Abstract
Background
We aimed to investigate whether varicocele (VC) in rats can cause Sertoli cell-only syndrome (SCOS).
Material/Methods
Forty adolescent SD rats were randomly divided into 4 groups: 4-weeks control group, 4-weeks experimental group, 12-weeks control group, and 12-weeks experimental group. Left varicocele models were introduced by partially ligating left kidney veins for the experimental groups, and the sham surgery groups as controls were executed with exactly the same surgery as in the experimental groups except for the ligation. Rats in control and experimental groups for 4 and 12 weeks were killed after laparotomy at 4 and 12 weeks, respectively, the testes were taken out and fixed in fixative containing 4% polyformaldehyde, then were stained by hematoxylin and eosin (HE). The density and viability of sperm were analyzed by computer-aided sperm analysis.
Results
Compared with rats in 4-weeks and 12-weeks control group, histological structures of bilateral testes in both experimental groups were impaired, most of them showing as focal focuses. The pathological changes of testes in rats of the 12-weeks experimental group were bilateral, and included atrophy of seminiferous tubules, turbulence of spermatogenic cells in seminiferous tubules, defluvium of most spermatogenic cells, abortion of spermatogenesis, and degradation of spermatogenic epithelia. One rat in the 12-weeks experimental group was shown having SCOS, with the spermatogenic cells in seminiferous tubules completely flaked, degraded, or absent, and only Sertoli cells lined the seminiferous tubules.
Conclusions
Laboratory VC caused progressive impairment of homolateral testes, and SCOS could be induced when the damage was severe. Our results indicate that asthenozoospermia, azoospermia, and SCOS can be prevented by the earlier treatment of VC.
MeSH Keywords: Sertoli Cell-Only Syndrome, Sperm Motility, Varicocele
Background
The incidence of varicocele is 15–20%, and many patients have low qualified semen, impaired testicle histology, and even worse fertility. Varicocele is defined as the condition in which the pampiniform venous plexus in the scrotum is enlarged abnormally because testicular veins near the testis are compressed by a nearby structure. It is reported that 15–20% of males have varicocele [1], most of them adolescence, and of these, 21–41% are diagnosed as sterile [2–4]. In addition, varicocele during adolescence has damaging effects on the testis in rats [5]. Varicocele leads to smaller [6,7] and softer [8] testes, progressive impairment of the ipsilateral testis [9], atrophy of the bilateral testis [10], stunt of testis [11], reduction of sperm counts [12], and even SCOS can occur when varicocele is serious. Tchovelidze et al. did testicular biopsy for 221 patients with bilateral varicocele, and reported that 20 of them had SCOS [13].
SCOS is characterized by normal secondary sex characteristic, but bilateral testis are smaller, and have azoospermia (complete lack of germ cells), and pathological biopsies show no spermatogenic epithelium in convoluted tubules, and only Sertoli cells exist in it. Varicocele might cause clinical SCOS, but evidence supporting this is conflicting [14,15]. Here, we report that SCOS can occur in experimental varicocele rats.
Material and Methods
Animals
Forty third-grade male rats, 6–7 week old, weighing 220–330 g, were purchased from the Laboratory Animal Centre of Zhengzhou University, Zhengzhou, China. All the experiments were authorized by the Ethics Committee of People’s Hospital of Henan Province. All rats were divided into 4 groups: 20 varicocele models were made by surgery, 10 of them were killed and sampled 4 weeks later, and the other 10 were killed and sampled after 12 weeks. Another 20 rats were used as sham operated groups after 4 and 12 weeks. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Henan Provincial People’s Hospital.
Surgery methods
Rats were anaesthetized by injecting 5% chloral hydrate into the abdomen. The upper abdomen was approached through a midline laparotomy incision. The abdominal contents were pushed to the right gently to expose the left kidney, left adrenal gland vein, inferior vena cava, left renal vein, and left spermatic vein. Adipose tissues and loose connective tissues circling left renal veins were dissected bluntly. The left renal veins wept sometimes when dissected bluntly, and the bleeding had to be stopped by pressing the wound for 2–4 min. A tunnel was made under the left adrenal gland vein and spermatic vein proximal to the vena cava with tearing, and a silk 3/0 was inserted through the tunnel. By using a metal pole of 0.55-mm diameter, the left renal vein was ligated up to 50%. After the ligation, the metal pole was removed and the rest of the renal vein was recovered, and rapid distension of left renal vein could be seen because of the increased pressure from the ligation. The increased pressure of the left renal vein was transmitted into the left spermatic vein, and induced the left varicocele [16,17]. The sham operated group rats underwent exactly the same surgery as the other groups except for the ligation. The incisions were sewed by silk 3/0 and the wounds were bound with sterilized dressing. Cefotaxime sodium was injected into the abdomen to prevent infection. Dressing was regularly changed and silk was broken up 7 days later. All rats were fed in different cages with standard granular feed after the laparotomy.
Observation of semen
The cauda epididymidis was cut after rats were killed, the adhering anadesma and adipose tissues were removed. After being washed by physiological saline, sperm were collected by an approach based on diffusion of sperm [18]. Epididymidis were put in petri dishes and deeply cut 5 times lengthways using a small scissors, then they were transferred to 3 mL of physiological saline preheated to 37, and were extruded gently using a pair of nippers to help the epididymal fluid diffuse out, then the petri dishes were incubated for 10 min in 37°C in a constant temperature incubator. Sperm suspension was mixed gently and 50 μL was diluted to 3 mL by physiological saline preheated to 37°C. Density and viability of sperm in 10 μL dilution were analyzed by use of a computer-assisted sperm analysis system (WLJY-9000 color semen analysis system, Beijing Weili New Century Science & Tech. Dev. Co. Ltd., China) on a blood cell counting chamber.
Testicular slices
Bilateral testis were taken out when rats were killed at the 4th or 12th week, and were fixed with 0.1 M Bouin’s solution (containing 5% acetic acid, 4% formaldehyde, and 0.9% picric acid) for more than 24 h after being washed with physiological saline. After fixation, testis were stepwise dehydrated by 50%, 70%, 80%, 90%, 95%, and 100% ethanol, at least 1 h each step. Then testicular tissues were hyalinized using dimethylbenzene, embedded in paraffin, and 5-μm slices of each testicle were made and stained with hematoxylin and eosin. The prepared slides were coded and examined with an optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data were analyzed by SPSS 13 for Windows (SPSS Inc, Chicago, IL, USA), all results are shown as mean ± standard deviation, and all the comparative analyses were done by ANOVA.
Results
Varicocele caused Sertoli-cell-only syndrome
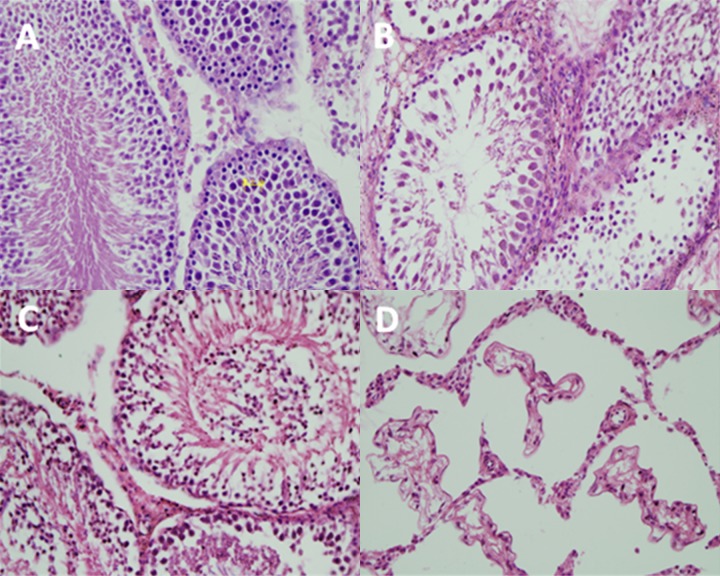
In rats of both 4-weeks and 12-weeks control groups, histological structures of the testis were normal, spermatogenic cells of each spermatogenic cycle in seminiferous tubules were arranged in an orderly manner and were highly distinguishable, sperm genesis was normal, and there were many sperms in the lumen (Figure 1A). Leydig cells in mesenchymal reign were distributed singly or in clusters, and were shown as regular triangle or polygon large cells. Cytoplasm was abundant and eosinophilic and had uniform color, the nuclei were round or oval, the chromatin was evenly distributed, and the nucleoli were obvious (Figure 1A).
Figure 1.
Slices of seminiferous tubules (magnification ×400). (A) The normal seminiferous tubules and Leydig cells in control group; (B) Seminiferous tubules impaired and in which spermatogenesis aborted; (C) Seminiferous tubules in which germ cells fell into lumen; (D) Seminiferous tubules in which only Sertoli cells were left.
In rats of both 4-weeks and 12-weeks experimental groups, histological structures of the bilateral testis were impaired, shown as focal or diffuse focuses, and most of them were focal (Figure 2). The histological structure impairment of testis in rats of the 4-weeks experimental group was light, spermatogenic cells of each spermatogenic cycle in seminiferous tubules were visible, the combinations of epithelia were almost normal or a little turbulent, caducous spermatogenic cells could be found in a few lumens, and artery and capillary in mesenchyme were ectatic and bloodshot. The histological structure of the testis in rats of the 12-weeks experimental group were impaired more heavily than in the 4-weeks experimental group, seminiferous tubules were atrophied, spermatogenic cells of each spermatogenic cycle in seminiferous tubules were a little turbulent, spermatogenesis stopped in the severe ones (most of them stopped at the stage of primary spermatocyte or androcyte, no mature sperm in lumens), most spermatogenic cells (spermatocyte and androcyte) were flaked, mesenchymal cells were denatured, diminished, and smaller, cytoplasm was reduced and granular, nuclear deformed and were deeply dyed, and distribution of chromatin became non-uniform (Figure 1B). This indicated that varicocele caused progressive histological impairment in rats.
Figure 2.
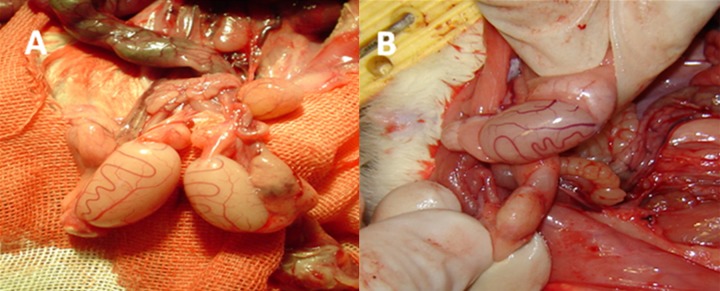
Left testicle of rat with varicocele was slightly (A) or severely (B) atrophied (magnification ×400).
The left testicle of 1 rat in the 12-weeks experimental group was seriously impaired, more heavily than the other ones in the same group. Seminiferous tubules atrophied drastically, spermatogenic cells in the lumen were completely flaked, degraded, or absent and, only Sertoli cells lined the seminiferous tubules (Figure 1C, 1D).
Varicocele caused low quality of semen
Dilution of semen was done by an approach based on diffusion of sperm (17) analyzed by computer-assisted sperm analysis system (WLJY-9000 color semen analysis system) on a blood cell counting chamber. As shown in Table 1, sperm density and viability in the 4-weeks experimental group are almost the same as in the control group, but sperm density and viability in experimental group 12 weeks were significantly lower than in the control group (P<0.01).
Table 1.
Sperm density and livability in control group and experimental group 4 and 12 weeks (χ̄±s, n=10).
| Indexes | Sperm density (106/ml) | Sperm livability (%) |
|---|---|---|
| Control group | 73.438±27.436 | 53.385±12.673 |
| Experimental group 4 weeks | 69.871±25.439 | 51.648±15.659 |
| Experimental group 12 weeks | 18.59±6.231* | 20.316±5.762* |
Stands for P<0.01 compared with control group.
Discussion
Our results indicate that left varicocele caused obvious impairment of the homolateral testicle in rats, most of them shown as focal focus, which looked like spots. We also found other histological damage, including degradation of spermatogenic epithelia, abortion of sperm genesis, flaking of spermatogenic cells, atrophy of seminiferous tubules, hydroncus of mesenchymal cells, and bloodshot capillary vessels. All these are similar to the pathological changes in adolescents with varicocele, some of which are sterile [9,19–22]. In our experiments, sperm genesis of some models still worked, but the spermatogenic epithelia and cells flaked dramatically, the caduceus epithelia and cells congested almost the whole lumen of the deferens, which is also consistent with the clinical symptoms of sufferers being sterile due to varicocele, usually showing as acceleration of abnormal sperm, few sperm, or azoospermia [23]. Experimental varicocele-caused histological impairment in animals has also been reported. The histological changes of the testis caused by varicocele were usually focal, and if it happened as diffusion in bilateral testis, the fertility was affected [24]. For instance, Choi et al. found that left varicocele in 7-week-old rats caused significant reduction of testicular weight, and decrease of seminiferous tubular diameter in the left testis 4 weeks after the varicocele were created [25]. Other histological changes, such as degeneration of germinal epithelium, tubular atrophy, Sertoli cell hyperplasia, and interstitial edema, were observed. Asci et al. also got similar results – there were severe histological abnormalities in the left testes in 2 of 8 rats with left varicocele, and changes in the right testis were detected in 1 rat [26].
In addition, varicocele caused progressive histological impairment in rats, because the 12-weeks experimental group had more severe histological changes than the 4-weeks experimental group; similar to the report of Zheng et al. on experimental varicocele in rats [9]. Clinically, incidence of varicocele was 35% of men with primary infertility (who never had children) and 81% of men with secondary infertility (who have children but are infertile now). Men with secondary infertility and varicocele had lower sperm concentration and more abnormal sperm compared with men with primary infertility and varicocele [27]. Chehval et al. did research on 13 men with varicoceles, and verified that the sperm density in semen became lower as they became older [28], suggesting that varicocele caused progressive damage.
Our results verified that sperm density and viability in the 12-weeks experimental group was significantly lower than in the control group and we inferred the reason might be the severe pathological changes of the testis. In other reports, the common pathological change of left varicocele in rats was abortion of spermatogenesis in the bilateral testis, but it is more severe in the left testicle and most of the spermatogenic cells aborted at the stage of primary spermatocyte or androcyte [20,21]. Clinically, it is reported that in 716 patients with varicocele, about 33.3% presented with normozoospermia, followed by asthenozoospermia (17.9%), oligoasthenoteratozoospermia syndrome (14.2%), and oligozoospermia (13.2%) [29]. It could lead to azoospermia when varicocele was serious [30]. These results and our results all show that varicocele can cause lower quality of semen.
It has been reported that left varicocele of rats caused acceleration of blood flow, higher temperature, and less oxygen in the testis, causing atrophy of the testis and reduction of sperm [12,31,32]. Varicocele surgery could improve the density, vitality, and shape of sperm, and reduce the response of oxidation and impairment of sperm DNA [33]. Abdel-Meguid proved that even a patient with azoospermia caused by varicocele could be fertile after surgery [34]. Ishikawa et al. reported that after varicocelectomy, spermatogenesis was induced in 2 of the 6 men with non-obstructive azoospermia, and 4 of the 54 men with severe oligospermia achieved paternity, with unassisted pregnancies [30]. It has been suggested that varicocelectomy in infertile men with palpable varicoceles and impaired semen quality could increase the rate of spontaneous pregnancy and improve semen characteristics within 1 year of follow-up.
However, there are few chances to recover the fertility of patients with azoospermia caused by varicocele after varicocelectomy; intracytoplasmic sperm injection was suggested to assist reproduction under these conditions [34]. If the patient with varicocele and azoospermia was diagnosed as SCOS, there were no sperm could be observe after varicocelectomy. Mehta et al. reported that it is controversial whether it is necessary to do varicocelectomy for patients with non-obstructive azoospermia, and they might use assisted reproductive technology directly [35].
Conclusions
Our results confirmed that experimental varicocele could cause homolateral testis being impaired progressively. The histological structure of the testis in rats with varicocele had progressively more severe pathological changes after varicocele, and SCOS could be induced when the damage was severe. Because varicocele can cause a progressive impairment to the testicle, we suggest that adolescents with varicocele should undergo varicocelectomy as early possible to reduce the chance of occurrence of azoospermia or SCOS.
Footnotes
Competing interests
None declared.
Source of support: Departmental sources
References
- 1.Jarow JP. Effects of varicocele on male fertility. Hum Reprod Update. 2001;7:59–64. doi: 10.1093/humupd/7.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Mostafa T, Anis TH, El-Nashar A, et al. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24:261–65. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 3.Cozzolino DJ, Lipshultz LI. Varicocele as a progressive lesion: positive effect of varicocele repair. Hum Reprod Update. 2001;7:55–58. doi: 10.1093/humupd/7.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–16. [PubMed] [Google Scholar]
- 5.Bryniarski P, Kaletka Z, Huk J, et al. Testicular volume and fertility potential in men operated due to varicocele and testicular hypotrophy in adolescence. Cent European J Urol. 2013;66:56–59. doi: 10.5173/ceju.2013.01.art18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto KJ, Kroovand RL, Jarow JP. Varicocele related testicular atrophy and its predictive effect upon fertility. J Urol. 1994;152:788–90. doi: 10.1016/s0022-5347(17)32710-6. [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Sigman M. Prevalence of testicular size discrepancy in infertile men with and without varicoceles. Urology. 2010;75:566–68. doi: 10.1016/j.urology.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 8.Cayan S, Akbay E, Bozlu M, et al. The effect of varicocele repair on testicular volume in children and adolescents with varicocele. J Urol. 2002;168:731–34. doi: 10.1016/s0022-5347(05)64735-0. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Zhang X, Zhou J, et al. Effects on the ipsilateral testis during progression of experimental varicocele in rat. Med Sci Monit. 2008;14(6):BR122–26. [PubMed] [Google Scholar]
- 10.Kass EJ, Stork BR, Steinert BW. Varicocele in adolescence induces left and right testicular volume loss. BJU Int. 2001;87:499–501. doi: 10.1046/j.1464-410x.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Paduch DA, Niedzielski J. Repair versus observation in adolescent varicocele: a prospective study. J Urol. 1997;158:1128–32. doi: 10.1097/00005392-199709000-00111. [DOI] [PubMed] [Google Scholar]
- 12.Sigman M, Jarow JP. Ipsilateral testicular hypotrophy is associated with decreased sperm counts in infertile men with varicoceles. J Urol. 1997;158:605–7. [PubMed] [Google Scholar]
- 13.Tchovelidze C, Sibony M, Callard P, et al. The testicular biopsy and spermatogenesis disturbance of infertile patients with bilateral varicocele. Arkh Patol. 2004;66:40–45. [PubMed] [Google Scholar]
- 14.Poulakis V, Ferakis N, de Vries R, et al. Induction of spermatogenesis in men with azoospermia or severe oligoteratoasthenospermia after antegrade internal spermatic vein sclerotherapy for the treatment of varicocele. Asian J Androl. 2006;8:613–19. doi: 10.1111/j.1745-7262.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu AK, Walsh TJ, Phonsombat S, et al. Bilateral but not unilateral testicular hypotrophy predicts for severe impairment of semen quality in men with varicocele undergoing infertility evaluation. Urology. 2008;71:1114–18. doi: 10.1016/j.urology.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 16.Liu JJ, Yang YR, Dong Q. The creation of experimental varicocele model in rats and its effect on the testis. West China Medical Journal. 2006;21:538–39. [Google Scholar]
- 17.Luo DY, Yang G, Liu JJ, et al. Effects of varicocele on testosterone, apoptosis and expression of StAR mRNA in rat Leydig cells. Asian J Androl. 2011;13:287–91. doi: 10.1038/aja.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinefelter GR, Jr, Gray LE, Suawz JD. The method of sperm collcetion significantly influences sperm motion parameters following ethane dimethanesulphonate administration in the rat. Repmd Toxicol. 1991;5:39–44. doi: 10.1016/0890-6238(91)90108-r. [DOI] [PubMed] [Google Scholar]
- 19.De Stefani S, Silingardi V, Micali S, et al. Experimental varicocele in the rat: early evaluation of the nitric oxide levels and histological alterations in the testicular tissue. Andrologia. 2005;37:115–18. doi: 10.1111/j.1439-0272.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Chang JS, Zhou XM, Chen DY. Varicocele in the rat: a new experimental model. Effect on histology, ultrastructure and temperature of the testis and the epididymis. Urol Res. 1991;19:319–22. doi: 10.1007/BF00299069. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Gao X, Liu X, et al. A new experimental inbred Wistar rat varicocele model: anatomy of the left spermatic vein and the effect on histology. Andrologia. 2008;40:13–17. doi: 10.1111/j.1439-0272.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- 22.Niedzidlske J, Paduch D, Raczynski P. Assessment of adolescent varicocele. Pediatr Surg Int. 1997;12:410–13. doi: 10.1007/BF01076952. [DOI] [PubMed] [Google Scholar]
- 23.Tchovelidze C, Sibony M, Callard P, et al. The testicular biopsy and spermatogenesis disturbance of infertile patients with bilateral varicocele. Arkh Patol. 2004;66:40–45. [PubMed] [Google Scholar]
- 24.Saypol DC, Howards SS, Turner TT, Miller ED., Jr Influence of surgically induced varicocele on testicular blood flow, temperature and histology in adult rats and dogs. J Clin Invest. 1981;68:39–45. doi: 10.1172/JCI110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi H, Kim KS, Kim KM. The effect of experimental varicocele on the testes of adolescent rats. J Urol. 1990;144:499–501. doi: 10.1016/s0022-5347(17)39502-2. [DOI] [PubMed] [Google Scholar]
- 26.Asci R, Sarikaya S, Büyükalpelli R, et al. The effects of experimental varicocele on testicular histology and fertility in monorchic adult rats. BJU International. 1999;83:493–97. doi: 10.1046/j.1464-410x.1999.00942.x. [DOI] [PubMed] [Google Scholar]
- 27.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–16. [PubMed] [Google Scholar]
- 28.Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992;57:174–77. doi: 10.1016/s0015-0282(16)54796-7. [DOI] [PubMed] [Google Scholar]
- 29.Al-Ali BM, Marszalek M, Shamloul R, et al. Clinical parameters and semen analysis in 716 Austrian patients with varicocele. Urology. 2010;75:1069–73. doi: 10.1016/j.urology.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Kondo Y, Yamaguchi K, et al. Effect of varicocelectomy on patients with unobstructive azoospermia and severe oligospermia. BJU Int. 2008;101:216–18. doi: 10.1111/j.1464-410X.2007.07279.x. [DOI] [PubMed] [Google Scholar]
- 31.Reyes JG, Farias JG, Henríquez-Olavarrieta S, et al. The hypoxic testicle: physiology and pathophysiology. Oxid Med Cell Longev. 2012;2012:929285. doi: 10.1155/2012/929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozhedomov VA, Lipatova NA, Rokhlikov IM, et al. Male fertility and varicocoele: role of immune factors. Andrology. 2014;2:51–58. doi: 10.1111/j.2047-2927.2013.00160.x. [DOI] [PubMed] [Google Scholar]
- 33.Kang DH, Lee JY, Chung JH, et al. Laparoendoscopic single site varicocele ligation: comparison of testicular artery and lymphatic preservation versus complete testicular vessel ligation. J Urol. 2013;189:243–49. doi: 10.1016/j.juro.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81:1585–88. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Mehta A, Goldstein M. Varicocele repair for nonobstructive azoospermia. Curr Opin Urol. 2012;22:507–12. doi: 10.1097/MOU.0b013e328358e27b. [DOI] [PubMed] [Google Scholar]