Abstract
The temporal variations in the pharmacokinetics and pharmacodynamics of methylprednisolone at 8 am versus 4 pm were investigated in six healthy male volunteers. Subjects completed three phases: no drug administration, 20 mg intravenous methylprednisolone at 8 am, and the same dose at 4 pm. Methylprednisolone clearance was 28% greater in the afternoon. The suppressive effects of methylprednisolone on basophils (measured as whole blood histamine), helper T lymphocytes, and cortisol concentrations, assessed by the ratio of the area under the curve (AUC) after methylprednisolone to the baseline AUC, were not different between the phases. The 50% inhibitory concentration values for methylprednisolone derived from pharmacodynamic models were also similar, indicating no difference in intrinsic responsiveness. However, cortisol concentrations returned to baseline about 4 hours earlier after the 4 pm compared with the 8 AM dose because of the enhanced afternoon methylprednisolonc clearance. These findings are in agreement with other studies that suggest adequate clinical effects and less disturbance of cortisol circadian behavior when methylprednisolone is administered as a single dose in the morning.
Corticosteroids are an important class of therapeutic agents because of their anti-inflammatory and immuno-suppressive properties. Ideal dosing regimens have yet to be elucidated for the treatment of numerous disease states. Currently, corticosteroids are most often administered as a single morning dose. However, these agents may be administered as divided doses or even as a single evening dose in those conditions requiring adrenal suppression.1
Numerous drugs, including theophylline,2 phenyloin,3 and cisplatin,4 have demonstrated temporal variations in their pharmacokinetics. This is not surprising because it has been shown in animals that both hepatic drug-metabolizing activities and renal clearance (CLR) mechanisms exhibit circadian variations.5,6 Human chronopharmacokinetic studies with exogenous corticosteroids are limited. Prednisolone has been evaluated in three studies with conflicting findings. The first study7 found no circadian differences in prednisolone clearance, volume of distribution (V), or half-life (t½). The second study8 found circadian differences in the clearance (CL) of free prednisolone and the free fraction when it was given as a low dose. The third study9 found circadian variation in all parameters investigated. To date, no human chronopharmacokinetic study with methylprednisolone has been reported. In male Norwegian rats, the maximum methylprednisolone t½ was noted to occur when the drug was administered at 6 pm, the commencement of the nocturnal activity period of the animals, whereas the shortest t½ was documented with noon administration, or midway through the diurnal rest span.10
The pharmacodynamics of exogenous corticosteroids may show temporal variations. The susceptibility of the hypothalamic-pituitary-adrenal axis to suppression in humans, as measured by 17-hydroxy-corticosteroid excretion, varies within the circadian cycle.1,11,12 When exogenous corticosteroids are administered between the hours of 8 am and 4 pm, the hypothalamic-pituitary-adrenal axis suppression is either minimized or absent altogether.1,11,12 It is not known whether such differences are the result of altered pharmacodynamics, altered pharmacokinetics, or a combination of both.
Many studies investigating corticosteroid therapy find in favor of a single morning dose, whereas others suggest that treatment of asthma and rheumatoid arthritis may be optimized by applying chronopharmacologic principles.l3–18
The purposes of this study were to determine whether methylprednisolone pharmacokinetics exhibits circadian variation and to determine if there are time-dependent differences in the direct suppressive effects of methylprednisolone on endogenous conisol concentrations and on helper T cell and basophil cell circulation. Pharmacodynamic models developed in this laboratory for these effects were used.19,20 Methylprednisolone, rather than prednisolone, was chosen for study because its kinetics is linear at low doses and thus less complicated to study.21
EXPERIMENTAL METHODS
Subjects
Six healthy, nonsmoking, male volunteers were enrolled in the study. Subjects were between the ages of 22 and 27 years, were within 20% of their ideal body weights, and had normal sleep-wake cycles. Subjects took no other drugs for 1 month before and for the duration of the study. Health status was assessed by medical history, physical examination, and routine blood chemistries and hematologic parameters. Subjects were excluded if they had histories of drug or alcohol abuse or if they had any contraindications to receiving corticosteroids. The study was approved by the lnvestigational Review Board of Buffalo General Hospital, and informed consent was obtained from each subject before enrollment in the study.
A randomized three-way crossover design was used, and each subject completed all three phases. The three phases were (1) no drug administration, (2) administration of a single 20 mg intravenous bolus of methylprednisolone at 8 am, and (3) administration of the same dose at 4 pm. Three subjects were randomized to begin their baseline phase at 8 am, whereas the others began at 4 pm. Each phase was conducted over a 24-hour period at the Buffalo General Hospital Clinical Research Facilities and were separated by 1 week. Subjects were required to fast a minimum of 7 hours before and for 2 hours after receiving the methylprednisolone dose. Three equicaloric meals with similar carbohydrate, protein, and fat content were served on each of the 3 study days, and subjects were requested to rest from midnight to 8 am.
A catheter was inserted into an arm vein approximately 15 minutes before the start of each study day and was kept patent by the instillation of about 2 ml sodium heparin solution (10 units/ml). The 20 mg methylprednisolone dose was administered as 25.4 mg of the sodium succinate ester (Solu-Medrol, The Upjohn Company, Kalamazoo, Mich.) by intravenous bolus injection in the contralateral arm. On the baseline day, approximately 8 ml blood was withdrawn every 2 hours and placed into heparinized tubes. During the study days involving drug administration, approximately 8 ml blood was obtained just before and at ¼, ½, 1, 1½, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 hours after drug administration. In addition, 4 ml blood was drawn into EDTA (Na2) tubes at 0, 1,2, 4, 8, 12, 16, 20, and 24 hour after drug administration for the determination of helper T cell (CD4 lymphocyte) counts. (On the baseline study day, the 1-hour time point was omitted.) The heparinized tubes were immediately placed on ice to prevent in vitro hydrolysis of the methylprednisolone succinate ester (MPS). On all study days 300 µl whole blood were removed from the heparinized tubes and then stored at −20° C for later determination of whole blood histamine concentration. The remaining blood was centrifuged and the plasma harvested and frozen at −20° C until assayed.
Assays
Plasma methylprednisolone and cortisol concentrations were simultaneously determined by slightly modifying the HPLC method of Ebling et al.22 Samples were extracted at 4° C and a sodium hydroxide wash was replaced by a water wash to prevent in vitro hydrolysis of MPS. The lower limit of quantitation was 10 ng/ml for both methylprednisolone and cortisol. Intraday coefficients of variation for methylprednisolone were 2.0% at 40 ng/ml and 4.2% at 600 ng/ml; for cortisol the intraday coefficients of variation were 2.3% at 40 ng/ml and 3.7% at 600 ng/ml. Interday coefficients of variation at 40 ng/ml were 7.3% and 7.5% for cortisol and methylprednisolone, respectively; interday coefficients of variation at 600 ng/ml were 6.2% and 5.7% for cortisol and methylprednisolone, respectively. In the assessment of the pharmacodynamics of cortisol, concentrations below the limit of quantitation were assigned a value of 5.0 ng/ml.
MPS plasma concentrations were measured by the HPLC method of Kong et al.23 The interday and intra-day assay variability was less than 8%. The lower limit of quantitation was 25 ng/ml.
Whole blood histamine was analyzed by a commercial radioimmunoassay procedure (Biomerica, Newport Beach, Calif.). The lower limit of quantitation was 2 ng/ml. Low- and high-quality control samples supplied by the company were within the specified ranges.
Total leukocyte counts were performed by an automated hemocytometer (Coulter Counter S. Plus IV; Coulter Electronics Inc., Hialeah, Fla.) on the whole blood samples collected for helper T cell analysis. Lymphocyte and monocyte proportions were determined microscopically, and the total number of circulating lymphocytes per cubic millimeter was determined. The whole blood samples were then lysed, reacted with mononuclear antibody (Coulter Cytostat anti-CD4, FITC), and analyzed on an automated flow cytometer (FACS 440; Becton Dickinson and Co., Rutherford, N.J.). Multiplication of the proportion of fluorescent cells by the number of circulating lymphocytes resulted in the total number of circulating helper T cells.
Pharmacokinetics
The nonlinear least-squares regression computer program PCNONL1N (Statistical Consultants, Lexington, Ky.) was used to fit the individual methylprednisolone plasma concentration-time curves to equation 1:
| (1) |
in which CMP is the plasma concentration of methylprednisolone at time t, kf is the first-order rate constant for the formation of methylprednisolone from MPS. k is the terminal slope, and V is the volume of distribution. This equation assumes negligible inter-conversion between methylprednisolone and its metabolite mcthylprcdnisone.
The CL of methylprednisolone was calculated from the following equation:
| (2) |
PCNONLJN was also used in the fitting of the individual MPS concentration-time data to equation 3:
| (3) |
in which CMps is the MPS concentration at time t,. is the MPS concentration at time zero, and k is the terminal slope. Noncompartmental analysis was used to obtain AUC, CL, and V, CL was determined as dose/AUC, and V was calculated as CL (AUMC/AUC), in which AUMC is the area under the moment curve. Perhaps because MPS is a high CL compound, its k value docs not closely match the appearance kf for methylprednisolone in equation 1; these parameters were thus calculated separately.
Pharmacodynamics
The net suppressive effects of methylprednisolone on helper T cell circulation, as well as on cortisol and histamine concentrations, were determined by calculating the ratio of AUC(0–24) of the pharmacodynamic parameter after administration of methylprednisolone to the respective baseline AUC(0–24), with a smaller ratio indicating greater suppression.
Immediately after the administration of corticosteroids, the number of circulating basophils (measured as whole blood histamine), helper T cells, and cortisol concentration begins to decline and later return to baseline when the drug is appreciably eliminated. Pharmacodynamic models that describe these direct suppressive effects are shown with the experimental data.19,30 These models account for both the pharmacokinetics of the administered corticosteroid along with the suppression and return to baseline of the measured pharmacodynamic parameter.
After intravenous administration of methylprednisolone, whole blood histamine (H) concentrations decline linearly with a first-order rate constant (kh). Corticosteroids arc postulated to inhibit the zero-order rate of return of basophils from the extra vascular compartment to the blood , whereas the movement of basophils from the blood is unaffected. Therefore, basophil cell trafficking after administration of methylprednisolone can be described by the following equation:
| (4) |
in which the baseline histamine concentration at time zero is Ho, CMP is the methylprednisolone concentration as a function of time (equation 1), and CMP) is the methylprednisolone concentration that produces a 50% reduction in . PCNONLIN was used to simultaneously fit each subject’s 8 am and 4 pm data to equation 3 and to generate the pharmacodynamic parameters , kh, and IC50. Because and kh were found to be the same for both phases during separate fittings, only the IC50 values were allowed to vary between treatments during the simultaneous fitting of the data.
Baseline cortisol concentrations exhibit a circadian rhythm described by the following cosine function:
| (5) |
| (6) |
in which Rcort is the circadian concentration of cortisol, Rm and Rb are the mean and amplitude of the cortisol concentrations, and tc. is time expressed in radians calculated according to equation 5, where T is the clock time within the 24-hour cycle, tz is the acrophase of the cortisol concentrations, and the ratio 15/57.3 converts the clock time into radians.
The cortisol model is based on the theory that exogenous corticosteroids inhibit the secretion of cortisol without affecting cortisol elimination from the body. Therefore, immediately after the intravenous dose of methylprednisolone, cortisol concentrations decline linearly with a first-order rate constant (kc). As CMP declines below the IC50, cortisol concentrations begin to return to the normal circadian rhythm. This process is illustrated in Fig. 2, and may be described by the following:
| (7) |
in which C is the cortisol concentration at time t, Co, is the initial cortisol concentration, and CMp, is the methylprednisolone concentration as described by the following:
| (8) |
In contrast to the basophii model, the cortisol model requires an instantaneous input function for CMP necessitating the use of the monocxponential fitting. By use of the PCNONLIN computer program, the cortisol data for alt three phases were simultaneously fitted to equations 5, 6, and 7, and the fitted parameters Rm, Rb, tz, kc (8 am), kc (4 pm), IC50 (8 am), IC50 (4 pm), Co (8 am), and Co (4 pm) were obtained.
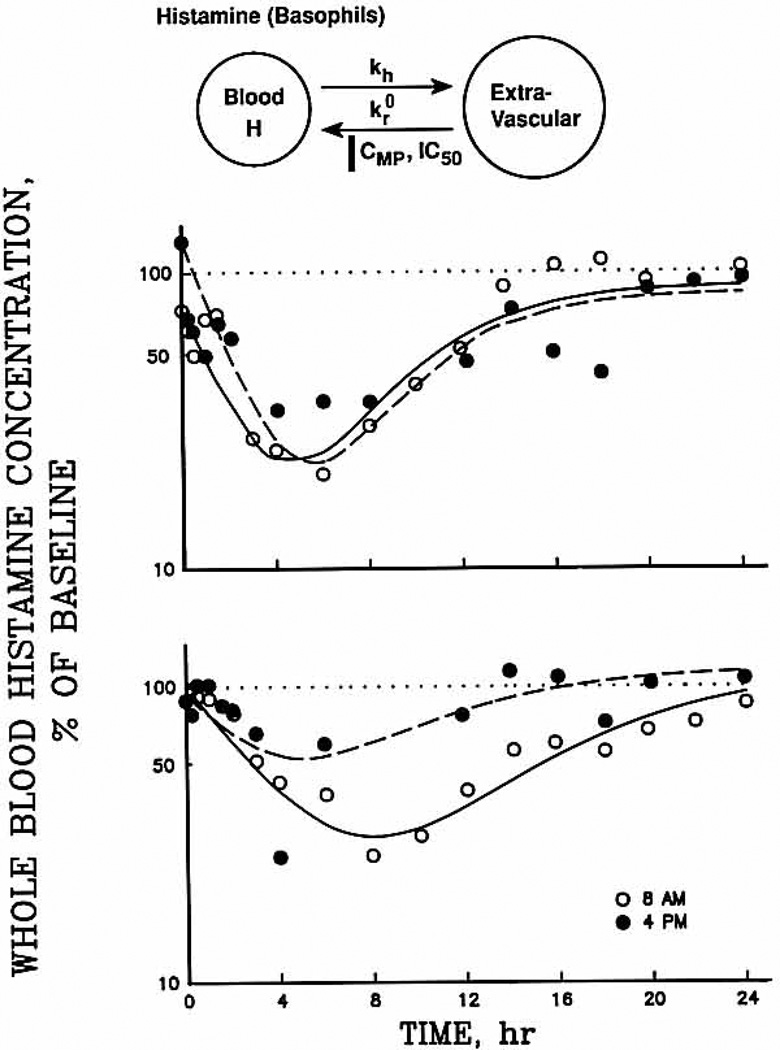
Fig. 2.
Whole blood histamine concentrations (expressed as a percentage of baseline) during all study phases for a representative responsive subject (upper panel), and a representative nonresponsivc subject (lower panel). Symbols are experimental data, and lines depict the fittings to the pharmacodynamic model presented above. Baseline whole blood histamine values are shown by the dotted line. 8 am data by the solid line, and 4 pm data by the broken line. Time 0 is the time of the dose. Symbols: kh, first-order rate constant; , zero-order rate of return of basophils from the extravascular compartment to the blood; CMP, methylprednisolone concentration as a function of time; IC50, methylprednisolone concentration that produces a 50% reduction in .
Helper T cells exhibit a circadian rhythm24 with the peak number of cells in the blood occurring at about 3 am and the nadir at about 4 pm. Methylprednisolone presumably inhibits the circadian rate of return of helper T cells to the vascular compartment (Fig. 4). The basophii model was modified to incorporate the natural circadian rhythm of helper T cells, and the following equations were developed: dTH
| (9) |
| (10) |
in which TH is the helper T cell count at time t, kf, is the first-order rate constant of helper T cell efflux from blood, tc is defined as in equation 6 with a different tz value, and CMP is determined by equation 8. The parameters Rm, Rb, and tz were kept constant, whereas kt and IC50 were allowed to vary during the simultaneous fitting of all three study phase data to equations 9 (baseline) and 10 (steroid dosing).
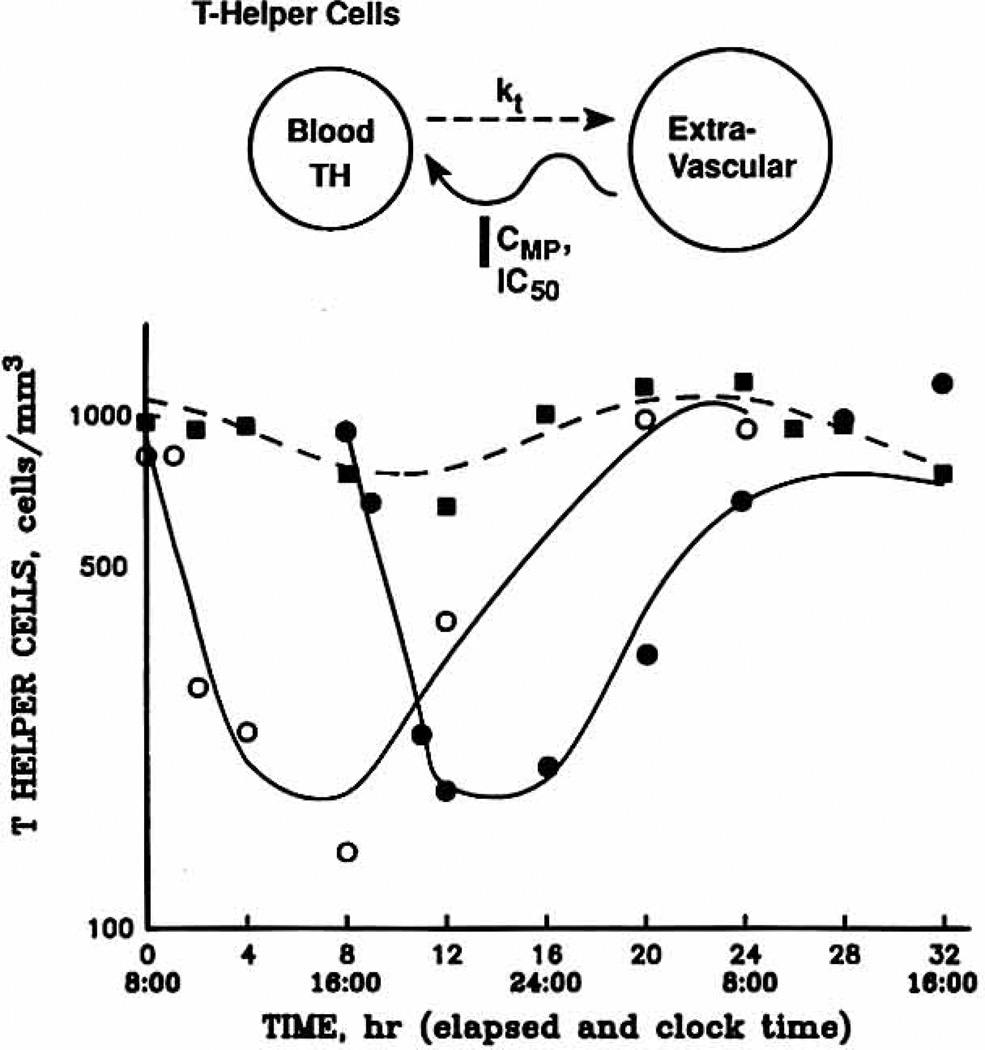
Fig. 4.
Helper T cell versus time profiles in one subject for baseline (squares), 8 am (open circles), and 4 PM (solid circles). Lines depict the fittings to the pharmacodynamic model shown above. TH, Helper T cell count at time t; kt, first-order rate constant of helper T cell efflux from blood.
Statistical analysis
The 8 am and 4 pm pharmaco-kinetic parameters, suppression ratios for each effect measurement, and IC50 values of each effect measurement were compared by use of a paired t test. Homogeneity of variance was determined by use of the F test. A one-way ANOVA for repeated measures was performed to detect differences between the phases for each effect measurement at each sample time point. All data were expressed as a percentage of baseline before analysis to correct for time variations. The Student Newman-Keuls test was carried out to identify mean values, which were significantly different. All findings were considered significant at the p < 0.05 level.
RESULTS
Pharmacokiitetics
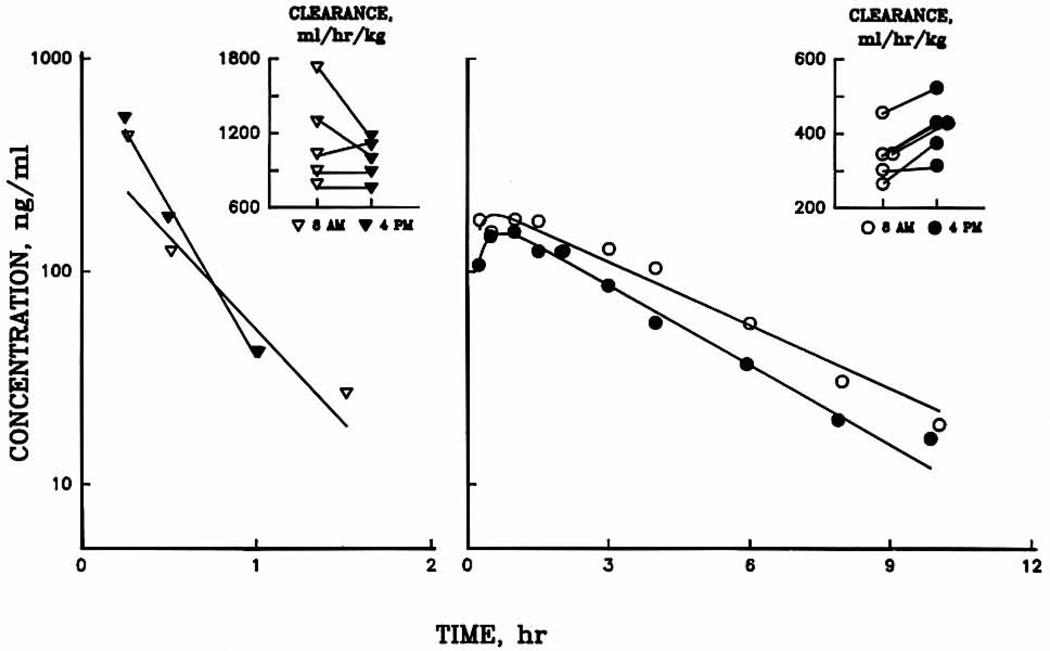
Fig. 1 illustrates the serum concentration–time profiles, showing linear decay of both MPS and methylprednisolonc after administration of MPS at 8 am and 4 pm in a representative subject. Plasma methylprednisolone concentrations were generally higher after the 8 am dose, whereas MPS concentrations were comparable.
Fig. 1.
Plasma concentration–time profiles for methylprednisolone succinate ester (left) and methylprednisolone (right) for a representative subject. Symbols are experimental data, and solid lines are the least-square regression fittings. Time 0 is the time of the dose. The insets show individual clearance values.
The mean phamnacokinetic parameters for MPS and methylprednisolone for five subjects are listed in Table I. After the 4 pm dose, methylprednisolone CL was 28% greater than after early morning administration. The methylprednisolone V was similar (about 1.24 L/kg) in each phase. Significant variability was observed in the kf parameter estimates at 8 am. Large values were observed in two subjects (65.7 and 75.5 hr−1). The few data points and rapid elimination of MPS partly account for this variability. The pharmacokinetic parameters for MPS were similar between both phases with mean CL values of approximately 1000 ml/hr/kg and mean V of about 0.6 L/kg.
Table I.
Pharmacokinetic parameters for methylprednisolone succinate and methylprednisolone
| Methylprednisolone succinate | Methylprednisolone | |||
|---|---|---|---|---|
| Treatment phase | 8 am | 4 pm | 8 am | 4 pm |
| AUC (ng · hr/ml) | 317 ± 103 | 349 ± 66 | 785 ± 114 | 645 ± 79* |
| V (L/kg) | 0.71 ± 0.36 | 0.49 ± 0.33 | 1.19 ± 0.09 | 1.28 ± 0.20 |
| CL (ml/hr/kg) | 1147 ± 375 | 952 ± 167 | 323 ± 72 | 414 ± 77* |
| k (hr−1) | 1.67 ± 0.34 | 2.47 ± 0.84 | 0.29 ± 0.05 | 0.32 ± 0.04 |
| kf (hr−1) | — | — | 30.7 ± 37 | 6.4 ± 2.6 |
Data are presented as mean values ± SD: n = 5 subjects.
AUC, Area under the plasma concentration–time curve; V, volume of distribution; CL, total body clearance; k, terminal slope; kf, first order rate constant for the formation of methylprednisolone from methylprednisolone succinate.
p < 0.05.
The methylprednisolone pharmacokinetic data for one subject was excluded from (he statistical analysis. A respiratory infection developed in this subject after the 4 pm study day, and his 4 pm AUC and CL of methyl-prednisolone (1140 ng · hr/ml and 235 ml/hr/kg) were outlier values deviating by more than two standard deviations from the mean values of the remaining subjects. Viral infections have been associated with increased plasma concentrations of drugs undergoing hepatic metabolism.25,26 Inclusion of five subjects in the pharmacokinetic data analysis still allowed Tor detection of a 17% difference between treatment means with α = 0.05 and β = 0.2.27
Basophil dynamics
Whole blood histamine concentration–time profiles for all 3 study days are shown for two subjects in Fig. 2. The top graph represents those subjects (n = 4) who showed marked suppression of their histamine concentrations on both treatment days; the bottom graph is typical of those subjects who had minimal suppression after afternoon administration. During both treatment phases, histamine concentrations began to decrease shortly after the methylprednisolone was administered, and they returned to baseline by the end of the study period. Histamine concentrations were significantly lower compared with the baseline from 3 hours after dose administration to 14 hours and again at 18 hours after the dose. Between the two dosing regimens, histamine concentrations were different at only the 6-hour time point, with the 8 am dosing regimen producing greater suppression. Baseline histamine concentrations were relatively constant over the 24-hour study period, although zero-time values varied from day to day. All histamine data were therefore analyzed as percentage change from baseline.
The pharmacodynamic parameters generated during the simultaneous computer fitting of the 8 am and 4 pm phases are summarized in Table II. Although the 1C50, values for these phases (10.14 ± 4.29 and 18.46 ± 17.59 ng/ml) were not statistically different, suppression was much more variable with the 4 pm dosing regimen (p < 0.01 with the F test). The net suppressive effects of methylprednisolone on histamine, as represented by the suppression ratios, were not statistically different between the two dosing regimens (Table II).
Table II.
Pharmacodynamic parameters for basophils from whole blood histamine
| Fitted jointly | |||
| kh (hr−1) | 0.36 ± 0.14 | ||
| (ng/hr) | 0.33 ± 0.11 | ||
| Treatment phase | |||
| 8 am | 4 pm | p Value | |
| Fitted separately | |||
| IC50 (ng/ml) | 10.4 ± 4.29 | 18.46 ± 17.59 | NS |
| Suppression ratio | 0.62 ± 0.16 | 0.64 ± 0.20 | NS |
Data are mean values ± SD: n = 6 subjects.
kh, First-order rate constant: , zero-order rate of return of basophils from the extravascular compartment to the blood: IC50, methylprednisolone concentration that produces a 50% reduction in : NS, not significant.
Cortisot dynamics
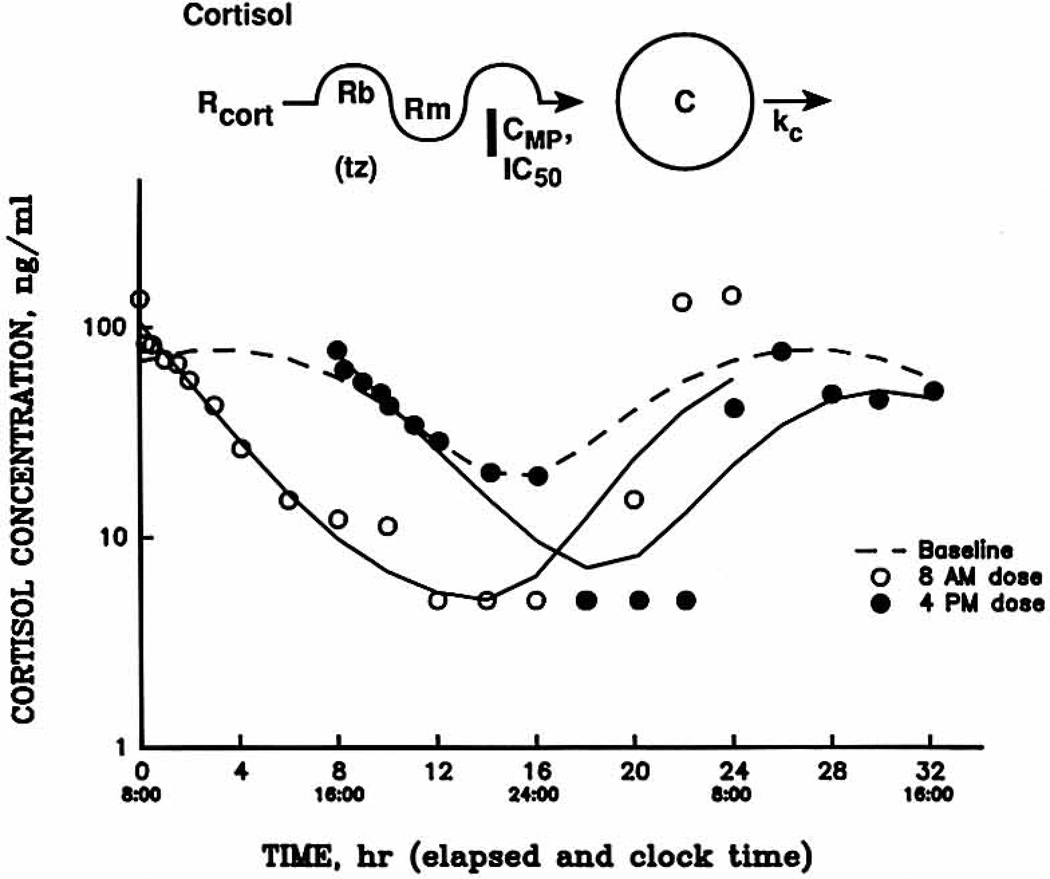
Representative cortisol data and curve fittings for all three phases are presented for one subject in Fig. 3. Evaluation of the various sample time points with the use of the repeated-measures ANOVA yielded significantly lower cortisol concentrations for the 8 am phase compared with baseline from 4 through 14 hours after the dose, as well as from 18 to 22 hours. The mean 4 pm phase cortisol concentrations differed significantly from baseline at 2 hours after the dose up to the 14-hour time point, as well as at 20 hours. Of particular note, the 8 am regimen produced greater cortisol suppression than that produced after the 4 pm regimen at 18 and 20 hours after the dose. This suggests that cortisol concentrations may rebound quicker after the 4 pm dosing regimen. This is supported by the model-fitted profiles in Fig. 3, which show that the return portions of the curve are 4 hours out of phase rather than 8 hours, the actual difference in the time of administration.
Fig. 3.
Cortisol plasma concentration–time profiles for all three study phases in one subject. Symbols show experimental data, and lines represent the fittings to the pharmacodynamic model shown above, Rcort, Circadian concentration of cortisol; Rb, amplitude of the cortisol concentrations; Rm, mean of the cortisol concentrations; tz, acrophase of the cortisol concentrations; C, cortisol concentration; ke, first-order rate constant.
As listed in Table III, no difference was detected in any of the model-fitted pharmacodynamic parameters for cortisol, compared by the paired t test, with the exception of the expected difference in the Co values of 152 (8 am) and 80 ng/ml (4 pm). The cortisol kc and IC50 values, approximately 0.33 hr−1 and 1.2 ng/ml, were simitar between phases and consistent with data reported previously.18 There were no differences in the net suppressive effects of methylprednisolone on cortisol concentrations as detected by comparison of the suppression ratios.
Table III.
Pharmacodynamic parameters for cortisol
| Fitted jointly | |||
| Rm (ng/ml) | 54.61 ± 15.36 | ||
| Rb (ng/ml) | 39.51 ± 0.85 | ||
| tz (hr) | 4.10 ± 1.15 | ||
| Treatment phase | |||
| 8 am | 4 pm | p Value | |
| Fitted separately | |||
| kc (hr−1) | 0.35 ± 0.09 | 0.29 ± 0.06 | NS |
| IC50 (ng/ml) | 1.19 ± 1.23 | 1.02 ± 1.0 | NS |
| Co (ng/ml) | 152 ± 39 | 80 ± 30 | <0.05 |
| Suppression ratio | 0.53 ± 0.11 | 0.64 ± 0.20 | NS |
Data are mean values ± SD; n = 6 subjects.
Rm, Mean of the cortisol concentrations; Rb, amplitude of the cortisol concentrations; tz, acrophase of the cortisol concentrations; kc, first-order rate constant; Co, cortisol concentration at time zero.
Helper T cell dynamics
Fig. 4 displays the helper T cell data fitted to the pharmacodynamic model for all three study phases in a representative subject. The typical circadian rhythm in helper T cell counts is evident on the baseline day, but the tz value differs from cortisol by about ½ day (2 am versus noon). On both of the study days that involved drug administration, the number of intravascular helper T cells decreased after the dose, with a nadir occurring at about 6 hours. After the morning and afternoon doses of methylprednisolone, helper T cell numbers were significantly lower than the baseline values at 4, 8, and 12 hours. However, cell numbers for the two treatment regimens were not different from each other.
The model-generated parameters for all phases can be found in Table IV. The IC50 values for the 8 am and 4 pm dosages were 7.22 and 8.12 ng/ml and, like the other model-fitted parameters, were not significantly different. Analogous to the cortisol and basophil results, the net suppressive effects of methylprednisolone on helper T cell circulation was almost identical between the two treatment phases. The dynamic data for the ill subject was included in the models because these parameters were consistent with the other subjects and the models allow for separation of pharmacokinetic and pharmacodynamic variables.
Table IV.
Pharmacodynamic parameters for helper T cells
| Fitted jointly | ||||
| Rm (cells/mm3) | 647 ± 224 | |||
| Rb (cells/mm3) | 138 ± 71 | |||
| tz (hr) | 16.6 ± 3.9 | |||
| Treatment phase | ||||
| 8 am | 4 pm | Baseline | p Value | |
| Fitted separately | ||||
| kt (hr−1) | 0.55 ± 0.07 | 0.56 ± 0.24 | 0.82 ± 0.30 | NS |
| IC50 (ng/ml) | 7.22 ± 3.73 | 8.12 ± 4.34 | — | NS |
| Supperssion ratio | 0.72 ± 0.18 | 0.78 ± 0.21 | — | — |
Data are mean values ± SD; n = 6 subjects.
kt, First-order rate constant of helper T cell efflux from blood.
DISCUSSION
Human studies that compare morning and evening disposition of drugs with CL values dependent on hepatic enzyme capacity are surprisingly limited. Both theophylline and free prednisolone, which are metabolized by the mixed function oxidase system, were found to exhibit lower CL values after morning administration than after afternoon administration, with CL values differing by 14% to 22%.8,9,28 No such differences were found in the metabolic CL of acetaminophen, but this drug is primarily cleared by way of conjugation as opposed to oxidation.29 In our study, the mean CL of methylprednisolone, which is eliminated by the mixed function oxidase system, was lower in the morning than in the afternoon, resulting in a larger AUC after the 8 am dose.
The chronopharmacokinetics of some high extraction drugs have been investigated and no consistent time dependence for CL was found.30–34 Our finding of no difference in disposition of the highly cleared pro-drug MPS is consistent with these studies.
The present models allow us to factor out the pharmacokinetic differences described above and to separately assess the pharmacodynamics. For basophil trafficking, the IC50 values were not statistically different between the two treatment phases. However, there was appreciable variability in the IC50 values for the afternoon dosing regimen, with two of the six subjects exhibiting almost no suppression. This suggests that there may be a subset of patients with decreased basophil sensitivity to corticosteroids in the afternoon. Perhaps the two subjects who showed limited suppression had a greater number of less-sensitive basophils35 circulating during the afternoon.
The cortisol pharmacodynamic model allowed us to determine that the intrinsic sensitivity of cortisol does not differ between morning and afternoon because the IC50 values were not different. However, the shape of the later portion of the concentration-time profiles was changed. The return to baseline concentrations occurred relatively earlier after the 4 pm dose. In fact, this return was only 4 hours out of synchronization with the 8 am dose despite the 8-hour difference in administration times (Fig. 3). This can be explained in part by the faster CL of methylprednisolone in the afternoon, resulting in quicker decline of methylprednisolone to below the IC50, value (Fig. 1).
The suppressive effects of corticosteroids on cortisol concentrations depend on the length of time inhibitory concentrations remain above the IC50, a function of the dose, CL, and potency of the administered steroid. Many older studies, which indirectly measured cortisol suppression by quantitating urinary or plasma 17-hydroxycorticosteroid concentrations and used a time point to time point comparison, found minimal suppression of these parameters.1,11,12 In addition, relatively small (the equivalent of about 4 to 5 mg methylprednisolone) single doses were given. Several studies have shown that 10 to 40 mg methylprednisolone administered in the morning produce appreciable suppression of cortisol concentrations.19,36,37 We found a similar degree of net suppression of cortisol concentrations in both phases; however, the normal circadian rhythm was significantly altered by the 4 pm dose. This dose produces nadir cortisol concentrations around the time that cortisol concentrations are normally increasing, thus perturbing the normal circadian cycle. Giving moderate doses of steroids in the morning allows dissipation of the suppressive effect in time to resume a normal circadian rhythm the following day. This may explain in part why it is preferable to administer corticosteroids predominantly as a single morning dose, which mimics the natural daily pattern of maximum steroid exposure during the morning hours. It is not only the degree of suppression that is of concern but also the preservation of the normal circadian rhythm.
In studies investigating T-cell responses to corticosteroids,24,38–40 T lymphocyte counts rapidly declined after a morning intravenous steroid dose, reached a nadir at around 6 hours, and then returned to baseline values within 24 hours. In this study, helper T cell response after 8 am and 4 pm doses was similar as determined by both the pharmacodynamic model and suppression ratios. However, the 4 pm dose results in a nadir of helper T cells close to the time of their normal peak in the early morning hours.
The clinical ramifications of this finding may be of interest because immune system activity undergoes a circadian rhythm. In addition to T-lymphocyte counts,24,41 in vitro studies of lymphocyte proliferation to soluble antigens and the allogeneic mixed lymphocyte reaction also reflect circadian variability.42–44 A periodic rhythmicity has also been suggested by in vivo immune function tests with the delayed cutaneous hypersensitivity reaction exhibiting maximal reactivity in the early morning.45
Interestingly, immune-mediated diseases also show circadian variations. In rheumatoid arthritis, the peak of disease activity occurs at about 3 am, and minimal activity occurs 12 hours later.46 Similarly, the greatest probability of renal allograft rejection occurs between 5 and 8 am.47 Those findings may be linked to the seemingly greater immune system activity during this period and may possibly be influenced by the occurrence of low concentrations of immunosuppressive agents, such as corticosteroids, during this critical interval. Further clinical studies are necessary to clarify whether afternoon or evening corticosteroid administration would improve therapeutic benefit without detrimental suppression of endogenous cortisol concentrations.
Acknowledgments
Supported in part by grant No. GM 24211 from the National Institutes of General Medical Sciences, National Institutes of Health (Bethesda, Md.). and by the Upjohn Company. (Kalamazoo, Mich.).
We appreciate the technical assistance of Ms. Nancy Pyszczynski. The Chemistry and Hematology Laboratories of the Buffalo General Hospital provided clinical chemistry, cell counting, and flow cytometry measurements.
Footnotes
Presented at the meeting of the American Society for Clinical Pharmacology and. Therapeutics. Orlando. Fla., March 18–20, 1992.
References
- 1.Reinberg A, Smolensky MH, D'Alonzo GE, McGovern JP. Chronobiology and asthma. III. Timing corticotherapy to biologic rhythms to optimize treatment goals. J Asthma. 1988;25:219–248. doi: 10.3109/02770908809071368. [DOI] [PubMed] [Google Scholar]
- 2.Scott PH, Tabachnik E, MacLeod S, Correia J, Newth C, Levison H. Sustained-release theophylline for childhood asthma: evidence for circadian variation of theophylline pharmacokinetics. J Pediatr. 1981;99:476–479. doi: 10.1016/s0022-3476(81)80354-x. [DOI] [PubMed] [Google Scholar]
- 3.Garrettson LK, Jusko WJ. Diphenylhydantoin elimination kinetics in overdosed children. Clin Pharmacol Ther. 1975;17:481–491. doi: 10.1002/cpt1975174481. [DOI] [PubMed] [Google Scholar]
- 4.Hrushesky W, Levi F, Kennedy BJ. Cis-diamine-dichloroplatinum (DDP) toxicity to the human kidney reduced by circadian timing [Abstract] Proc Am Soc Clin Oncol. 1980;21:C45. [Google Scholar]
- 5.Belanger PN, Labrecque G. Temporal aspects of drug metabolism. In: Lemmer B, editor. Chronopharmacology cellular and biochemical interactions. New York: Marcel Dekker; 1989. pp. 15–34. [Google Scholar]
- 6.Waterhouse JM, Minors DS. Temporal aspects of renal drug elimination. In: Lemmer B, editor. Chronopharmacology cellular and biochemical interactions. New York: Marcel Dekker; 1989. pp. 35–50. [Google Scholar]
- 7.McAllister WAC, Mitchell DM, Collins JV. Prednisolone pharmacokinetics compared between night and day in asthmatic and normal subjects. Br J Clin Pharmacol. 1981;11:303–304. doi: 10.1111/j.1365-2125.1981.tb00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English J, Dunne MB, Marks V. Diurnal variation in prednisolone kinetics. Clin Pharmacol Ther. 1983;33:381–385. doi: 10.1038/clpt.1983.49. [DOI] [PubMed] [Google Scholar]
- 9.Meffin PJ, Brooks PM, Sallustio BC. Alterations in prednisolone disposition as a result of time of administration, gender and dose. Br J Clin Pharmacol. 1984;17:395–404. doi: 10.1111/j.1365-2125.1984.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English J, Marks V. Diurnal variation in methylprednisolone metabolism in the rat [Abstract] IRCS Med Sci. 1981;9:721. [Google Scholar]
- 11.Nichols T, Nugent CA, Tyler FH. Diurnal variation in suppression of adrenal function by glucocorticoids. J Clin Endocrinol. 1965;25:343–349. doi: 10.1210/jcem-25-3-343. [DOI] [PubMed] [Google Scholar]
- 12.Segre EJ, Klaiber EL. Therapeutic utilization of the diurnal variation in pituitary-adrenocortical activity. Calif Med. 1966;104:363–365. [PMC free article] [PubMed] [Google Scholar]
- 13.Reinberg A, Halberg F, Falliers C. Circadian timing of methylprednisolone effects in asthmatic boys. Chronobiologia. 1974;1:333–349. [PubMed] [Google Scholar]
- 14.Reinberg A, Gervais P, Chaussade M, Fraboulet G, Duburque B. Circadian changes in effectiveness of corticosteroids in eight patients with allergic asthma. J Allergy Clin Immunol. 1983;71:425–433. doi: 10.1016/0091-6749(83)90073-8. [DOI] [PubMed] [Google Scholar]
- 15.Reinberg A, Guillet P, Gervais P, Ghatu J, Vignaud D, Abulker C. One month chronocorticotherapy (Dutimelan 8 15 mite). Control of the asthmatic condition without adrenal suppression and circadian rhythm alteration. Chronobiologia. 1977;4:295–312. [PubMed] [Google Scholar]
- 16.Kowanko IC, Pownall R, Knapp MS, Swannell AJ, Mahoney PGC. Time of day of prednisolone administration in rheumatoid arthritis. Ann Rheum Dis. 1982;41:447–452. doi: 10.1136/ard.41.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant SD, Fursham PH, DiRaimondo VC. Suppression of 17-hydroxycorticosteroids in plasma and urine by single and divided doses of triamcinolone. N End J Med. 1965;273:1115–1118. doi: 10.1056/NEJM196511182732101. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari E, Bussolo PA, Montalbetti N, Kuhl JFW, Halberg F. Circadian variation of urinary 17-hydroxy-corticosteroids excretion in relation to dexamethasone-induced suppression. Int J Chronobiol. 1974;2:17–23. [PubMed] [Google Scholar]
- 19.Kong A, Ludwig EA, Slaughter RL, et al. Pharmacokinetics and pharmacodynamic modeling of direct suppression effects of methylprednisolone on serum cortisol and blood histamine in human subjects. Clin Pharmacol Ther. 1989;46:616–628. doi: 10.1038/clpt.1989.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald JA, Salazar DE, Cheng H, Jusko WJ. Two-compartment basophil cell trafficking model for methylprednisolone pharmacodynamics. J Pharmacokinet Biopharm. 1991;19:521–536. doi: 10.1007/BF01062961. [DOI] [PubMed] [Google Scholar]
- 21.Szefler SJ, Ebling WF, Georgitis JW, Jusko WJ. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol. 1986;30:323–329. doi: 10.1007/BF00541537. [DOI] [PubMed] [Google Scholar]
- 22.Ebling WJ, Szefter SJ, Jusko WJ. Analysis of cortisol, methylprednisolone, and methylprednisolone hemisuccinate: absence of effects of troleandomycin on ester hydrolysis. J Chromatogr. 1984;305:271–280. [PubMed] [Google Scholar]
- 23.Kong A, Slaughter RL, Jusko WJ. Simultaneous analysis of methylprednisolone hemisuccinate, cortisol, and methylprednisolone by normal-phase high-performance liquid chromatography in human plasma. J Chromatogr. 1988;430:241–248. doi: 10.1016/s0378-4347(00)80658-1. [DOI] [PubMed] [Google Scholar]
- 24.Levy FA, Canon C, Touitou Y, et al. Circadian rhythms in circulating T lymphocytes subtypes and plasma testosterone, total and free cortisol in five healthy men. Clin Exp Immunol. 1988;71:329–335. [PMC free article] [PubMed] [Google Scholar]
- 25.Peetham JA, Nakatsu K, Munt PW. Theophylline pharmacokinetics and respiratory infections. Lancet. 1978;2:898. doi: 10.1016/s0140-6736(78)91612-4. [DOI] [PubMed] [Google Scholar]
- 26.William SJ, Farrell GC. Inhibition of antipyrine metabolism by interferon. Br J Clin Pharmacol. 1987;22:610–612. doi: 10.1111/j.1365-2125.1986.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolley P, Strom BL. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39:489–490. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- 28.Giacona N, Elvin AT, Seligsohn R, et al. Diurnal variation in theophylline elimination [Abstract] Drug Intell Clin Pharm. 1983;17:452. [Google Scholar]
- 29.Shively CA, Vessell ES. Temporal variations in acetaminophen and phenacetin half-life in man. Clin Pharmacol Ther. 1975;18:413–424. doi: 10.1002/cpt1975184413. [DOI] [PubMed] [Google Scholar]
- 30.Cipolle RJ, Canafax DM, Rabatin J, Bowers LD, Sutherlan DER, Hrushesky WJM. Time-dependent disposition of cyclosporine after pancreas transplantation, and application of chronopharmacokinetics to improve immunosuppression. Pharmacotherapy. 1988;8:47–51. doi: 10.1002/j.1875-9114.1988.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakano S, Hollister LE. Chronopharmacology of amitriptyline. Clin Pharmacol Ther. 1983;33:453–459. doi: 10.1038/clpt.1983.61. [DOI] [PubMed] [Google Scholar]
- 32.Bougerolle AM, Chabard JL, Jbilou M, et al. Chronopharmacokinetic and bioequivalence studies of two formulations of trimipramine after oral administration in man. Eur J Drug Metab Pharm. 1989;14:139–144. doi: 10.1007/BF03190854. [DOI] [PubMed] [Google Scholar]
- 33.Langer B, Lemmer B. Circadian changes in the pharmacokinetics and cardiovascular effects of oral propranolol in healthy subjects. Eur J Clin Pharmacol. 1938;33:619–624. doi: 10.1007/BF00542498. [DOI] [PubMed] [Google Scholar]
- 34.Bruguerolle B, Prat M. Circadian phase-dependent pharmacokinetics and acute toxicity of mepivicaine. J Pharm Pharmacol. 1988;40:592–594. doi: 10.1111/j.2042-7158.1988.tb05314.x. [DOI] [PubMed] [Google Scholar]
- 35.Leonard EJ. Two populations of human blood basophils: effect of prednisone on circulating number. J Allergy Clin Immunol. 1987;79:775–780. doi: 10.1016/0091-6749(87)90210-7. [DOI] [PubMed] [Google Scholar]
- 36.Reiss WG, Slaughter RL, Ludwig EA, Middleton E, Jusko WJ. Steroid dose sparing: pharmacodynamic responses to single versus divided doses of methylprednisolone in man. J Allergy Clin Immunol. 1990;85:1058–1066. doi: 10.1016/0091-6749(90)90051-5. [DOI] [PubMed] [Google Scholar]
- 37.Kondrotas RJ, Slaughter RL, Brass C, Jusko WJ. Ketoconazole effects on methylprednisolone disposition and their joint effects on endogenous cortisol. Clin Pharmacol Ther. 1987;42:465–470. doi: 10.1038/clpt.1987.179. [DOI] [PubMed] [Google Scholar]
- 38.Slade JD, Hepburn B. Prednisone-induced alterations of circulating human lymphocyte subsets. J Lab Clin Med. 1983;101:479–487. [PubMed] [Google Scholar]
- 39.Zweiman B, Atkins PC, Bedard P, Flaschen SL, Lisak RP. Corticosteroid effects on circulating lymphocyte subset levels in normal humans. J Clin Immunol. 1984;4:151–155. doi: 10.1007/BF00915049. [DOI] [PubMed] [Google Scholar]
- 40.Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49:536–549. doi: 10.1038/clpt.1991.64. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie AWS, Oswald I, Micklem HS, et al. Circadian variation of lymphocyte subpopulations: a study with monoclonal antibodies. Br Med J. 1983;286:1773–1775. doi: 10.1136/bmj.286.6380.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan MS, Byers US, Levin AS. Circadian rhythm of stimulated lymphocyte blastogenesis. J Allergy Clin Immunol. 1976;58:180–189. doi: 10.1016/0091-6749(76)90153-6. [DOI] [PubMed] [Google Scholar]
- 43.Tavadia HB, Fleming KA, Hume PD, Simpson HW. Circadian rhythmicity of human plasma cortisol and PHA-induced lymphocyte transformation. Clin Exp Immunol. 1975;22:190–193. [PMC free article] [PubMed] [Google Scholar]
- 44.Eskola J, Frey H, Molnar G, Soppi E. Biological rhythm of cell-mediated immunity in man. Clin Exp Immunol. 1976;26:253–257. [PMC free article] [PubMed] [Google Scholar]
- 45.Cove-Smith JR, Kabler P, Pownall R, Knapp S. Circadian variation in an immune response in man. Br Med J. 1978;2:253–254. doi: 10.1136/bmj.2.6132.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harkness JAL, Richter MB, Panayi GS, Van DePette K, Unger A, Pownall R. Circadian variation in activity in rheumatoid arthritis. Br Med J. 1981;284:551–554. doi: 10.1136/bmj.284.6315.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knapp MS, Cove-Smith JR, Dugdale R, Mackenzie N, Pownall R. Possible effect of time on renal allograft rejection. Br Med J. 1979;1:75–77. doi: 10.1136/bmj.1.6156.75. [DOI] [PMC free article] [PubMed] [Google Scholar]