Abstract
Prednisolone pharmacokinetics (PK) and pharmacodynamics (PD) were investigated in relation to sex and race in white males, black males, white females, and black females (n = 8/group) after a single oral dose (0.27 mg/kg) of prednisone. The study consisted of baseline and prednisone phases with 32-hour sampling in each phase. Women were studied during the luteal phase of their menstrual cycle. Total and free plasma prednisolone concentrations were assayed by HPLC and ultrafiltration, and pharmacokinetic data were analyzed by compartmental fitting using WinNonlin. Plasma cortisol concentrations were assayed by HPLC; T-helper, T-suppressor lymphocyte, and neutrophil cell counts were determined by FACS and hemocytometry, and these pharmacodynamic data were evaluated by basic and extended indirect response models using ADAPT II. Total body weight–normalized free prednisolone oral clearance and apparent volume of distribution were higher in men compared with women, regardless of race (by22%in whites and40%in blacks for oral clearance, p < 0.01; by32%in whites and38% in blacks for apparent volume of distribution, p < 0.01). The 50% inhibitory concentration (IC50) values for T-suppressor cell-trafficking inhibition were higher in whites than in blacks, regardless of sex (by 125% in men and 208% in women, p < 0.01). The IC50 or SC50 values for effects of prednisolone on cortisol secretion and T-helper lymphocyte or neutrophil trafficking were not statistically different between men and women, blacks and whites. The findings of this study suggest that there are some prednisolone PK/PD differences related to sex and race. However, these differences do not suggest the need for dosage adjustments, and additional experiments with repeat dosing are needed to fully evaluate the clinical implication of these findings.
The role of sex as a factor in the pharmacokinetics and the pharmacodynamics (PK/PD) of drugs has become well appreciated.1 Women often exhibit modestly more rapid clearances of drugs metabolized by the CYP3A4 pathway (e.g., methylprednisolone2). They may also show alterations in the disposition of drugs in relation to the phase of the menstrual cycle (e.g., theophylline3), pregnancy (e.g., caffeine4), or after menopause (e.g., verapamil5). Women receiving oral contraceptives are likely to show more rapid clearances of conjugated drugs (e.g., oxazepam) but have reduced clearances of many oxidized compounds (e.g., prednisolone6). Interpretation of data is sometimes complicated by the need to assess whether pharmacokinetic parameters are properly normalized for body weight differences and if the phase of the menstrual cycle was monitored.
The assessment of pharmacodynamic differences between men and women requires control of pharmacokinetic factors and use of appropriate methodology to relate responses to plasma or biophase drug concentrations. There are some notable examples of marked sex differences in drug efficacy. Aspirin is less effective in women in prevention of stroke, perhaps related to sex hormone–dependent differences in platelet aggregation.7 Women are more prone to develop life-threatening ventricular arrhythmia (torsades de pointes) from cardiovascular drugs that prolong repolarization (e.g. quinidine, procainamide).8 Opioids such as pentazocine show greater efficacy for pain relief in women, but the NSAID ibuprofen exhibits better responses in men with no sex-associated differences in kinetics.9
Racial differences in pharmacokinetics of several drugs have been demonstrated.10,11 White patients were found to have 50% higher methylprednisolone clearances than black patients in a sex- and age-matched study in renal transplant recipients.12 Black renal transplant patients were also found to have different toxicity profiles compared with whites.13 Measurements of lymphocyte responsiveness to corticosteroids have demonstrated a diminished reactivity in cells from African Americans.14 A study with the newer immunosuppressant, mycophenolate mofetil, has shown the need for higher dosages in African American renal allograft recipients.15 In general, blacks exhibit higher renal allograft loss rates (15%) compared with whites (7%).16 These findings suggest that differences in genetic composition may result in clinically important interethnic variation in drug disposition and responsiveness. Furthermore, differences in ethnicity may mask potential sex-related effects if ethnic background is not evaluated as a contributing factor.
Corticosteroids, used extensively for their anti-inflammatory and immunosuppressive properties, represent a class of drugs whose PK/PD can be influenced by sex and race. Women were found to have a significantly higher clearance of methylprednisolone and thus have a lower drug exposure than do men.2 However, greater sensitivity to several biomarkers in women was found. Thus, pharmacodynamic differences offset a pharmacokinetic difference. Prednisolone, the active moiety metabolized from prednisone, differs in structure from methylprednisolone in lacking a 6 α-methyl group. Two studies have observed 23% and 21% greater clearance of free prednisolone in female compared with male adult subjects.17,18 Neither study, however, measured any pharmacodynamic markers to examine the possible difference in biological response, which may accompany these clearance differences. Hence, it is the purpose of this study to evaluate the role of sex and race in affecting prednisolone kinetics and responses after single oral doses of prednisone.
METHOD
Subjects
Four groups of 8 subjects per group gave written informed consent to participate in the study according to the Declaration of Helsinki. Using previous data,19 a sample size of 8 per group was calculated to provide a 90%power with an alpha level of 0.05 in detecting a 25 L/h (26% of the reported mean) difference in free prednisolone oral clearance with a standard deviation of 15 L/h. The study was approved by the Kaleida Health Millard Fillmore Hospital Institutional Review Board (Buffalo, NY). All 32 subjects were within 20% of ideal body weight, between 18 and 45 years of age, and had a normal sleep-wake cycle (nightshift workers were excluded). All subjects were determined healthy by assessment of medical history, physical examination, blood chemistry, and hematological profile. None of the subjects had a documented allergy to corticosteroids, and none were receiving any concurrent medications known to alter prednisolone metabolism. All subjects were descendents of same-race parents. All 16womenwere premenopausal, as defined by the presence of monthly menstruation and absence of menopausal symptoms of the climacteric. None of the women were using any oral or parental hormonal contraceptives, and all were determined not pregnant by a urine human chorionic gonadotropin pregnancy test at the beginning of both baseline and prednisone phases. The study attempted to evaluate all women during the luteal phase of their menstrual cycles, during which estradiol and progesterone concentrations in the blood are relatively elevated and constant. This was imposed during both baseline and prednisone phases and was accomplished by a calendar method along with a urine ovulation testing method with the use of the Answer One-Step Ovulation Predictor Kit (Carter Products, New York), a monoclonal antibody test that detects a luteinizing hormone surge in the urine.
Procedures
Each subject was confined in the clinical research center during both the baseline phase (32 hours, no drug) and the prednisone phase (32 hours) of the study to control for identical eating and sleeping times. Each study phase began at 8 a.m. and was separated by a 2-week period for men and an approximately 4-week period for women. Subjects were required to fast from 10 p.m. the evening before and for 2 hours after receiving the prednisone dose. On each study day, an 18-gauge angiocatheter was inserted into an arm vein to facilitate blood sample collections. The device was kept patent with the frequent use of a dilute heparin (10 units/ml) solution.
During the baseline phase, plasma cortisol concentrations, T-helper and T-suppressor lymphocyte counts, and neutrophil cell counts were obtained. Approximately 7 ml blood samples were drawn into heparin-containing collection tubes at 0, 1, 2, 3, 4, 6, 8, 12, 14, 16, 18, 20, 24, 28, and 32 hours. The blood was centrifuged at 2000 rpm for 15 minutes and the plasma harvested and frozen at −20°C until assayed. In addition, 3 ml blood was drawn into EDTA-containing tubes at 0, 1, 2, 4, 6, 8, 12, 18, 24, and 32 hours for the determination of T-helper and T-suppressor lymphocyte and neutrophil cell counts.
During the prednisone phase, each subject received a total body weight (TBW)–based prednisone dose (given as Deltasone, Pharmacia-Upjohn Co., Kalamazoo, MI) orally to provide similar initial blood concentrations of prednisolone in each subject and to ensure detectable responses. Dosing adjustment to TBW rather than to ideal body weight was chosen because of the strong correlation of free prednisolone clearance with the degree of obesity.20 The dosing scheme was as follows: 40 to 49 kg, 12.5 mg; 50 to 59 kg, 15 mg; 60 to 69 kg, 17.5 mg; 70 to 79 kg, 20 mg; and 80 to 89 kg, 22.5 mg. Blood samples of approximately 7 ml were obtained at predose; at 20 and 40 minutes; and at 1, 1.5, 2, 3, 4, 6, 8, 12, 14, 16, 18, 20, 24, 28, and 32 hours postdose to determine plasma prednisolone and cortisol concentrations. The blood samples were processed as described above. In addition, 3 ml blood was drawn into EDTA-containing tubes at predose and at 1, 2, 4, 6, 8, 12, 18, 24, and 32 hours postdose for the determination of T-helper and T-suppressor lymphocyte and neutrophil cell counts.
Bioanalysis
Plasma Corticosteroid Concentrations
Plasma prednisolone and cortisol concentrations were determined using the normal-phase HPLC procedure of Rose and Jusko,21 as updated by Jusko et al.22 Lower limits of quantitation were 5 ng/ml for prednisolone and 4.9 ng/ml for cortisol. Samples analyzed below the limit of quantitation were not used for data analysis. Intra- and interday coefficients of variation were less than 7.5%.
Plasma Protein Binding of Prednisolone
Plasma protein binding of prednisolone was determined by ultrafiltration at 37°C using the Amicon Centrifree Device after spiking each plasma sample with trace amounts of purified 3H-prednisolone and using liquid scintillation counting (Packard model A1900, Downers Grove, IL) to obtain the free fraction.
Cell Responses
Differential and complete leukocyte counts were performed on an automated hemocytometer (CELL-DYN 1700, Abbott Laboratories, Abbott Park, IL). Total lymphocyte and segmented neutrophil counts were obtained. T-helper and T-suppressor cell counts were determined by reacting the whole-blood samples with anti-CD3, anti-CD4, and anti-CD8 antibodies and subsequently measuring fluorescence by flow cytometry (FACS Calibur, Becton Dickinson Immunocytometry Systems, San Jose, CA). T-helper cells were immunoreactive as CD3+, CD4+, and CD8−; T-suppressor cells were CD3+, CD4−, and CD8+.
Pharmacokinetics
The pharmacokinetics of prednisolone was described by monoexponential or biexponential disposition with or without an absorption time lag (tlag). Individual fittings of the pharmacokinetics of free prednisolone concentrations (Cp) were chosen based on the best fit of the following equations:
| (1) |
| (2) |
| (3) |
in which
| (4a,b) |
Parameters obtained from the above compartmental analysis include ka (first-order absorption rate constant), tlag (lag time in absorption), Vc/F (apparent volume of the central compartment), CL/F (apparent systemic clearance), and k12 and k21 (first-order distribution rate constants between the central and peripheral compartments).
WinNonlin (Pharsight Corp., Apex, NC) was used for pharmacokinetic modeling of total and free prednisolone concentration data, with 1/y weighting used to obtain parameter estimates.
Pharmacodynamics
Cortisol
The suppressive effects of prednisolone on cortisol secretion were characterized by an indirect response model23 that was extended to consideration of biexponential disposition of cortisol concentrations.24 The response (R) is represented as a combination of responses of the system each with monoexponential disposition:
| (5) |
Rn is a solution of the following differential equation:
| (6) |
| (7) |
In the above equations, Ln are coefficients of the biexponential function, where L1 and L2 > 0 and L1 + L2 = 1. The λn are slopes of the decline of R versus time plotted on a log scale, IC50 is the concentration of free prednisolone causing 50% cortisol suppression, and Cp(t) is the free prednisolone concentration at time t. The kin(t) is an asymmetric input rate function for endogenous circadian cortisol secretion, as described by
| (8) |
where km is the mesor or mean value of cortisol secretion rate, t is the time of sampling following dosing, kb1 and kb2 are the first and second amplitudes of the cortisol secretion rate, tp1 and tp2 are the first and second acrophases or peak times of the cortisol secretion rate, and the ratios 2π/24 and 2π/12 convert the 24-hour and 12-hour periods into radians. The 12-hour period for the second cosine is chosen by visualization using FOURPHARM.25 The periodic input function kin(t) produces one solution Rcort(t) of the same periods for the baseline:
| (9) |
The Rm is the mesor of baseline cortisol concentrations and relates to the input function (kin(t)) via
| (10) |
where Rb1 and Rb2 are the first and second amplitudes of the baseline cortisol concentrations, and tz1 and tz2 are the first and second acrophases of the baseline cortisol concentrations.
Cortisol concentrations from baseline and treatment periods were fitted simultaneously by the maximum-likelihood method using the ADAPT II26 program to generate the L1, L2, λ1, λ2, IC50, km, kb1, kb2, tp1, and tp2 parameters. Estimates of km, L1, L2, λ1, and λ2 were then used to calculate Rm using equation (10) to examine the sex and racial differences in mean values of baseline cortisol concentrations.
To assess the net suppression of cortisol secretion following prednisone administration, the area under the cortisol concentration curves was calculated using the linear trapezoidal rule. These values were used to calculate the area between baseline and effect curves (ABEC), determined by the following equation:
| (11) |
where is the area under the baseline curve, and is the area under the prednisolone response curve from 0 to 24 hours. Larger ABEC values indicate greater net suppression.
T-Helper and T-Suppressor Lymphocytes
Under normal physiologic conditions, lymphocytes equilibrate between the blood pool and extravascular compartment (such as lymph nodes, spleen, and bone marrow). T-lymphocyte trafficking has been shown to exhibit a circadian rhythm.27 The suppression of T-lymphocyte influx into blood appears to be the primary mechanism28 for the observed lymphocytopenic effect of prednisolone. In this study, after administration of prednisolone, T-lymphocyte cell counts decreased in relation to inhibition of the lymphocyte trafficking between the peripheral lymphoid tissues and the central blood compartment by prednisolone.29 This results in accumulation of lymphocytes in the peripheral lymphoid tissues. As prednisolone concentrations dissipate from blood, lymphocyte buildup in the peripheral compartment is dumped into the blood compartment, resulting in a rebound phase observed in both T-helper and T-suppressor lymphocyte cell counts in most subjects.
The rate of change of the T-helper and T-suppressor cell counts in the precursor (P, extravascular compartment) and in the central blood compartment (R) over time was characterized as indirect and described as
| (12) |
| (13) |
| (14) |
| (15) |
where Cp is the estimated free plasma prednisolone concentration, IC50 is the free plasma prednisolone concentration inhibiting kp(t) by 50%, and Imax is the extent of inhibition that was fixed at 0.8 for both types of cells on the basis of previous observations.30 The ko is defined as the apparent zero-order rate constant for the production of the precursor, while kout represents the first-order rate constant for loss of response. The kp(t) is a time-dependent periodic influx rate of lymphocytes into blood:
| (16) |
where km is the mean input rate of lymphocytes into blood, kb is the amplitude of this rate and tp is the time of occurence of the peak input.
Baseline and treatment periods for each cell count were fitted simultaneously by the maximum-likelihood method using theADAPTII26 program to generate the ko, kout, km, kb, tz, and IC50 parameters. Prednisolone plasma concentrations and T-helper and T-suppressor cell counts were shifted by 96 hours to allow zero time values to be fitted. Achievement of steady state prior to time zero (i.e., prior to 96 hours on adjusted scale) was ensured by visual inspection of fitted curves in all individual fittings. The ABEC was not calculated due to the rebound.
An additional first-order elimination rate constant was added to the precursor compartment. The rate constant value generated was negligible. We also explored the option of letting the zero-order production rate constant (ko) be the time-dependent periodic process and letting the first-order distribution rate constant (kp) be time independent. This model did not generate an appreciable difference in curve fitting compared with the final model. The evaluation of goodness of fit was done by comparing the sum of squared residuals as well as ADAPT II forms of the Akaike Information Criterion and Schwarz Criterion.
Neutrophils
Prednisolone elevates blood neutrophils.31 Accelerated release from the pool of mature neutrophils in the bone marrow is thought to account for neutrophilic leukocytosis. 31 The neutrophil model is the basic indirect response model III23 with initial condition, Ro = kin/kout:
| (17) |
The Smax value for prednisolone was fixed at 3.0 according to earlier observations.32 Baseline levels of neutrophils were assumed to be constant with no biorhythm. Individual ABEC values were calculated according to equation (11). Neutrophil cell counts from treatment were fitted by the maximum-likelihood method using the ADAPT II26 program to generate the kin, kout, and SC50 parameters.
Statistical Methods
Sex- and race-based comparisons were determined by two-way ANOVA after log transformation of parameter estimates. Data are expressed as mean values ± SD. Statistical significance was defined as p < 0.05. All statistical analyses were done using SYSTAT for Windows, version 9.01 (SPSS, Chicago).
RESULTS
Subject Characteristics
Thirty-two healthy subjects (white males [WM], black males [BM], white females [WF], black females [BF]; n = 8/group) completed the study. Table I lists their de-mographic characteristics. All 16 females reported positive readings on ovulation predictor kits at least 2 days prior to day 1 of the study for both baseline and treatment phases. The timing of entry into the study in relation to their menstrual cycle averaged 21 ± 2 days during baseline and 20 ± 4 days during treatment phase. These timings were within the usual range of the luteal phase.
Table I.
Subject Demographics
| White Males | Black Males | Black Females | White Females | |
|---|---|---|---|---|
| Age (years) | 28.1 ± 8.9 | 33.3 ± 5.3 | 29.9 ± 6.6 | 30.1 ± 8.5 |
| TBW (kg) | 77.1 ± 6.6 | 74.0 ± 11.6 | 72.5 ± 10.6 | 65.3 ± 10.3 |
| Dose/TBW (mg/kg) | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 |
Data are mean ± SD (n = 32). TBW, total body weight.
Pharmacokinetics
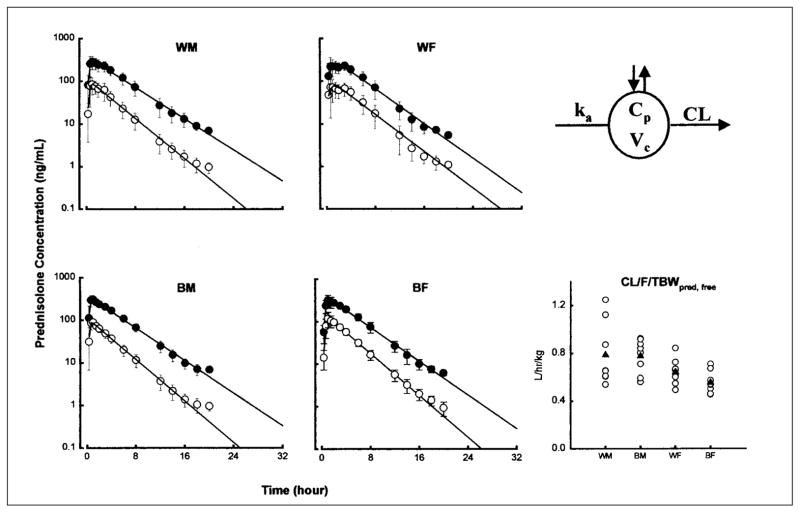
The mean plasma (± SD) concentration versus time profiles of prednisolone (total and free) with fitted equations following a TBW-based single oral dose of prednisone are shown in Figure 1 for the four groups. The pattern reflects rapid appearance of total and free prednisolone and their parallel decline. The PK profiles of 15/32 subjects were best fitted with monoexponential disposition and 17/32 with biexponential disposition. The four profiles are essentially superimposible, indicating that there are no appreciable sex- or race-based differences in the pharmacokinetic patterns of prednisolone.
Figure 1.
Time course of mean ± SD and fitted prednisolone concentrations (total prednisolone, filled circles; free prednisolone, open circles). The pharmacokinetic model used (top right) and comparative CL/F/TBW values of free prednisolone are also shown for individual subjects (open circles) as well as group mean values (filled triangles).
The compartmental pharmacokinetic parameters of total and free prednisolone in the four groups and the two-way ANOVA results are summarized in Table II. The AUC (area under the concentration vs. time curve) of free prednisolone was significantly larger (p < 0.01) in the female group in both races (434 ± 69 ng•h/ml vs. 375 ± 111 ng•h/ml in whites, 497 ± 84 ng•h/ml vs. 364 ± 66 ng•h/ml in blacks). The clearance normalized to TBW was 22% higher in WM and 40% higher in BM compared with females (sex effect p < 0.01). The volume of distribution was also higher in men, resulting in a similar elimination half-life. Norace-based difference was noted except for total prednisolone elimination half-life.
Table II.
Pharmacokinetic Absorption and Disposition Parameters of Total and Free Prednisolone
| Parameter | White Males | Black Males | White Females | Black Females |
p-Value
|
||
|---|---|---|---|---|---|---|---|
| Gender | Race | Gender × Race | |||||
| Total prednisolone | |||||||
| Absorption | |||||||
| ka (h−1) | 5.62 ± 5.78 | 9.71 ± 8.13 | 4.80 ± 4.44 | 2.98 ± 1.86 | NS | NS | NS |
| tlag (h) | 0.283 ± 0.0861 | 0.294 ± 0.0341 | 0.263 ± 0.139 | 0.243 ± 0.0963 | NS | NS | NS |
| Disposition | |||||||
| Vc/F (L) | 44.8 ± 12.1 | 45.5 ± 15.2 | 29.1 ± 8.85 | 42.2 ± 8.46 | < 0.05 | NS | NS |
| Vc/F/TBW (L/kg) | 0.590 ± 0.196 | 0.613 ± 0.169 | 0.453 ± 0.136 | 0.586 ± 0.101 | NS | NS | NS |
| CL/F (L/h) | 13.5 ± 2.91 | 12.6 ± 2.03 | 11.8 ± 2.16 | 11.7 ± 1.89 | NS | NS | NS |
| CL/F/TBW (L/h/kg) | 0.178 ± 0.05 | 0.172 ± 0.0181 | 0.181 ± 0.0266 | 0.165 ± 0.0399 | NS | NS | NS |
| t1/2 (h) | 2.33 ± 0.523 | 2.51 ± 0.813 | 1.74 ± 0.467 | 2.54 ± 0.546 | NS | < 0.05 | NS |
| AUC (ng•h/ml) | 1638 ± 426 | 1628 ± 239 | 1544 ± 239 | 1708 ± 298 | NS | NS | NS |
| Free prednisolone | |||||||
| Absorption | |||||||
| ka (h−1) | 6.65 ± 6.26 | 9.74 ± 6.41 | 7.94 ± 6.98 | 4.01 ± 5.07 | NS | NS | NS |
| tlag (h) | 0.315 ± 0.108 | 0.338 ± 0.123 | 0.343 ± 0.178 | 0.279 ± 0.0525 | NS | NS | NS |
| Disposition | |||||||
| Vc/F (L) | 165 ± 46.6 | 162 ± 43.0 | 107 ± 34.0 | 114 ± 29.4 | < 0.01 | NS | NS |
| Vc/F/TBW (L/kg) | 2.18 ± 0.745 | 2.183 ± 0.357 | 1.65 ± 0.462 | 1.58 ± 0.321 | < 0.01 | NS | NS |
| CL/F (L/h) | 59.4 ± 14.9 | 57.6 ± 13.8 | 42.1 ± 9.59 | 39.8 ± 6.40 | < 0.01 | NS | NS |
| CL/F/TBW (L/h/kg) | 0.788 ± 0.265 | 0.778 ± 0.143 | 0.644 ± 0.108 | 0.554 ± 0.093 | < 0.01 | NS | NS |
| t1/2 (h) | 1.92 ± 0.264 | 2.01 ± 0.538 | 1.76 ± 0.264 | 2.01 ± 0.466 | NS | NS | NS |
| AUC (ng•h/ml) | 375 ± 111 | 364 ± 65.6 | 434 ± 68.5 | 497 ± 84.2 | < 0.01 | NS | NS |
NS, not significant (p > 0.05). Data are mean values ± SD (n = 8/group). ka, first-order absorption rate constant; tlag, time lag in absorption; Vc/F, apparent volume of the central compartment; TBW, total body weight; CL/F, apparent systemic clearance; t1/2, half-life; AUC, area under the prednisolone concentration versus time curve.
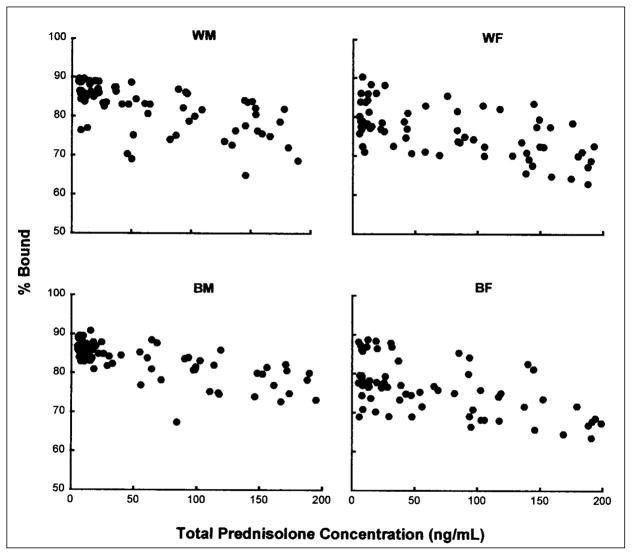
Figure 2 illustrates the plasma protein-binding profiles of prednisolone. Data from four groups superimpose well, indicating the lack of sex- or race-based differences in plasma protein binding of prednisolone. The concentration range for prednisolone was low, and binding was modestly nonlinear.
Figure 2.
Relationship between total prednisolone concentration and percentage of prednisolone bound to plasma proteins. Each data point represents the percentage bound at the corresponding total prednisolone concentration in a subject.
Pharmacodynamics
Endogenous Cortisol
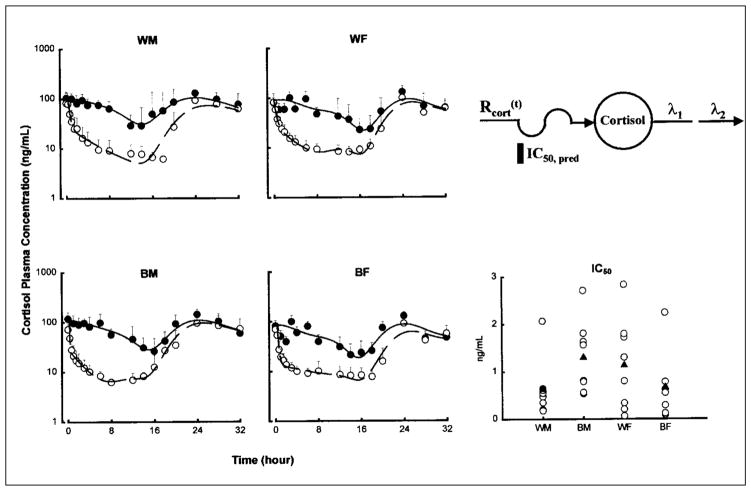
The mean plasma (± SD) concentration versus time profiles with fitted curves of cortisol are shown in Figure 3 for the four groups. The circadian rhythm of cortisol concentration is evident from the baseline, with peak concentrations occurring around 8 a.m. (0 and 24 hours after dosing) and a nadir around midnight (16 hours after dosing). After prednisone dosing, the decline of cortisol was immediate and biexponential in nature before reaching a prolonged trough and subsequently resuming a circadian rhythm.
Figure 3.
Time course of mean ± SD and fitted plasma cortisol concentrations. Symbols show experimental data, and lines show the fittings to the pharmacodynamic model shown above. The baseline phase is displayed by the solid symbols and solid lines. The prednisone phase is displayed by the open symbols and broken lines. The comparative free prednisolone IC50 values of cortisol suppression are also shown for individual subjects (open circles) as well as group mean values (filled triangles).
The pharmacodynamic parameter estimates for the cortisol model are listed in Table III. The baseline cortisol data for 1 white male subject were excluded because of his lack of circadian rhythm. Hence, his treatment cortisol data were fitted alone to generate pharmacodynamic parameters.
Table III.
Pharmacodynamic Parameters for Cortisol Suppression
| Parameter | White Males | Black Males | White Females | Black Females |
p-Value
|
||
|---|---|---|---|---|---|---|---|
| Gender | Race | Gender × Race | |||||
| λ1 (h−1) | 2.38 ± 1.51 | 5.58 ± 3.55 | 2.92 ± 1.21 | 4.75 ± 2.44 | NS | < 0.01 | NS |
| λ2 (h−1) | 0.117 ± 0.081 | 0.324 ± 0.14 | 0.0816 ± 0.054 | 0.128 ± 0.109 | < 0.05 | < 0.05 | < 0.05 |
| L1 (ng/ml) | 0.983 ± 0.016 | 0.980 ± 0.020 | 0.991 ± 0.0067 | 0.985 ± 0.019 | NS | NS | NS |
| L2 (ng/ml) | 0.0197 ± 0.016 | 0.0263 ± 0.019 | 0.009044 ± 0.0067 | 0.0170 ± 0.0193 | NS | NS | NS |
| Rm (ng/ml) | 67.0 ± 23.9 | 67.8 ± 9.51 | 58.0 ± 12.3 | 52.4 ± 7.54 | < 0.05 | NS | NS |
| IC50 (ng/ml) | 0.631 ± 0.607 | 1.30 ± 0.763 | 1.13 ± 0.961 | 1.90 ± 3.55 | NS | NS | NS |
| ABEC (ng•h/ml) | 1160 ± 701 | 1270 ± 372 | 899 ± 314 | 891 ± 183 | < 0.05 | NS | NS |
Data are mean values ± SD (n = 8/group). λ1, λ2, slopes of biexponential decline of cortisol concentration; L1, L2, coefficients of biexponential function; Rm, mean baseline cortisol concentration; IC50, free prednisolone concentration producing 50% of the maximum inhibition of cortisol excretion; ABEC, area between the baseline and effect curves; NS, not significant.
The IC50 value, a measure of intrinsic sensitivity to the suppressive effects of free prednisolone, was the lowest (most sensitive) in WM (0.631 ± 0.607 ng/ml). IC50 values for the other three groups were 2 to 3 times that of WM, with the highest (least sensitive) in BF (1.90 ± 3.55 ng/ml). However, this difference was not significant. There was also greater suppression observed in the men from both races, as indicated by the significantly higher ABEC values. The Rm values were higher in men than in women in both races. This parameter reflects the baseline mean cortisol concentrations.
T-Helper and T-Suppressor Lymphocytes
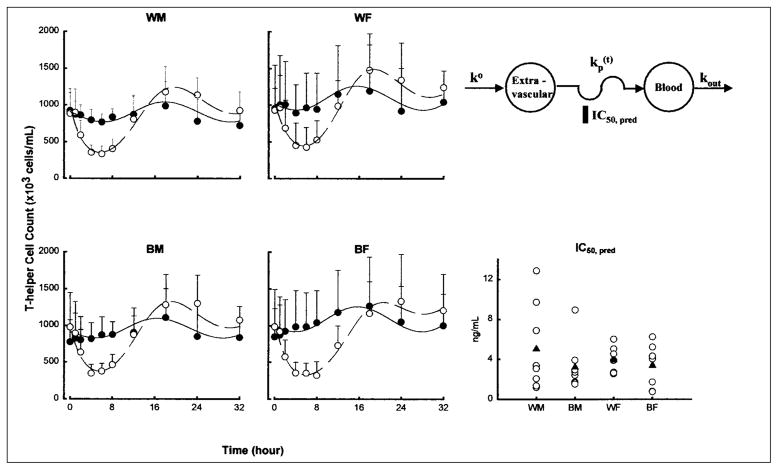
The mean whole-blood cell count profiles with fitted curves of T-helper and T-suppressor cells are shown in Figures 4 and 5 for the four groups. The baseline cell counts showed a circadian rhythm and were generally well characterized as a cosine function. The nadir occurred at 8 a.m. (0 and 24 hours after dosing), and the acrophase was found at 2 a.m. (18 hours after dosing). This pattern is somewhat opposite to that of cortisol concentrations during baseline. Prednisone was given near the low point of the baseline circadian rhythm; nonetheless, cell concentrations declined rapidly, reaching a nadir after about 5 hours. Following the nadir, cell counts returned toward baseline, reaching the acrophase at about 18 hours. A rebound is evident in Figures 4 and 5, with peak cell counts being about 116% of those during baseline. After the acrophase, cell counts trended toward but did not reach the baseline at the time of last sampling.
Figure 4.
Time course of mean ± SD and fitted blood T-helper lymphocyte counts. Symbols show experimental data, and lines show the fittings to the pharmacodynamic model shown. Symbols and lines are defined in the legend of Figure 3. The comparative free prednisolone IC50 values of T-helper cell inhibition are also shown for individual subjects (open circles) as well as group mean values (filled triangles).
Figure 5.
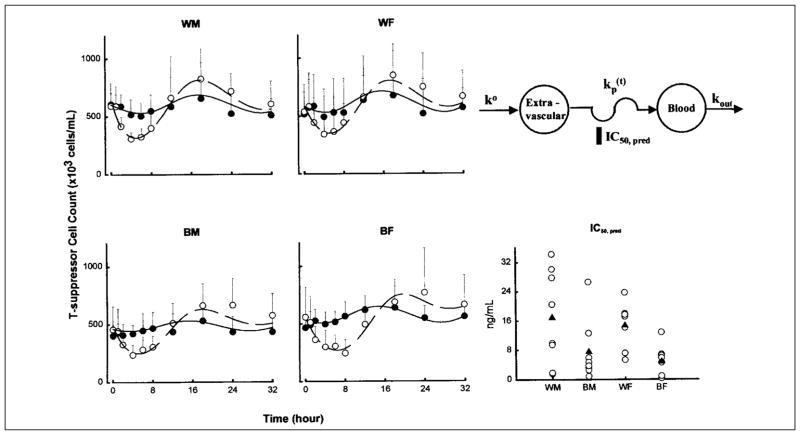
Time course of mean ± SD and fitted blood T-suppressor lymphocyte counts. Symbols show experimental data, and lines show the fittings to the pharmacodynamic model shown. Symbols and lines are defined in the legend of Figure 3. The comparative free prednisolone IC50 values of T-suppressor cell inhibition are also shown for individual subjects (open circles) as well as group mean values (filled triangles).
The pharmacodynamic parameter estimates for cell trafficking are shown in Table IV. The T-helper, T-suppressor, and neutrophil data from 1 white woman were excluded from the analysis because she showed no suppressive/stimulatory response after a 15mg dose of prednisone. In contrast to cortisol, WM was the least sensitive group to the suppressive effects of prednisolone on both T-helper and T-suppressor cell trafficking, as evident from their higher IC50 values. The only parameters that achieved statistical significance (p < 0.05) were IC50 and ko for T-suppressor cells between white and black subjects.
Table IV.
Pharmacodynamic Parameters of Cell Trafficking
| Parameter | White Males | Black Males | White Females | Black Females |
p-Value
|
||
|---|---|---|---|---|---|---|---|
| Gender | Race | Gender × Race | |||||
| T-helper cell trafficking | |||||||
| ko (cells/ml•h−1) | 403 ± 66.0 | 402 ± 191 | 555 ± 182 | 541 ± 322 | NS | NS | NS |
| kout (h−1) | 0.435 ± 0.077 | 0.412 ± 0.118 | 0.504 ± 0.088 | 0.470 ± 0.141 | NS | NS | NS |
| km (h−1) | 0.146 ± 0.16 | 0.168 ± 0.075 | 0.087 ± 0.063 | 0.114 ± 0.087 | NS | NS | NS |
| kb (h−1) | 0.0567 ± 0.10 | 0.0454 ± 0.030 | 0.0192 ± 0.015 | 0.0280 ± 0.022 | NS | NS | NS |
| tp (h) | 13.4 ± 8.14 | 15.4 ± 2.38 | 13.6 ± 1.37 | 12.2 ± 6.81 | NS | NS | NS |
| IC50 (ng/ml) | 5.05 ± 4.32 | 3.22 ± 2.44 | 3.90 ± 1.36 | 3.38 ± 2.06 | NS | NS | NS |
| T-suppressor cell trafficking | |||||||
| ko (cells/ml•h−1) | 236 ± 99.5 | 171 ± 64.8 | 283 ± 186 | 182 ± 97.4 | NS | < 0.05 | NS |
| kout (h−1) | 0.369 ± 0.117 | 0.366 ± 0.151 | 0.431 ± 0.205 | 0.305 ± 0.143 | NS | NS | NS |
| km (h−1) | 0.198 ± 0.206 | 0.215 ± 0.182 | 0.114 ± 0.086 | 0.292 ± 0.393 | NS | NS | NS |
| kb (h−1) | 0.0845 ± 0.132 | 0.0505 ± 0.035 | 0.0241 ± 0.021 | 0.139 ± 0.225 | NS | NS | NS |
| tp (h) | 18.3 ± 5.01 | 17.0 ± 9.60 | 17.8 ± 8.30 | 11.1 ± 8.32 | NS | NS | NS |
| IC50 (ng/ml) | 16.9 ± 12.9 | 7.50 ± 8.47 | 14.7 ± 6.48 | 4.77 ± 4.20 | NS | < 0.01 | NS |
| Neutrophil trafficking | |||||||
| kout (h−1) | 0.0841 ± 0.025 | 0.121 ± 0.075 | 0.106 ± 0.026 | 0.102 ± 0.033 | NS | NS | NS |
| kin (cells/ml•h−1) | 0.281 ± 0.11 | 0.331 ± 0.258 | 0.346 ± 0.063 | 0.243 ± 0.059 | NS | NS | NS |
| SC50 (ng/ml) | 57.7 ± 59.8 | 28.4 ± 34.2 | 26.9 ± 20.1 | 24.9 ± 18.2 | NS | NS | NS |
| ABEC (106 cells•h/ml) | 32.4 ± 19.3 | 32.3 ± 11.2 | 51.9 ± 19.4 | 38.0 ± 12.4 | NS | NS | NS |
Data are mean values ± SD (white males, n = 8; black males, n = 8; white females, n = 7; black females, n = 8). ko, zero-order rate of production of T-helper or T-suppressor cells into the extravascular compartment; kout, first-order rate constant describing T-helper or T-suppressor or neutrophil cell movement out of the blood compartment; km, first-order rate of T-helper or T-suppressor cells entering the blood from the extravascular compartment per unit time; kb, amplitude of the circadian entry rate; Tp, peak time of the circadian function; IC50, free drug concentration producing50%of maximum inhibitory on kin; SC50, free drug concentration producing 50% of maximum stimulation of kin; ABEC, area between the baseline and effect curves.
Neutrophils
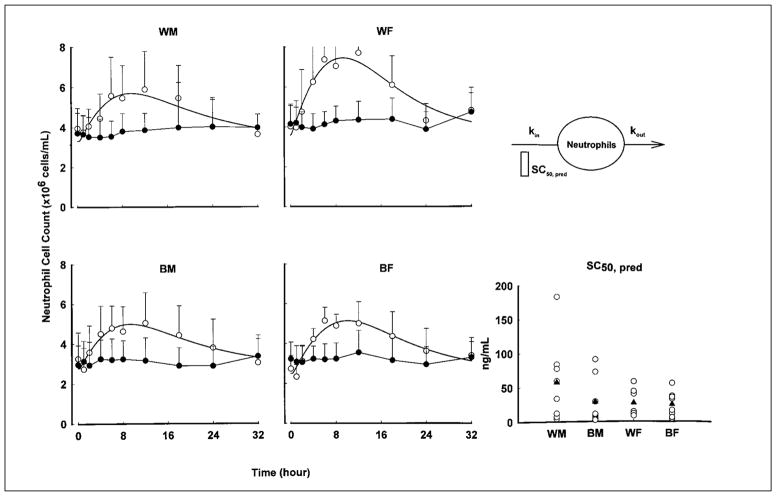
The mean whole-blood cell count profiles with fitted curves of neutrophils are shown in Figure 6, and the parameter estimates are listed in Table IV for the four groups. Consistent with T-helper and T-suppressor lymphocyte modeling results, WM is the least sensitive group to the stimulatory effects of prednisolone, as evident from their highest SC50 value. However, none of these parameter differences achieved statistical significance.
Figure 6.
Time course of mean ± SD and fitted blood neutrophil counts. Symbols show experimental data, and lines show the fittings to the pharmacodynamic model shown. The comparative prednisolone SC50 values of neutrophil stimulation are also shown for individual subjects (open circles) as well as group mean values (filled triangles).
DISCUSSION
To assess sex and race as covariates in the use of prednisone, we investigated the pharmacokinetics and various pharmacodynamic markers in male, female, black, and white healthy subjects following a single oral dose of prednisone. Basic and extended indirect response models were used to allow quantitation of the underlying physiologic and genetic differences in these subjects.
The mean pharmacokinetic parameter values agree well with previous investigations.17,19 In our study, the TBW-normalized mean free prednisolone oral clearance in male subjects was significantly higher than the corresponding mean in female subjects, regardless of race (by 22% in whites, 40% in blacks; p < 0.01, Table II). The TBW-normalized apparent volume of distribution was also higher in men than in women (by 32% in whites, 38% in blacks; p < 0.01). The similarity in the direction and magnitude of the difference in oral apparent clearance and volume of distribution results in similar half-lives in men and women in this study. Meffin et al17 have reported a 21% higher free prednisolone clearance in females (p = 0.036) after intravenous administration of prednisolone. However, this finding of a sex difference was weakened by confounders such as not controlling for menstrual cycle phase and a small sample size. It was suggested that young women have approximately 1.4 times the CYP3A4 activity of men.1 One would therefore expect a higher CYP3A4 substrate clearance (such as for prednisolone) in women than in men. However, prednisolone undergoes a variety of biotransformation pathways in the liver and possibly other organs, and thus it cannot be determined conclusively which enzyme is responsible for noted differences. Interestingly, the hepatic expression of the transport protein P-glycoprotein that seems to be functionally associated with CYP3A is higher in men compared with women.33 Steroid hormones, including prednisolone, have been found to interact with P-glycoprotein as substrates or inhibitors in a complex manner.34
The plasma prednisolone concentration reflects the absorption/metabolism of its parent compound, prednisone, as well as their interconversion. Bioavailability of prednisone may account for, in part, the observed differences in apparent prednisolone clearance. The similarity in the decrease of both CL/F and Vc/F (and the resulting lack of change in half-life) in female subjects may therefore be a consequence of bioavailability (F), the common denominator of both parameters. This implies that women may exhibit increased bioavailability of prednisone relative to men. If this is true, it may also explain the opposite results obtained from previous investigations in which women exhibited higher clearance after intravenous administration of prednisolone.17,18
As mentioned above, prednisolone undergoes a variety of biotransformation processes, including CYP3A-catalyzed 6β-hydroxylation. A recent study, using IV and oral midazolam as a probe, has shown a similarity in CYP3A activity in black and white men.35 This finding concurs with our observation of a lack of significant racial differences in total or free prednisolone clearance. However, the similar clearance of prednisolone between blacks and whites differs from another study that showed a 37% lower methylprednisolone clearance in blacks.13 The two compounds often exhibit differing sensitivity to various drug interactions as well.20
We chose to study prednisolone PK/PD during the luteal phase of the menstrual cycle based on the premise that any perturbation caused by hormonal differences would be most apparent when the female hormone concentrations were relatively high. In fact, sex hormones have been shown to affect maturation and function of lymphocytes, influence monocyte and macrophage activity, and modulate immunoregulatory mechanisms.36–38 Estrogen and progesterone may inhibit T-suppressor and natural killer cell functions, as shown by an insignificant drop in activity during the luteal phase.36,39,40 Neutrophil and total T-lymphocyte counts correlate inversely with estrogen level: leukocyte counts are lowest at the follicular estrogen peak, while monocytes and granulocytes rise during the luteal phase and are maximal at the progesterone peak.41
An important feature of this study is the application of indirect response models for quantitation of the diverse pharmacodynamic effects of prednisolone. It was necessary to assign Imax or Smax values because only one dose level was studied and maximal responses were not attained. However, these parameters were evident from previous studies, and the use of moderate doses allowed better opportunity to assess changes in response.
The cortisol pharmacodynamic model used in this study differed from our previous studies.42,43 This model extended the basic indirect response models by considering the biexponential decline in cortisol concentrations. This differs from the monoexponential patterns after doses of methylprednisolone.44 This may be caused by dual effects of prednisolone on adrenal suppression as well as displacement of cortisol from plasma protein-binding sites. The other steroids do not bind to transcortin as do cortisol and prednisolone.45 Another feature of this model, the dual cosine function, permitted asymmetric input rates of cortisol, allowing us to capture the nonmidpoint nadir of cortisol as well as the abrupt increase in cortisol concentrations seen between 18 and 20 hours.
The lymphocyte pharmacodynamic model used in this study also differed from our previous studies.2,42,43 In this study, a model that is capable of encompassing a substantial rebound component, as demonstrated by the return of T-helper and T-suppressor lymphocytes beyond corresponding baseline cell counts, was needed. This was accomplished by use of a modified precursor-dependent indirect pharmacodynamic response model.46 As such, the pharmacodynamic parameters of km and kb have first-order (h−1) rather than zero-order units (cells/ml/h), and the values from the previous studies have to be converted for direct comparison. Such converted km and kb values are similar to those obtained in the present study.43
The cortisol IC50 values for the men were similar to those obtained previously.42,43 Within sex, blacks have higher IC50 values (106% in men,68%in women; Table III), indicating a potential race-based difference in sensitivity to prednisolone’s suppressive effect on cortisol secretion. However, this observed difference did not achieve statistical significance. Women in our study showed significantly (p < 0.05) lower Rm values, indicating a lower mean baseline cortisol concentration. This was not observed in our previous study,2 which also showed a threefold lower mean Rm value compared with our mean value.
In our T-helper lymphocyte model, no statistically significant differences in parameters were found; however, the mean T-helper lymphocyte IC50 values were higher in whites, regardless of sex (57% in men,15%in women). Consistent with this observation, the T-suppressor lymphocyte IC50 was more than twofold higher in white men than in black men and more than three-fold higher in white women than in black women. This difference was statistically significant. Interestingly, we did not detect any sex difference in any of the parameters.
Burton et al32 reported pronounced neutrophilia after dexamethasone treatment, which may be the result of down-regulation of adhesion receptors, L-selectin, and CD18 expression on circulating neutrophils. We observed a substantial neutrophilic effect similar to those reported in previous studies.32,42 Compared with the SC50 value reported in a study done in 11 white men and 1 black man, our mean SC50 value for white men was nearly threefold higher. However, the variability of parameters in both studies was high. In our neutrophil model, no statistically significant differences in parameter values were found. Consistent with IC50 values in the T-helper and T-suppressor cell model, the mean neutrophil SC50 values were higher in whites, especially in white men, with a substantial twofold difference compared with black men.
The findings of this study indicate that PK/PD differences exist for prednisolone based on race and gender. However, the clinical importance of these findings is difficult to conclude. Women have a smaller clearance of free prednisolone with or without normalizing to TBW. This results in higher systemic exposure to prednisolone, as shown by higher AUC values. However, the volume of distribution of prednisolone is also higher in women, resulting in similar half-lives between men and women. Along with this, the failure to detect significantly different sensitivity values (IC50 or SC50) in any of the pharmacodynamic measurements between the two sexes implies that men and women should receive the same prednisone dose because of similarity in net response. The opposite was observed for race-based effects in prednisolone PK/PD. No significant racial difference was observed in any of the pharmacokinetic parameters of free prednisolone. However, whites have shown consistently lower sensitivities to prednisolone effects on T-helper, T-suppressor, and neutrophil trafficking, suggesting the need for higher doses to maintain the same immunosuppressive/therapeutic effects of prednisone. However, if implemented, this higher dosage requirement, compounded by their greater sensitivity to the adrenal suppressive effects as shown by a lower cortisol IC50 value, may expose whites to a greater frequency of untoward side effects from prednisone therapy. Last, due to the chronic nature of corticosteroid therapy, additional experiments with repeat dosing are desired to further evaluate the clinical implications of these findings.
Acknowledgments
Supported by grant GM 24211 from the National Institutes of General Medical Sciences (NIH) and fellowship support for M. H. Magee from Clinical Pharmacology Unit, SmithKline Beecham Pharmaceuticals, Philadelphia.
The authors would like to thank the clinical staff of the Clinical Pharmacokinetics Laboratory at the Millard Fillmore Hospital and Ms. Nancy Pyszczynski and Ms. Suzette Mis for providing valuable support in this study. The authors would also like to thank Dr. Wojciech Krzyzanski for his insightful discussion.
References
- 1.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lew KH, Ludwig EA, Milad MA, Donovan D, Middleton E, Jr, Ferry J, Jusko WJ. Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 1993;54:402–414. doi: 10.1038/clpt.1993.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998;34:203–218. doi: 10.2165/00003088-199834030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Brazier JL, Ritter J, Berland M, Khenfer D, Faucon G. Pharmacokinetics of caffeine during and after pregnancy. Dev Pharmacol Ther. 1983;6:315–322. doi: 10.1159/000457332. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz JB, Capili H, Daugherty J. Aging of women alters S-verapamil pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 1994;55:509–517. doi: 10.1038/clpt.1994.64. [DOI] [PubMed] [Google Scholar]
- 6.Boekenoogen SJ, Szefler SJ, Jusko WJ. Prednisolone disposition and protein binding in oral contraceptive users. J Clin Endocrinol Metab. 1983;56:702–709. doi: 10.1210/jcem-56-4-702. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- 8.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 9.Walker JS, Carmody JJ. Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesth Analg. 1998;86:1257–1262. doi: 10.1097/00000539-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Wood AJ, Zhou HH. Ethnic differences in drug disposition and responsiveness. Clin Pharmacokinet. 1991;20:350–373. doi: 10.2165/00003088-199120050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci. 1997;86:1328–1333. doi: 10.1021/js9702168. [DOI] [PubMed] [Google Scholar]
- 12.Tornatore KM, Reed KA, Venuto RC. Racial differences in the pharmacokinetics of methylprednisolone in black and white renal transplant recipients. Pharmacotherapy. 1993;13:481–486. [PubMed] [Google Scholar]
- 13.Tornatore KM, Biocevich DM, Reed K, Tousley K, Singh JP, Venuto RC. Methylprednisolone pharmacokinetics, cortisol response, and adverse effects in black and white renal transplant recipients. Transplantation. 1995;59:729–736. doi: 10.1097/00007890-199503150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Spahn JBE, Covar R, Leung D. Do African Americans display a diminished response to glucocorticoids? J Allergy Clin Immunology. 1999;104:S62. [Google Scholar]
- 15.Neylan JF. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1997;64:1277–1282. doi: 10.1097/00007890-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Valente JF, Hariharan S, Peddi VR, et al. Causes of renal allograft loss in black vs. white transplant recipients in the cyclosporine era. Clin Transplant. 1997;11:231–236. [PubMed] [Google Scholar]
- 17.Meffin PJ, Brooks PM, Sallustio BC. Alterations in prednisolone disposition as a result of time of administration, gender and dose. Br J Clin Pharmacol. 1984;17:395–404. doi: 10.1111/j.1365-2125.1984.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey FJ, Frey BM. Urinary 6 beta-hydroxyprednisolone excretion indicates enhanced prednisolone catabolism. J Lab Clin Med. 1983;101:593–604. [PubMed] [Google Scholar]
- 19.Chakraborty A, Blum RA, Mis SM, Cutler DL, Jusko WJ. Pharmacokinetic and adrenal interactions of IL-10 and prednisone in healthy volunteers. J Clin Pharmacol. 1999;39:624–635. doi: 10.1177/00912709922008137. [DOI] [PubMed] [Google Scholar]
- 20.Jusko WJ, Ludwig EA. Corticosteroids. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics: Principle of Therapeutic Drug Monitoring. Vancouver, WA: Applied Therapeutics; 1995. [Google Scholar]
- 21.Rose JQ, Jusko WJ. Corticosteroid analysis in biological fluids by high-performance liquid chromatography. J Chromatogr. 1979;162:273–280. doi: 10.1016/s0378-4347(00)81514-5. [DOI] [PubMed] [Google Scholar]
- 22.Jusko WJ, Pyszczynski NA, Bushway MS, D’Ambrosio R, Mis SM. Fifteen years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. J Chromatogr B Biomed Appl. 1994;658:47–54. doi: 10.1016/0378-4347(94)00218-5. [DOI] [PubMed] [Google Scholar]
- 23.Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–478. doi: 10.1007/BF01061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krzyzanski W, Jusko WJ. Indirect pharmacodynamic models for responses with multicompartmental distribution or polyexponential disposition. J Pharmacokin Pharmacodyn. 2001;28:57–78. doi: 10.1023/a:1011517718990. [DOI] [PubMed] [Google Scholar]
- 25.Krzyzanski W, Chakraborty A, Jusko WJ. Algorithm for application of Fourier analysis for biorhythmic baselines of pharmacodynamic indirect response models. Chronobiol Int. 2000;17:77–93. doi: 10.1081/cbi-100101034. [DOI] [PubMed] [Google Scholar]
- 26.D’Argenio DZ, Schumitzky A. ADAPT II Users’ Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: Biomedical Simulations Resource; 1997. [Google Scholar]
- 27.Miyawaki T, Taga K, Nagaoki T, Seki H, Suzuki Y, Taniguchi N. Circadian changes of T lymphocyte subsets in human peripheral blood. Clin Exp Immunol. 1984;55:618–622. [PMC free article] [PubMed] [Google Scholar]
- 28.Milad MA, Ludwig EA, Anne S, Middleton E, Jr, Jusko WJ. Pharmacodynamic model for joint exogenous and endogenous corticosteroid suppression of lymphocyte trafficking. J Pharmacokinet Biopharm. 1994;22:469–480. doi: 10.1007/BF02353790. [DOI] [PubMed] [Google Scholar]
- 29.Bloemena E, Weinreich S, Schellekens PT. The influence of prednisolone on the recirculation of peripheral blood lymphocytes in vivo. Clin Exp Immunol. 1990;80:460–466. doi: 10.1111/j.1365-2249.1990.tb03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ten Berge RJ, Sauerwein HP, Yong SL, Schellekens PT. Administration of prednisolone in vivo affects the ratio of OKT4/OKT8 and the LDH-isoenzyme pattern of human T lymphocytes. Clin Immunol Immunopathol. 1984;30:91–103. doi: 10.1016/0090-1229(84)90010-2. [DOI] [PubMed] [Google Scholar]
- 31.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 32.Burton JL, Kehrli ME, Jr, Kapil S, Horst RL. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J Leukoc Biol. 1995;57:317–325. doi: 10.1002/jlb.57.2.317. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995;275:1011–1018. [PubMed] [Google Scholar]
- 34.Nakayama A, Eguchi O, Hatakeyama M, Saitoh H, Takada M. Different absorption behaviors among steroid hormones due to possible interaction with P-glycoprotein in the rat small intestine. Biol Pharm Bull. 1999;22:535–538. doi: 10.1248/bpb.22.535. [DOI] [PubMed] [Google Scholar]
- 35.Wandel C, Witte JS, Hall JM, Stein CM, Wood AJ, Wilkinson GR. CYP3A activity in African American and European American men: population differences and functional effect of the CYP3A4*1B5′-promoter region polymorphism. Clin Pharmacol Ther. 2000;68:82–91. doi: 10.1067/mcp.2000.108506. [DOI] [PubMed] [Google Scholar]
- 36.Ansar Ahmed SPW, Talal N. Sex hormones, immune responses, and autoimmune disease: mechanism of a sex hormone action. American Journal of Pathology. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 37.Polan ML, Kuo A, Loukides J, Bottomly K. Cultured human luteal peripheral monocytes secrete increased levels of interleukin-1. J Clin Endocrinol Metab. 1990;70:480–484. doi: 10.1210/jcem-70-2-480. [DOI] [PubMed] [Google Scholar]
- 38.Leslie CA, Dubey DP. Increased PGE2 from human monocytes isolated in the luteal phase of the menstrual cycle. Implications for immunity? Prostaglandins. 1994;47:41–54. doi: 10.1016/0090-6980(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 39.Sulke AN, Jones DB, Wood PJ. Variation in natural killer activity in peripheral blood during the menstrual cycle. Br Med J (Clin Res Ed) 1985;290:884–846. doi: 10.1136/bmj.290.6472.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moldofsky H, Lue FA, Shahal B, Jiang CG, Gorczynski RM. Diurnal sleep/wake-related immune functions during the menstrual cycle of healthy young women. J Sleep Res. 1995;4:150–159. doi: 10.1111/j.1365-2869.1995.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 41.Mathur S, Mathur RS, Goust JM, Williamson HO, Fudenberg HH. Cyclic variations in white cell subpopulations in the human menstrual cycle: correlations with progesterone and estradiol. Clin Immunol Immunopathol. 1979;13:246–253. doi: 10.1016/0090-1229(79)90069-2. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty A, Blum RA, Cutler DL, Jusko WJ. Pharmacoimmunodynamic interactions of interleukin-10 and prednisone in healthy volunteers. Clin Pharmacol Ther. 1999;65:304–318. doi: 10.1016/S0009-9236(99)70110-4. [DOI] [PubMed] [Google Scholar]
- 43.Wald JA, Law RM, Ludwig EA, Sloan RR, Middleton E, Jr, Jusko WJ. Evaluation of dose-related pharmacokinetics and pharmacodynamics of prednisolone in man. J Pharmacokinet Biopharm. 1992;20:567–589. doi: 10.1007/BF01064420. [DOI] [PubMed] [Google Scholar]
- 44.Kong AN, Ludwig EA, Slaughter RL, DiStefano PM, DeMasi J, Middleton E, Jr, Jusko WJ. Pharmacokinetics and pharmacodynamic modeling of direct suppression effects of methylprednisolone on serum cortisol and blood histamine in human subjects. Clin Pharmacol Ther. 1989;46:616–628. doi: 10.1038/clpt.1989.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocci ML, Jr, D’Ambrosio R, Johnson NF, Jusko WJ. Prednisolone binding to albumin and transcortin in the presence of cortisol. Biochem Pharmacol. 1982;31:289–292. doi: 10.1016/0006-2952(82)90172-1. [DOI] [PubMed] [Google Scholar]
- 46.Sharma A, Ebling WF, Jusko WJ. Precursor-dependent indirect pharmacodynamic response model for tolerance and rebound phenomena. J Pharm Sci. 1998;87:1577–1584. doi: 10.1021/js980171q. [DOI] [PubMed] [Google Scholar]