Abstract
In this case report, we describe an “uncommon” case of axial gouty arthropathy in a 69-year-old woman with bilateral sciatica that was thoroughly evaluated with conventional radiography, CT scan, magnetic resonance imaging, bone scintigraphy, and PET-CT. Axial gouty arthropathy should be included in the differential diagnosis of chronic low back pain, mainly when several risk factors for gout are present.
Keywords: Spine, sacroiliac, gouty arthropathy, radiography, bone scintigraphy, CT scan, MRI, PET-CT
Introduction
Gout is a metabolic disorder and the most common form of microcrystalline arthropathy (1). Gouty arthritis is usually monoarticular, more frequently found in men, and usually affects peripheral joints (1). Although axial involvement of gout is generally considered to be rare or unusual (2 –6), recent publications, mostly in the rheumatologic literature, have pointed out that axial gouty arthropathy may be, in fact, much more common than initially perceived (6 –9). With that in mind, we report an “uncommon” case of a 69-year-old woman, with spinal and sacroiliac involvement of gout, thoroughly evaluated with conventional radiography, computed tomography (CT) scan, magnetic resonance imaging (MRI), bone scintigraphy, and PET-CT.
Case report
A 69-year-old obese woman (BMI: 40) with previous history of constrictive pericarditis, chronic renal insufficiency, high blood pressure, and diabetes mellitus was sent by her family physician to the urgent care department of our institution due to low fever and exacerbation of her chronic back pain, uncontrolled with non-steroidal anti-inflammatory drugs (NSAIDs) and tramadol.
She suffered from low back pain irradiating to the buttocks and hips, preventing her to bear weight and walk. Her physical examination did not show any motor or sensitive deficits, but sacroiliac provocative tests were positive. She did not have any other articular symptoms or signs.
Her medical treatment included daily doses of lisinopril, sinvastatine, carvedilol, acetylsalicylic acid, and furosemide.
Laboratory tests found a moderate renal insufficiency with a creatinine clearance of 43 mL/min/1.73 m2 (normal >90 mL/min.1.73 m2); a discrete neutrophilic leukocytosis (10.350 /µL); CRP, 22.5 mg/dl (normal <1.0 mg/dl); total seric calcium, 10.01 mg/dl (normal, 8.80–10.40 mg/dl); alkaline phosphatase, 192 UI/L (normal, 30–120 UI/L); and uric acid, 13.2 mg/dl (normal, 2.7–7.7 mg/dl).
Clinically, she was diagnosed with bilateral sciatic and had a history of lumbar vertebral canal stenosis.
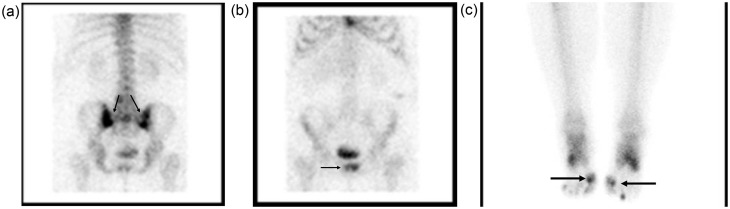
Bone scan showed increased uptake of both SI joints (Fig. 1a), left L4/L5 interapophyseal joint, symphysis pubis (Fig. 1b), and first metatarsophalangeal joints (Fig. 1c).
Fig. 1.
Bone scan shows strong radiophamaceutical uptake (black arrows) in both sacroiliac joints (a), and less intense in the symphysis pubis (b), and first metatarsophalangeal joints (c).
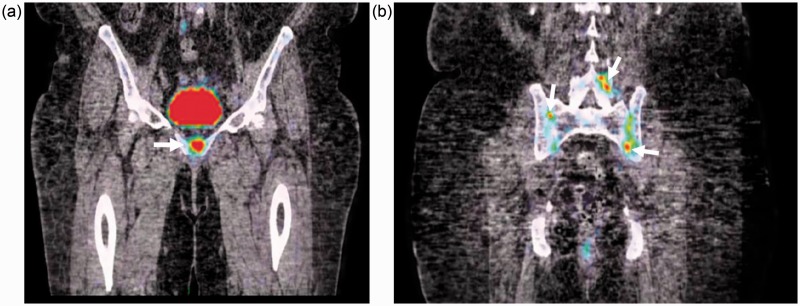
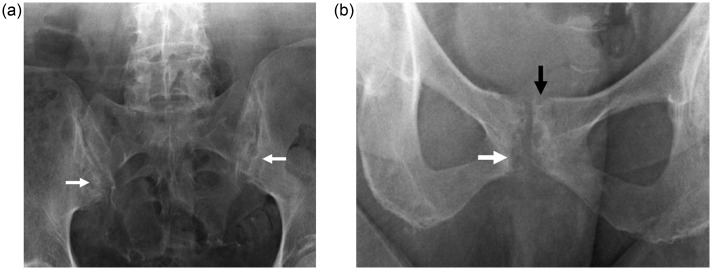
PET-CT also showed increased FDG uptake in the same areas (Fig. 2), and conventional radiography presented typical intra-articular and para-articular “punch-out” bone erosions, well delimited by discrete sclerosis on the SI joints (Fig. 3a), symphysis pubis (Fig. 3b), and fifth proximal interphalangeal joint of the left foot.
Fig. 2.
Coronal PET-CT fusion reformats of the pelvis showing increased 18F-FDG uptake in the symphysis pubis (arrow; (a)), both sacroiliac joints and left L4–L5 interapophyseal joints (arrows; (b)).
Fig. 3.
(a) Radiograph of pelvis (incidence of Ferguson) showing well-defined subchondral bone erosions on both sacroiliac joints (arrows), with discrete adjacent sclerosis. (b) Localized antero-posterior radiography of the symphysis pubis showing similar “punched-out” subchondral (white arrow) and para-articular erosions (black arrow) typically seen on gouty arthropathy.
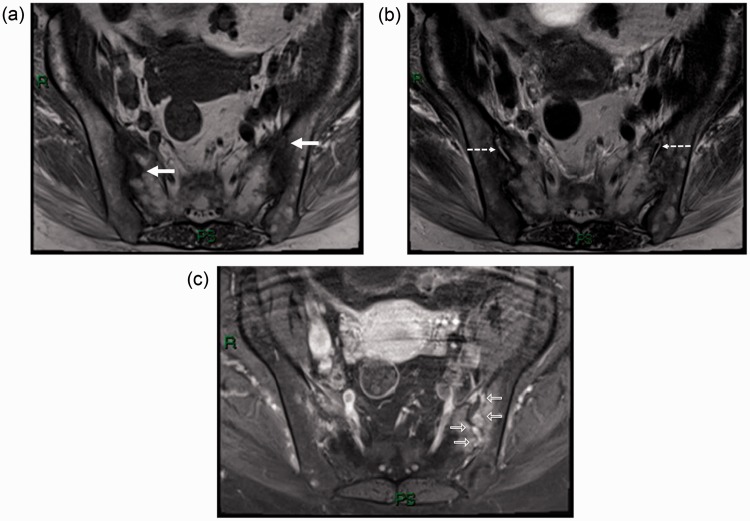
MRI of the SI joints displayed bony erosions filled with a discrete material of intermediate-low signal intensity on T1-weighted (T1W) sequences (Fig. 4a), intermediate signal intensity on T2-weighted (T2W) images (Fig. 4b), and avid enhancement after intravenous gadolinium injection (Fig. 4c). Only a little amount of fluid was found within the articular spaces, and adjacent bone marrow edema was identified on T2W fat-saturated sequences (Fig. 4b).
Fig. 4.
Axial MR T1W (a), T2W (b) and T1 contrast-enhanced fat-suppressed (c) images through the sacroiliac joints, exhibiting bilateral bone erosions (white arrows), intra-articular fluid (dashed arrows), and enhancement after gadolinium injection (open arrow).
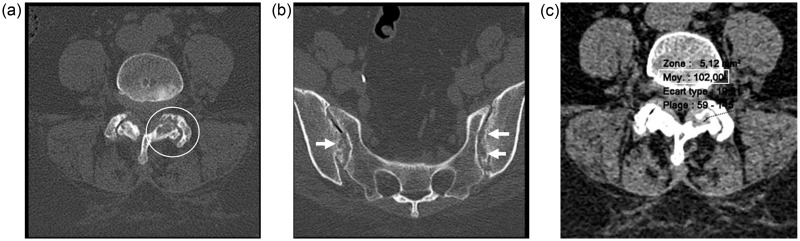
Besides the initial diagnosis of spinal canal stenosis, para-articular bone erosions with thin sclerotic margins were identified on the left interapophyseal facets of L4/L5 (Fig. 5a) and in both SI joints (Fig. 5b), and a high density material was found within these bony lesions, with a mean density of 106 HU (Fig. 5c).
Fig. 5.
Axial CT scan on bone windows showing subchondral and para-articular bone erosions on the left L4–L5 interapophyseal joint (circle on (a)) and both sacroiliac joints (arrows on (b)). Small region of interest disclosing the high density material (102 HU) within one of these bone erosions on the left interapophyseal joint (c).
After thorough evaluation of these exams, a radiologic diagnosis of gouty arthritis with axial involvement was made, and as a differential diagnosis, amyloidosis associated with secondary hyperparathyroidism, which was further discarded since she did not present any other findings compatible with these disorders.
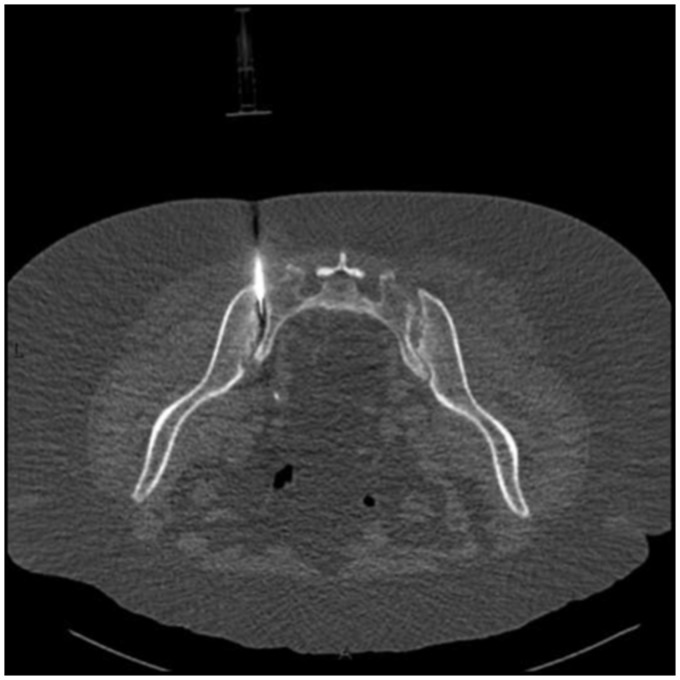
Nevertheless, since she did not present clinical findings consistent with peripheral microcrystalline arthropathy, a CT-guided sacro-iliac biopsy was performed (Fig. 6) to exclude septic arthritis due to an indolent pathogen, such as brucella or mycobacteria. The aspiration revealed a white chalky material that exhibited negatively birefringent monourate sodium crystals under polarized light microscopy.
Fig. 6.
CT-guided biopsy needle placed on the left sacroiliac joint revealing a white chalky material with negatively birefringent monourate sodium crystals under polarized light macroscopy (not shown).
She was treated with colchicine, clinically improved from her back pain, and after 15 days of treatment her CRP and uric acid levels dropped from 22.5 mg/dl and 13.2 mg/dl, to 0.4 mg/dl and 9.9 mg/dl, respectively.
The patient was eventually discharged and her dose of colchicine interrupted, maintaining a daily allopurinol intake.
Discussion
Gout results from the deposition of uric acid salts and crystals in and around the joints and soft tissues (1). It affects 3.9% of the total North-American adult population (10), with a peak age incidence occurring between 30 and 50 years, being 5 times more common in men than premenopausal women. After the age of 60 years, the prevalence of the disease in women tends to approach that seen in men (1).
The first attack of gouty arthritis is monoarticular in about 85–90% of cases, with the first metatarsophalangeal joint being affected in over 50% of patients (11).
It is more frequently seen in the appendicular skeleton, mainly in the lower limbs, despite the fact that with chronic disease, any joint in the body may be affected (1).
There are also several risk factors associated with hyperuricemia, such as obesity, hypertension, cardiomyopathy, chronic renal failure, and intake of diuretics and salicylates in low doses (12), all of which our patient had.
In the acute phase, conventional radiography can demonstrate soft tissue swelling and joint effusion. During the chronic phase, or tophaceous gout, which is characterized by the presence of deposits of monosodium urate (tophus), well-defined intra-articular or para-articular erosions delimited by thin sclerotic borders can be identified. Another characteristic feature is the presence of an “overhanging edge”, a thin bony expansion at the periphery of the erosion protruding into the soft tissues and partially covering the tophus (11).
The joint space is remarkably well preserved until late in the disease, and there is no periarticular osteopenia, although with progression of the arthropathy, disuse osteoporosis may be seen (11).
CT scan is more useful to evaluate the spine and sacroiliac joints than X-rays, and can also be used to measure tophi density, which present an attenuation of 160–170 HU. Recently, dual-energy CT was shown to detect urate deposits with an overall accuracy that was in the range of 87–94% (13).
Radiographic analyses have shown a prevalence of sacroiliac gouty arthropathy in the range of 13–17% (1,7), with a recent publication showing that axial involvement in gout could be as high as 35% with CT scan evaluation (8), although a bias of patient selection could, at least in part, explain these results.
The tophus has iso- to low signal intensity on T1W images in relation to muscle, but it is quite variable on T2W images, due to differences in calcium concentration within a tophus (14).
The differentiation of gouty arthritis from infectious arthritis or osteomyelitis is not always easy. As recently described in the literature, the bone marrow edema pattern is usually mild with uncomplicated gout, compared to cases with concomitant osteomyelitis (15). However, sometimes it can be troublesome to differentiate the two diagnoses, since we identified extensive bone marrow edema in T2W fat-saturated images. Furthermore, there is increased radiopharmaceutical uptake in gouty arthritis when assessed with bone scan, as seen in any inflammatory condition, making it very difficult to differentiate from osteomyelitis (16).
To our knowledge, this is the first report to evaluate gouty arthritis with FDG PET-CT, although tophaceous gout has already been described with conventional FDG-PET (17 –19), unveiling the hypermetabolic activity in and around the joints in these patients. Nevertheless, it is imperative to clarify, that this is costly and involves unnecessary radiation levels. However, it was ordered in our patient evaluation, since the attending physician did not agree with our first diagnosis of gouty arthropathy, making the awareness of less common joints affection by gout important not only to radiologist, but also to clinicians.
In conclusion, despite spinal and sacroiliac gouty arthritis is considered to be unusual, or rare, radiologists should take into account this possibility, especially when associated to other risk factors, such as obesity, hypertension, cardiomyopathy, chronic renal failure, or intake of diuretics and salicylates.
Conflict of interest
None declared.
References
- 1. Monu JU, Pope TL, Jr. Gout: a clinical and radiologic review. Radiol Clin North Am 2004; 42: 169–184 [DOI] [PubMed] [Google Scholar]
- 2. Duprez TP, Malghem J, Vande Berg BC, et al. Gout in the cervical spine: MR pattern mimicking diskovertebral infection. Am J Neuroradiol 1996; 17: 151–153 [PMC free article] [PubMed] [Google Scholar]
- 3. Barrett K, Miller ML, Wilson JT. Tophaceous gout of the spine mimicking epidural infection: case report and review of the literature. Neurosurgery 2001; 48: 1170–1172. discussion 1172–1173 [DOI] [PubMed] [Google Scholar]
- 4. Cabot J, Mosel L, Kong A, et al. Tophaceous gout in the cervical spine. Skeletal Radiol 2005; 34: 803–806 [DOI] [PubMed] [Google Scholar]
- 5. Wazir NN, Moorthy V, Amalourde A, et al. Tophaceous gout causing atlanto-axial subluxation mimicking rheumatoid arthritis: a case report. J Orthop Surg (Hong Kong) 2005; 13: 203–206 [DOI] [PubMed] [Google Scholar]
- 6. Justiniano M, Colmegna I, Cuchacovich R, et al. Spondyloarthritis as a presentation of gouty arthritis. J Rheumatol 2007; 34: 1157–1158 [PubMed] [Google Scholar]
- 7. Jajic I. Gout in the spine and sacro-iliac joints: radiological manifestations. Skeletal Radiol 1982; 8: 209–212 [DOI] [PubMed] [Google Scholar]
- 8. Konatalapalli RM, Lumezanu E, Jelinek JS, et al. Correlates of axial gout: a cross-sectional study. J Rheumatol 2012; 39: 1445–1449 [DOI] [PubMed] [Google Scholar]
- 9. Lumezanu E, Konatalapalli R, Weinstein A. Axial (spinal) gout. Curr Rheumatol Rep 2012; 14: 161–164 [DOI] [PubMed] [Google Scholar]
- 10. Dalbeth N, Doyle A, McQueen FM. Imaging in gout: insights into the pathological features of disease. Curr Opin Rheumatol 2012; 24: 132–138 [DOI] [PubMed] [Google Scholar]
- 11. Gentili A. Advanced imaging of gout. Semin Musculoskelet Radiol 2003; 7: 165–174 [DOI] [PubMed] [Google Scholar]
- 12. Spieker LE, Ruschitzka FT, Luscher TF, et al. The management of hyperuricemia and gout in patients with heart failure. Eur J Heart Fail 2002; 4: 403–410 [DOI] [PubMed] [Google Scholar]
- 13. Glazebrook KN, Guimaraes LS, Murthy NS, et al. Identification of intraarticular and periarticular uric acid crystals with dual-energy CT: initial evaluation. Radiology 2011; 261: 516–524 [DOI] [PubMed] [Google Scholar]
- 14. Yu JS, Chung C, Recht M, et al. MR imaging of tophaceous gout. Am J Roentgenol 1997; 168: 523–527 [DOI] [PubMed] [Google Scholar]
- 15. Poh YJ, Dalbeth N, Doyle A, et al. Magnetic resonance imaging bone edema is not a major feature of gout unless there is concomitant osteomyelitis: 10-year findings from a high-prevalence population. J Rheumatol 2011; 38: 2475–2481 [DOI] [PubMed] [Google Scholar]
- 16. Pickhardt PJ, Shapiro B. Three-phase skeletal scintigraphy in gouty arthritis: an example of potential diagnostic pitfalls in radiopharmaceutical imaging of the extremities for infection. Clin Nucl Med 1996; 21: 33–39 [DOI] [PubMed] [Google Scholar]
- 17. Blumer SL, Scalcione LR, Ring BN, et al. Cutaneous and subcutaneous imaging on FDG-PET: benign and malignant findings. Clin Nucl Med 2009; 34: 675–683 [DOI] [PubMed] [Google Scholar]
- 18. Steiner M, Vijayakumar V. Widespread tophaceous gout demonstrating avid F-18 fluorodeoxyglucose uptake. Clin Nucl Med 2009; 34: 433–434 [DOI] [PubMed] [Google Scholar]
- 19. Sato J, Watanabe H, Shinozaki T, et al. Gouty tophus of the patella evaluated by PET imaging. J Orthop Sci 2001; 6: 604–607 [DOI] [PubMed] [Google Scholar]