of caging chromophores with other fluorescent probes is another consideration when designing an experiment. Nitroaromatic chromophores are not fluorescent5, and can be used in conjunction with a wide range of probes such as fura-2, fluo-4 and all GFPs22. There are potential problems of using fluorescent caging chromophores (7-diethylaminocoumarinyl-4-methyl (DEAC), 6-bromo-7-hydroxycoumarin-4-methyl (Bhc) and others) as these have high fluorescent quantum yields and emit light in the same region as many analytical fluorescent probes.
Light sources for uncaging
The majority of uncaging experiments use flash lamps (for example, pulsed xenon or mercury arc lamps focused with a parabolic mirror) or lasers as the light source74, and there are advantages and disadvantages to both. Flash lamps are robust, reasonably cheap and can efficiently uncage compounds (up to 80%) in a 1-ms pulse69. Their relatively long pulse-width permits multiple rounds of excitation of the same molecule, as the excited state lifetime of aromatic molecules is only a few nanoseconds; hence the chemical yield from flash-lamp excitation normally exceeds the quantum yield. Being pulsed, flash lamps also conveniently obviate the need of a shutter.
The near-UV lasers that are used for uncaging are much more expensive to purchase and maintain than flash lamps. Lasers can be pulsed or continuous wave light sources. Many confocal microscopes are supplied with a continuous wave Ar-Kr laser (output 354|[ndash]| 363 nm), which can be used for uncaging in conjunction with a shutter. Pulsed lasers have been used in many experiments with caged compounds. The ideal pulsed laser (frequencydoubled ruby, pulse-width 35 ns at 347 nm) is unfortunately no longer available commercially. The widely available frequency-tripled Nd-YAG laser has a very short pulsewidth (3 ns) that limits the chemical yield to the quantum yield for a single pulse and the slow repetition rate does not permit closely spaced (less than 1 ms) multiple pulses.
Compared to flash lamps or near-UV lasers, two-photon excitation offers some potential intrinsic advantages in terms of depth of uncaging in tissues and axial confinement. Such performance is dependent, however, on the characteristics of the caged compound (Box 1 and Fig. 3). For two-photon uncaging, solid-state mode locked Ti:sapphire lasers are used. These lasers are in effect pseudo-continuous, so must also be shuttered when used for photorelease experiments.
Shutters for light sources can be mechanical or optical. Optical shutters are most often supplied with commercial confocal or two-photon microscopes and laser launches. These shutters are either acousto-optical tunable filters (AOTF) or electro-optical modulators (EOM). If very fast, precise control of uncaging is required (submillisecond timing), then AOTF or EOM must be used. But if periods of >1 ms are adequate, then mechanical shutters work well.
Use with cells
Caged compounds need to be applied to cells in a controlled manner when hormones and transmitters are being used for extracellular uncaging or loaded into the cytosol if second messengers are being released. For quantitative understanding of the effects of uncaging, the amount released per light pulse needs to be measured.
Cell loading and extracellular application
Caged second messengers have been loaded into cells by various means. The most precise way to do this is via a patch pipette. A known concentration of caged compound can be dialyzed into the cell cytosol using this method, with a defined concentration of any other compound such as a Ca2+ dye. This technique is especially useful with caged Ca2+ compounds, as a Ca2+ dye and a known amount of Ca2+ typically accompany the probe69, 70, 71, 72. Caged compounds can be made cell permeable using the acetoxymethyl (AM) esterification technique popular with Ca2+ dyes. Cells are loaded by exposing them to a relatively low (< 5 |[mu]|M) extracellular concentration of the cell-permeable AM version of the compound. The caged compound is de-esterified by intracellular esterases. This loading method will lead to qualitative results because the amount of caged compound in the cells is unknown75, 76. These two approaches are essentially complementary in their advantages and disadvantages: dialyzing the cell gives a known concentration of caged compound but also robs the cytosol of its normal milieu, whereas the AM technique preserves the latter at the expense of precision.
Microinjection and the use of cell penetrating peptides (CPPs) offer alternatives to whole-cell patch clamp and AM esters. Microinjection, like patch clamp, is a skill that requires much practice to perfect. It is less precise than the patch clamp technique in defining the final concentration of caged compound, as the cells' cytosolic volume always varies. It does, however, have the advantages that the normal cytosolic milieu is maintained and many cells can be loaded in a few minutes19, 51, 54. CPPs have been extensively studied as a means of transporting molecules into cells, as they are an especially attractive means of potentially selectively loading drugs into cells. The mechanisms of loading are far from being completely understood; endocytosis and simple plasma membrane permeation are two possible routes into cells, and it is sometimes thought that both mechanisms occur in parallel. This technique has much potential, but has only been used in one experiment with caged compounds so far53.
Passive diffusion and detergent plasma membrane permeablization77 are the remaining methods that have been used to load caged compounds into cells. The latter is quite disruptive to cells, and sometimes cannot be tolerated by them at all. Passive diffusion can only be used when the plasma membrane is actually permeable to the caged compound, and the compound is sufficiently water soluble as to be useful inside the cell30; this is clearly a very fine balance. Almost no caged compounds actually satisfy these criteria. These methods require the caged compound to be continuously applied to cells at a high concentration in a relatively large volume, as in both techniques the cytosol is homeostatic with the extracellular fluid (unlike CPP and AM techniques, these passive loading methods do not concentrate caged compounds inside the cell).
Caged peptides and proteins usually require microinjection or loading into cells via a patch pipette (reviewed in ref. 46). This requirement can be overcome by linking the caged peptide to a CPP. Inclusion of a fluorescent label can allow easy visualization of cellular uptake53).
Extracellular application of caged hormones and neurotransmitters is far simpler than intracellular loading. The caged compound can be applied to the cell-bathing solution7, 9, 78 or locally to one part of the preparation via a picospritzer10. The former method consumes large quantities of caged compounds, but probably defines the concentration more accurately than the latter method.
Quantification of uncaging
Quantification of the amount of photolysis in situ is often an important part of the use of photorelease technology. One solution to this challenging problem is to couple a fluorescence change to the uncaging event; this is really only feasible for large molecules such as peptides or proteins. An elegant realization of inherent quantification of uncaging has been realized with a method for visualizing 14-3-3 activation (that is, phosphorylation) by synthesis of a fluorescent NB-caged phosphopeptide that changes its fluorescence emission upon binding to 14-3-3 (ref. 52).
The extent of uncaging of other types molecules may be measured by fluorescence imaging or from a calibrated bio-response, or estimated from the photochemical properties of the caged compound and the measured flux density in the image plane. Fluorescent Ca2+ dyes have been used extensively for quantification of Ca2+ uncaging in situ69, 70, 71, 72, 79, 80, 81, 82, 83. Good indicators have not been made for other caged compounds, so the extent of uncaging of these probes must be estimated from a calibration of the flux density of the uncaging beam in the sample, along with the known photophysical properties of the caged compound (quantum yield, extinction coefficient and concentration). If uncaging is performed on the stage of a fluorescent microscope, a simple means of quantification of uncaging has been devised. NPE-caged ATP releases one proton for every molecule of ATP, so from the pH change in a droplet (not a cell) containing a known concentration of NPEATP, the amount of ATP liberated can be estimated, and provide a simple means to estimate the photon flux (only knowledge of the relative flux is required). The percentage photolysis of caged compound (X) actually used is then a ratio of the concentrations and properties of NPE-ATP and caged X, and the relative photon fluxes84.
Features and advantages of uncaging
Uncaging has many useful features and advantages compared to other methods for changing the concentration of a molecule inside or on living cells. Specifically, uncaging can be: intracellular, extremely rapid, controlled in time or space, and quantitatively controlled and repeated. There have been many hundreds of studies published using caged compounds. I have selected a few of these to illustrate the advantages of uncaging (see Box 2 for tips on handling caged compounds). Of course, many of these experiments use more than one of the aspects mentioned above simultaneously.
Box 2. Handling Caged Compounds.
Commercially available caged compounds (Table 1) are stored as solids and are sold in small quantities (1–10 mg aliquots). The most convenient way to handle these is to dissolve the entire aliquot in water, dividing this solution into aliquots and storing them at –80°C until needed. Of the compounds listed in Table 1, only caged cAMP and fluorescein are not highly soluble in water, solutions of at least 100 mM can be made of the other compounds. Solutions of calcium cages are stable for many years frozen. MNI-Glu is more stable than CNB-Glu: CNB-Glu has a half-life at room temperature of about 27 h, whereas no hydrolysis of MNI-Glu can be detected after 8 h at room temperature (pH 7.4). Solutions of MNI-D-aspartate can be stored at 4°C for 2 d without detectable hydrolysis99. NPE-caged phosphates have also been found to be highly stable in solution. Exposure to white light should be kept to a minimum. In practice it has been found that caged compounds are not hypersensitive to low levels of room light, but the epi-illumination path of microscopes must be filtered appropriately. I use Roscolux number 10 yellow light filters for all white lights in my lab.
Speed of release
The rate of photorelease of the caged substrate from biologically inert precursor is generally much faster than rapid changing of the solution. This is especially true of complex biological preparations in which it is very difficult to displace the nonactivating solution with a new mixture containing the crucial activating component. In part, this is due to the tortuous nature of many biological preparations and their delicate nature, but also there are inevitable diffusional delays that arise from simple mass action. Use of caged compounds permits the intimate proximity of effector and receptor that bypasses these difficulties. Thus, calcium uncaging in single skeletal muscle fibers activates contraction about five times faster than the most rapid solution change85. Multidisciplinary techniques have become so refined that NPE-ATP has been used to measure the rate of force generation by a single dynein molecule (Fig. 4a).
Figure 4. Examples of biological applications of caged compounds.
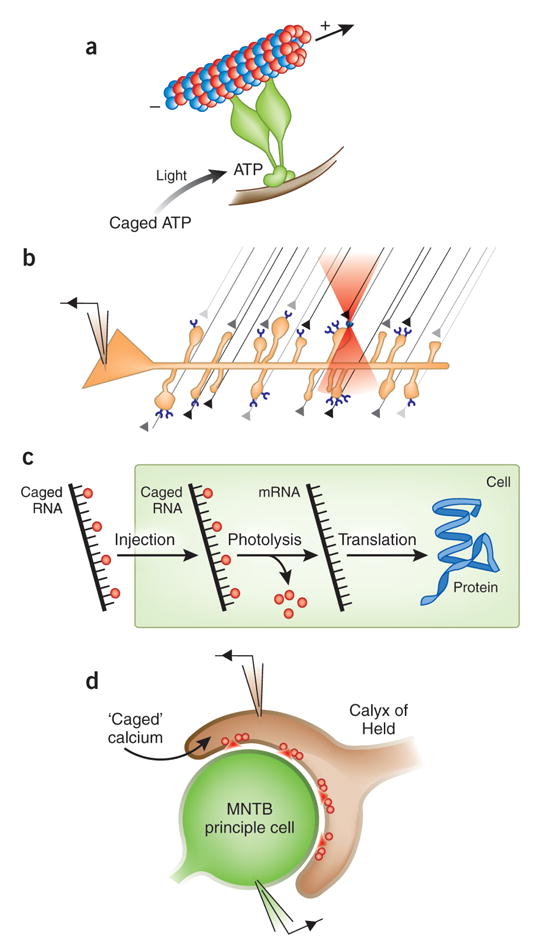
(a) The rate of force development generated by a single dynein molecule can be measured using an optical trap after photolysis of NPE-ATP96. Uncaging of ATP develops 6 pN of force from one dynein arm moving rapidly. (b) Strategy for mapping of APMA-receptor density by two-photon uncaging of glutamate. (c) Scheme for uncaging of mRNA in zebrafish. mRNA encoding the protein of interest is caged, injected into zebrafish at the single-cell stage and uncaged in a discrete volume of the zebrafish at a selected time during development. (d) Quantitative, rapid uncaging of Ca2+ in the calyx of Held allows correlation of presynaptic [Ca2+] with evoked postsynaptic currents82.
Location of release
Another powerful aspect of uncaging technology is that release only occurs where light is incident. Thus, filling the cytosolic compartment of cells with caged compounds permits either global or local concentration changes of the caged compound to be effected simply by using whole-cell or subcellular illumination. For example, an important advantage of intracellular Ca2+ uncaging mentioned above was that since Ca2+ release was evenly distributed throughout the cytosol (that is, global uncaging), Ca2+ microfluorometry could be used to measure the [Ca2+], giving an estimate of the [Ca2+] at the plasma membrane from channel opening. Local (subcellular) uncaging of Ca2+ in longitudinally projecting motor neurons slows rapid growth, consistent with previous observations on pathfinding that growth cone stalling and axon retraction are associated with high-frequency [Ca2+] transients75, 86. Similar types of experiments have been performed with caged IP3 (refs. 84,87). A focused UV beam permits such local uncaging, especially in already small intracellular compartments, but extracellular confinement of uncaging cannot be assisted by cellular geometry, so experimenters have turned to the inherent three-dimensionality of two-photon excitation to achieve localized release of neurotransmitters in the extracellular space78 such that diffraction-limited uncaging of glutamate is now possible (Fig. 4b). This technique is starting to find wide application10, 88, 89, 90, 91.
Timing of release
Since uncaging releases its active component independently of the normal biological source, the timing of release can be dictated solely by experimenter and imposed upon the cell in any temporal pattern. Thus, uncaging is orthogonal to the metabolic history of the cell or animal. A striking example of this is the use of caged mRNA that was injected into zebrafish at the one-cell embryonic stage, then uncaged at least 24 h later, at a particular point during development (Fig. 4c).
Intracellular release
Since its inception, the uncaging technique has been used for intracellular release13, 30 as light passes through the plasma membrane, enabling concentration changes in the otherwise inaccessible intracellular compartment (Fig. 3c,d). This aspect of the uncaging method is probably the single most powerful advantage compared to other methods of changing solute concentrations. A classic and important example of intracellular uncaging was the photorelease of IP3 in smooth and skeletal muscle when biological uncaging was still in its infancy11. Loading of caged IP3 into relaxed muscle, followed by rapid uncaging showed that only in smooth muscle could this second messenger mobilize intracellular Ca2+ fast enough to mimic physiological stimulations. This definitively settled a hotly debated question of the time. Intracellular uncaging of Ca2+ has also been widely used to study secretory processes in many cell types69, 70, 71, 72, 79, 80, 81, 82, 83.
Quantitation of release
Since the 'calcium hypothesis' for neurotransmitter release92 was proposed, obtaining a quantitative relationship between presynaptic [Ca2+] and postsynaptic response has been something of a 'Holy Grail' for synaptic physiologists. A fundamental technical difficulty with obtaining the correlation has been the size of the Ca2+ microdomains that result from channel opening near the plasma membrane; namely, they are so small that they are beyond the resolution of the light microscope. Global uncaging of Ca2+ circumvented this problem, allowing precise correlations between synaptic [Ca2+] and postsynaptic current to be measured at a central synapse for the first time82, 83 (Fig. 4d).
Future directions
Photolysis of caged compounds is now a well-established technique for studying living cells. A vast array of biomolecules have been caged by chemists and used in biological experiments. The most important of these caged compounds are commercially available (Table 1). The great majority of applications of these compounds use regular near-UV light for uncaging. Recently, two-photon excitation of caged compounds has become practical using mode-locked, Ti:sapphire lasers. However, relatively few caged biomolecules (glutamate10, 93 and calcium43) undergo useful, let alone exceptional two-photon excitation. Development of new chromophores with excellent two-photon cross-sections is one frontier for probe development. This is especially important if biologists want to apply uncaging to living animals as the complex biological tissue of the body is highly scattering to light, especially at short wavelengths (350|[ndash]|550 nm). A second frontier for probe development is photoreversible caged compounds. All the probes discussed in this review are activated unidirectionally. Recently techniques for photochemical switching of ion channel conduction94, and the |[alpha]|-helical content of peptides have been developed95. These studies are signposts for new directions for caged compounds.
Acknowledgments
The Ellis-Davies laboratory is supported by grants from the US National Institutes of Health GM53395, GM65473 and MH0717505.
References
- 1.Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem Int Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Lipp P. Calcium-life and death signal. Nature. 1998;395:645–649. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 3.Adams SR, Kao JPY, Grynkiewicz G, Minta A, Tsien RY. Biologically useful chelators that release Ca2+ upon illumination. J Am Chem Soc. 1988;110:3212–3220. [Google Scholar]
- 4.Ellis-Davies GCR, Kaplan JH. A new class of photolabile chelators for the rapid release of divalent cations: generation of caged Ca and caged Mg. J Org Chem. 1988;53:1966–1969. [Google Scholar]
- 5.Ellis-Davies GCR, Kaplan JH. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci USA. 1994;91:187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis-Davies GCR. Synthesis of photolabile EGTA derivatives. Tetrahedr Lett. 1998;39:953–957. [Google Scholar]
- 7.Walker JW, McCray JA, Hess GP. Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of onitrobenzyl derivatives and their effects on receptor function. Biochemistry. 1986;25:1799–1805. doi: 10.1021/bi00355a052. [DOI] [PubMed] [Google Scholar]
- 8.Milburn T, et al. Synthesis, photochemistry and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989;28:49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- 9.Wieboldt R, et al. Photolabile precursors of glutamate: Synthesis, photochemical properties, activation of glutamate receptors in the microsecond time scale. Proc Natl Acad Sci USA. 1994;91:8752–8756. doi: 10.1073/pnas.91.19.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki M, et al. Dendritic spine morphology is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JW, et al. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987;327:249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- 12.Walker JW, Feeney J, Trentham DR. Photolabile precursors of inositol phosphates. Preparation and properties of 1-(2-nitrophenyl)ethyl esters of myoinositol 1,4,5-trisphosphate. Biochemistry. 1989;28:3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan JH, Forbush B, Hoffman JF. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 14.Walker JH, Reid GP, McCray JA, Trentham DR. Photolabile 1-(2-nitrophenyl) ethyl phosphate esters of adenine nucleotide analogues. Synthesis and mechanism of photolysis. J Am Chem Soc. 1988;110:7170–7177. [Google Scholar]
- 15.Walker JW, et al. Signaling pathways underlying eosinophil cell motility revealed by using caged peptides. Proc Natl Acad Sci USA. 1998;95:1568–1573. doi: 10.1073/pnas.95.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman DM, et al. Caged phosphoproteins. J Am Chem Soc. 2005;127:846–847. doi: 10.1021/ja043875c. [DOI] [PubMed] [Google Scholar]
- 17.Marriott G. Caged protein conjugates and light-directed generation of protein activity: preparation, photoactivation, and spectroscopic characterization of caged Gactin conjugates. Biochemistry. 1994;33:9092–9097. doi: 10.1021/bi00197a010. [DOI] [PubMed] [Google Scholar]
- 18.Mendel D, Elman JA, Schultz PG. Construction of light-activated protein-protein interactions. J Am Chem Soc. 1991;113:2758–2760. [Google Scholar]
- 19.Ghosh M, et al. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 20.Ando H, Furuta T, Tsien RY, Okamoto H. Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos. Nat Genet. 2001;28:317–325. doi: 10.1038/ng583. [DOI] [PubMed] [Google Scholar]
- 21.Monroe WT, McQuain MM, Chang MS, Alexander JS, Haselton FR. Targeting expression with light using caged DNA. J Biol Chem. 1999;274:20895–20900. doi: 10.1074/jbc.274.30.20895. [DOI] [PubMed] [Google Scholar]
- 22.Kasai H. Comparative biology of Ca-dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci. 1999;22:88–93. doi: 10.1016/s0166-2236(98)01293-4. [DOI] [PubMed] [Google Scholar]
- 23.Judkewitz B, Roth A, Hausser M. Dendritic enlightenment: using patterned twophoton uncaging to reveal the secrets of the brain's smallest dendrites. Neuron. 2006;50:180–183. doi: 10.1016/j.neuron.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 25.McGall GH, et al. The efficiency of light-directed synthesis of DNA arrays on glass substrates. J Am Chem Soc. 1997;119:5081–5090. [Google Scholar]
- 26.McGall GH, Christians FC. High-density genechip oligonucleotide probe arrays. Adv Biochem Eng Biotechnol. 2002;77:22–42. doi: 10.1007/3-540-45713-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Ellis-Davies GCR. In: Caged Glutamate For Use in the CNS New Encyclopedia of Neuroscience. Squire L, Albright T, Bloom F, Gage F, Spritzer N, editors. Elsevier; Oxford: in the press. [Google Scholar]
- 28.Gee KR, Wieboldt R, Hess GP. Synthesis and photochemistry of a new photolabile derivative of GABA. J Am Chem Soc. 1994;116:8366–8367. [Google Scholar]
- 29.Molnar P, Nadler JV. γAminobutyrate, a-carboxy-2-nitrobenzyl ester selectively blocks inhibitory synaptic transmission in rat dentate gyrus. Eur J Pharm. 2000;391:255–262. doi: 10.1016/s0014-2999(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 30.Engels J, Schlaeger EJ. Synthesis, structure and reactivity of adenosine cyclic 3', 5'-phosphate benzyl triesters. J Med Chem. 1977;20:907–911. doi: 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]
- 31.Dantzig JA, Higuchi H, Goldman YE. Studies of molecular motors using caged compounds. Methods Enzymol. 1998;291:307–348. doi: 10.1016/s0076-6879(98)91021-7. [DOI] [PubMed] [Google Scholar]
- 32.Ogden D, Khodakhah K. Intracellular Ca2+ release by InsP3 in cerebellar Purkinje neurones. Acta Physiol Scan. 1996;157:381–394. doi: 10.1046/j.1365-201X.1996.28252000.x. [DOI] [PubMed] [Google Scholar]
- 33.Bamberg E, Clarke RJ, Fendler K. Electrogenic properties of the Na+,K+-ATPase probed by presteady state and relaxation studies. J Bionerg Biomembr. 2001;33:401–405. doi: 10.1023/a:1010667407003. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Corrie JT, Wootton JF. Photolabile precursors of cyclic nucleotides with high aqueous solubility and stability. J Org Chem. 2002;67:3474–3478. doi: 10.1021/jo020040g. [DOI] [PubMed] [Google Scholar]
- 35.Thirlwell H, et al. Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys J. 1994;67:2436–2447. doi: 10.1016/S0006-3495(94)80730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geibel S, et al. P(3)-[2-(4-hydroxyphenyl)-2-oxo]ethyl ATP for the rapid activation of the Na+,K+-ATPase. Biophys J. 2000;79:1346–1357. doi: 10.1016/S0006-3495(00)76387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantevari S, Egger M, Hoing C, Niggli E, Ellis-Davies GCR. Synthesis and 2- photon photolysis of 6-(ortho-nitroveratryl)-caged IP3. ChemBioChem. 2006;7:174–180. doi: 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- 38.Hagen V, et al. Highly efficient and ultrafast phototriggers for cAMP and cGMP by using long-wavelength UV/vis-activation. Angew Chem Int Ed. 2001;40:1045–1048. doi: 10.1002/1521-3773(20010316)40:6<1045::aid-anie10450>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Furuta T, et al. Bhc-cNMPs as either water-soluble or membrane-permeant photoreleasable cyclic nucleotides for both one- and two-photon excitation. ChemBioChem. 2004;5:1119–1128. doi: 10.1002/cbic.200300814. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester analog shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 41.Ellis-Davies GCR. Development and application of calcium cages. Meth Enzymol. 2003;360A:226–238. doi: 10.1016/s0076-6879(03)60112-6. [DOI] [PubMed] [Google Scholar]
- 42.Adams SR, Lev-Ram V, Tsien RY. A new caged Ca2+, azid-1, is far more photosensitive than nitrobenzyl-based chelators. Chem Biol. 1997;4:867–878. doi: 10.1016/s1074-5521(97)90119-8. [DOI] [PubMed] [Google Scholar]
- 43.Momotake A, Lindegger N, Niggli N, Barsotti RJ, Ellis-Davies GCR. The nitrodibenzofuran chromophore-a new caging group for ultra efficient photolysis in living cells. Nat Methods. 2006;3:35–40. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- 44.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 45.Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca2+-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- 46.Marriott G, Roy P, Jakobson K. Preparation and light-directed activation of caged proteins. Methods Enzymol. 2003;360:274–288. doi: 10.1016/s0076-6879(03)60115-1. [DOI] [PubMed] [Google Scholar]
- 47.Chang C, Fernandez T, Panchal R, Bayley H. Caged catalytic subunit of cAMPdependent protein kinase. J Am Chem Soc. 1998;120:7661–7662. [Google Scholar]
- 48.Curley K, Lawrence DS. Photoactivation of a signal transduction pathway in living cells. J Am Chem Soc. 1998;120:8573–8574. [Google Scholar]
- 49.Petersson EJ, Brandt GS, Zacharias NM, Dougherty DA, Lester HA. Caging proteins through unnatural amino acid mutagenesis. Methods Enzymol. 2003;360:258–273. doi: 10.1016/s0076-6879(03)60114-x. [DOI] [PubMed] [Google Scholar]
- 50.Muralidharan V, Muir TW. Protein ligation: an enabling technology for the biophysical analysis of proteins. Nat Methods. 2006;3:429–438. doi: 10.1038/nmeth886. [DOI] [PubMed] [Google Scholar]
- 51.Hahn ME, Muir TW. Photocontrol of Smad2, a multiphosphorylated cell-signaling protein, through caging of activating phosphoserines. Angew Chem Int Ed. 2004;43:5800–5803. doi: 10.1002/anie.200461141. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez ME, Nitz M, Stehn J, Yaffe MB, Imperiali B. Fluorescent caged phosphoserine peptides as probes to investigate phosphorylation-dependent protein associations. J Am Chem Soc. 2003;125:10150–10151. doi: 10.1021/ja0351847. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen A, Rothman DM, Stehn J, Imperiali B, Yaffe MB. Caged phosphopeptides reveal a temporal role for 14–3-3 in G1 arrest and S-phase checkpoint function. Nat Biotechnol. 2004;22:993–1000. doi: 10.1038/nbt997. [DOI] [PubMed] [Google Scholar]
- 54.Wood JS, Koszelak M, Liu J, Lawrence DS. A caged protein kinase inhibitor. J Am Chem Soc. 1998;120:7145–7146. [Google Scholar]
- 55.Bosques CJ, Imperiali B. Photolytic control of peptide self-assembly. J Am Chem Soc. 2003;125:7530–7531. doi: 10.1021/ja035360b. [DOI] [PubMed] [Google Scholar]
- 56.Ottl J, Gariel D, Marriott G. Preparation and photoactivation of caged fluorophores and caged proteins using a new class of heterobifunctional, photocleavable cross-linking reagents. Bioconjug Chem. 1998;9:143–151. doi: 10.1021/bc970147o. [DOI] [PubMed] [Google Scholar]
- 57.Ando H, Okamoto H. Practical procedures for ectopic induction of gene expression in zebrafish embryos using Bhc-diazo-caged mRNA. Methods Cell Sci. 2003;25:25–31. doi: 10.1023/B:MICS.0000006846.13226.38. [DOI] [PubMed] [Google Scholar]
- 58.Okamoto H, Hirate Y, Ando H. Systematic identification of factors in zebrafish regulating the early midbrain and cerebellar development by ordered differential display and caged mRNA technology. Front Biosci. 2004;9:93–99. doi: 10.2741/1205. [DOI] [PubMed] [Google Scholar]
- 59.Ando H, et al. Lhx2 mediates the activity of Six3 in zebrafish forebrain growth. Dev Biol. 2005;287:456–468. doi: 10.1016/j.ydbio.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 60.Khan S, et al. Excitatory signaling in bacterial probed by caged chemoeffectors. Biophys J. 1993;65:2368–2382. doi: 10.1016/S0006-3495(93)81317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Namiki S, Arai T, Fujimori K. High-performance caged nitric oxide: a new molecular design, synthesis and photochemical reaction. J Am Chem Soc. 1997;119:2840–3841. [Google Scholar]
- 62.Goeldner M, Givens R, editors. Dynamic Studies in Biology. Wiley-VCH; Weinheim, Germany: 2005. [Google Scholar]
- 63.Furuta T, et al. Brominated 7-hydroxycoumarin-4-ylmethyls: photolabile protecting groups with biologically useful cross-sections for two-photon photolysis. Proc Natl Acad Sci USA. 1999;96:1193–1200. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papageorgiou G, Ogden DC, Barth A, Corrie JT. Photorelease of carboxylic acids from 1-acyl-7-nitroindolines in aqueous solution: rapid and efficient photorelease of L-glutamate. J AM Chem Soc. 1999;121:6503–6504. [Google Scholar]
- 65.Wettermark G. Photochromism of o-nitrotoluenes. Nature. 1962;194:677. [Google Scholar]
- 66.Gaplovsky M, et al. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: 2-nitrobenzyl alcohols form 2-nitroso hydrates by dual proton transfer. Photochem Photobiol Sci. 2005;4:33–42. doi: 10.1039/b409927c. [DOI] [PubMed] [Google Scholar]
- 67.Hirayama Y, Iwamura M, Furuta T. Design, synthesis and photochemical properties of caged bile acids. Bioorg Med Chem Lett. 2003;13:905–908. doi: 10.1016/s0960-894x(02)01074-0. [DOI] [PubMed] [Google Scholar]
- 68.Havinga E, De Jongh RO, Dorst W. Photochemical acceleration of the hydrolysis of nitrophenyl phosphates and nitrophenyl sulfates. Recl Trav Chim Pays-Bas. 1956;75:378–383. [Google Scholar]
- 69.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 70.Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 71.Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca binding and Ca clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 73.Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotidegated channels. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rapp G, et al. Lasers and flashlamps in research on the mechanism of muscle contraction. Ber Bunsenges Phys Chem. 1989;93:410–415. [Google Scholar]
- 75.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1988. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 76.Fellin T, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2005;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 77.Zimmermann B, Ellis-Davies GCR, Kaplan JH, Somlyo AV, Somlyo AP. Kinetics of contractions initiated by photolysis of caged calcium and caged ATP in smooth muscle. J Biol Chem. 1995;270:23966–23874. doi: 10.1074/jbc.270.41.23966. [DOI] [PubMed] [Google Scholar]
- 78.Denk W. Two-photon scanning photochemical microscopy: mapping ligand-gated ion channel distributions. Proc Natl Acad Sci USA. 1994;91:6629–6633. doi: 10.1073/pnas.91.14.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 80.Thomas P, Wong PG, Lee A, Almers WW. A low affinity Ca receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993;11:93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- 81.Kasai H, et al. Two components of exocytosis and endocytosis in phaeochromocytoma cells studied using caged Ca compounds. J Physiol. 1996;494:53–65. doi: 10.1113/jphysiol.1996.sp021475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 83.Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 84.Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci USA. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaplan JH, Ellis-Davies GCR. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci USA. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomez TM, Spitzer NC. In vivo regulation of axon and pathfinding by growth cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 87.Finch EA, Augustine GJ. Local calcium signaling by IP3 in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 88.Smith MA, Ellis-Davies GCR, Magee JC. Synaptic mechanisms of the distancedependent scaling of Schaffer collateral synapses in CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Sobczyk A, Scheuss V, Svoboda K. NMDA Receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Béïque JC, Da-Ting Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katz B, Miledi R. The effect of calcium on acetylcholine release from motor nerve terminals. Proc Roy Soc B Biol Sci. 1965;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- 93.Ellis-Davies GCR, Momotake M, Paukert M, Kasai H, Bergles DW. 4-carboxymethoxy-5,7-dinitroindolinyl-Glu: an improved caged glutamate for expeditious ultraviolet and 2-photon photolysis in brain slices. J Neurosci. 2007;27:6601–6604. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumita JR, Smart OS, Woolley GA. Photo-control of helix content in a short peptide. Proc Natl Acad Sci USA. 2000;97:3803–3808. doi: 10.1073/pnas.97.8.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shingyoji C, Higuchi H, Yoshimura M, Katayama E, Yanagida T. Dynein arms are oscillating force generators. Nature. 1998;393:711–714. doi: 10.1038/31520. [DOI] [PubMed] [Google Scholar]
- 97.Denk W, Stricker JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 98.Brown EB, et al. Photolysis of caged calcium in femtoliter volumes using two-photon excitation. Biophys J. 1999;76:489–499. doi: 10.1016/S0006-3495(99)77217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang YH, Sinha SR, Fedoryak OD, Ellis-Davies GCR, Bergles DE. Synthesis and characterization of MNI-D-aspartate, a caged compound for selective activation of glutamate transporters and NMDA receptors in brain tissue. Biochemistry. 2005;44:3316–3326. doi: 10.1021/bi048051m. [DOI] [PubMed] [Google Scholar]