Abstract
Background
Cyclosporine (CsA), prednisolone (Pred), and sirolimus (Sir) are inimunosuppressive compounds inhibiting lymphocyte proliferation at the cytokine gene transcription (CsA and Pred) or signal transduction (Sir) levels.
Methods
Double- and triple-drug interactions were simultaneously studied using lectin-induced proliferation of isolated cell lymphocytes (ICLP) and whole blood lymphocytes from men and women as well as two-way mixed lymphocyte reaction assays. Drug interactions were described with isobolograms and quantitated with the universal response surface approach by estimating the interaction parameter α.
Results
All compounds inhibited more than 89% of control proliferative responses. In each assay, CsA was less potent than Pred (3- to 14-fold) and Sir (5- to 11-fold). Sir was of similar or higher potency than Pred and 1.5-fold more potent in men than women. Pred was 1.4 times more potent in women but this was found only in the ICLP assay. All combinations were synergistic (α>0), with greater synergism found for combinations involving Sir, especially in the ICLP (α>13) and two-way mixed lymphocyte reaction (α>40) assays. Moreover, the Sir/Pred interaction in the ICLP assay was two to five times more synergistic in women, because their mean α was 56 compared with 13 in men. Double-combination α values were able to reasonably describe CsA/Pred/Sir triple-interaction effects.
Conclusions
These studies indicate that CsA, Pred, and Sir act and synergistically interact in vitro, with gender and assay as additional factors, and that whole blood lymphocyte proliferation cultures are useful in assessing the nature and intensity of drug interactions.
The immunologic response is one barrier to successful transplantation and T cell-mediated rejection is the major contributor to acute and chronic rejections leading to graft failure (1). Thus, to avoid or delay rejection, inimunosuppressive therapy is used to decrease cell-mediated reactivity and relies on specific (e.g., OKT3, cyclosporine [CsA*], and tacrolimus) and nonspecific (e.g., steroids and azathioprine) immunosuppressive drugs (2). Combination therapy is required to produce adequate immunosuppression with acceptable toxicity. The classic maintenance therapy involves CsA, prednisolone (Pred), and azathioprine (1).
Sirolimus (Sir) is a new macrolide immunosuppressive compound acting at the mid-late G1 phase through an original mechanism blocking transductional signals produced by the fixation of cytokines (e.g., interleukin [IL]-1, IL-2, and IL-6) to their membrane receptors (3,4). Sir differs from CsA and steroids in its mode of action because at the G0 phase, CsA acts by inhibiting IL-2 gene transcription and steroids act by decreasing cytokine (e.g. IL-1, -2, -6) and cell surface molecule (e.g., intercellular adhesion molecule-1, lymphocyte function-associated antigen-1) gene transcription (3, 5, 6). Thus, as CsA, Pred, and Sir act through different mechanisms at the cytokine gene transcription or signal transduction levels, we hypothesize that their combination may produce additive or synergistic therapeutic effects.
To assess this issue, double- and triple-drug interaction experiments were conducted in vitro on human lymphocytes. Mixed lymphocyte reaction (MLR) as well as lectin-induced isolated cell lymphocyte proliferation (ICLP) assays have been used as in vitro models for cell-mediated rejection (7–10), but they may not properly reflect in vivo phenomena as the separation of mononuclear cells from whole blood is required. De Groote et al. (11) demonstrated that diluted whole blood culture, as it mimics the natural environment, may be the most appropriate milieu to study cytokine production and, thus, lymphocyte function (12). Therefore, CsA/Pred/Sir actions and interactions were simultaneously studied in these three lymphocyte proliferation assays to uncover possible assay-related differences. Moreover, as gender may affect the response to immunosuppressive drugs, the present studies used cells from both men and women. Indeed, higher rates of rejection have been observed in female recipients of cardiac allografts (13) including those receiving CsA and azathioprine maintenance therapy (14). Also, early withdrawal of maintenance steroids is less often achievable in female allograft recipients (15).
Currently, the nature and intensity of drug interactions can be assessed by several methods as thoroughly examined in a recent review (16). The isobologram method (17) was selected to describe drug interactions because no assumptions are required on the constancy of the interaction across drug concentrations. Because this method cannot predict the combined effect for new conditions, the universal response surface approach (URSA) (18) was used to quantify the interaction. URSA describes the interaction surface with a single statistical summary parameter named α and possesses interpolating and extrapolating capabilities.
This article presents the results of CsA/Pred/Sir interactions in three in vitro human lymphocyte proliferation assays. Differences were found between assays but all demonstrated the synergistic interactions between CsA, Pred, and Sir in double or triple combinations. Gender differences were found especially for Sir and its combination with Pred. These results are promising indications for the therapeutic application of CsA/Pred/Sir combinations, because these agents display nonoverlapping side effects. Also, the use of whole blood lymphocyte proliferation (WBLP) culture in assessing the nature and intensity of drug interactions is extended.
MATERIALS AND METHODS
Reagents
CsA and Sir were obtained as gifts from Sandoz Research Institute (East Hanover, NJ) and Wyeth-Ayerat Research (Princeton, NJ). Pred and phytohemagglutinin-L were purchased from Sigma Chemical Co. (St. Louis, MO). Stock solutions of each drug were made in cthanol and kept at −20°C (CsA and Pred) or −80°C (Sir) for the duration of the study. Serial dilutions of the stock solutions with complete media were made fresh on each experimentation day in borosilicate sterile glasB tubes. Final ethanol concentrations in incubation wells were always less than 0.23%.
Study subjects
Caucasian male (n=6) and female (n=3) drug-free volunteers were included; women receiving birth control pills were excluded. Volunteers were in good health, ranging in age between 25 and 40 years. Women were not within 5 days of ovulation on study day because lower cell-mediated immune response occurs during this period (19). Blood was collected in heparinized glass tubes at 9 a.m. on the day of experiment and kept at room temperature for use within 6 hr.
ICLP and two-way MLR
The two-way MLR was selected instead of the usual one-way MLR, because less than 20 different drug combinations can be studied at once with the latter method. As stated later in the section Interaction assays, 96 combinations were needed to fully characterize drug actions and interactions, and the two-way MLR made it possible. Peripheral blood mononuclear cells were isolated from blood diluted 1/1 (v/v) with RPMI 1640 (LiFe Technologies, Grand Island, NY) by Ficoll-Paque 400 (Pharmacia LKB, Uppsala Sweden) density centrifugation. The interface containing lymphocytes and monocytes was washed three times with RPMI 1640, and the cells were resuspended in isolated lymphocyte complete medium (RPMI 1640 supplemented with 2 mM l-glutamine, 20 mM HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.25 mM 2-mercaptoethanol [Life Technologies], and 10% heat-inactivated human serum). The number of cells was determined by trypan blue exclusion using a hemocytometer, and viability >95% was found in all cases, Mononuclear cells (50000/well) were cultured in 96-well flat-bottom microtiter plates (Falcon 3072; Becton Dickinson, Lincoln Park, NJ) after the addition of different drug combinations. Proliferation was induced by the addition of phytohemagglutinin-L (ICLP) at an optimized final concentration of 3 µg/ml or by the addition of 50000 cells obtained from another donor (MLR) to each well. All cultures were performed in triplicate in a total volume of 200 µl per well. After incubating phytohemagglutinin-stimulated cultures for 72 hr or two-way MLRs for 6 days at 37°C in a 7.5% CO2-humidified air incubator, cultures were pulsed with 1 µCi of [3H]thymidine per well (New England Nuclear, Boston, MA) for 20 hr. Cells were then harvested onto microplates, dried, and counted in liquid scintillation fluid using a Top Count Microplate Scintillation Counter (Packard Instruments Co., Meriden, CT).
WBLP
Blood was diluted 1/20 (v/v) with whole blood complete medium (ICLP medium with 0.25 mM 2-mercaptoethanol). This dilution factor was proven to generate optimum proliferation. Diluted blood (160 µl) was plated per well. All cultures were performed in triplicate after the ICLP procedure. The only difference was in the addition of a 3% hydrogen peroxide washing step before drying the microplates. This step decreased the color quenching due to the presence of red blood cells on the micicroplate filters.
Interaction assays
Interaction experiments were conducted in 96-well plates. Each plate contained 96 different conditions (1/well): 90 drug combinations, 4 maximum stimulation (Smax) controls, and 2 background samples. Triplicates were determined by preparing three identical plates as suggested by Levasseur et al. (20). Drug combinations were distributed as follows. To properly quantify CsA/Pred/Sir double and triple interactions, single-drug inhibition curves have to be defined accurately. Therefore, for each compound, 10 different concentrations spanning over 4 log-units were studied (30 conditions). Plates included samples with no drug-producing maximum responses (Smax) and with no mitogen and no [3H]thymidine (Back). To fully define each interaction, five combination ratios at the drug concentration producing a response equal to 50% (IC50) of Smax-Back were studied and characterized with three combination concentrations (15/interaction). They were chosen to theoretically produce 25%, 50%, and 75% inhibition of Smax, assuming additive interaction. The five ratios at the IC50 (1:1,1:2, 2:1,1:4, and 4:1) were selected to reasonably describe each interaction with the isobologram method. Their adequacy was checked by performing experiments with a factorial design containing 17 ratios. For triple interactions, the five ratios at the IC50 were 1:1:1, 4:1:1, 1:4:1, 1:1:4, and 4:1:4. All drug combinations were prepared in a stock plate and dispensed using multichannel pipettes. The use of stock plates allowed for studying ICLP and WBLP for two donors along with their MLR using the exact same conditions.
Data analysis
Single drug inhibition
Observed effects were directly expressed as percent of Smax, as background noise was negligible (<0.1% Smax), With increased drug concentrations, lower proliferative responses were obtained. The concentration (C)-effect (E) relationship was modeled with the sigmoid Imax: model:
| (1) |
Smax is the maximum proliferative response and is fixed to 100%. The percent of Smax at infinite concentration is the background or Back. Thus, the maximum inhibitory effect (Imax) of the drug is equal to (Smax – Back), The slope of the curve is given by γ and IC50 is the drug concentration producing 50% of Imax.
Double and triple interaction with URSA
Combination data were analyzed with the isobologram method (17) and URSA (18). The equation applied to the CsA/Pred double interaction is:
| (2) |
The first and second part of equation 2 can be recognized as equation 1 written for CsA and Pred. The last part is the combination term and contains the interaction parameter α. If α is equal to zero, the interaction between CsA and Pred is Loewe additive and the combination effect is the simple addition of the effects of CsA and Pred alone. When α is positive, Loewe synergism is indicated, meaning that a similar effect can be achieved with lesser drug concentrations. When α is negative, antagonism is indicated. The magnitude of α is directly related to the degree of bowing of isobols. Increased synergism will be translated into greater bowing of isobols and larger positive α values.
An extension of equation 2 was used for the triple-drug combination data. It included the three double combinations (αCsA/Pred, αCSA/Sir, αPred/Sir) as well as the triple-combination term containing the interaction parameter βCsA/Pred/Sir. If β is equal to zero, there is no alteration of the three double-drug interactions inside the triple combination. If β is positive, additional synergism is found within the triple interaction; if β is negative, antagonism is present.
Statistical analysis
URSA analysis was performed by means of nonlinear regression fitting using the ADAPT II program (Biomedical Simulations Resource, Los Angeles, CA), applying the General Least Squares procedure and the bisection technique previously described (18) with CsA, Pred, and Sir concentrations treated as independent variables. Single-drug parameters were obtained initially and used as initial estimates in the simultaneous quantitation of the three double-drug interactions (Back, IC50, γ, and α). These parameter values were fixed during the estimation of β. Assuming a normal distribution of the errors from the observed data and predicted values, the variance was defined as σ1·M(θ,Ci)σ2, where σ1, and σ2 are the vectors of variance, θ is the vector of dynamic parameters, and M(θ,Ci) are the predicted values from the URSA at combined concentration Ci.
Statistical results are expressed as mean ± SD. Parametric and nonparametric tests were used to assess assay- and gender-related differences. The MLR results were not included in the statistical analysis, because larger variabilities were obtained in replicates and parameter estimates. Relationships between parameters were quantified using orthogonal regressions, because each estimated parameter has a certain degree of error. Data analysis was performed using SAS software (version 6; SAS Institute, Cary, NC) and P<0.05 was chosen as the level of significance.
RESULTS
Single-drug inhibition
Single-drug inhibition curves for two selected individuals are presented in Figure 1. At negligible drug concentrations, Smax was achieved. With increased concentrations, lower mitogenic responses were obtained. The Imax was observed at drug concentrations of 1,000–10,000 nM. Concentrations needed to achieve 50% of Imax (IC50) were greater for CsA than for Pred or Sir. Single-drug parameters (Back, IC50, and γ) for the three drugs are reported in Table 1 for the MLR, ICLP, and WBLP assays in men and women.
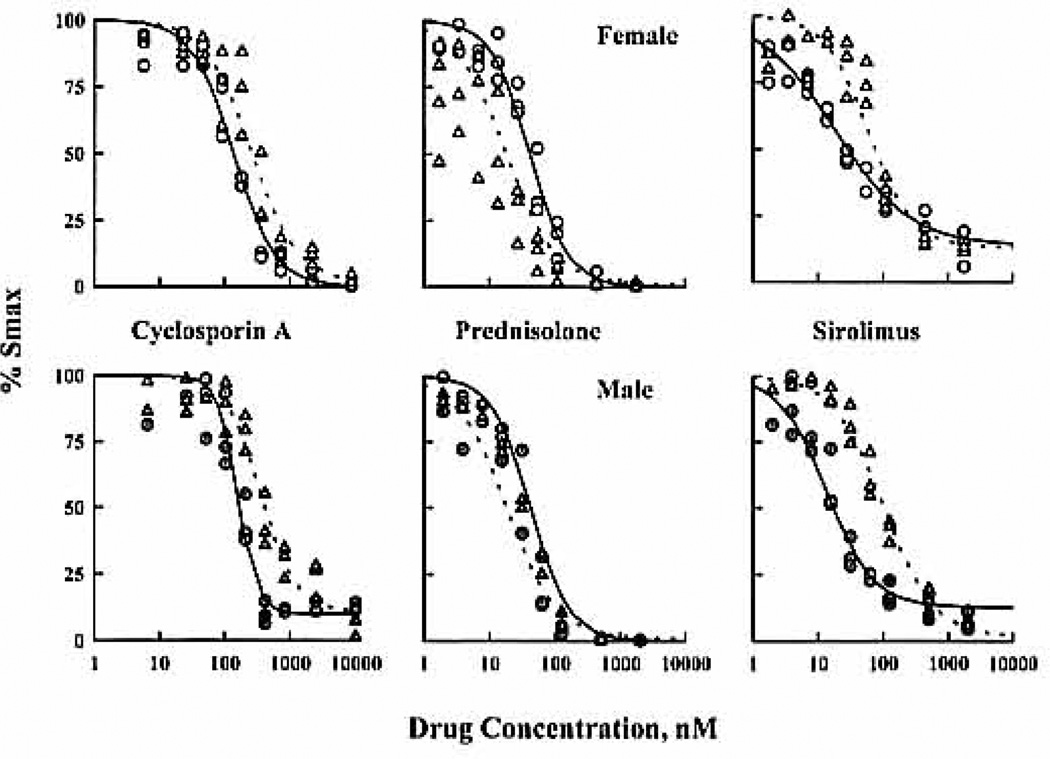
Figure 1.
CsA, Pred, and Sir inhibition of lymphocyte proliferation induced by lectins in ICLP (○) and WBLP (△) assays. The upper and lower panels display results from representative female and male subjects, respectively. Both assays were performed the same day using identical conditions.
Table 1.
Summary parameters for single-drug inhibition of lymphocyte proliferation in three systems
| CsA | Pred | Sir | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Back (%Smax) |
IC50 (nM) |
γ | Back (%Smax) |
IC50 (nM) |
γ | Back (%Smax) |
IC50 (nM) |
γ | |
| MLR (n) | (3) | (3) | (3) | ||||||
| Mean | 0.13 | 157 | 1.55 | 2.52 | 27.0 | 1.68 | 5.59 | 43.4 | 1.47 |
| SD | 0.17 | 22 | 0.25 | 4.1 | 3.9 | 0.72 | 4.4 | 18 | 0.10 |
| ICLP | |||||||||
| Female (n) | (5) | (6) | (6) | ||||||
| Mean | 10.9 | 192a,c | 3.26a,c | 1.64 | 39.8 | 1.13a | 6.33 | 26.5a | 0.95 |
| SD | 17 | 32 | 1.2 | 2.5 | 5.7 | 0.33 | 4.3 | 21 | 0.29 |
| Male | (5) | (5) | (5) | ||||||
| Mean | 9.27 | 129a,b,c | 1.48 | 4.93a | 54.8a,c | 1.15 | 7.47 | 15.9c | 0.97 |
| SD | 6.1 | 20 | 0.88 | 4.8 | 25 | 0.32 | 8.2 | 15 | 0.36 |
| WBLP | |||||||||
| Female (n) | (7) | (10) | (8) | ||||||
| Mean | 0.67 | 265b,c | 1.22c | 1.06 | 31.6 | 1.46c | 1O.2c | 55.2 | 1.05c |
| SD | 0.68 | 61 | 0.14 | 0.98 | 16 | 0.31 | 4.5 | 14 | 0.37 |
| Male (n) | (6) | (11) | (10) | ||||||
| Mean | 2.68 | 396c | 1.05 | 0.85 | 29.3 | 1.37 | 4.44 | 37.8 | 1.13 |
| SD | 4.5 | 107 | 0.30 | 0.89 | 15 | 0.28 | 11 | 29 | 0.48 |
Significant difference between ICLP and WBLP cultures (P<0.05).
Significant difference between gender for the same culture type (P<0.05).
Significant difference between compounds for the same gender and culture type (P<0.05).
CsA inhibits lymphocyte proliferation to a greater extent in WBLP and MLR, as mean Imax were 100% compared with 91% in ICLP, A 1.5- to 3-fold lower potency was found in WBLP for both men and women producing a shift of the concentration-effect curve to the right (Fig. 1). The gender difference in CsA potency was inconsistent between assays: IC50 values were lower in men in ICLP (129 nM compared with 192 nM in women) but higher in WBLP (396 nM compared with 265 nM). Moreover, the steepness of the concentration-effect relationship was higher in ICLP and MLR than in WBLP, possibly related to decreased cell heterogeneity during the lymphocyte isolation process.
Pred inhibits on average 94% to 99% of Smax regardless of assays and gender. Lower Pred potency was observed in ICLP, in which the mean IC50 was 55 nM in men compared with 29 nM in WBLP and 27 nM in MLR. The WBLP concentration-effect curves were shifted to the left of ICLP curves (Fig. 1). No significant gender differences were found; however, higher Pred potency was obtained in women for ICLP.
Sir acts later in the cell-cycle phase than CsA and Pred. Even added 8 hr after the initiation of lymphocyte proliferation, Sir retains its activity but lower IC50 values were found. These findings are due to Sir instability, as the decrease in IC50 with an increased time-delay occurs due to Sir degradation in culture medium as measured by high-performance liquid chromatography (our unpublished data). These findings occur in ICLP and WBLP cultures, and the rate of degradation was not modified by the presence of the few red blood cells. To render the assay practical, Sir was added at the same time as CsA and Pred. Sir inhibited lymphocyte proliferation from 90% to 94%, and the inhibition was greater in WBLP in men. IC50 values were significantly 2-fold lower in ICLP and 1.5-fold lower in men compared with women in both assays. Values obtained in MLR were closer to WBLP values.
When comparing the three compounds, all inhibited on average more than 89% of Smax, but CsA potency was significantly lower than Pred and Sir in the three assays. Sir potency was greater than Pred (threefold) and CsA (eightfold) in ICLP. Similar potency of Sir and Pred was observed in WBLP and MLR. An interesting pattern for the steepness parameter (γ) was obtained in ICLP (Table 1). The later the compound acts during the cell cycle, the larger the cell heterogeneity and the lower the γ value. Indeed, γ was 0.95 for Sir, 1.1 for Pred, and 3.3 for CsA in women. This pattern was not clearly observed in WBLP and may be due to the greater cell heterogeneity.
Double- and triple-drug interactions
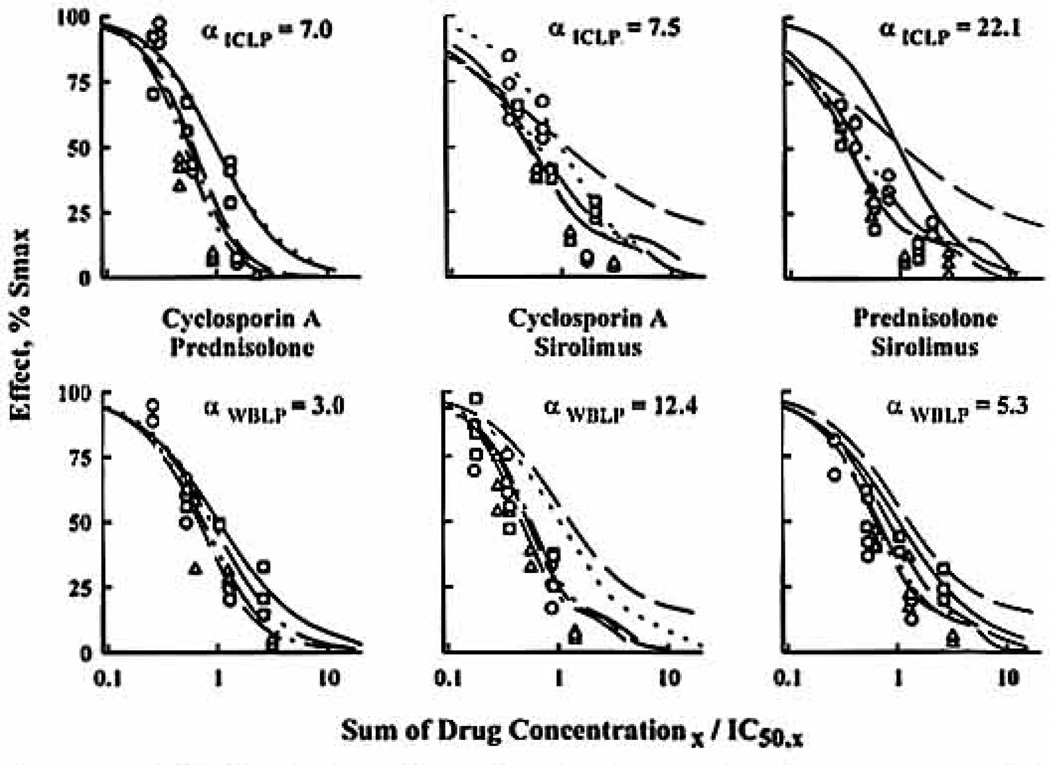
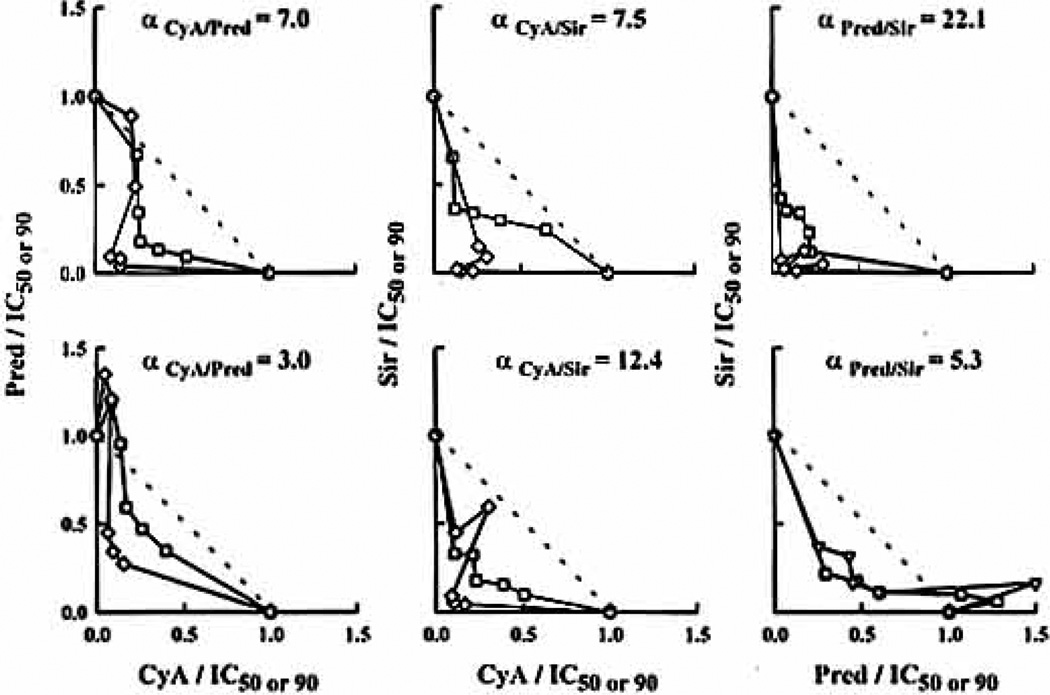
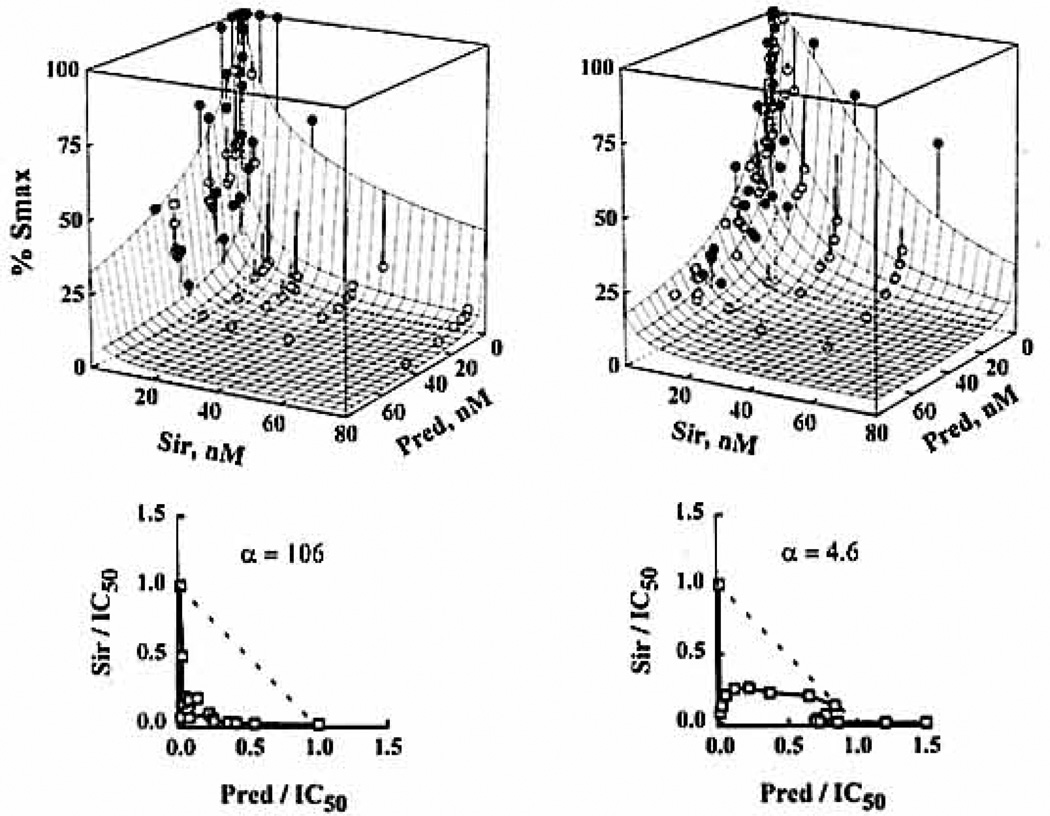
In analyzing double-drug interaction data, all combinations were found to be mainly synergistic because the combination effect curves drawn with IC50-normalized concentrations were shifted to the left of single-drug effect curves (Fig. 2). Applying the isobologram method for each interaction, a majority of ratios were found in the synergistic region (Fig. 3), but most isobols were asymmetric as confirmed by the full factorial design (Fig. 4). The relation between isobol shapes and the URSA α values can be seen in Figure 3, where greater bowing in the isobols translated into larger α values. The α values obtained for the different assays in men and women were always greater than zero and are summarized in Table 2. Higher synergism was obtained with isolated lymphocyte assays, especially for the Pred/Sir combination as presented in Figure 4: the greater synergism in ICLP can be seen in the tightening of the three-dimensional fishnet into the bottom far left corner of the cube.
Figure 2.
URSA for the three double-drug interactions constructed with the 9 drug combinations covering three ratios at the IC50 (△, 1:1; □, 1:4; and ○, 4:1) in a female subject using ICLP (upper panel) and WBLP (lower panel) assays. The concentrations are normalized by the IC50 values for each compound. A shift of the interaction curve to the left of single-drug curves (curves without data points) indicates synergism (α>0) and the absence of shift indicates additivity (α=0). Alpha values are reported.
Figure 3.
Isobolograms at the IC50 (□)and IC50 (◇) for the three double-drug interactions constructed with the five drug combination ratios at the IC50 (1:1, 1:2, 2:1, 1:4, and 4:1) in a female subject using ICLP (upper panel) and WBLP (lower panel) assays. Diagonal lines depict additivity.
Figure 4.
Three-dimensional representation of the URSA for the Pred/Sir interaction in ICLP (left) and WBLP (right) along with the isobolograms at the IC50. Seventeen different ratios were studied with five to eight points per ratio. The fishnets are the best fitting surface. The 90-combination data set is plotted ort the same graph, with vertical lines indicating the distance between the mean data point and the surface. ●, Above the surface; ○, below the surface.
Table 2.
Summary of α values for double-drug combinations
| αCsA/Pred | αCsA/Sir | αPred/Sir | |
|---|---|---|---|
| MLR (n) | (3) | ||
| Mean | 9.35 | 40.7 | 41.0 |
| SD | 13 | 48 | 29 |
| ICLP assay | |||
| Female (n) | (5) | ||
| Mean | 4.72 | 21.7 | 56.3a,b,c |
| SD | 2.3 | 23 | 30 |
| Male(n) | (5) | ||
| Mean | 10.6 | 25.2 | 12.5a |
| SD | 12 | 40 | 9.0 |
| WBLP assay | |||
| Female (n) | (6) | ||
| Mean | 2.87c | 6.93 | 6.68b |
| SD | 2.0 | 4.1 | 3.0 |
| Male (n) | (6) | ||
| Mean | 5.65 | 7.12 | 3.68 |
| SD | 4.1 | 5.2 | 2.4 |
Significant difference between culture types for the same combination and gender (P<0.05).
Significant difference between gender for the same combination and culture type (P<0.05).
Significant difference between combinations for the same gender and culture type (P<0.05).
Comparing the three double interactions, greater synergism was obtained for combinations involving Sir, especially in ICLP (α>13) and MLR (α>40). In addition to assay differences, a significant gender effect was observed for Pred/Sir, because their interaction was two to five times more synergistic in women. This was unrelated to gender differences in individual Pred and Sir potencies, because there was no correlation between IC50 and α values.
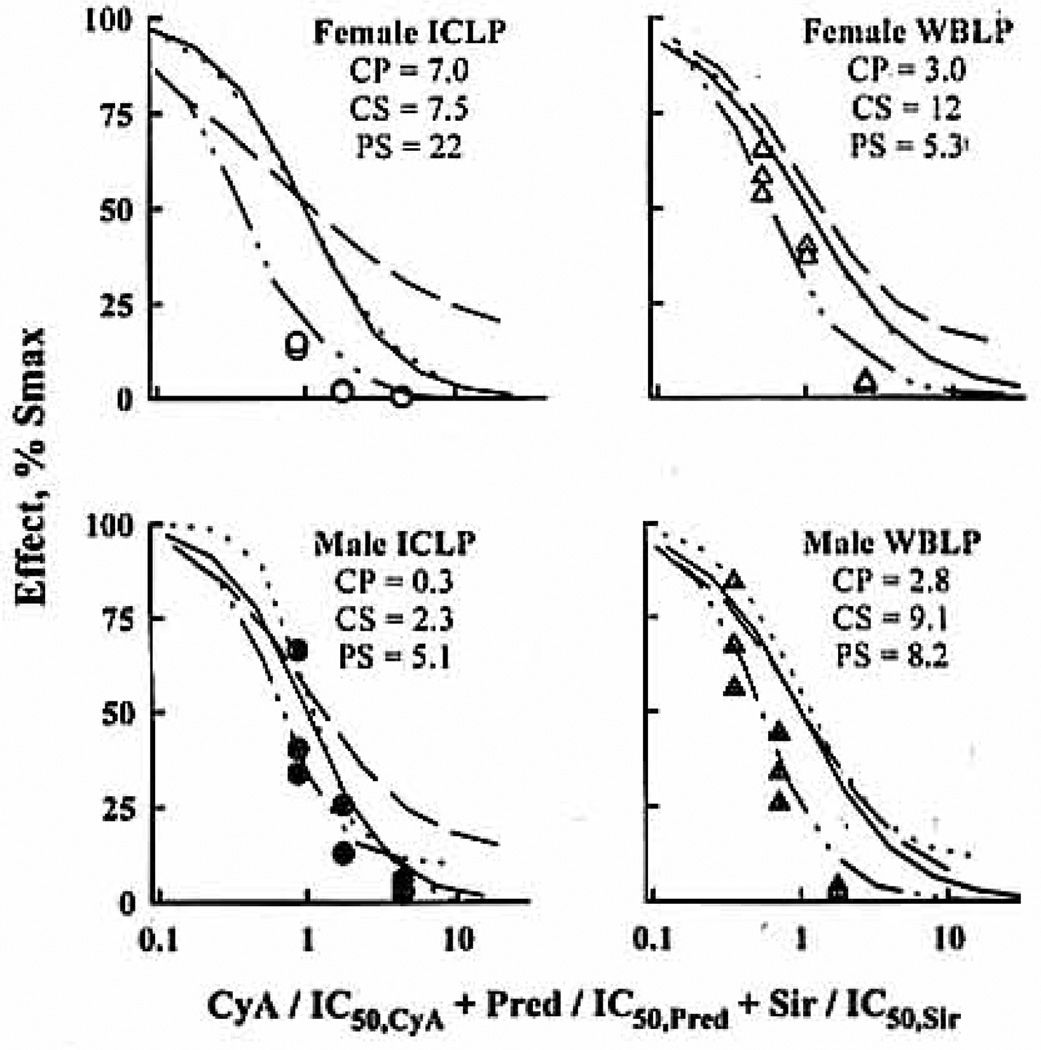
In describing the triple interaction, all confidence intervals on β estimates were wide and contained zero, because no additional synergism was found within the triple combination. Thus, double combination α values reasonably predicted triple interaction effects (Fig. 5).
Figure 5.
URSA applied to the triple-drug interaction. CsA (·····), Pred (——), and Sir (— — —) single-agent effects are simulated along with the triple-combination effects assuming double-drug interaction a values. Observed effects are reported for the ratio 1:1:1 at the IC50. Drug concentrations were normalized by their IC50 values. A shift of the interaction curve to the left (or right) of predicted combined effects will have indicated additional synergism (or antagonism) within the triple combination. Double-interaction α values are reported for CsA/Pred (CP), CsA/Sir (CS), and Pred/Sir (PS).
DISCUSSION
Successful transplantation requires sufficient immunosuppression, and T-cell-mediated reactivity is the major contributor to acute and chronic rejections leading to graft failure (1). Therefore, immunosuppressive therapy is used to decrease humoral and cell-mediated reactivity by partially blocking rate-limiting steps in the immune response (2). Combination therapy is required to produce adequate immunosuppression with acceptable toxicity, because these compounds display severe side effects such as nephrotoxicity (CsA and tacrolimus), neurotoxicity (CsA), myelotoxicity (azathioprine), diabetes mellitus (steroids), and hyperlipidemia (Sir) (1, 21, 22). Therefore, combination therapy should include compounds that are additive or synergistic only in their therapeutic effects and display nonoverlapping side effects, leading to the choice of CsA, Pred, and Sir for the present studies.
A convenient and reasonable way to assess drug interactions is by studying in vitro systems mimicking in vivo settings. MLR as well as ICLP assays have been successfully used in studying double or triple combinations involving most immunosuppressive agents (7–10). However, the WELP assay may better reflect in vivo conditions as suggested by De Groote et al. (11), and its applicability in defining single drug effects has been assessed previously (12, 23, 24), Some differences were found between ICLP and WBLP especially for Pred and Sir (24). The present article extends the comparison between MLR, ICLP, and WBLP assays from single-drug to double- and triple-drug interactions using CsA, Pred, and Sir.
As expected, assay-related differences were found with single drugs, especially for IC50 values. All compounds inhibited more than 89% of control proliferative responses. In each assay, CsA was less potent than Pred (3- to 14-fold) and Sir (5- to 11-fold), and Sir was of similar or higher potency than Pred. The present IC50 values are similar to published results (24). Assay discrepancies may be related to the presence of red blood cells or to the greater cell heterogeneity inherent in WBLP assays. Indeed, all three drugs distribute into red blood cells and in vitro blood/plasma ratios are close to 2 for CsA and Pred, and 10 for Sir (25). As blood was diluted 27 times and hematocrit values averaged 0.41 in females to 0.47 in males, red blood cell distribution explains less than 10% of the 2- to 3-fold and larger WBLP IC50 values of CsA and Sir, The greater cell heterogeneity observed in WBLP cultures may be another important parameter in explaining assay-related differences, as CsA, Pred, and Sir may have different activities on each leukocyte subset (3, 5). Therefore, it may be important to study and compare drug effects in WBLP and ICLP cultures, especially for drugs acting on different leukocyte populations.
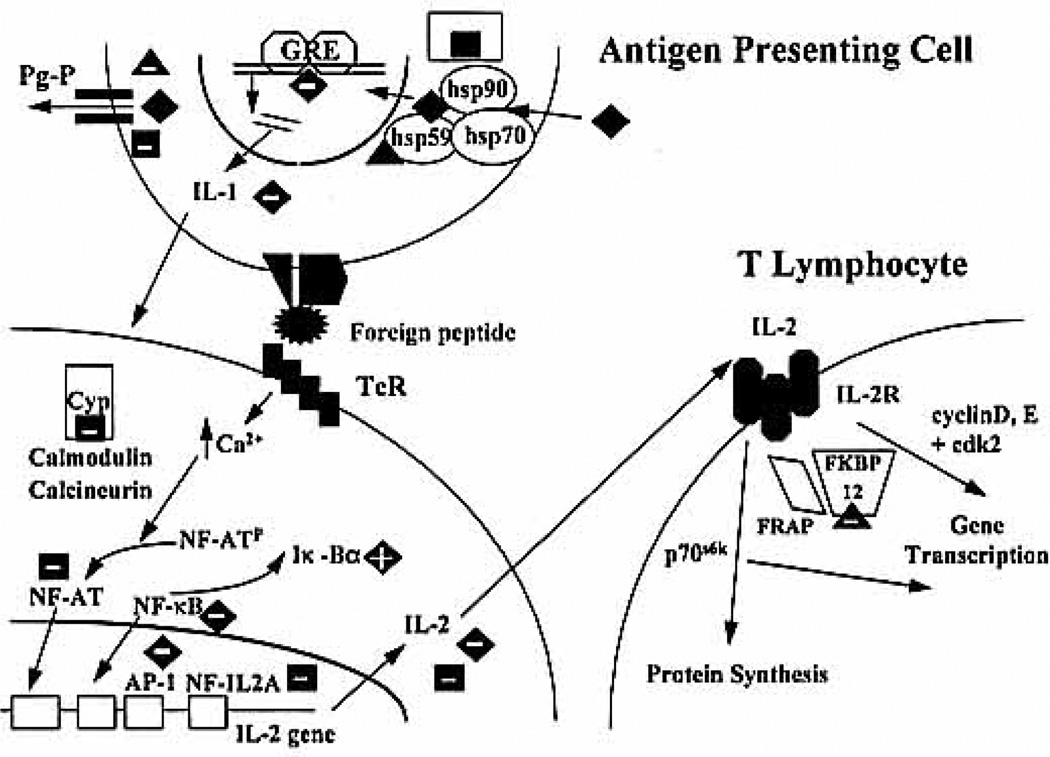
Analysis of interaction data using the isobologram and URSA methods was successful owing to the careful study design. All combinations were synergistic, with greater synergism found for combinations involving Sir, especially in ICLP and MLR assays in which only mononuclear cells are present (Fig. 4). During the induction of proliferation, T-helper lymphocytes are activated after the recognition of foreign molecules by T-cell receptors (Fig. 6). This activation creates an increase in intracellular calcium concentrations, leading to activation of calcineurin/calmodulin activity. This complex is responsible for the dephosphorylation of the T-cell specific IL-2 transcription factor called nuclear factor of activated T cell or NF-AT. Initiation of IL-2 gene transcription requires the presence of several transcription factors: NF-AT, NF-κB, AP-1, and NP-IL-2A. Both CsA and Pred act on these initial activation steps (Fig. 6). After binding to its cytosolic cyclophilin protein, CsA inhibits calcineurin/calmodulin activity, decreasing the translocation of NF-AT into the nucleus (3). The mechanism of action of corticosteroids is more intricate and incompletely understood (5,6,26,27). After binding to their cytosolic receptor complex containing heat shock proteins (hsp90 and hsp59), they enter the nucleus after the cytoplasmic release of hsp90. After nuclear loss of hsp59, steroids alter gene transcription by binding to DNA glueocorticoid-responsive elements. In lymphocytes, steroids decrease the availability of NF-κB by increasing the production of its inhibitory molecule, Iκ-Bα, thus decreasing IL-2 gene transcription (26, 27). In addition, CsA produces increased intracellular steroid concentrations by interacting with P-glyco-proteins (28). Therefore, as CsA and Pred act at a similar level, they interact with only slight synergism, possibly in relation to increased Pred intracellular concentrations.
Figure 6.
Detailed mechanism of action of CsA (square), Pred (diamond), and Sir (triangle) in antigen-presenting cells and T lymphocytes. Inhibition is represented by a negative sign; stimulation is represented by a positive sign within the drug symbol. Abbreviations used in the figure: Cyp, cyclophilin; FRAP, FKBP-rapamycin-associated protein; GRE, glucocorticoid repressive element; Pg-P, P-glyco-protein; TcR, T cell receptor.
Newly synthesized IL-2 binds to its membrane receptor and activates a cascade of events partially consisting of kinase (p70s6k, cdk2) and cyclin (cyclins D, E, A, and B) activations and leading the cell to S-phase (4) (Fig. 6). After binding to the cytosolic protein FKBP12, Sir blocks lymphocyte reactivity by inhibiting kinase and cyclin activations produced by the fixation of cytokines to their membrane receptors (3, 4). Moreover, Sir binds to FKBP59 or hsp59, possibly altering normal corticosteroid receptor activity. Tacrolimus, a Sir-related compound binding to hsp59 with similar affinity, modifies glucocorticoid binding, increasing receptor affinity but decreasing the separation rate and transfer of the steroid-receptor complex to the nucleus (29, 30). In addition, Sir produces increased intracellular steroid concentrations by interacting with P-glycoproteins (28). Thus, as Sir acts at different levels than Pred and CsA, combinations with Sir display intense synergism, confirming in vitro and in vivo results obtained in humans (7,10, 31).
Gender-related differences were observed with single drugs as well as in interactions. Sir was 1,5-fold more potent in men. A gender effect of corticosteroids4 occurs in lymphocyte trafficking (32), but similar differences were only observed in I CLP, in which Pred was 1.4 times more potent in women. Interestingly, a gender effect was obtained with the Sir/Pred combination, for which synergism was two to five times higher in women independently of the gender effect on Sir and Pred alone. However, for the same combined concentrations, similar effects were obtained for both genders in WBLP culture. Therefore, gender may not be of major importance for double and triple combinations of CsA, Pred, and Sir.
As CsA, Pred, and Sir display nonoverlapping side effects, their triple combination is promising in transplantation as well as in controlling autoimmune diseases or allergic reactions (4,9). Moreover, the combination with Sir is of particular interest because Sir may reduce chronic rejection by inhibiting arterial smooth muscle cell proliferation (1, 33). Additional pharmacodynamic and pharmacokinetic interactions may arise in vivo because CsA, Pred, and Sir are metabolized by CYP-3A4 enzymes and Pred alters lymphocyte trafficking, decreasing the number of T-helper lymphocytes present in blood (32). Decreased Pred clearance with increased Sir exposure was observed in renal transplant patients (34). Thus, greater synergistic interactions between the three compounds may occur in vivo (31).
In conclusion, CsA/Pred/Sir double and triple interactions were examined in human lymphocyte proliferation assays. Differences were found between assays, but all demonstrated synergistic interactions, especially for combinations containing Sir. Gender differences were found especially for Sir and its combination with Pred, These results are promising indications for the therapeutic application of CsA/Pred/Sir combinations, because they display nonoverlapping side effects. Moreover, WBLP cultures are extremely useful in assessing drug actions and interactions in vitro.
Acknowledgments
We thank Dr, William Greco and Dr. Hélène Faessel for synergistic discussions.
Footnotes
This work was supported by grant GM 24211 from the National Institutes of Health, Bethesda, MD. G.M. Ferron received Fellowship support from Wyeth-Ayerst Research, Radnor, PA.
Abbreviations: α, double-drug interaction parameter using URSA; Back, % of Smax not affected by the drug; β, triple-drug interaction parameter using URSA; CsA, cyclosporine; γ, slope of the concentration-effect relationship; hsp, heat shock protein; IC50, drug concentration producing response equal to 50% of Smax-Back; ICLP, isolated cell lymphocyte proliferation; IL, interleukin; Imax, maximum inhibitory effect; MLR, mixed lymphocyte reaction; NF-AT, nuclear factor of activated T cell; Pred, prednisolone; Sir, sirolimus (rapamycin); Smax, maximum stimulation (100%); URSA, universal response surface approach; WBLP, whole blood lymphocyte proliferation.
Meno-Tetang GML, Jusko WJ. Gender-related differences in prednisolone inhibition of phytohemagglutinin-induced rat lymphocyte proliferation: role of estradiol-17β. Submitted for publication.
REFERENCES
- 1.Shoker AS. Immunopharmacologic therapy in renal transplantation. Pharmacotherapy. 1996;16:562. [PubMed] [Google Scholar]
- 2.Halloran PF. Molecular mechanisms of new immunosuppressants. Clin Transplant. 1996;10:118. [PubMed] [Google Scholar]
- 3.Metclafe SM, Richards FM. Cyclosporine, FK506, and rapamycin: some effects on early activation events in serum-free, mitogen-stimulated mouse spleen cells. Transplantation. 1990;49:798. [PubMed] [Google Scholar]
- 4.Sehgal SN. Rapamune (sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17:660. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Scudeletti M, Castagnetta L, Imbimbo B, Puppo F, Pierri I, Indiveri F. New glucocorticoids: mechanisms of immunological activity at the cellular level and in the clinical setting. Ann N Y Acad Sci. 1990;595:368. doi: 10.1111/j.1749-6632.1990.tb34310.x. [DOI] [PubMed] [Google Scholar]
- 6.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids: glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151:4081. [PubMed] [Google Scholar]
- 7.Kahan BD, Gibbons S, Tejpal N, Stepkowski SM, Chou T-C. Synergistic interactions of cyclosporine and rapamycin to inhibit immune performances of normal human peripheral blood lymphocytes in vitro. Transplantation. 1991;51:232. doi: 10.1097/00007890-199101000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Kahan BD, Tejpal N, Gibbons-Stubbers S, et al. The synergistic interactions in vitro and in vivo of brequinar sodium with cyclosporine or rapamycin alone and in triple combinations. Transplantation. 1993;55:894. doi: 10.1097/00007890-199304000-00039. [DOI] [PubMed] [Google Scholar]
- 9.Haczku A, Alexander A, Brown P, et al. The effect of dexamethasone, cyclosporine, and rapamycin on T-lymphocyte proliferation in vitro: comparison of cells from patients with glucocorticoid-sensitive and glucocorticoid-resistant chronic asthma. J Allergy Clin Immunol. 1994;93:510. doi: 10.1016/0091-6749(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 10.Weaver JL, Pine PS, Aszalos A. The interaction of immunosuppressive compounds in tandem stimulated peripheral human lymphocytes. Immunopharmacol Immunotox. 1994;16:179. doi: 10.3109/08923979409007089. [DOI] [PubMed] [Google Scholar]
- 11.DeGroote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-2, IL-6, INF-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 12.Bloemena E, Roos MTL, Van Heijst JLAM, Vossen JMJJ, Schellekens PTA. Whole-blood lymphocyte cultures. J Immunol Methods. 1989;122:161. doi: 10.1016/0022-1759(89)90260-3. [DOI] [PubMed] [Google Scholar]
- 13.Esmore D, Keogh A, Spratt P, Jones B, Chang V. Heart transplantation in females. J Heart Lung Transplantation. 1991;10:335. [PubMed] [Google Scholar]
- 14.Crandall BG, Renlund DG, O’Connell JB. Increased cardiac allograft rejection in female heart transplant recipients. J Heart Transplantation. 1988;7:419. [PubMed] [Google Scholar]
- 15.Steinmuller TM, Graf KJ, Scheicher J, Leder K, Bechstein WO. The effect of FK506 versus cyclosporine on glucose and lipid metabolism: a randomized trial. Transplantation. 1994;58:669. [PubMed] [Google Scholar]
- 16.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331. [PubMed] [Google Scholar]
- 17.Gcssncr PK. The isobolographic method applied to drug interactions. In: Moselli PL, Garattini S, Cohen SN, editors. Drug interactions. New York: Raven Press; 1974. p. 349. [Google Scholar]
- 18.Greco WR, Park HS, Rustum YM. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinium and 1-beta-D-arabinofurano-sylcytosine. Cancer Res. 1990;50:5318. [PubMed] [Google Scholar]
- 19.Marchetti B, Morale MC, Gallo F, Batticane N, Farinella Z, Cioni M. Neuroendocrineimmunology (NEI) at the turn of the century: towards a molecular understanding of basic mechanisms and implications for reproductive physiopathology. Endocrine. 1995;3:845. doi: 10.1007/BF02738890. [DOI] [PubMed] [Google Scholar]
- 20.Levasseur L, Faessel H, Slocum H, Greco WR. Precision and pattern in 96-well plate cell growth experiments. Proc Biostat Sec Am Statist Ass. 1995;227 [Google Scholar]
- 21.Hricik DE, Almawi WY, Strom TB. Trends in the use of glucocorticoids in renal transplantation. Transplantation. 1994;57:979. [PubMed] [Google Scholar]
- 22.Murgia MG, Jordan S, Kahan BD. The side effect profile of sirolimus: a phase I study in quiescent cyclosporin-predmsone-treated renal transplant patients. Kidney Int. 1996;49:209. doi: 10.1038/ki.1996.28. [DOI] [PubMed] [Google Scholar]
- 23.Piekoszewski W, Chow FS, Jusko WJ. Pharmacokinetic and pharmacodynamic effects of coadministration of methylprod-nisolone and tacrolimus in rabbits. J Pharmacol Exp Ther. 1994;269:103. [PubMed] [Google Scholar]
- 24.Piekoszewski W, Chow FS, Jusko WJ. Inhibition of phytohaem-agglutinin-induced lymphocyte proliferation by immunosuppressive drugs: use of whole blood culture. Immunopharmacol Immunotoxicol. 1994;16:389. doi: 10.3109/08923979409007100. [DOI] [PubMed] [Google Scholar]
- 25.Yatscoff R, Legatt D, Keenen R, Chackowsky P. Blood distribution of rapamycin. Transplantation. 1993;56:1202. doi: 10.1097/00007890-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS. Role of transcriptiona1 activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 27.Auphan N, Didonato JA, Rosette C, Helmberg A, Karin M. Immuno suppress ion by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 28.Ning YM, Sanchez ER. Immunophilins, heat shock proteins, and glucocorticoid receptor actions in vivo. Methods: A Companion to Methods Enzymol. 1996;9:188. doi: 10.1006/meth.1996.0025. [DOI] [PubMed] [Google Scholar]
- 29.Ning YM, Sanchez ER. Potentiation of glucocorticoid receptor-mediated gene expression by the immunophilin ligands FK506 and rapamycin. J Biol Chem. 1993;268:6073. [PubMed] [Google Scholar]
- 30.Ning YM, Sanchez ER. Stabilization in vitro of the untransformed glucocorticoid receptor complex of S49 lymphocytes by the immunophilin ligand FK506. J Steroid Mol Biol. 1994;52:187. doi: 10.1016/0960-0760(94)00162-f. [DOI] [PubMed] [Google Scholar]
- 31.Kahan BD. Sirolimus: a new agent for clinical renal transplantation. Transplant Proc. 1997;29:48. doi: 10.1016/s0041-1345(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 32.Lew KH, Ludwig EA, Milad MA, et al. Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 1993;54:402. doi: 10.1038/clpt.1993.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidbauer G, Hancock WW, Wasowska B, Badger AM, Kupiec-Weglinski JW. Abrogation by rapamycin of accelerated rejection in sensitized rats by inhibition of alloantibody responses and selective suppression of intragraft mononuclear and endothelial cell activation, cytokine production, and cell adhesion. Transplantation. 1994;57:933. doi: 10.1097/00007890-199403270-00028. [DOI] [PubMed] [Google Scholar]
- 34.Jusko WJ, Ferron GM, Mis SM, Kahan BD, Zimmerman JJ. Pharmacokinetics of prednisolone during administration of sirolimus in renal transplant patients. J Clin Pharmacol. 1996;36:1100. doi: 10.1002/j.1552-4604.1996.tb04162.x. [DOI] [PubMed] [Google Scholar]