Summary
Background
Primary progressive aphasia (PPA) is a progressive language disorder associated with atrophy of the dominant language hemisphere, typically left. Current PPA criteria divide PPA into three variants: non-fluent (nfvPPA), semantic (svPPA) and logopenic (lvPPA). The classification of PPA into one of the three variants may be performed at 3 levels: I) clinical, II) imaging-supported, III) definite pathologic diagnosis. This paper aimed at assessing the feasibility of the imaging-supported diagnostics of PPA variants in the Polish clinical setting with access to magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) examinations.
Case Report
We present the clinical and neuroimaging data on 6 patients (4 women, 2 men) clinically diagnosed with PPA (3 with nfvPPA and 3 with lvPPA) in whom MRI and SPECT were performed in order to determine if imaging-supported diagnosis could be established in those cases. In 4 individuals (2 with nfvPPA and 2 with lvPPA) clinical diagnosis was supported by neuroimaging (SPECT, albeit not MRI), thus level II of PPA diagnosis could be established in those cases. MRI results were either inconsistent with the clinical diagnosis (Patients 1 and 2) or a mixed pattern of atrophy was observed (Patients 3–6).
Conclusions
Imaging-supported diagnosis of PPA variant is more feasible with quantitative analysis of SPECT images than with purely qualitative visual analysis of MRI. Hypoperfusion abnormalities evidenced by SPECT are more variant-specific than patterns of atrophy.
Keywords: Primary Progressive Aphasia, Frontotemporal Dementia, Magnetic Resonance Imaging (MRI), Single Photon-Emission Computerized Tomography (SPECT)
Background
Primary progressive aphasia (PPA) is a rare progressive language disorder associated with atrophy of the dominant language hemisphere, usually left. Its epidemiology is not established [1]. This clinical entity, first reported in the XIX century by Arnold Pick (see: [2]), has been more recently re-introduced by Marsel Mesulam [3,4] as well as Julie Snowden [5] and John Hodges [6,7]. Based on the constellation of clinical symptoms, PPA is now typically divided into three variants: non-fluent (nfvPPA) with agrammatism or apraxia of speech, semantic (svPPA) with a progressive and selective loss of lexical semantics, and logopenic (lvPPA) with frequent word-finding pauses and phonemic paraphasias [1,8]. Importantly, the clinical diagnosis of specific variant has been associated with a relatively distinct neuroimaging pattern. Specifically, most cases of nfvPPA present with progressive atrophy within the left inferior frontal, opercular and insular regions, whereas svPPA is practically always associated with bilateral atrophy of the anterior temporal lobes, more prominent on the left side. In contrast, most patients with lvPPA have atrophy extending beyond the fronto-temporal regions (e.g. parietal lobule). Of note, whereas the first two variants have been linked with frontotemporal lobar degeneration (FTLD), the logopenic variant has been primarily related to Alzheimer’s disease (AD) [9].
Current PPA criteria were established by a panel of experts between 2006 and 2009 and published in 2011 [1]. PPA diagnosis is a 2-step process. Initial PPA diagnosis is based on neuropsychological, neurological and radiological examinations. A patient diagnosed with PPA has a predominant language deficit in the absence of significant visuospatial impairment, visual and episodic memory deficits or behavioural disturbances. Most patients with PPA present with no abnormalities in general neurological examination at the time of diagnosis. As PPA progresses, parkinsonism, apraxia and upper motor neuron involvement are often observed [10].
European Federation of Neurological Societies (EFNS) recommends structural magnetic resonance imaging (MRI) in the diagnostic work-up of patients with dementia in order to exclude other underlying conditions and to assess the pattern of cerebral atrophy. According to EFNS recommendations, the essential MRI sequences providing the important minimum set of information required in case of a subject with dementia are 3D T1-weighted gradient echo; turbo/fast spin echo T2-weighted and fluid-attenuated inversion recovery (FLAIR) and T2-gradient echo. A multiplanar reformatting tool can be applied if 3D T1-weighted techniques are unavailable. DWI and post-contrast 2D T1-weighted spin echo images are recommended to identify recent infarcts (e.g. in vascular dementia, transient global amnesia, vasculitis), neocortical or striatal abnormalities in Creutzfeldt-Jakob disease, infections (e.g. herpes simplex virus encephalitis), demyelinating changes in multiple sclerosis or other inflammatory diseases (e.g. vasculitis, sarcoidosis). However, if MRI is unavailable or contraindicated, computed tomography (CT) is sufficient in terms of neuroradiological assessment, as it serves mainly to exclude major space-occupying lesions (tumor, subdural haematoma), hydrocephalus or cerebrovascular disease [11]. Additionally, hypothyroidism, B12 deficiency, and other metabolic causes of dementia should be ruled out using blood tests at the initial stage of the diagnostic work-up. All these procedures refer to initial diagnostics of PPA.
The next stage of PPA diagnostics requires more detailed neuropsychological and optionally neuroradiological and genetic/histopathological examinations. The classification of PPA into one of the three variants (nfvPPA, svPPA or lvPPA) may be performed at 3 levels: I) clinical, II) imaging-supported, III) definite pathological diagnosis. The clinical diagnosis is based on comprehensive language examination assessing: speech production features (grammar, motor speech, sound errors and word-finding pauses), repetition, single-word and sentence comprehension (in terms of length and syntax), confrontation naming, semantic knowledge and reading/spelling. The imaging-supported diagnosis is based on the clinical diagnosis and the identification of PPA variant specific pattern of either atrophy on MRI or hypoperfusion/hypometabolism on single photon emission computed tomography (SPECT) or positron emission tomography (PET). SPECT and PET both rely on detection of radioactive compound that selectively binds in the brain: fluorine-18 (18F)-2-fluoro-2-doexy-D-glucose (FDG) – marker of cerebral glucose metabolism in PET and 99m-Tc-hexamethylpropylene amine oxime (HMPAO) – one of the most commonly used tracers to examine cerebral blood flow in SPECT. The magnitude of hypometabolism observed in FDG-PET is greater than the amplitude of hypoperfusion seen in SPECT. However, in general, SPECT is more widely available and costs much less [11]. The description of neuroimaging abnormalities typical for each PPA variant is presented in Table 1. Diagnosis with definite pathology requires clinical diagnosis and identification of either histopathological evidence of a specific neurodegenerative pathology (e.g. AD, FTLD-tau, FTLD-ubiquitin/TDP) or specific gene mutations.
Table 1.
Neuroimaging abnormality patterns in three variants of primary progressive aphasia for imaging-supported diagnosis (see: [1]).
| Variant | MRI | SPECT/PET | |
|---|---|---|---|
| Non-fluent | Predominant left posterior fronto-insular atrophy | or | Predominant left posterior fronto-insular hypoperfusion or hypometabolism |
| Semantic | Predominant anterior temporal lobe atrophy | or | Predominant anterior temporal hypoperfusion or hypometabolism |
| Logopenic | Predominant left posterior perisylvian or parietal atrophy | or | Predominant left posterior perisylvian or parietal hypoperfusion or hypometabolism |
MRI – magnetic resonance imaging; PET – positron emission tomography; SPECT – single-photon emission computerized tomography.
This paper aimed at assessing the feasibility of the imaging-supported diagnostics of PPA variants in the clinical setting with access to MRI and SPECT examinations.
Material and methods
Between 2007 and 2012, ten patients were clinically diagnosed with PPA at Neurology Department and Outpatient Neurology and Memory Clinics, St. Adalbert Hospital in Gdansk, Poland. Four patients were excluded from imaging analysis: one with PPA complicated by a history of a serious head trauma, and the other ones did not undergo either SPECT or MRI due to different reasons (lack of consent and/or medical complications making further examinations impossible). Finally, 6 out of 10 PPA patients were included for the verification of imaging-supported diagnostics of PPA variants. Patients’ basic clinical data are presented in Table 2.
Table 2.
The clinical and neuroimaging-based diagnosis of PPA variant.
| Patient | Age at onset | Age at clinical diagnosis | Clinical diagnosis | MRI (disease duration) | SPECT (disease duration) | Level of diagnosis – I (clinical)/ II* (neuroimaging) |
|---|---|---|---|---|---|---|
| 1 | 74 | 76 | lvPPA | svPPA (2 yrs) | lvPPA/svPPA (2 yrs) | I |
| svPPA (6 yrs) | lvPPA/svPPA (4 yrs) | |||||
| svPPA (6 yrs) | ||||||
|
| ||||||
| 2 | 70 | 75 | nfvPPA | lvPPA (5 yrs) | II (SPECT) | |
| lvPPA (6 yrs) | nfvPPA (6 yrs) | |||||
| lvPPA/nfvPPA (9 yrs) | nfvPPA (9 yrs) | |||||
|
| ||||||
| 3 | 64 | 68 | lvPPA | lvPPA/nfvPPA (4 yrs) | lvPPA (4 yrs) | II (SPECT) |
|
| ||||||
| 4 | 69 | 72 | lvPPA | lvPPA/nfvPPA (3 yrs) | lvPPA(3 yrs) | II (SPECT) |
|
| ||||||
| 5 | 62 | 65 | nfvPPA | lvPPA/nfvPPA (3 yrs) | lvPPA (3 yrs) | I |
|
| ||||||
| 6 | 54 | 56 | nfvPPA | lvPPA/nfvPPA (2 yrs) | nvfPPA (2 yrs) | II (SPECT) |
lvPPA – logopenic variant of primary progressive aphasia; nfvPPA – non-fluent variant of primary progressive aphasia.
Methods
Neuropsychological assessment addressed all aspects of language required for PPA diagnosis [1]. The implemented tasks were derived from Boston Diagnostic Aphasia Examination [12], a set of clinical trials by Łucki and Maruszewski [13] and Progressive Aphasia Language Scale [14]. Selected results of language assessment are presented in Table 3.
Table 3.
Language function assessment results.
| Type of PPA | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| lvPPA | nfvPPA | lvPPA | lvPPA | nfvPPA | nfvPPA | |
| Naming | ||||||
| Boston Naming test (max. 15) | 1 | 8 | 1 | 2 | 8 | 13 |
| Responsive naming1 (max. 30) | 8 | 24 | 5 | 5 | 30 | 22 |
| Confrontation naming 1 (max. 114=100%)2 | 71% | 99% | 54% | 46% | 91% | 88% |
| Single-word and sentence comprehension | ||||||
| Word discrimination1 (max. 72=100%)2 | 97% | 100% | 88% | 63% | 100% | 100% |
| Commands1 (max. 15) | 10 | 13 | 10 | 7 | 14 | 13 |
| Yes/no answers3 (max. 10) | 8 | 10 | 9 | 6 | 10 | – |
| Space relations3 (max. 10) | 9 | 10 | 9 | – | 10 | 10 |
| Syntax comprehension3 (max. 10) | 7 | 8 | NA | – | 10 | 9 |
| Repetition | ||||||
| Syllable strings (+ preserved, − impaired) | + | + | − | − | − | − |
| Sentences1 high/low frequency (max. 8/8) | 2/0 | 7/7 | 4/1 | NA | 4/5 | 4/4 |
Boston Diagnostic Aphasia Examination;
the results are presented as percentage values to enable the comparison of naming with word discrimination which is based on the same set of stimuli;
set of clinical trials by Łucki and Maruszewski;
NA – not administered.
In all patients, magnetic resonance imaging (MRI) was performed on a 1.5 T scanner. Slices of the brain were taken in the transverse, coronal and sagittal planes based on multiple MR sequences, including T1-, T2-weighted, and fluid attenuated inversion recovery.
In all patients, brain perfusion was assessed with SPECT examination at the Department of Nuclear Medicine at Medical University of Gdansk, Poland. SPECT imaging was performed 30 min after intravenous injection of 740 (20 mCi) MBq of 99mTc-HMPAO (Ceretec) using a standard double-head, low-energy, high-resolution gamma camera (Symbia T6, SPECT/CT, Siemens). Sixty-four projections of 20s were gathered. Each patient was immobilized during the acquisition with a head holder. Reconstruction was performed by an iterative method (flash 3D) on 128×128 pixel matrix. Chang’s attenuation correction method was applied to the data (0.12/cm). In order to assess the study, a more objective, Scenium software by Siemens was used. It compares a patient’s scan to a predefined reference database, calculating a statistical Z-score on a voxel-by-voxel basis.
Structural MRI scans were visually evaluated by an experienced radiologist (AMK) to determine the atrophy pattern. Similarly, every SPECT scan was assessed by an experienced nuclear medicine specialist (BB). All of the raters were blinded to the clinical diagnosis of PPA variant.
In Patient 1 and 2 neuroimaging was performed several times in the disease course and all MRI and SPECT results were presented in this paper. Patients had no history of familial dementia and genetic testing was performed in only 2 of the reported patients and yielded negative results.
Patient 1 (Figure 1)
Figure 1.
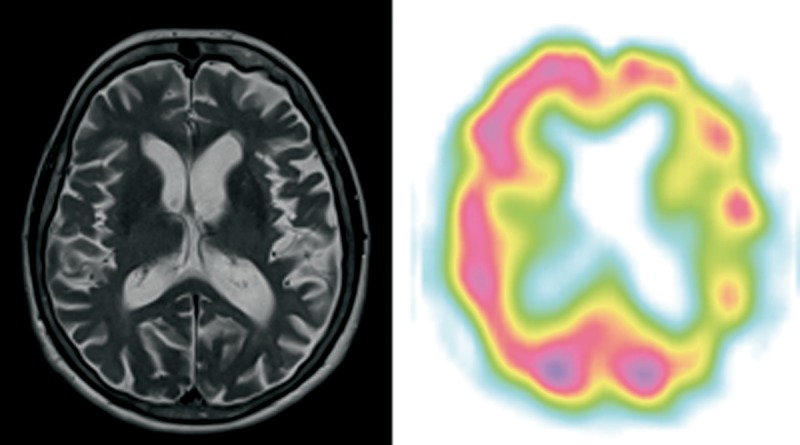
Patient 1 – clinical diagnosis – lvPPA, MRI – svPPA predominant anterior temporal lobe atrophy (on the left side in this case) and dilatation of the temporal horn of the lateral ventricle, SPECT – lvPPA/svPPA predominant left posterior perisylvian, temporal and frontal (posterior part, on the left side) hypoperfusion.
A right-handed woman, retired knitter, aged 76, came to the outpatient clinic with a diagnosis of AD and a two-year history of language deterioration. The patient was aware of her language problems and presented to the neurologist on her own initiative.
At the time of the assessment, the patient had severe anomia and impaired long sentence repetition due to phonological loop impairment. Word comprehension and object knowledge were better preserved than naming (see: Table 3). There was no agrammatism or apraxia of speech. She was diagnosed with lvPPA. The patient’s cognitive function deteriorated significantly in two years after PPA diagnosis, and she required 24-hour supervision due to episodic and semantic memory problems at that time. She died at the age of 81, after 7 years of the disease, due to complications of deep vein thrombosis.
Patient 2 (Figure 2)
Figure 2.
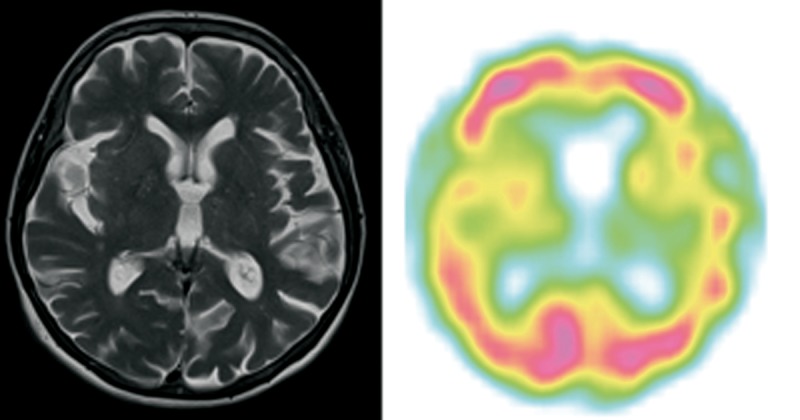
Patient 2 – clinical diagnosis – nfvPPA, MRI – lvPPA/nfvPPA on this transverse section predominant left posterior perisylvian and parietal atrophy, SPECT – nfvPPA predominant left posterior fronto-insular hypoperfusion.
A right-handed woman, retired accountant, aged 75, complained of difficulties in spontaneous speech that had started five years earlier. There was a prominent progression of aphasia within two years preceding her referral. She was referred to the Memory Clinic from general Neurology Clinic.
The patient’s speech was non-fluent and effortful with long pauses and shortened phrases. Conversation discourse was moderately impaired. However, grammatical and phonological errors were more common in writing than in spontaneous speech as the speech output was very scarce. She had problems with syntax comprehension. Naming and repetition were mildly disturbed with preserved word comprehension and object knowledge. She was diagnosed with nfvPPA. Daily function of that patient was quite stable during 4-year observation following diagnosis. After 11 years of disease duration, at the age of 81 she presented severe apathy, marked cognitive deterioration and required 24-hour supervision.
Patient 3 (Figure 3)
Figure 3.
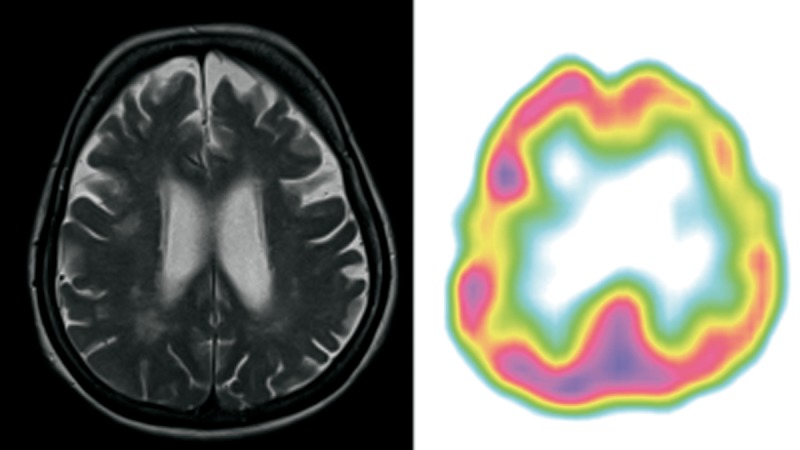
Patient 3 – clinical diagnosis – lvPPA, MRI – lvPPA/nfvPPA bilateral atrophy of frontal lobes and subtle atrophy of parietal lobe. The ventricles were enlarged in proportion to atrophy with left-sided predominance. SPECT – lvPPA predominant left posterior frontal, perisylvian and parietal hypoperfusion.
A 68-year-old right-handed woman, retired clerk with a four-year history of cognitive problems was referred to the Memory Clinic at her son’s request. At the time of diagnosis she was independent in the activities of daily living, but suffered from severe word-finding problems.
Her speech output was quite fluent but with impoverished content due to shortage of nouns. There was prominent anomia accompanied by impaired sentence repetition. Single-word comprehension, object knowledge and sentence comprehension were better preserved. Motor aspects of speech and syntax were not affected. She was clinically diagnosed with lvPPA. In that patient follow-up was not available.
Patient 4 (Figure 4)
Figure 4.
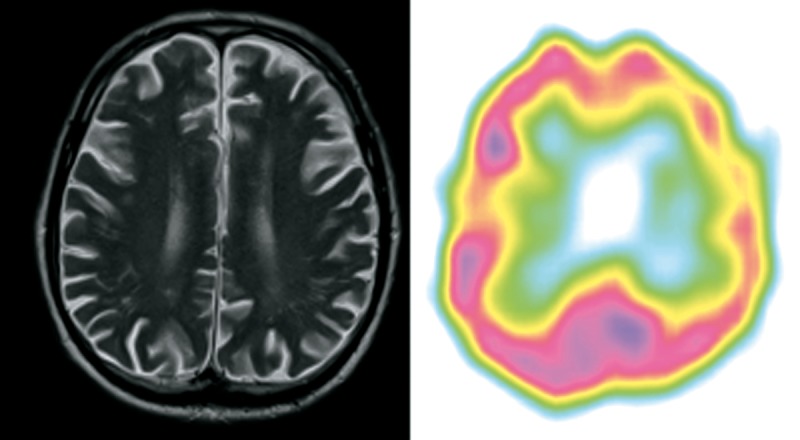
Patient 4 – clinical diagnosis – lvPPA, MRI – lvPPA/nfvPPA on this transverse section slightly left posterior fronto-insular atrophy, SPECT – lvPPA predominant left posterior perisylvian and parietal hypoperfusion.
A 72-year-old right-handed man, retired manager, was referred to the Memory Clinic at his wife’s request because of prominent word-finding difficulties and short-term memory problems. The symptoms appeared at least 3 years before diagnosis.
The patient’s spontaneous speech was severely anomic, albeit no apraxia of speech or agrammatism was observed. Repetition was severely affected even at the word level. The patient had problems with sentence comprehension. Single-word comprehension and object knowledge were relatively preserved when the array of presented stimuli was limited to 10. Formal word comprehension testing was difficult due to severe short-term memory impairment. The patient frequently got distracted during the tasks. Because of aphasia profile and short-term memory impairment he was clinically diagnosed with lvPPA. A year following PPA diagnosis the patient developed severe behavioural disturbance.
Patient 5 (Figure 5)
Figure 5.
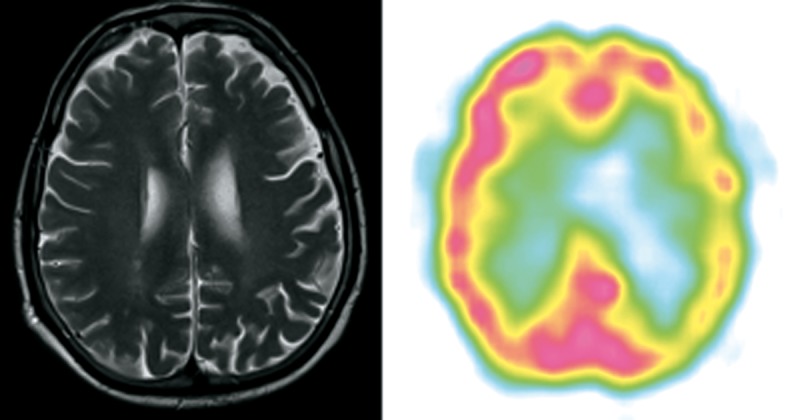
Patient 5 – clinical diagnosis – nfvPPA, MRI – lvPPA/nfvPPA on this transverse section slightly cortical and subcortical atrophy predominant in left frontal and parietal atrophy, SPECT – lvPPA diffuse hypoperfusion of left frontal, temporal and parietal lobe.
A 65-year-old right-handed man, technician, reported at least 3-year history of language impairment. He stopped working due to apraxia, 1 year prior to PPA diagnosis.
The patient’s speech was non-fluent with apraxia of speech and agrammatism. Anomia was mild. Single-word comprehension and object knowledge were preserved. Syntax comprehension impairment was mild, but occasionally appeared during examination. Repetition of syllable strings was impaired. The patient was diagnosed with nfvPPA. He died due to cardiovascular problems approximately a year after he was diagnosed with PPA.
Patient 6 (Figure 6)
Figure 6.
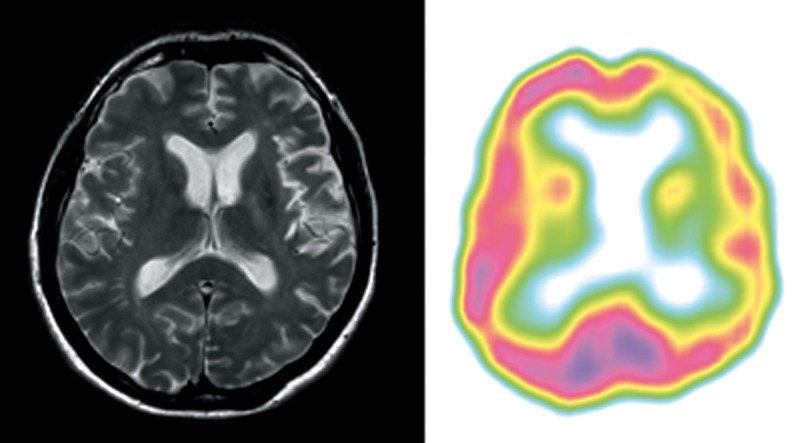
Patient 6 – clinical diagnosis – nfvPPA, MRI – lvPPA/nfvPPA on this transverse section subtle left posterior fronto-insular atrophy, SPECT – nfvPPA predominant left posterior fronto-insular and temporal hypoperfusion.
A 56-year-old originally left-handed (with forced right-handedness) woman, retired teacher, who a couple of months prior to the examination worked as a babysitter, reported a 2-year history of speech impairment. Her family reported that a couple of months before, executive symptoms added to the clinical picture.
The patient’s spontaneous speech output was scarce, non-fluent, with agrammatism and apraxia of speech. The patient had problems with syntax comprehension and repetition of strings of syllables. Single-word comprehension and object knowledge were preserved. Anomia was mild. The patient was diagnosed with nfvPPA. A year later severe behavioural symptoms emerged. At the age of 58, after 4 years of disease duration, she presented with symptoms typical for behavioral variant of FTD and required 24 h supervision.
Results
Patient 1
MRI performed at the age of 75 (after 2 years of disease duration) showed bilateral frontal and temporal atrophy. MRI repeated after six years of the disease showed predominant atrophy of the anterior part of the frontal lobe with enlarged temporal horn of the lateral ventricle. SPECT evidenced hypoperfusion of the anterior temporal lobe, posterior frontal lobe and superior part of the parietal lobe on the left.
Patient 2
MRI performed at the age of 74 and 75 years (4 and 5 years of disease duration) showed subtle diffuse cortical and subcortical atrophy, predominantly in the perisylvian sulcus and parietal lobes on the left. The SPECT scans performed at the same time showed predominant left-sided hypoperfusion of the posterior frontal lobe and above the fronto-parietal area.
Patient 3
MRI performed at the age of 68 (4 years of disease duration) showed bilateral atrophy of temporal and frontal lobes with the left temporal lobe predominance. The hippocampal atrophy was noted mainly on the left. Subtle atrophy of both parietal lobes was also observed. SPECT performed at the same time showed bilateral atrophy of frontal and temporal lobes with left-sided predominance.
Patient 4
In MRI study performed after 3 years of disease duration very subtle atrophy of left posterior frontal and temporal lobes was observed. SPECT performed at the same time showed predominant temporal lobe hypoperfusion and very subtle hypoperfusion in the left frontal lobe.
Patient 5
MRI performed at the age of 65 showed subtle diffuse cortical and subcortical atrophy, more prominent in the parietal and fronto-parietal area with left-sided asymmetry. SPECT evidenced significant hypoperfusion of left frontal, temporal and parietal lobes.
Patient 6
MRI performed at the 2nd year of disease duration showed subtle left posterior fronto-insular atrophy. SPECT performed at the same time evidenced very prominent hypoperfusion in frontal and temporal lobes.
Three patients were clinically diagnosed with nfvPPA and the other three subjects received a diagnosis of lvPPA (see: Table 2). No patients were clinically diagnosed with svPPA, although Case 1 presented with atrophy and hypoperfusion patterns quite typical for svPPA. In 4 individuals (2 with nfvPPA and 2 with lvPPA) the clinical diagnosis was supported by neuroimaging (SPECT but not MRI) (see: Table 2). MRI results were either inconsistent with the clinical diagnosis (in Patients 1 and 2) or a mixed pattern of atrophy was observed (in Patients 3–6). A mixed hypoperfusion pattern was observed in Patient 1 only, relatively early in the disease course.
Discussion
Current clinical criteria of PPA offer three levels of diagnostic confidence: clinical, imaging-supported and with definite pathology [1]. In 4 out of 6 of our patients, the imaging-supported PPA variant was established. Two patients were only clinically diagnosed with PPA variant, although both revealed neuroimaging abnormalities characteristic for PPA. Visual analysis of MRI scans produced mixed and rather variant non-specific results. In contrast, both the quantitative and the qualitative assessment of SPECT scans permitted us to ascribe hypoperfusion pattern to the PPA variant in all but one patient.
The observation that neuroimaging did not support the clinical diagnosis in each case may reflect the fact that delineation between lvPPA and nfvPPA is not clear and, thus, has been often questioned [15; see: 2]. Specifically, the overlap between these two variants is substantial, as speech fluency is a dynamic and multidimensional feature, and logopenia is a stage-related phenomenon, also seen with other FTLD pathology, e.g. caused by progranulin mutation [10,16]. Thus, the observed discrepancies between clinical diagnosis and neuroimaging might have been related to the more advanced disease stage at which the examination was conducted. Also, our results seem to support previous research indicating that in automatized MRI-based classification of 86 PPA patients the differentiation between nfvPPA and lvPPA cases was the most difficult [17]. Furthermore, the following biomarkers have recently been studied in 32 PPA individuals [18]: neuroimaging (MRI and SPECT or PET), serum progranulin levels, APOE genotype and cerebrospinal fluid (CSF) biomarkers in AD. It was found that all svPPA and lvPPA patients, in comparison to only 66% of nfvPPA patients, showed at least 1 positive neuroimaging-supported diagnostic biomarker, either structural (MRI) or functional (SPECT or PET). In this study, MRI classifications were not less specific than the ones based on SPECT. However, in a small case series (8 patients) [19] it was shown that predictions made on the basis of functional neuroimaging (SPECT and PET) were more accurate in the diagnosis of PPA variant than those based on MRI, providing partial support for our results. The concordance of clinical and FDG-PET examination diagnosis of PPA variant was also demonstrated in a group of 15 patients [20]. Nonetheless, a recent prospective MRI study showed that the specificity of atrophy rapidly decreases as the disease progresses [21], helping to explain the inconsistence of our MRI findings with the clinical classification of specific PPA variants. Of note, in this study, substantial progression of atrophy was noted over a two-year period, with atrophy at follow-up being less distinctive and encompassing all 3 major components of the language network: the inferior frontal gyrus, the temporoparietal junction, and lateral temporal cortex. Accordingly, neuropsychological profile was less specific. Thus, MRI imaging as a supported factor of the PPA diagnosis seems more feasible at relatively early disease stages.
Difficulties with the classification of PPA variants can also stem from heterogeneity of frontotemporal lobar degeneration (FTLD), as PPA patients may also present with a mixed pattern of deficits. The emergence of behavioural problems in Patient 6 and the co-occurrence of apraxia and non-fluent speech in Case 5 are consistent with the view that different FTLD syndromes (e.g. PPA, behavioural variant of FTD, corticobasal syndrome) may be stage-related and represent the continuum of Pick Complex [10,22–24]. Thus, the same patient may present with behavioural disturbances, apraxia or language impairment, and the clinical diagnosis may depend on the moment of the assessment, when a given cluster of symptoms predominates in the clinical picture.
In the present study, in all (Patient 2–4, 6) but two cases SPECT allowed for earlier and more precise PPA variant classification than MRI. This finding may be explained by earlier perfusion abnormalities preceding the atrophy [25]. Moreover, larger abnormalities on functional cerebral imaging with SPECT or PET may be also explained by deafferentation of the region connected to the atrophic area [26,27]. Additionally, this phenomenon could explain why it is difficult in some cases to ascribe the image to only one PPA variant (e.g. Patient 1, where larger area of atrophy typical for logopenic and semantic variants, was observed). However, it allowed imaging to support the diagnosis of particular PPA variants in four cases, where changes were less prominent.
Although, to our knowledge, this is the first paper dealing with the applicability of the revised PPA criteria in the Eastern European clinic, the lack of histopathologic and genetic examination is an important shortcoming of our study. Also, the MRI scans were only visually inspected. Thus, the comparison of visual MRI assessment against the volumetric analysis could not have been performed. However, the study aimed at showing if visual assessment of MRI scans can be useful in the PPA variant imaging-supported diagnosis as volumetric analysis is not used in the clinical practice.
What is more, neuropsychological assessment was not performed with the same set of trials in all patients as the disease severity influenced the choice of measures and data was retrospectively collected. However, all the core aspects of language function were assessed in each individual.
Our patients did not have lumbar puncture, and cerebrospinal fluid AD biomarkers were not evaluated, as PPA diagnostic criteria do not require CSF evaluation [1]. Cerebrospinal fluid (CSF) biomarkers available in the European Union are related to Alzheimer disease pathology: amyloid-β1–42 (Aβ1–42), total tau (T-tau) and tau phosphorylated at threonine 181 (P-tau). Previous data on patients with PPA highlighted the usefulness of AD-CSF biomarkers to differentiate AD-associated PPA from non-AD cases. There was a strong association between clinical diagnosis and AD-CSF biochemical markers in the lvPPA suggesting the AD pathology [18]. However, PPA diagnostic criteria do not require CSF evaluation [1].
Another limitation of our study is the fact that none of the patients from our case series was clinically diagnosed with svPPA. However, the differential diagnosis is much more challenging when nfvPPA and lvPPA are considered, as in svPPA both clinical and neuroimaging findings are most homogenous and relatively prominent [8,28,29].
Conclusions
Imaging-supported diagnosis of PPA variant is more feasible with quantitative analysis of SPECT images than with purely qualitative visual analysis of MRI. Hypoperfusion abnormalities evidenced by SPECT are more variant-specific than patterns of atrophy, although this may change as the disease progresses. In some cases with nfvPPA and patients with lvPPA, a clinical diagnosis of PPA variant may not be consistent with neuroimaging data and, unless specific biomarkers or genes are determined, only level I diagnosis can be established. Quantitative hypoperfusion assessment on SPECT seems more useful than visual analysis of structural MRI for imaging-supported PPA variant diagnosis.
Acknowledgements
Emilia J. Sitek and Michał Harciarek received the scholarship for young scientists awarded by Polish Ministry of Science and Higher Education during the preparation of the manuscript.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harciarek M, Kertesz A. Primary progressive aphasias and their contribution to the contemporary knowledge about the brain-language relationship. Neuropsychol Rev. 2011;21(3):271–87. doi: 10.1007/s11065-011-9175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11(6):592–98. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49(4):425–32. [PubMed] [Google Scholar]
- 5.Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. BehavNeurol. 1989;2(3):167–82. [Google Scholar]
- 6.Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 7.Hodges J, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6(11):1004–14. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 8.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kertesz A, Davidson W, McCabe P, et al. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsych Soc. 2003;9(5):710–19. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, Agosta F, Barkhof F, et al. EFNS task force: the use of neuroimaging in the diagnosis of dementia. Eur J Neurol. 2012;19(12):e131–40. 1487–501. doi: 10.1111/j.1468-1331.2012.03859.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) Lea and Febiger, Distributed by Psychological Assessment Resources; Philadelphia, Odessa, FL: 1983. [Google Scholar]
- 13.Łucki W. Zestaw prób do badania procesów poznawczych u pacjentów z uszkodzeniami mózgu-podręcznik. [Set of tasks to diagnose cognitive functions in brain-damaged patients]. Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego; Warszawa: 1995. [in Polish] [Google Scholar]
- 14.Leyton CE, Villemagne VL, Savage S, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using β-amyloid imaging. Brain. 2011;134(Pt10):3030–43. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 15.Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59(1):156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 16.Snowden JS, Neary D. Progressive anomia with preserved oral spelling and automatic speech. Neurocase. 2003;9(1):27–43. doi: 10.1076/neur.9.1.27.14368. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SM, Ogar JM, Laluz V, et al. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage. 2009;47(4):1558–67. doi: 10.1016/j.neuroimage.2009.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil-Navarro S, Lladó A, Rami L, et al. Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dement Geriatr Cogn Disord. 2013;35(1–2):106–17. doi: 10.1159/000346289. [DOI] [PubMed] [Google Scholar]
- 19.Panegyres PK, McCarthy M, Campbell A, et al. Correlative studies of structural and functional imaging in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2008;23(2):184–91. doi: 10.1177/1533317507312621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinovici GD, Jagusi WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogalski E, Cobia D, Harrison TM, et al. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–10. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kertesz A, Hudson L, Mackenzie IR, et al. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44(11):2065–72. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- 23.Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: the Pick complex. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):46–49. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- 24.Kertesz A, Martinez-Lage P, Davidson W, et al. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55(9):1368–75. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 25.Soriani-Lefevre M-H, Hannequin D, Bakchine S, et al. Evidence of bilateral temporal lobe involvement in PPA: SPECT study. J Nucl Med. 2003;44(7):1013–22. [PubMed] [Google Scholar]
- 26.Caselli RJ, Jack CR, Petersen RC, et al. Asymmetric cortical degenerative syndromes: Clinical and radiological correlations. Neurology. 1992;42(8):1462–48. doi: 10.1212/wnl.42.8.1462. [DOI] [PubMed] [Google Scholar]
- 27.Sinnatamby R, Antoun NA, Freer CE, et al. Neuroradiological findings in primary progressive aphasia: CT MRI and cerebral perfusion SPECT. Neuroradiology. 1996;38(3):232–38. doi: 10.1007/BF00596535. [DOI] [PubMed] [Google Scholar]
- 28.Kertesz A, Jesso S, Harciarek M, et al. What is semantic dementia?: a cohort study of diagnostic features and clinical boundaries. Arch Neurol. 2010;67(4):483–89. doi: 10.1001/archneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 29.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]


