Abstract
Objectives
To evaluate whether daytime sleepiness, poor sleep quality and morningness and eveningness preferences are associated with common mental disorders (CMDs) among college students.
Methods
A total of 963 college students completed self-administered questionnaires that collected information about socio-demographic characteristics, sleep quality characteristics, CMDs, and other lifestyle behaviors.
Results
The prevalence of CMDs was 24.3% (95% CI: 21.5-27.1%) among all students. Prevalence estimates of both excessive daytime sleepiness and poor sleep quality were higher among females (35.4% and 54.4%) than males (22.0% and 45.8%). Cigarette smoking was statistically significantly and positively associated with having CMDs (p=0.034). Excessive daytime sleepiness (OR= 3.65; 95% CI: 2.56-4.91) and poor sleep quality (OR=4.76; 95% CI: 3.11-7.29) were associated with increased odds of CMDs.
Conclusion
Given the adverse health consequences associated with both sleep disorders and CMDs, improving sleep hygiene among college students is imperative to public health.
Keywords: Chile, college students, daytime sleepiness, mental disorders, sleep quality
Introduction
Sleep is a part of the body's daily cycle. Observational and experimental studies have shown that short sleep duration and sleep disturbances are associated with poor performance of the body's daily tasks, 1 depressive disorders, 2 impaired memory, 3 poor academic performance, 1,4,5 decreased motivation, 1 suicidal thoughts, 6 obesity, 4,7 and cardiac morbidity, 4,7-9
Psychiatric disorders, which include mood and anxiety disorders, have been suggested as the leading causes of disability globally. 10 Sleep disturbances are common features of mood disorders, the most frequent of which is excessive daytime sleepiness.11 Daytime sleepiness occurs when there is constant background sleepiness throughout the day. 12 In addition to its impact on neuropsychiatric disorders, daytime sleepiness can result in injury at work or home, car accidents, decreased alertness, and lower overall productivity.11
Horne and Ostberg13 demonstrated that individuals have preferences for times of day when they are most alert and productive. This circadian preference is often referred to as morningness/eveningness Chronotype.13 Disturbances in circadian rhythms play a role in the pathogenesis of mental disorders.14 Early chronotypes are referred to as morning types and have shown to be associated with a good attitude towards life, physical and mental health, self-esteem, familial relationships, and school functioning. 15 However, evening chronotypes, those who prefer later bed and wake/rise times, are associated with higher risk of infections, poor sleep quality, tobacco consumption, depression and other psychiatric disorders. 2,15,16
Characteristics of sleep among college-age students—daytime sleepiness, sleep quality, chronotype, and mental disorders—have all been studied individually. However there is little data that evaluated the association between sleep characteristics and mental health disorders among college-age students. In this study we sought to examine the extent to which daytime sleepiness, poor sleep quality and evening chronotype are associated with common mental disorders (CMDs) among Chilean college students.
Methods and Materials
Study Setting and Sample
The study was conducted among four universities in Chile between December 2010 and June 2011. Details of research methodology, including participants sampling methods and data collection procedures, have been described previously.17 Briefly, flyers were posted in each department to invite participants. Students who expressed an interest in participating in the study were invited to meet in a large classroom or an auditorium where they were informed about the purpose of the study and were invited to participate in the survey. Students consenting to participate were asked to complete paper-and-pencil self-administered individual surveys. There was no time limit for completing the survey. Students who could not read the survey (i.e., were blind) were excluded, as were those enrolled in correspondence, extension, or night school programs. A total of 994 undergraduate students participated in the study. For this study, 68 students with incomplete questionnaires were excluded. Complete data were available for 928 students and were included in these analyses. Approximately 70% (n=652) of participants were females and the overall mean age was 21.9 ± 3.4 years. The majority of students (68.9%) were freshmen or sophomores.
Participant information was collected by self-administered questionnaires. The questionnaires were anonymous and with no personal identifiers. The procedures used in this study were approved by the institutional review boards of participating institutions.
Variable Specification
The questionnaire included information about their age, gender, education level, physical activity, and behavioral and sleep characteristics. The questionnaires also collected information about consumption of energy drinks or other caffeinated beverages during the past month. Brands of energy drinks included: Red Bull, Dark Dog, Burn, Shark, Red Devil and Battery. Other caffeinated beverages included: coffee, tea, yerba mate (llex paraguariensis) and cola drinks. Use of any energy drinks or caffeinated beverages were combined into a single “any stimulant drinks” category. Alcohol consumption was classified as low (≤1 alcoholic beverages per month), moderate (1-19 alcoholic beverages per month), and high (≥20 alcoholic beverages per month). Body mass index (BMI) was calculated as weight (kg)/height (m2).
Following the completion of the self-administered questionnaire, participants' anthropometric measurements were taken by research nurses using standard approaches 18. Height (cm) and mass (kg) were measured without shoes or outerwear. Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg with subjects standing on a scale. Three measurements were taken on each anthropometric characteristic, and averages of the three measurements were used in our analyses. BMI was grouped according to the World Health Organization (WHO) protocol as underweight (BMI<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (BMI≥30 kg/m2) 18.
Epworth Sleep Scale (ESS)
The ESS is an 8-item questionnaire that measures a person's general level of daytime sleepiness. 18 The questions include details about an individual's tendency to fall asleep during commonly faced situations. Scores for all 8-items can range from 0 to 24. In adults, an ESS score ≥10 is taken to indicate increased daytime sleepiness. 12 The ESS has been used successfully in cross-cultural settings including Latin American adults and college students. 15,19
Pittsburgh Sleep Quality Index (PSQI)
The PQSI is a self-reported questionnaire with 19 items evaluating sleep quality over the past month(Cronbach's α =0.83).20 The PSQI instrument includes seven components of sleep: duration of sleep, sleep disturbance, sleep latency, habitual sleep efficiency, use of sleep medicine, daytime dysfunction due to sleepiness, and overall sleep quality. 20 The score for each of these components ranges from 0 to 3, three being the greatest dysfunction. The values of the seven components are then summed, giving a range from 0 to 21, with higher scores representing poorer sleep quality in the past month 20. Prior literature shows that an overall score greater than 5 indicates poor sleepers and scores of 5 or less indicate good sleepers. Studies among college students have shown the validity of these cut-points, 21 therefore the same cutoffs were used in this study.
Morningness-Eveningness Questionnaire (MEQ)
Circadian preference of morningness/eveningness were assessed with a 19-item Horne and Osberg questionnaire (Cronbach's α =0.82).13,22 The scores range from 16 to 86 and participants can be classified in five categories: definite and moderate evening (E)-type, neutral type, and moderate and definite morning (M)-type. Higher scores indicate a higher preference towards morningness. In this study we used the following cut offs: (1) 16 to 41 for evening type; (2) 42 to 58 for intermediate type; and (3) 59 to 86 for morning.13 The MEQ scores have been shown to correlate well with melatonin onset as a physiological marker of the circadian period supporting the validity of the questionnaire. 23,24
General Health Questionnaire12 (GHQ-12)
The GHQ-12 is a 12-item questionnaire used for screening of non-pathological common mental disorders (Cronbach's α =0.86) including depression, anxiety and somatoform disorders. The GHQ has been commonly used worldwide, including in Chile.25 The GHQ-12 asks respondents to report how they felt during last four weeks on a range of variables including problems with sleep and appetite, subjective experiences of stress, tension, or sadness, mastering of daily problems, decision making and self-esteem. The maximum possible score was 12 with higher scores suggesting higher mental distress. In this study, presence of CMDs was defined using previously established cut points in other study populations. Scores of 5 or higher on the GHQ-12 scale were considered as having CMDs. 26-28
Statistical Analysis
Continuous variables were presented using means (±standard deviation) and categorical and binary variables using counts and percentages for basic univariate summaries. Bivariate associations of PSQI and ESS scores with GHQ-12 items were summarized using Spearman's correlation coefficient. Logistic regression analysis was performed to investigate the association between sleep disorder characteristics and CMDs. Odds ratios (OR) and 95% CI of CMDs were calculated to summarize these associations. The following characteristics were identified a priori as potential confounding factors for the association between CMDs and sleeping disorders: age, gender, smoking, alcohol consumption, stimulant use, body mass index, and physical activity. These confounders were adjusted for in our multivariable logistic models. All analyses were performed using SPSS Statistical Software for Windows (SPSS Version 19, Chicago, Illinois, USA). All reported test statistics and their corresponding p-values are two-tailed.
Results
Daytime Sleepiness
A total of 963 Chilean college students were included in this study. Daytime sleepiness (ESS≥10) prevalence for all participants was 31.3% (95%CI; 28.3-34.4%). Female students were more often classified as having daytime sleepiness (35.3%; 95%CI 31.6-39.1%) than males (22.0%; 95%CI 17.0-27.0%) (p < 0.001). Overall, 20 year-old males had the lowest prevalence of daytime sleepiness (17.2%), while 21 year-old females had the highest prevalence (39.1%) (Figure 1).
Figure 1. Prevalence of Daytime Sleepiness according to Age and Sex.
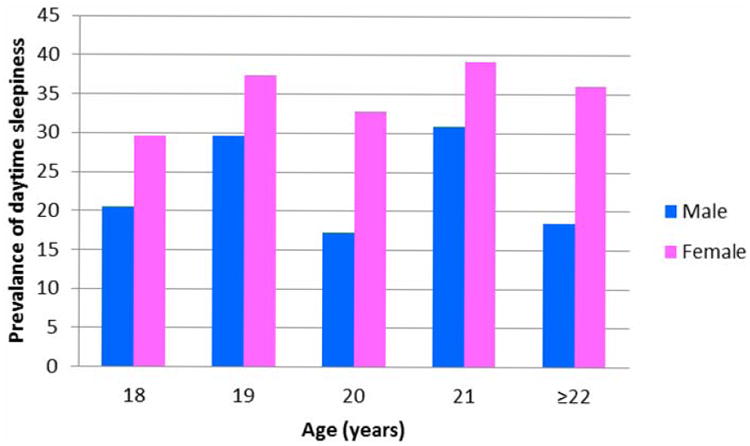
*Daytime sleepiness (ESS≥10)
Common Mental Disorders (CMDs)
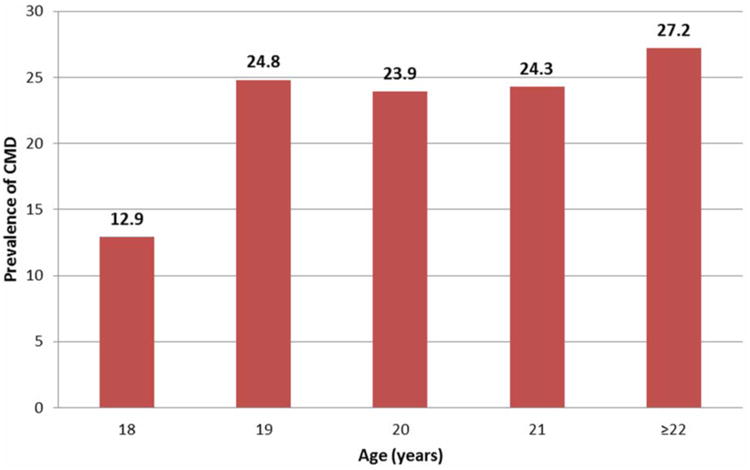
The prevalence of CMDs among all students was 24.3% (95%CI: 21.5-27.1%). Male students had higher prevalence of CMDs (26.1%; 95%CI: 22.7-29.4%) compared to females (20.1; 95%CI: 15.3-24.9%). The prevalence of CMDs was higher for older students, with students age 22 years or older at 27% (95% CI: 22.8-31.4%) and 18 year olds at 13% (95% CI: 6.5-19.4%) (Figure 2).
Figure 2. Prevalence (%) of Common Mental Disorders (CMDs) according to Age.
Participant Characteristics by CMDs
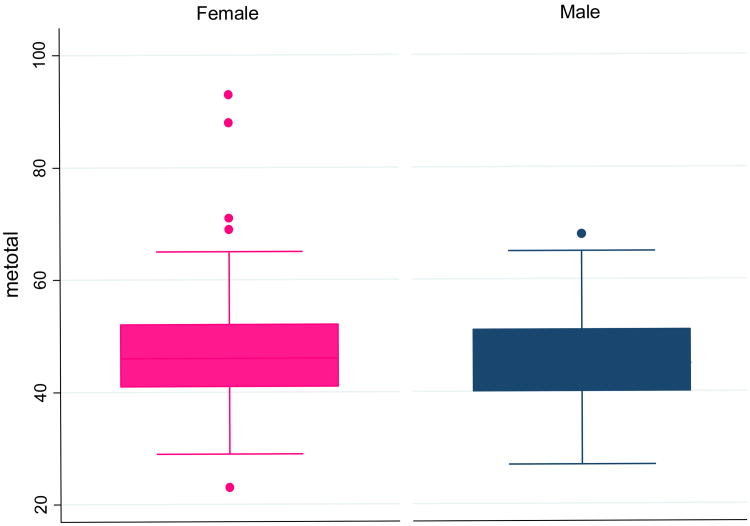
Behavioral and demographic characteristics of participants according to CMDs are shown in Table 1. In these bivariate analyses, the prevalence of CMDs among current smokers was significantly higher (28.3%) as compared with non-smokers (19.7%). There were marginal associations between CMDs and gender and between CMDs and age. No other behavioral and demographic characteristics were associated with common mental disorders. A comparison of morning and evening type scores to gender is shown in Figure 3. The distributions of MEQ scores were similar for both males and females.
Table 1. Common Mental Disorders (CMDs) by Demographic and Lifestyle Characteristics.
| CMDs | |||
|---|---|---|---|
|
| |||
| Yes (n=225) | No (n=701) | p-value | |
| Age (years) | % | % | |
| 18 (n=108) | 12.9 | 87.0 | 0.051 |
| 19 (n=157) | 24.8 | 75.1 | |
| 20 (n=134) | 23.9 | 76.1 | |
| 21 (n=111) | 24.3 | 75.6 | |
| ≥ 22 (n=416) | 27.1 | 72.8 | |
| Sex | |||
| Female (n=652) | 20.2 | 79.8 | 0.055 |
| Male (n=173) | 26.1 | 73.9 | |
| Cigarette smoking status | |||
| Never (n=360) | 19.7 | 80.3 | 0.034 |
| Former (n=127) | 26.9 | 73.1 | |
| Current (n=407) | 28.3 | 71.7 | |
| Alcohol consumption | |||
| <1 drink/month (n=407) | 21.4 | 78.6 | 0.128 |
| 1-19 drinks/month (n=375) | 25.6 | 74.4 | |
| ≥ 20 drinks/month (n=144) | 29.2 | 70.8 | |
| Use of any stimulant drinks | |||
| No (n=343) | 42.5 | 45.8 | 0.434 |
| Yes (n=420) | 57.5 | 54.2 | |
| Body mass index (kg/m2) | |||
| Underweight (n=13) | 7.7 | 92.3 | 0.079 |
| Normal (n=519) | 23.5 | 76.5 | |
| Overweight (n=248) | 29.4 | 70.6 | |
| Obese (n=115) | 20.0 | 80.0 | |
| Any physical activity | |||
| No (n=422) | 24.9 | 75.1 | 0.720 |
| Yes (n=504) | 23.8 | 76.2 | |
Figure 3. Distribution of Morningness and Eveningness Questionnaire (MEQ) Scores by Sex.
Box plots comparing MEQ scores by sex. The central box shows the data between the upper and lower quartiles, with the median represented by the middle line. The “whiskers” (lines on either side of central box) extend from the upper and lower quartiles to a distance of 1.5×IQR (interquartile range) away of the most extreme data point within that range, whichever is smaller.
GHQ-12 items with Total PSQI and ESS Scores
Table 2 shows that all of the GHQ-12 items are significantly and positively correlated with total PSQI and ESS scores. The highest correlation for PSQI score was “enjoying normal activities” (r=0.449) and “able to concentrate” for ESS (r=0.318). The lowest correlations for PSQI and ESS score were “playing useful part” (r=0.188) and “thinking of self as worthless” (r=0.143) respectively.
Table 2. Correlations of Epworth Sleepiness Scale (ESS) score and Pittsburgh Sleep Quality Index (PSQI) total score with General Health Questionnaire (GHQ-12) items.
| Spearman's Correlation | ||
|---|---|---|
| GHQ-12 Items | ESS score* | PSQI score* |
| Able to concentrate | 0.318 | 0.373 |
| Lost much sleep | 0.148 | 0.211 |
| Playing useful part | 0.198 | 0.188 |
| Capable of making decisions | 0.295 | 0.348 |
| Under stress | 0.201 | 0.208 |
| Could not overcome difficulties | 0.246 | 0.322 |
| Enjoy normal activities | 0.294 | 0.449 |
| Face up to problems | 0.311 | 0.378 |
| Feeling unhappy and depressed | 0.243 | 0.28 |
| Losing confidence | 0.209 | 0.274 |
| Thinking of self as worthless | 0.143 | 0.193 |
| Feeling reasonably happy | 0.151 | 0.219 |
p-values for each of the GHQ 12 items <0.001
Relation between Sleep Disorders and CMDs
We investigated the association between sleep disorders (daytime sleepiness, poor sleep quality and evening chronotype) and CMDs using logistic regression (Table 3). Those with daytime sleepiness were associated with 3.54-fold higher odds of CMDs (OR=3.54; 95%CI: 2.56-4.91) compared to those without daytime sleepiness in an unadjusted analysis. The magnitude of association was slightly attenuated after adjusting for age, gender, alcohol consumption, stimulant use, cigarette smoking, BMI, and physical activity (OR =3.06; 95%CI: 2.05-4.57). In an unadjusted analysis, participants with poor sleep quality had 4.65-fold higher odds (OR=4.65; 95%CI: 3.22-6.73) of experiencing CMDs compared with those with good sleep quality. The association remained in multivariate adjusted model (OR=4.76; 95%CI: 3.11-7.29). No statistically significant association was noted between evening chronotype and CMD (OR=0.97; 95%CI: 0.43-2.61).
Table 3. Daytime Sleepiness, Poor Sleep Quality and Evening Chronotype in Relation to Common Mental Disorders.
| Characteristic | Unadjusted OR (95% CI) | Age and sex adjusted OR (95% CI) | Multivariate adjusted* OR (95% CI) |
|---|---|---|---|
| Daytime sleepiness | |||
| No | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 3.54 (2.56-4.91) | 3.46 (2.49-4.81) | 3.06 (2.05-4.57) |
| Sleep quality | |||
| Good | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Poor | 4.65 (3.22-6.73) | 4.57 (3.16-6.62) | 4.76 (3.11-7.29) |
| Chronotype | |||
| Morning type | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Evening type | 0.97 (0.43-2.61) | 0.95 (0.65-1.41) | 0.92 (0.64-1.33) |
Adjusted for age, sex, smoking, alcohol drinking, stimulant use, body mass index, and physical activity;
Presence of common mental disorder was defined as having GHQ total score of 5 and higher
Discussion
Our study is the first to comprehensively examine the relationship between sleep characteristics and CMDs among Chilean college students. We showed that the prevalence of CMDs is quite high among Chilean college students at 25% (95% CI: 21.5-27.1%), and that poor sleep quality and daytime sleepiness were positively associated with CMDs.
Daytime sleepiness was reported by nearly a third of study participants, poor sleep quality was reported by over half of them, and evening chronotype by approximately 10%. We observed higher odds of CMD in relation to daytime sleepiness (OR: 3.53; 95% CI 2.56-4.91) and poor sleep quality (OR: 4.65; 95%CI 3.22-6.73). Our results demonstrating a strong association between sleep disorders and CMDs are consistent with existing literature. 16,29-31 Kang et al. reported that depression, a common mental disorder, was significantly associated with having poor sleep quality.32 Taylor et al. reported significant associations between sleep disorders and depression, anxiety, and stress in a sample of 1,074 college students from the University of North Texas.33 Using two large population-based studies conducted 11 years apart in Norway, Sivertsen et al. (2012) found that participants with insomnia are associated with a 6-fold higher odds of depression (OR = 6.21, 95% CI = 4.45–8.68).34 The authors noted that both insomnia and depression predicted each other and that effect sizes were markedly similar. Previous research of college students in the United States found a higher risk of depressive symptoms among evening chronotypes.16 In our study, there was no association between CMD and eveningness chronotype (OR: 0.97; 95%CI 0.43-2.6). We don't have a clear explanation for this. It is possible that our limited sample size might have contributed to the lack of statistical power. Taken as a whole, the body of evidence suggests that sleep disorders, including daytime sleepiness and poor sleep quality, increase the risk for common mental disorders.
Mechanisms for the relation between sleep disorders and CMDs have not been fully elucidated but are likely to be multiple. Sleep and wake cycles are controlled by large amounts of cortical neurons in the brainstem and hypothalamic area of the brain.35 Sleep deprivation inhibits activity of the hypothalamic-pituitary-adrenal (HPA) axis.36 The dysregulation of the HPA axis observed among individuals with depression and anxiety disorders could be one mechanism linking the two comorbidities 9 In addition, melatonin concentrations, a hormone that controls sleep wake patterns and induces sleepiness, have been found to be lower in adolescents with unipolar depression 37 as compared with controls. Misaligned melatonin secretion could be another shared pathophysiologic mechanism linking the two disorders.
There are several limitations with our study. First, we used cross-sectional data, which doesn't allow us to establish the temporal relationship between sleep disorders and CMDs. It is possible that those with depressed mood and anxiety disorders are more likely to have sleep disorders. Longitudinal studies of these characteristics and factors would be invaluable to clarify the temporality and examine the effects of sleep disorders on the development of CMDs. Second, the questionnaires were self-administered, which could have introduced some error or bias due to the subjective nature of sleep characteristics and CMDs. The concordance of our results with those that used objective assessments serves to mitigate this concern 38,39. Third, in our study we adjusted for putative confounders. However, we cannot exclude the possibility that there is residual confounding by factors such as history of mental disorders. Finally, the population we studied was not a random selection which limits the generalizability our study findings.
In summary, we have found noteworthy associations between sleep disorders such as excessive daytime sleepiness and poor sleep quality with CMDs. Both sleep disorders and common mental disorders are highly prevalent in Chilean college students. Our study has important clinical and public health implications. College often presents students with increased social commitments, academic pressures and demands, voluntary sleep deprivation, peer pressure, or financial pressure. These intense lifestyle changes influence sleep behaviors with adverse health effects. However, our study suggests that by decreasing daytime sleepiness and improving overall sleep quality, the risk for common mental disorders could be reduced. Given the adverse health consequences associated with both sleep disorders and CMDs, improving sleep hygiene among college students is imperative. Educational programs designed to promote sleep hygiene among college students, can in turn, lead to reduced risk of CMDs. More empirical research, however, is needed to evaluate such programs in Chile and other settings characterized by high prevalence of sleep disorders and CMDs.
Acknowledgments
This research was completed while T.C. was a research training fellow with the Harvard School of Public Health Multidisciplinary International Research Training (HSPH-MIRT) Program. The HSPHMIRT Program is supported by an award from the National Institute for Minority Health and Health Disparities (T37-MD000149). The authors thank the Centro de Rehabilitación Club de Leones Cruz del Sur for providing facilities and logistic support throughout the research process. The authors also thank the participating universities for supporting the conduct of this study.
References
- 1.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Randler C. Association between morningness-eveningness and mental and physical health in adolescents. Psychol Health Med. 2011;16:29–38. doi: 10.1080/13548506.2010.521564. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra S, Kushida CA. Primary hypersomnias of central origin. Continuum (Minneap Minn) 2013;19:67–85. doi: 10.1212/01.CON.0000427212.05930.c4. [DOI] [PubMed] [Google Scholar]
- 4.Arora T, Hosseini-Araghi M, Bishop J, Yao GL, Thomas GN, Taheri S. The complexity of obesity in UK adolescents: relationships with quantity and type of technology, sleep duration and quality, academic performance and aspiration. Pediatr Obes. 2012 doi: 10.1111/j.2047-6310.2012.00119.x. [DOI] [PubMed] [Google Scholar]
- 5.Gaultney JJ. The prevalence of sleep disorders in college students: impact on academic performance. College Health Sep. 2010;59:91–97. doi: 10.1080/07448481.2010.483708. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73:e1160–1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 7.Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, Csako G, Cizza G. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8:e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Partonen T. Evening types are prone to depression. Chronobiol Int. 2013;30:719–725. doi: 10.3109/07420528.2013.784770. [DOI] [PubMed] [Google Scholar]
- 10.Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, Sawyer SM, Mathers CD. Global burden of disease in young people aged 10-24 years: a systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 11.Millman RP. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–1786. doi: 10.1542/peds.2005-0772. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 14.Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- 15.Schneider ML, Vasconcellos DC, Dantas G, Levandovski R, Caumo W, Allebrandt KV, Doring M, Hidalgo MP. Morningness-eveningness, use of stimulants, and minor psychiatric disorders among undergraduate students. Int J Psychol. 2011;46:18–23. doi: 10.1080/00207594.2010.513414. [DOI] [PubMed] [Google Scholar]
- 16.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. J Affect Disord. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 17.Velez JC, Souza A, Traslavina S, Barbosa C, Wosu A, Andrade A, Frye M, Fitzpatrick AL, Gelaye B, Williams MA. The Epidemiology of Sleep Quality and Consumption of Stimulant Beverages among Patagonian Chilean College Students. Sleep Disord. 2013;2013:910104. doi: 10.1155/2013/910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Report of a WHO Expert Committee. World Health Organizations; Geneva: 1995. Physical status: the use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 19.Bouscoulet LT, Vazquez-Garcia JC, Muino A, Marquez M, Lopez MV, de Oca MM, Talamo C, Valdivia G, Pertuze J, Menezes AM, Perez-Padilla R, Group P. Prevalence of sleep related symptoms in four Latin American cities. J Clin Sleep Med. 2008;4:579–585. [PMC free article] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Carney CE, Edinger JD, Meyer B, Lindman L, Istre T. Daily activities and sleep quality in college students. Chronobiol Int. 2006;23:623–637. doi: 10.1080/07420520600650695. [DOI] [PubMed] [Google Scholar]
- 22.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 23.Roeser K, Meule A, Schwerdtle B, Kubler A, Schlarb AA. Subjective sleep quality exclusively mediates the relationship between morningness-eveningness preference and self-perceived stress response. Chronobiol Int. 2012;29:955–960. doi: 10.3109/07420528.2012.699124. [DOI] [PubMed] [Google Scholar]
- 24.Griefahn B, Künemund C, Bröde P, Mehnert P. Zur Validität der deutschen Übersetzung des Morningness-Eveningness-Questionnaires von Horne und Östberg. Somnologie. 2001;5:71–80. [Google Scholar]
- 25.Araya R, Wynn R, Lewis G. Comparison of two self administered psychiatric questionnaires (GHQ-12 and SRQ-20) in primary care in Chile. Soc Psychiatry Psychiatr Epidemiol. 1992;27:168–173. doi: 10.1007/BF00789001. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes AC, Hayes RD, Patel V. Abuse and other correlates of common mental disorders in youth: a cross-sectional study in Goa, India. Soc Psychiatry Psychiatr Epidemiol. 2013;48:515–523. doi: 10.1007/s00127-012-0614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, Rutter C. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med. 1997;27:191–197. doi: 10.1017/s0033291796004242. [DOI] [PubMed] [Google Scholar]
- 28.Patel V, Araya R, Chowdhary N, King M, Kirkwood B, Nayak S, Simon G, Weiss HA. Detecting common mental disorders in primary care in India: a comparison of five screening questionnaires. Psychol Med. 2008;38:221–228. doi: 10.1017/S0033291707002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besoluk S, Onder I, Deveci I. Morningness-eveningness preferences and academic achievement of university students. Chronobiol Int. 2011;28:118–125. doi: 10.3109/07420528.2010.540729. [DOI] [PubMed] [Google Scholar]
- 30.Borisenkov MF, Perminova EV, Kosova AL. Chronotype, sleep length, and school achievement of 11- to 23-year-old students in northern European Russia. Chronobiol Int. 2010;27:1259–1270. doi: 10.3109/07420528.2010.487624. [DOI] [PubMed] [Google Scholar]
- 31.Moo-Estrella J, Perez-Benitez H, Solis-Rodriguez F, Arankowsky-Sandoval G. Evaluation of depressive symptoms and sleep alterations in college students. Arch Med Res. 2005;36:393–398. doi: 10.1016/j.arcmed.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Kang JM, Lee JA, Jang JW, Kim YS, Sunwoo S. Factors associated with poor sleep quality in primary care. Korean J Fam Med. 2013;34:107–114. doi: 10.4082/kjfm.2013.34.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor DJ, Bramoweth AD, Grieser EA, Tatum JI, Roane BM. Epidemiology of insomnia in college students: relationship with mental health, quality of life, and substance use difficulties. Behav Ther. 2013;44:339–348. doi: 10.1016/j.beth.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, Nordhus IH, Overland S. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74:758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 35.Zeitzer JM. Control of sleep and wakefulness in health and disease. Prog Mol Biol Transl Sci. 2013;119:137–154. doi: 10.1016/B978-0-12-396971-2.00006-3. [DOI] [PubMed] [Google Scholar]
- 36.Aydin A, Selvi Y, Besiroglu L, Boysan M, Atli A, Ozdemir O, Kilic S, Balaharoglu R. Mood and metabolic consequences of sleep deprivation as a potential endophenotype' in bipolar disorder. J Affect Disord. 2013;150:284–294. doi: 10.1016/j.jad.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, Hickie IB. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: Comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013 doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Brooks AT, Krumlauf MC, Whiting BP, Clark RJ, Wallen GR. Are you Sleeping? Pilot Comparison of Self-Reported and Objective Measures of Sleep Quality and Duration in an Inpatient Alcoholism Treatment Program. Subst Abuse. 2012;6:135–139. doi: 10.4137/SART.S10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramar K, Olson EJ. Management of common sleep disorders. Am Fam Physician. 2013;88:231–238. [PubMed] [Google Scholar]