Abstract
Important for energy metabolism, neurotransmission, bone stability, and other cellular functions, Mg2+ has well-established and undisputedly critical roles in adult tissues. Its contributions to early embryonic development are less clearly understood. For decades it has been known that gestational Mg2+ deficiency in rodents produces teratogenic effects. More recent studies have linked deficiency in this vital cation to birth defects in humans, including spina bifida, a neural fold closure defect in humans that occurs at an average rate of 1 per 1000 pregnancies. The first suggestion that Mg2+ may be playing a more specific role in early development arose from studies of the TRPM7 and TRPM6 ion channels. TRPM7 and TRPM6 are divalent-selective ion channels in possession of their own kinase domains that have been implicated in the control of Mg2+ homeostasis in vertebrates. Disruption of the functions of these ion channels in mice as well as in frogs interferes with gastrulation, a pivotal process during early embryonic development that executes the emergence of the body plan and closure of the neural tube. Surprisingly, gastrulation defects produced by depletion of TRPM7 can be prevented by Mg2+ supplementation, indicating an essential role for Mg2+ in gastrulation and neural fold closure. The aim of this review is to summarize the data emerging from molecular genetic, biochemical and electrophysiological studies of TRPM6 and TRPM7 and provide a model of how Mg2+, through these unique channel-kinases, may be impacting early embryonic development.
Keywords: TRPM6, TRPM7, ion channel, kinase, magnesium, gastrulation
Introduction
Magnesium is essential for life. It is the fourth most abundant cation in the body and the second most abundant intracellular cation [1]. Involved in numerous biological functions, including cell cycle, channel regulation, ATPase activity, metabolic regulation, it is estimated that at least 350 enzymes are directly or indirectly regulated by Mg2+ [2]. While Mg2+ is linked to a growing number of physiological and pathological roles in adult animals and humans, the cation’s contribution to embryonic development is less understood. Unlike Ca2+, whose influence over embryonic development is firmly established [3,4], only a handful of studies have implicated Mg2+ in this process. Studies of the effects of gestational Mg2+ deficiency in rats were among the first reports to demonstrate that Mg2+ is required for embryonic development [5,6]. Pregnant females fed a Mg2+ deficient diet between days 6 and 14 of gestation showed a high incidence of resorptions and gross malformation in the full term fetuses [6]. Pups born from Mg2+ deficient pregnant females also displayed growth retardation, an increased incidence of abnormal fat metabolism, insulin resistance and diabetes [7]. More recent epidemiological studies have associated Mg2+ deficiency with a negative impact on human fetal growth and development as well. A decrease in basal [Mg2+]i from cord blood platelets is associated with small for gestational age (SGA) babies [7]. Oral Mg2+ supplementation given before the 25th week of gestation is also associated with a lower frequency of preterm births, a lower frequency of low birth weight, and fewer SGA infants compared with placebo [8]. The recommended dietary allowance (RDA) in the United States for magnesium is 410–420 mg per day for a male adult and 320–360 mg per day for a female; the magnesium requirement is increased during pregnancy and lactation (320–400 mg/day), depending on age [9]. A maternal Mg2+ intake below 378 mg/day has been associated with a 2- to 3-fold higher risk for the neural tube defect, spina bifida in offspring, suggesting a pivotal role for Mg2+ not just in growth but in morphogenesis as well [10].
Xenopus laevis as a model system to study the role of Mg2+ in early development
The advantages of using Xenopus laevis (African clawed frog) to study early development include large egg size, large numbers of eggs from a single female frog, and most importantly rapid development ex-utero, which allows the concentration of ions in the medium to be easily manipulated. Early studies by Brown showed that Xenopus laevis embryos reared in a medium with low Mg2+ ion levels (≤10−6 M) will be arrested in their growth and exhibit edema, paralysis and death at a time their normally reared sibling are beginning to feed [11]. A more detailed description of the effect of Mg2+ deficiency on Xenopus laevis embryogenesis was conducted later by Miller and Lanesman [12]. The authors reported that Xenopus laevis embryos reared in media with Mg2+ concentrations of 10−2 to 10−4 M develop normally while those grown in media with initial Mg2+ ion concentrations of 10−5 M, 10−6 M, 10−7 M, or lower often exhibited edema and gastrulated poorly, showing a curved axis and reduced rates of tail expansion, limited coiling of the gut, slowed head enlargement and restricted heart growth. Magnesium-deficient embryos can also be distinguished from their normal siblings because their melanophores are smaller and more punctate than normal [12]. In addition, Mg2+ deficiency also causes incomplete formation of the pigmented layer of the eye [12]. In general, tissues that undergo the greatest part of their growth and differentiation after hatching are the most severely affected by Mg2+ deficiency. For example, somites that are well organized at stage 31 (early tadpole stage) in the magnesium-deficient embryo become severely disorganized by stage 42 (late tadpole stage) [12]. While this study was the first to indicate a critical requirement for Mg2+ in early development, it did not necessarily suggest a role for Mg2+ in actively controlling morphogenesis. The notion that Mg2+ may have signaling functions akin to those uncovered for Ca2+ emerged from functional studies of the TRPM7 and TRPM6 ion channels [13].
TRPM7 and TRPM6 are required for early development
TRPM7 and TRPM6 are unique bifunctional proteins with ion channel and kinase domains (for a more detailed review see [14]). The channels are permeable to a wide range of divalent cations, including Mg2+, Ca2+ and Zn2+ [15,16,17]. Deletion of TRPM7 from mice disrupts embryonic development, with the mice dying before day 7.5 of embryogenesis [18,19]. Mutations in TRPM6 have been clearly linked to familial hypomagnesemia with secondary hypocalcemia (HSH) [20,21]. HSH is characterized by very low Mg2+ and low Ca2+ serum levels. Shortly after birth affected individuals exhibit neurologic symptoms of hypomagnesemic hypocalcemia, including seizures and muscle spasms, but otherwise develop normally if treated with magnesium supplements. In contrast, mice defective in TRPM6 showed early embryonic mortality and neural tube defects: 10% of TRPM6-null homozygotes had spinabifida occulta, 0% had spina bifida aperta, and 30% had exencephaly [22]. No mutations in TRPM7 have reported for HSH patients, however, heterozygous mice engineered to lack the kinase domain (TRPM7ΔKINASE) developed signs of hypomagnesaemia and exhibited a defect in intestinal Mg2+ absorption [18]. Cells derived from heterozygous TRPM7ΔKINASE mice had reduced TRPM7 currents, which likely reduced intestinal Mg2+ uptake in the animals [18]. A more severe phenotype was observed for homozygous TRPM7ΔKINASE null embryos, which were able to initiate gastrulation and mesoderm formation, but died between day 7.5 and 8.5 of embryogenesis. Thus, studies in mice have indicated a critical role for TRPM6 and TRPM7 in early embryogenesis, but how the channels may have contributed to morphogenesis remained unclear. Investigation of TRPM7’s functions in Xenopus laevis provided the first indication that these channels’ ability to permeate Mg2+ may be a key factor in their control over early embryogenesis.
TRPM7 regulates gastrulation during vertebrate embryogenesis
Following the blastula stage of development, coordinated movements of gastrulation begin, which transform a simple hollow ball of cells into a multilayered structure with a central gut tube and bilateral symmetry, containing the three germ layers from which the tissues of the adult vertebrate body are generated. During gastrulation, cells in the dorsal marginal zone undergo convergent extension movements, in which polarized cell movements establish the physical body axis of the developing embryo [23]. During gastrulation the non-canonical Wnt pathway (planar cell polarity (PCP) pathway) regulates convergent extension movements through activation of the small GTPases Rho and Rac via the cytoplasmic phospho protein Dishevelled [24]. Depletion of TRPM7 from developing Xenopus laevis embryos using anti-sense morpholino technology resulted in gastrulation defect phenotypes [25]. In Xenopus laevis TRPM7 depleted embryosaxial extension was impaired, resulting in a severe dorsal-flexure and failure of the blastopore and neural folds to close. In addition, anterior structures, including the head, eyes and cement glands, were reduced or absent. One of the advantages of the Xenopus laevis system is the ability to conduct gain-of-function analysis. Strikingly, the phenotypes caused by depletion of TRPM7 were prevented by Mg2+ supplementation, by expression of TRPM6, as well as by expression of the Mg2+ transporter SLC41A2. In contrast, elevating levels of Ca2+ in the media failed to rescue the phenotype caused by depletion of TRPM7. Surprisingly, no function for TRPM7’s kinase domain was revealed in these studies.
As mentioned above the non-canonical Wnt pathway regulates convergent extension movements through the cytoplasmic phosphoprotein Dishevelled (Dvl) [26]. Activation of Dvl stimulates the coordinated activation of Rho and Rac to control cytoskeletal remodeling required for convergent extension cell movements [27]. Expression of a constitutively active form of Dvl suppressed the gastrulation phenotype caused by depletion of TRPM7, indicating that TRPM7 is likely functioning upstream of Dvl to regulate convergent extension movements. Dvl controls activation of the Rho pathway through the form in protein Daam1 [28]. A constitutively active form of Daam1 failed to rescue the XTRPM7 MO-induced phenotype, indicating that TRPM7 does not influence convergent extension movements through Rho [25]. Rather, Dvl’s control of Rac, which regulates convergent extension movements via the c-Jun N-terminal kinase (JNK) pathway, was impaired in XTRPM7 depleted embryos.
Investigation of TRPM7’s role in cell migration using fibroblasts gave additional insight into the results obtained in Xenopus laevis. Depletion of TRPM7 from fibroblasts interfered with cells’ ability to polarize and migrate directionally [29]. Similar to what was observed in Xenopus laevis, TRPM7’s channel activity was essential to its control of polarized cell movements via Rac. In addition, activation of the small GTPase Cdc42, which governs cell polarity, was impaired in TRPM7-depleted fibroblasts. In both systems, expression of the Mg2+ transporter SLC41A2 was effective in reversing the phenotype produced by reduced TRPM7 expression. Together, these results indicate that the embryonic lethality caused by loss of TRPM7 is dependent, at least in part, upon the channel’s ability to control polarized cell movements through Mg2+ and Rac. Studies in mice, however, have revealed that TRPM7 disruption can also result in the demise of embryonic stem cells and induced pluripotent stem cells [18,30]. These results mirror those originally obtained by Schmitz and colleagues who found that deletion of TRPM7 from DT40 cells causes cell cycle arrest [31]. Importantly, the loss of cell viability and cell cycle arrest in embryonic stem cells and DT40 cells in which TRPM7 is ablated can be suppressed by supplementation of the growth medium with Mg2+ [18,31]. Therefore, some of the effects of TRPM7 on early development may also be in part due to loss of populations of stem cells that depend on TRPM7 for survival. Furthermore, studies have also suggested that TRPM7’s ability to permeate Ca2+ can contribute to the channel’s control of cell adhesion and cell motility [32,33,34,35]. Thus, it still remains possible that both Mg2+ and Ca2+ are contributing to TRPM7’s actions in early development through both of these cations’ effects on cell adhesion [36]. For example, changes in intracellular Mg2+ could also potentially be affecting intracellular Ca2+ homeostasis and thus indirectly influencing cell migration [37]. Therefore, additional studies are required to determine whether TRPM7-dependent in flux of Ca2+ is also affecting morphogenesis. Even though more work is required to understand how Mg2+ influences gastrulation in the developing embryo, recent studies have yielded tantalizing clues to how Mg2+ may be controlling signaling events in cells.
Signaling properties of Mg2+
Of the two major intracellular divalent cations, Ca2+ is now well accepted as a major intracellular regulator [38]. Whether Mg2+ also serves as a second messenger in intracellular signaling is controversial [39,40,41]. Recently, however, Li and colleagues uncovered that mutations in the Mg2+ transporter MAGT1 is responsible for a novel X-linked human immunodeficiency characterized by CD4 lymphopenia, severe chronic viral infections, and defective T-lymphocyte activation [42]. Their study revealed that MAGT1 deficiency abolishes Mg2+ influx required for activation of phospholipase Cγ1 (PLC-γ1) and Ca2+ influx in T cells. How Mg2+ is influencing PLC-γ1 activation is not clear. Nor is it understood how Mg2+ is affecting the activity of Disheveled in Xenopus laevis embryos. One possibility is that Mg2+ acts by directly binding to the enzymes involved in these signal transduction processes to modulate catalysis [43]. Alternatively, Mg2+ may be influencing these pathways indirectly through Ca2+, as discussed earlier, or through other pathways. In support of the latter hypothesis, depletion of TRPM7 in fibroblasts has been recently shown to lower the concentration of cellular reactive oxygen species (ROS), which can be reversed by Mg2+ supplementation of the cellular growth media and by expression of the Mg2+ transporter SLC41A2 [44]. ROS are now well regarded as important regulators of cell migration and have been shown to act through several effectors [45,46]. Redox signaling has been shown to modulate Wnt-β-catenin signaling through ROS-dependent binding of nucleoredoxin to Dishevelled [47]. ROS could potentially be affecting Wnt signaling by targeting downstream effectors of Dishevelled such as JNK [48]. Interestingly, a connection between TRPM7, ROS, and JNK has already been made. Overexpression of TRPM7 in HEK-293 cells activates JNK [36]. Whereas depletion of the channel, which lowers ROS levels in a Mg2+-dependent manner, suppresses JNK activity [44]. Perturbations in extracellular Mg2+ levels can also impact intracellular ROS levels. Wolf and colleagues reported that HC11 mouse mammary epithelial cells cultured in medium containing low extracellular Mg2+ have decreased ROS levels [49]. Adding Mg2+ back to the growth medium was able to reestablish the concentration of ROS in HC11 cells to a normal level. Thus, Mg2+ is able to affect ROS levels in multiple cell types. However, the mechanism by which Mg2+ influences cellular ROS levels remains poorly understood. Lower intracellular Mg2+ may be impeding ROS production, stimulating ROS turnover, or affecting both processes simultaneously. For example, HC11 cells cultured in low Mg2+ had increased glutathione transferase activity [49]. Glutathione transferases are a family of scavenger enzymes involved in the detoxification of free radical species [50]. In addition, other studies have reported that Mg2+ can stimulate the activity of NADPH oxidase, a major source of ROS within cells [51,52]. It is also conceivable that Mg2+ may be affecting ROS production by mitochondria [53]. At this point, the role of Mg2+ in cell signaling remains poorly understood. Additional work is needed to give a clearer picture of how Mg2+ is affecting the pathways controlling morphogenesis and to determine whether changes in redox status in the embryo are involved.
Conclusions and Future Work
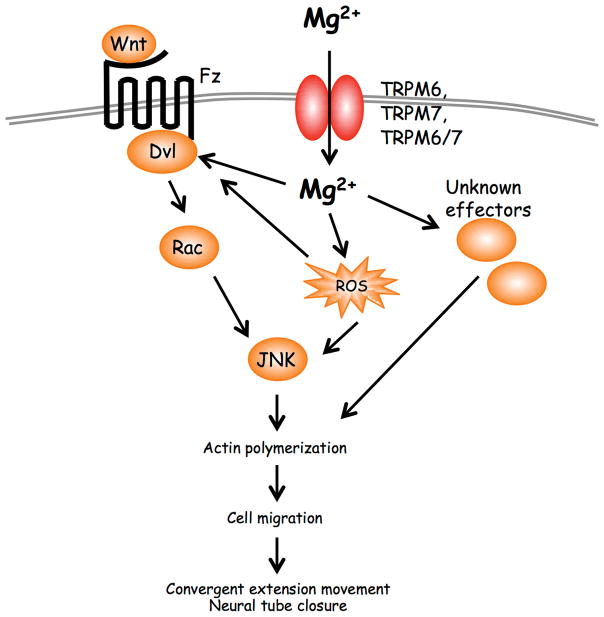
Studies in Xenopus laveis have uncovered a critical role for TRPM7 in the regulation of convergent extension movements and have demonstrated that TRPM7 functions within the PCP pathway to regulate gastrulation and neural fold closure. These defects likely explain the early embryonic lethality of TRPM7’s deletion in mice, which occurs between embryonic day 6.5 and 7.5 [19]. The phenotypes caused by loss of TRPM7 in Xenopus laevis could be prevented by Mg2+ supplementation and by expression of a Mg2+ transporter in the developing embryos, giving the first evidence that Mg2+ plays a critical role in gastrulation and neural fold closure. Interestingly, mice deficient in TRPM6, which is mutated in HSH disease, also show embryonic mortality and neural tube defects. However, as reported by Walder and colleagues, “the anatomical basis for the neural tube defects is not clear” [22]. The investigators concluded that because they never saw an embryo with complete absence of neural tube closure (craniorachischiss), “the primary defect seems not to involve disordered planar cell polarity (PCP).” Yet, craniorachischiss in more recent mouse PCP mutants was not revealed until they were combined with other PCP mouse mutants [54]. One possible explanation for this unusual result is that the functions of TRPM7 and TRPM6 overlap during neural fold closure and that a loss of TRPM6 could be partially compensated by TRPM7, which would have the effect of masking the full phenotype produced by TRPM6 deletion. This would lead to a model in which the two channels are functioning together to regulate gastrulation and neural fold closure (Figure 1). In addition to functioning as homo-oligomers, TRPM6 and TRPM7 can also combine to form hetero-oligomeric channels [17,55]. Xenopus laevis is an ideal system to tease apart the respective roles of TRPM6 and TRPM7 during morphogenesis and determine whether these two channels are in fact acting in concert. A simultaneous study of both channels together will also help elucidate how these channels influence Mg2+ homeostasis in developing embryo. These experiments are needed in order shed much needed light on how Mg2+ itself is regulated during gastrulation and how it is employed to regulate Wnt signaling. In addition to its effects on early embryogenesis, TRPM7 has also been implicated in organogenesis [30]. It will be important to determine whether TRPM7 is also functioning within the non-canonical Wnt pathway to regulate organ development in a Mg2+-dependent manner. Despite the fact we do not yet know how Mg2+ exerts its effects during morphogenesis, a preponderance of evidence now supports the conclusion that this divalent cation should be regarded as a regulatory cation in its own right. Mg2+ can at long last emerge from Ca2+’s shadow.
Figure 1. Model of TRPM7 and TRPM6 in non-canonical Wnt signaling.
Binding of Wnt ligands to Frizzled (Fz) receptors leads to the activation of Dishevelled (Dvl). Dvl through Daam1 mediates activation of Rho, which stimulates Rho kinase (ROCK) (not shown). Dvl also controls activation of Rac, which in turn activates JNK, to initiate cytoskeletal changes required for polarized cell movements during gastrulation and neural fold closure. TRPM7 and TRPM6, functioning either as homo-oligomers and/or hetero-oligomers, mediate influx of Mg2+ to control activation of Rac and JNK. Mg2+ may be influencing Wnt signaling through Dvl or its downstream effectors or may be regulating levels of reactive oxygen species (ROS) to modulate Dvl and/or JNK signaling.
Acknowledgments
This work was supported by the generous support of the National Institutes of Health, NIGMS (GM080753) to LWR and NIGMS (GM086377) to RH.
References
- 1.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:D720–734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- 3.Webb SE, Miller AL. Calcium signalling during embryonic development. Nat Rev Mol Cell Biol. 2003;4:539–551. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- 4.Markova O, Lenne PF. Calcium signaling in developing embryos: focus on the regulation of cell shape changes and collective movements. Semin Cell Dev Biol. 2012;23:298–307. doi: 10.1016/j.semcdb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang FL, Wang R, Khairallah EA, Schwartz R. Magnesium depletion during gestation and lactation in rats. J Nutr. 1971;101:1201–1209. doi: 10.1093/jn/101.9.1201. [DOI] [PubMed] [Google Scholar]
- 6.Hurley LS, Cosens G, Theriault LL. Teratogenic effects of magnesium deficiency in rats. J Nutr. 1976;106:1254–1260. doi: 10.1093/jn/106.9.1254. [DOI] [PubMed] [Google Scholar]
- 7.Venu L, Padmavathi IJ, Kishore YD, Bhanu NV, Rao KR, et al. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obesity (Silver Spring) 2008;16:1270–1276. doi: 10.1038/oby.2008.72. [DOI] [PubMed] [Google Scholar]
- 8.Makrides M, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2001:CD000937. doi: 10.1002/14651858.CD000937. [DOI] [PubMed] [Google Scholar]
- 9.Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): 1997. [PubMed] [Google Scholar]
- 10.Groenen PM, van Rooij IA, Peer PG, Ocke MC, Zielhuis GA, et al. Low maternal dietary intakes of iron, magnesium, and niacin are associated with spina bifida in the offspring. J Nutr. 2004;134:1516–1522. doi: 10.1093/jn/134.6.1516. [DOI] [PubMed] [Google Scholar]
- 11.Brown DD. Rna Synthesis during Amphibian Development. J Exp Zool. 1964;157:101–117. doi: 10.1002/jez.1401570115. [DOI] [PubMed] [Google Scholar]
- 12.Miller JC, Landesman R. Magnesium deficiency in embryos of Xenopus laevis. J Embryol Exp Morphol. 1977;39:97–113. [PubMed] [Google Scholar]
- 13.Wolf FI. TRPM7: channeling the future of cellular magnesium homeostasis? Sci STKE. 2004:pe23. doi: 10.1126/stke.2332004pe23. [DOI] [PubMed]
- 14.Runnels LW. TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr Pharm Biotechnol. 2011;12:42–53. doi: 10.2174/138920111793937880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, et al. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 21.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 22.Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, et al. Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet. 2009;18:4367–4375. doi: 10.1093/hmg/ddp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 24.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Su LT, Khadka DK, Mezzacappa C, Komiya Y, et al. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev Biol. 2011;350:348–357. doi: 10.1016/j.ydbio.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Form in homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 29.Su LT, Liu W, Chen HC, Gonzalez-Pagan O, Habas R, et al. TRPM7 regulates polarized cell movements. Biochem J. 2011;434:513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, Wu LJ, Jun J, Cheng X, Xu H, et al. The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci U S A. 2012;109:E225–233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 32.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. Embo J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser D, Langeslag M, Kedziora KM, Klarenbeek J, Kamermans A, et al. TRPM7 triggers Ca(2+) sparks and invadosome formation in neuroblastoma cells. Cell Calcium. 2013;54:404–415. doi: 10.1016/j.ceca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei C, Wang X, Chen M, Ouyang K, Song LS, et al. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su LT, Chen HC, Gonzalez-Pagan O, Overton JD, Xie J, et al. TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol. 2010;396:858–869. doi: 10.1016/j.jmb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpe P, Vezu L. Intracellular magnesium and inositol 1,4,5-trisphosphate receptor: molecular mechanisms of interaction, physiology and pharmacology. Magnes Res. 1993;6:267–274. [PubMed] [Google Scholar]
- 38.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6:113–127. [PubMed] [Google Scholar]
- 40.Murphy E. Mysteries of magnesium homeostasis. Circ Res. 2000;86:245–248. doi: 10.1161/01.res.86.3.245. [DOI] [PubMed] [Google Scholar]
- 41.Takaya J, Higashino H, Kobayashi Y. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes Res. 2000;13:139–146. [PubMed] [Google Scholar]
- 42.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowan JA. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15:225–235. doi: 10.1023/a:1016022730880. [DOI] [PubMed] [Google Scholar]
- 44.Chen HC, Su LT, Gonzalez-Pagan O, Overton JD, Runnels LW. A Key Role for Mg2+ in TRPM7’s Control of ROS Levels During Cell Stress. Biochem J. 2012 doi: 10.1042/BJ20120248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012;22:107–115. doi: 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tochhawng L, Deng S, Pervaiz S, Yap CT. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013;13:246–253. doi: 10.1016/j.mito.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 48.Hoogeboom D, Burgering BM. Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta. 2009;1796:63–74. doi: 10.1016/j.bbcan.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Wolf FI, Trapani V, Simonacci M, Boninsegna A, Mazur A, et al. Magnesium deficiency affects mammary epithelial cell proliferation: involvement of oxidative stress. Nutr Cancer. 2009;61:131–136. doi: 10.1080/01635580802376360. [DOI] [PubMed] [Google Scholar]
- 50.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi T, Kaneda M, Kakinuma K. Essential requirement of magnesium ion for optimal activity of the NADPH oxidase of guinea pig polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1983;115:261–267. doi: 10.1016/0006-291x(83)90998-1. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H, Pabst MJ, Johnston RB., Jr Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J Biol Chem. 1985;260:3635–3639. [PubMed] [Google Scholar]
- 53.Nunez-Villena F, Becerra A, Echeverria C, Briceno N, Porras O, et al. Increased expression of the transient receptor potential melastatin 7 channel is critically involved in lipopolysaccharide-induced reactive oxygen species-mediated neuronal death. Antioxid Redox Signal. 2011;15:2425–2438. doi: 10.1089/ars.2010.3825. [DOI] [PubMed] [Google Scholar]
- 54.Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- 55.Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]