Abstract
Rett syndrome (RTT) is a rare neurodevelopmental disorder, characterized by severe behavioral and physiological symptoms. Mutations in the methyl CpG-binding protein 2 gene (MECP2) cause >95% of classic cases, and currently there is no cure for this devastating disorder. The serotonin receptor 7 (5-HT7R) is linked to neuro-physiological regulation of circadian rhythm, mood, cognition, and synaptic plasticity. We presently report that 5-HT7R density is consistently reduced in cortical and hippocampal brain areas of symptomatic MeCP2–308 male mice, a RTT model. Systemic repeated treatment with LP-211 (0.25 mg/kg once/day for 7 days), a brain-penetrant selective 5-HT7R agonist, was able to rescue RTT-related defective performance: anxiety-related profiles in a Light/Dark test, motor abilities in a Dowel test, the exploratory behavior in the Marble Burying test, as well as memory in the Novelty Preference task. In the brain of RTT mice, LP-211 also reversed the abnormal activation of PAK and cofilin (key regulators of actin cytoskeleton dynamics) and of the ribosomal protein (rp) S6, whose reduced activation in MECP2 mutant neurons by mTOR is responsible for the altered protein translational control. Present findings indicate that pharmacological targeting of 5-HT7R improves specific behavioral and molecular manifestations of RTT, thus representing a first step toward the validation of an innovative systemic treatment. Beyond RTT, the latter might be extended to other disorders associated with intellectual disability.
INTRODUCTION
Rett syndrome (RTT) is a rare neurodevelopmental disorder, characterized by severe behavioral and physiological symptoms (Hagberg et al, 2002; Ricceri et al, 2012). One essential feature of RTT is the apparently normal perinatal development until about 6–18 months of age, when RTT patients start losing their acquired cognitive, social, and motor skills and develop a wide variety of symptoms, including autistic-like behaviors, anxiety, motor disturbances, stereotypic hand movements, and severe cognitive dysfunction (Hagberg, 2002). Mutations in the methyl CpG binding protein 2 gene (MECP2) cause >95% of classic cases (Amir et al, 1999; Chahrour and Zoghbi, 2007; Guy et al, 2011). MECP2 encodes a multifunctional protein that binds to methylated DNA and mainly acts as a key transcriptional regulator (Guy et al, 2011). How mutations in the MECP2 gene lead to the neurobehavioural features of RTT is still unknown and there is no cure for this devastating disorder.
Serotonin receptor 7 (5-HT7R) is among the most recently discovered G-coupled serotonin receptors (Hedlund and Sutcliffe, 2004; Matthys et al, 2011). The relevance of this receptor in neuro-physiological phenomena like regulation of the circadian rhythm, mood, cognitive processes and in synaptic plasticity has been established (Adriani et al, 2012; Cifariello et al, 2008; Hedlund et al, 2003; Leopoldo et al, 2011; Matthys et al, 2011). Previous in vitro studies demonstrated that the 5-HT7R regulates synaptic morphology and function in cultured hippocampal neurons through selective activation of the small Rho GTPases, RhoA and Cdc42, key regulators of actin cytoskeleton dynamics (Kobe et al, 2012; Kvachnina et al, 2005).
Aberrations in Rho GTPases signaling pathways account for several forms of syndromic and non-syndromic intellectual disabilities and result in abnormal neuronal connectivity and deficient cognitive function (De Filippis et al, in press; Ramakers, 2002). By cycling between an active GTP-bound state and an inactive GDP-bound state, Rho GTPases integrate extracellular and intracellular signals to coordinate dynamic changes in the actin cytoskeleton, thereby stimulating a variety of processes, including morphogenesis, migration, neuronal development, cell division, and adhesion (Auer et al, 2011; Govek et al, 2005; Hall, 2005). Despite Rho GTPases being critical for several cellular key processes and being involved in many pathological conditions (Ba et al, 2013; Ramakers, 2002; van Galen and Ramakers, 2005), their direct pharmacological manipulation still remains troublesome because of the paucity of specific drugs.
Recently, we demonstrated that the activation of brain Rho GTPases by the CNF1 bacterial protein rescues the neurobehavioral phenotype in a RTT mouse model (De Filippis et al, 2012). The therapeutic approach we followed, however, does currently bear low translational value, due to the need for an intracerebroventricular (icv) route of administration. Novel systemic treatments targeting brain Rho GTPases are therefore required.
In this study, we verified whether repeated systemic intraperitoneal (i.p.) treatment with LP-211, a novel brain-penetrant 5-HT7R selective agonist that binds with high affinity at the human cloned 5-HT7R (Hedlund et al, 2010; Leopoldo et al, 2011) affects brain Rho GTPases activation state in vivo. Based on the positive results of this first study, we argued that modulation of Rho GTPases signaling by 5-HT7R stimulation might represent a potential therapeutic approach for RTT. To test this hypothesis, we investigated the functional and molecular consequences of i.p. treatment with LP-211 in symptomatic (8–10 months old) MeCP2–308 male mice, a RTT model carrying a truncating mutation of the MeCP2 gene (De Filippis et al, 2010; Shahbazian et al, 2002).
MATERIALS AND METHODS
Subjects
The experimental subjects were fully symptomatic (8–10 months of age) hemizygous MeCP2–308 male mice and wt littermates (B6.129S-MeCP2tm1Heto/J, stock number: 005439; backcrossed to C57BL/6J mice for at least 12 generations from the Jackson Laboratories, USA) (De Filippis et al, 2010; Shahbazian et al, 2002). To assess whether LP-211 affects RhoGTPases activation state in mouse brain, adult male mice weighting 30–40 g were purchased from Charles River Italia (Calco, Italy). All procedures were carried out in accordance with the European Communities Council Directive (86/609/EEC) and were formally approved by Italian Ministry of Health.
Drug and Treatment
LP-211 was prepared following the same procedure described in (Leopoldo et al, 2008). The compound was dissolved in vehicle solution, ie, 1% dimethyl sulfoxide (DMSO) in saline.
To assess the effects of LP-211 on brain RhoGTPases activation, mice were randomly assigned to be injected with one of the following drug dosages: LP-211 at 0.125, 0.25 mg/kg or vehicle (DMSO 1% in saline; N=3 per experimental groups). This range of drug dosages was chosen according to the previous experience by our group in mice. Mice were injected with the assigned dosage across 7 successive days (Adriani et al, 2012; Romano et al, 2014). After 4 hours from the last 7th injection, mice were killed and brains were dissected and rapidly frozen in dry ice.
RTT mice and wt littermate controls were daily i.p.-injected (between 0900 and 1100 hours) for 7 consecutive days with either LP-211 (0.25 mg/kg) or vehicle (1% of DMSO in saline). After a 4-hour wash-out period from the 7th i.p. injection, four mice per experimental group were killed by decapitation and their brains were dissected and rapidly frozen for biochemical analyses. The remaining mice were transcardially perfused 24 h after the last i.p. injection and brains subsequently processed for immunohistochemistry analyses (N=4 per experimental group).
Rho GTPases Activation Assay
This analysis is based on the use of effectors (GST–PAK70_106 for Cdc42 and GST-Rhotekin for Rho, Millipore, Temecula, CA, USA) capable of binding activated Cdc42 or Rho. Samples were homogenized in 50 mM Tris (pH 7.4), 1 mM EDTA (pH 8.0), 0.5% Nonidet P-40, 150 mM NaCl, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF and processed as in the study by (Diana et al, 2007). For western blotting following pull-down analysis, proteins were resolved on 12% SDS-polyacrylamide gel and electrically transferred onto polyvinylidene difluoride membranes (Bio-Rad). Following blocking in TBS-T containing 5% non-fat milk, membranes were incubated overnight at 4 °C with the following primary antibodies: mouse anti-Rho (1:500; Millipore), mouse anti Cdc42 (1:1000; Santa Cruz). After washing in TBS-T, immunocomplexes were detected with HRP-conjugated species-specific secondary antibodies (Jackson Laboratory, Bar Harbor, ME, USA) followed by enhanced chemiluminescence reaction. Immunoreactive bands were normalized with respect to the total Rho or Cdc42.
Behavioral Analyses
To unravel the effects of the LP-211 treatment on RTT-related behavioral alterations, two cohorts of RTT mice and wt littermate controls were used (1st experiment: N=8–9; 2nd experiment: N=8–11 per experimental group).
Experiment 1
Light/dark test
To evaluate the effects of LP-211 on anxiety-like behaviors we compared mice from the 4 experimental groups in the light/dark paradigm, 15 min after the 6th i.p. injection, as previously described (De Filippis et al, 2010).
The experimental apparatus consisted of an opaque Plexiglas rectangular box (20 × 14 × 27 cm) with smooth walls, subdivided into two compartments of equal size. One compartment had white walls and was brightly illuminated (250 lux), whereas the other one was black and topped to prevent the light from entering. Mice were individually placed into the dark area and a 9-min session was performed. Tests were videotaped and scored by a trained observer blind to the genotype of mice, using dedicated software (The Observer v2.0 for DOS, Noldus Information Technology, Wageningen, the Netherlands) to obtain the time spent in each compartment, the number of transitions between the two compartments and latency to enter the light compartment.
Marble burying test
To evaluate the effects of LP-211 on exploratory behavior, 50 min after the 6th i.p. injection with either LP-211 or vehicle, mice were tested in the marble burying task, as previously described (Deacon, 2006). Briefly, a transparent Plexiglas box (38 × 22 × 30 cm) was filled with ∼5 cm of sawdust bedding, lightly tamped down to make a flat, even surface over which nine marbles were evenly distributed. At the end of a 30-min session during which mice were allowed to freely explore the cage, the number of buried marbles (at least 2/3 of their depth) was counted. The test was carried out under dim lights.
Dowel test
To evaluate the effects of LP-211 on motor learning capacities, the dowel test was performed 2 h after the 6th i.p. injection as in (De Filippis et al, 2010). The hardwood round dowel used, was 9.0 mm in diameter and 35 cm long. The dowel was mounted horizontally 50 cm above a 5 cm dept bedding of sawdust. At the beginning of the testing, each mouse was placed in the middle of the dowel so that the length of its body was parallel to the dowel. Latency to fall from the dowel into a cage of bedding was recorded (30-s criterion). Each mouse repeated the test twice, with an intertrial interval of at least 15 min. If mice were able to walk across the dowel and off of the dowel, they received the maximum score of 30 s.
Experiment 2
General health score
The general health of the experimental mice was qualitatively evaluated by a trained observer blind to the genotype and treatment of the experimental mice as previously described (Guy et al, 2007), with little modification. Briefly, mice received a score (ranging from 0—normal appearance to 2—highly compromised) for each of the following symptoms: gait, mobility, breathing, kyphosis, fur, hindlimb clasping, tremors, and general condition. Body weight of the animals was also recorded at each scoring session. The individual scores for each category were subsequently summed up to obtain a semiquantitative measure of symptom status, called throughout the text ‘the general health score'. The qualitative evaluation was carried out on the last day of treatment (the 7th) and after a 6-day-long wash-out period (on the 13th day of the schedule).
Wire hanging test
To evaluate limb strength, the four-paw wire hanging test was performed at the end of the general health scoring session on the 7th and 13th day of the treatment schedule (Klein et al, 2012). A wire cage top (25 × 15 cm) with its edges taped off was used for this experiment. The mouse was placed on the center of the wire lid and the lid was slowly inverted and placed at ∼40 cm above the sawdust bedding (5-cm depth). Latency to fall from the wire was recorded and scored. The time out period was 30 s.
Dowel test
The second cohort of mice was tested twice in the Dowel test: on the 6th day of treatment (after 2 h from the injection) and on the 12th day of the schedule (after a 5-day long wash-out period). The same protocol above described was applied (see Experiment 1), apart from the fact that mice were left undisturbed in their home cages for at least 2 h before being tested.
Spontaneous behavior in a novel cage
After 15 min from the 7th i.p. injection, mice were placed singly into a transparent plastic cage (26 cm × 16 cm × 17 cm) with a wire cage top. Mice were left in a quiet room under dim light condition (about 20 lux) during a 75 min period (until 90 min from the i.p. injection) and their spontaneous activity was video-recorded. The behavior of the mice was subsequently scored by means of the Noldus Observer software v3.0, during three 5-min intervals (from the beginning of the videos: 0–5; 35–40; 70–75). At the time of videotape analysis, the long side of the cage was subdivided into three sections of the same length by two lines placed on the video screen. The following items were scored: Crossing (number of line crossings with both forepaws), Rearing, Grooming, Inactivity, and Bar holding. This approach also allowed the evaluation of the potential appearance of spontaneous stereotypic behaviors, a sound feature of RTT, and LP-211 effects thereon.
Spontaneous spatial novelty preference task
On the 10th day of the schedule (after a 3-day long wash-out period), mice were tested on a spontaneous, spatial novelty preference task as previously described (Lyon et al, 2011; Sanderson et al, 2007). The Y-maze was made from transparent Perspex, and consisted of three 30 cm long, 8 cm wide arms with 20 cm high walls, connected by a central junction. A thin layer of sawdust covered the floor of the maze. Each mouse was assigned two arms (the ‘start arm' and the ‘other arm') to which they were exposed during the first phase of the task (the ‘exposure phase'). Allocation of arms to specific spatial locations was counterbalanced within each experimental group. During the 5-min ‘exposure' phase, the entrance to the third, ‘novel', arm was closed off by the presence of a large Perspex white block. The mouse was placed at the end of the start arm, facing the experimenter, and allowed to explore the start arm and the other arm freely for 5 min, beginning as soon as the mouse left the start arm. At the end of 5 min, the mouse was removed from the maze and returned to the home cage for 1 min. During this time, the Perspex block closing off the novel arm was removed and the sawdust redistributed throughout the maze to minimize the use of odor cues. The mouse was then returned immediately to the start arm, facing the experimenter, for the 2-min test phase. This consisted of 2 min free exploration during which the mouse could enter all three arms, beginning as soon as the mouse left the start arm.
The test was video-recorded and the number of entries and the length of time spent into each arm, during both the exposure and the test phase, were subsequently scored by means of the Noldus Observer software v3.0. For the test phase, a discrimination ratio ((novel arm/(novel+other arm)) was calculated for time spent in each arm. Previous work has demonstrated that wt mice display a preference for the novel arm during the test phase (Lyon et al, 2011), and that this preference relies on extramaze cues, whereas preference for the novel arm is abolished in mice with cytotoxic hippocampal lesions (Deacon et al, 2002).
Neurobiological Analyses
Immunohistochemistry
The 5-HT7 immunoreactivity experiments were conducted on brain coronal sections using a conventional streptavidin–biotin technique. Free floating sections (15-μm) were stained using a polyclonal rat IgG antibody against 5-HT7 receptor (Lifespan Biosciences, USA) using a dilution of 1:500, which was determined in preliminary experiments. Briefly, sections were incubated with 3% hydrogen peroxide for 30 min, and followed by a 30-min incubation with 10% goat serum to block non-specific protein binding. Negative control sections were treated with non-immunized goat immunoglobulin serum under the same conditions. Following PBS washing, biotinylated goat IgG (dilution 1:1000; GE Healthcare, UK) was applied for 2 h, then followed by streptavidin peroxidase conjugate incubation (GE Healthcare, UK). The color reaction was developed with a solution of 0.02% diaminobenzidine in 0.003% hydrogen peroxide for 15 min. The sections were mounted onto gelatin-chrome alum-coated glass slides and viewed through a white light microscope (Leitz, Germany). Immunohistochemistry procedures were simultaneously carried out on a number of brain sections (at least four sections /animal) from each experimental group.
Optical density analysis
Positive staining was evaluated by calculating the optical density (OD) of areas of interest in each region and subregion (ie sensorimotor, piryform and motor cortex), as previously described with minor modifications (Nativio et al, 2012). Standard outline digital images were captured using a SPOT-RT CCD videocamera (SPOT-RT Image Software V 3.0 SPOT Diagnostic Instruments, Sterling Height, Michigan) for each region of interest. Semiquantitative analysis of positive immunoreactivity was performed on brain multiple sections/region. Final OD values were calculated as a function of the specific signal relative to external background measure (taken from outside the section). Constant optical conditions were maintained throughout the morphometric and densitometric evaluation.
Western Blot Analyses
Hippocampal tissues were isolated and homogenized in lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% NP40, 0.1% SDS) and a mix of phosphatase and protease inhibitors (Complete Mini, Roche) using an Ultra-Turrax homogenizer at 4 °C. Lysates were sonicated and then clarified by centrifugation for 15 min at 10 000 g. Protein concentration was determined using bovine serum albumin (BSA) as a standard in a Bradford reagent assay. Total lysates were boiled in SDS sample buffer, separated by SDS-PAGE electrophoresis and blotted to nitrocellulose membrane (Bio-Rad). Filters were blocked in PBS-Tween-20 (0.1%) plus 5% non-fat milk (Fluka) and incubated with primary antibodies for 16 h at 4 °C. The following primary antibodies were used: rabbit polyclonal anti-5HT7 (1:100, cod. LS-C39057, Lifespan Biosciences), rabbit polyclonal anti-PAK3 (1:1000, cod. 06902, Upstate), rabbit polyclonal anti-phospho-PAK1/2/3 (pS141) (1:500, cod. 44940G, Invitrogen), rabbit polyclonal anti-cofilin (1:5000, cod. ab1106250, Abcam), rabbit polyclonal anti-phospho-cofilin (pS3) (1:100, cod. ab1286650, Abcam), rabbit polyconal anti-rpS6 (1:1000, cod. 2217, Cell Signaling), rabbit polyclonal anti-phospho-rpS6 (1:200, cod. 2211 (Ser 235/236) and cod. 2215 (Ser 240/244), Cell Signaling), mouse monoclonal anti β-actin (1:1000, cod. sc-81178, Santa Cruz). After washing three times with PBS-Tween, filters were incubated with peroxidase-conjugated secondary antibodies (anti-mouse or rabbit IgG; 1:4000; Amersham) for 1 h at room temperature. Detection was performed by Enhanced Chemiluminescence kit (EuroClone). For quantitative measurements, western blot signals were acquired and analyzed by a Fluor-S densitometer and the Quantity One software (Bio-Rad); OD from at least three different experiments were calculated for each sample and normalized with the corresponding β-actin signal OD; the OD ratios were then compared and expressed as the average fold increase, with 1 (wt control) as the control value. OD values of β-actin appeared unaffected by genotype and treatment. Similar results were also obtained with membranes hybridized with 14,3,3β and β-tubulin antibodies (not shown; sc-1657 and sc-5274, respectively, both from Santa Cruz).
Statistical Analysis
Data were analyzed with either parametric or non-parametric analysis of variance, depending on distribution of the response variable considered. The Levene test was applied to confirm that variance did not differ between groups. To unravel the presence of outliers, the Grubbs' test was applied. Post hoc comparisons were performed by Tukey HSD, by Fisher's PLSD post hoc comparisons or by Mann–Whitney test with Bonferroni's correction. Specifically, ANOVA models included genotype and LP-211 treatment as between-subject factor and repeated measurements as within-subject factor.
RESULTS
5-HT7 Receptor Stimulation by LP-211 Modulates Rho GTPases Activation In Vivo
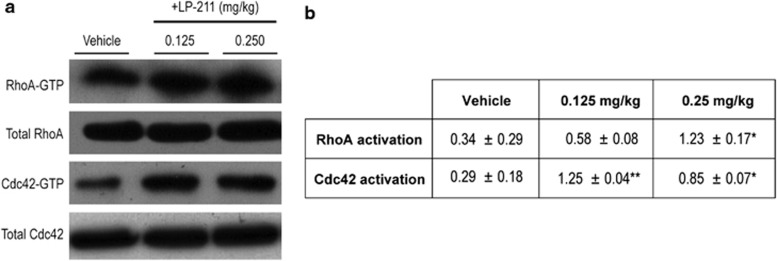
To verify whether 5-HT7R modulates Rho GTPases in vivo, we first assessed whether treatment with LP-211 (0.125, 0.25 mg/kg or vehicle, seven consecutive daily i.p. injections) affects Rho GTPases activation state in mouse brain. A three- to fourfold increase in the activation levels of Cdc42 was confirmed in the brain of mice that received both LP-211 doses compared with vehicle-injected mice (Figure 1). The high LP-211 dose (0.25 mg/kg) also induced a threefold increase in RhoA brain activation (Figure 1), thus resembling the molecular effects on Rho GTPases activation observed in neuronal cultures (Kobe et al, 2012).
Figure 1.
Dose-response evaluation of LP-211 effects on RhoGTPases RhoA and Cdc42 activation state in mouse brain. (a) Representative immunoblots showing the amount of both total and activated Rho and Cdc42 (RhoA-GTP and Cdc42-GTP) in mouse cortex. (b) Densitometric analysis of the pull-down experiments. Data are expressed as relative GTPase activity normalized for the amount of total RhoA and Cdc42 protein loaded±SEM. LP-211 or vehicle were i.p. administered for 7 consecutive days. Statistical significance was calculated by two-way ANOVA with Fisher's PLSD post hoc comparisons. *p<0.05 and **p<0.01 vs vehicle-injected mice.
LP-211 Treatment Rescues the Behavioral Phenotype in a RTT Mouse Model
Based on these results and on previous behavioral studies (Adriani et al, 2012), we then investigated LP-211 effects (seven daily i.p. injections, at 0.25 mg/kg dose) in the RTT mouse model.
Experiment 1
A tailored battery of validated behavioral tests was carried out on the 6th day of the treatment schedule.
Light/Dark test
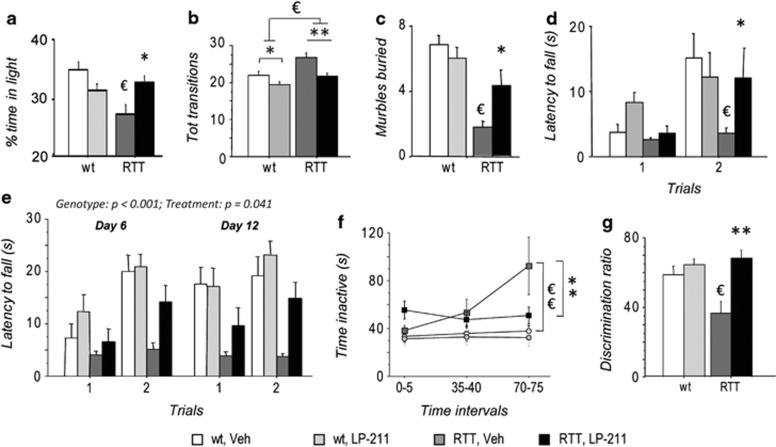
Increased anxiety-like behavior was confirmed in vehicle-injected RTT mice in the Light/Dark test (De Filippis et al, 2012; De Filippis et al, 2010) compared with wt controls, as demonstrated by the reduced time spent in the bright/intimidating part of the apparatus (wt, Veh vs RTT, Veh: p<0.05 after post hoc comparisons on the significant genotype*treatment interaction: F(1,31)=7.97; p=0.008; Figure 2a). Notably, LP-211 treatment selectively increased the time RTT mice spent in the lit compartment (RTT, Veh vs RTT LP-211: p<0.05 after post hoc comparisons on the significant genotype*treatment interaction; Figure 2a), thus rescuing their otherwise anxious-like phenotype.
Figure 2.
Stimulation of the 5-HT7 receptor improves the behavioral phenotype in a mouse model of Rett syndrome. (a) Vehicle-injected RTT mice (RTT, Veh) spent significantly less time in the light compartment in the Light/Dark test compared with wild-type controls (wt, Veh), thus confirming increased anxiety-like behaviors. Treatment with LP-211 restored wt-like levels of anxiety-like behaviors in RTT mice. (b) RTT mice displayed a higher number of transitions between the two compartments during the Light/Dark test compared with wt controls. The LP-211 treatment rescued the hyperactive phenotype of RTT mice, thus confirming the anxiolytic properties of the treatment. (c) RTT mice buried significantly less marbles in the Marble Burying test compared with wt controls, thus suggesting decreased digging/exploratory behavior in a novel environment. LP-211 significantly increased this parameter. A normalization of performance was thus observed in RTT mice who received the LP-211 treatment. (d) In the Dowel test, RTT mice displayed significantly shorter latencies to fall from a dowel during the second trial compared to wt controls, thus confirming impaired motor learning capacities. LP-211 significantly improved the performance of RTT mice in this test. (e) Dowel test results were confirmed on a second cohort of mice. Comparable genotype and treatment effects were found on days 6 and 12, after a 5-day long wash-out period, thus suggesting lasting effects of a 7-day long treatment with LP-211 in RTT mice. (f) RTT mice showed increased inactivity after 70 min of exposure to a novel cage compared with wt controls. The LP-211 treatment completely rescued the hypoactive phenotype of RTT mice. (g) In the spatial novelty preference task, RTT mice showed decreased discrimination of the novel arm compared to wt controls. The LP-211 treatment completely restored the hippocampal-dependent short-term spatial memory of RTT mice in this non-aversive task. LP-211 or vehicle were i.p. injected for 6 (a–d) or 7 (e–g) consecutive days. Data are mean±SEM. Statistical significance was calculated by two-way ANOVA with Tukey's post hoc test. €, wt, Veh vs RTT, Veh; p<0.05; *, RTT, Veh vs RTT, LP-211, p<0.05, after post hoc comparisons on the genotype*treatment or genotype*treatment*repeated measures interactions.
Mutant mice also performed a higher number of transitions compared with wt mice during the test (main effect of genotype: F (1,31)=7.46; p=0.010, wt (Veh+LP-211 pooled)=61.88±2.41, N=17; RTT (Veh+LP-211 pooled)=72.61±3.50, N=18, Figure 2b), thus excluding that hypoactivity or motivational deficits might explain the reduced time mutant mice spent in the light compartment of the apparatus. A main effect of treatment was also evident as for total number of transitions, with LP-211-treated mice of either genotype performing fewer transitions compared with vehicle-injected mice (F(1,31)=9.35; p=0.005, Veh (wt+RTT pooled)=73.65±3.27, N=17; LP-211(wt+RTT pooled)=61.50±2.64, N=18, Figure 2b). When treated with LP-211, RTT mice were thus undistinguishable from wt littermates for this parameter. No differences were found as for latency to leave the start compartment (data not shown).
Marble burying test
The 5-HT7R has been linked to the expression of marble burying in mice (Hedlund and Sutcliffe, 2007), a species-typical exploratory behavior (Deacon, 2006). In the Marble Burying test, RTT mice displayed decreased digging and buried a lower number of marbles compared with wt littermates (main effect of genotype: F(1,31)=29.54; p<0.001; Figure 2c), thus indicating decreased environment-directed exploratory behavior. RTT mice significantly improved their performance upon treatment with LP-211, thus reaching wt-like levels (RTT, Veh vs RTT, LP-211: p<0.05 after post hoc comparisons on the genotype*treatment interaction: F(1,31)=11.91; p=0.002).
Dowel test
Motor learning ability was assessed in the Dowel test. As expected, wt control mice significantly improved their performance from the first to the second trial (p<0.05, after post hoc comparisons on the genotype*treatment*repeated measures interaction: F(1,31)=5.06; p=0.032; Figure 2d). By contrast, no changes in latency to fall from the dowel along consecutive trials was evident in RTT mice, which fell from the dowel significantly earlier than wt controls on the second trial (p>0.05 after post hoc comparisons; Figure 2d). A worse performance of RTT mice was thus confirmed in this test (De Filippis et al, 2012; De Filippis et al, 2010). Treatment with LP-211 significantly improved the motor learning ability of mutated mice and restored wt-like levels of performance on the second trial (p<0.05 after post hoc comparison; Figure 2d).
Experiment 2
General health score
The evaluation of the general health status of the experimental mice confirmed that significant differences were evident among the four experimental groups (H=21.03; p<0.001; median (inter-quartile range): wt, veh: 1.25 (0.63–2); wt, LP-211: 0.75 (0.5–1.25); RTT, veh: 4.5 (4–5.25); RTT, LP-211: 3.38 (2.75–4.87)). Post hoc comparisons by the Mann–Whitney test with Bonferroni correction revealed that subtle but consistent gross phenotypic alterations can be detected in 10-month-old MeCP2–308 male mice (wt, veh vs RTT, veh: U=98.5; p=0.008). The LP-211 treatment did not affect the general health status of either wt or RTT mice. No genotype or treatment interactions with the day of testing (7th and 13th day of the schedule) were evidenced for any of these parameters.
We found that RTT mice weighted less compared with wt littermates (wt: 30.09±0.36; RTT: 26.15±0.49; main effect of genotype: F(1,36)=23.46; p<0.001). No treatment effects, as well as no genotype or treatment interactions with the day of testing (7th and 13th day of the treatment schedule) were evidenced.
Wire hanging test
In the four-paw wire hanging test, post hoc comparisons on the significant Kruskal–Wallis analysis (H=10.85; p=0.013) uncovered a worse performance of RTT mice compared with wt controls (wt, veh vs RTT, veh: U=82.0; p=0.013; median (inter-quartile range): wt, veh: 30 (30–30); RTT, veh: 17 (13.5–24.5)), thus suggesting reduced limb strength in RTT mice. Given that RTT mice weighted less than wt controls at this age (see above), we cannot rule out that this difference may be attributed to differences in body weight. A 7-day long treatment with LP-211 did not significantly affect these parameters in neither wt (wt, LP-211: median (inter-quartile range): 30 (30–30)) nor RTT mice (RTT, LP-211: median (inter-quartile range): 24 (9.38–30)). No genotype or treatment interactions with the day of testing (7th and 13th day of the treatment schedule) were evidenced for any of these parameters.
Dowel test
In the first experiment, behavioral testing was conducted on a single cohort of mice within a 2-h interval on the 6th day of treatment. We reasoned that, in case of interference by stress upon behavioral results, the Dowel test, the last test carried out in the previous cohort of mice, should have been the most affected. To exclude that the adopted protocol of repeated testing biased the results, we have carried out the Dowel test in a second cohort of mice on the 6th (after 2 h the i.p. injection) and on the 12th day of the schedule (after a 5-day long wash-out period). The same profile we reported in Experiment 1 was confirmed (Figure 2e), with RTT mice showing a worse performance compared with wt controls (main effect of genotype: F(1,36)=17.84; p=0.002) and the LP-211 treatment significantly improving their performance (main effect of treatment: F(1,36)=4.50; p=0.041). No genotype or treatment interactions with the day of testing were evidenced, thus demonstrating that the same profile of improved motor learning capacities in LP-211-treated RTT mice compared with veh-treated RTT controls persisted on the 13th day of the treatment schedule. Lasting beneficial effects of the LP-211 treatment on motor learning performance in RTT mice are thus suggested.
Spontaneous behavior in a novel cage
On the 7th day of treatment, starting 15 min after the i.p. injection, mice were exposed to a novel cage and their spontaneous locomotor activity was analyzed at three 5-min intervals: 0–5; 35–40; 70–75. As expected, the number of crossings decreased as time passed in all the experimental groups (main effect of repeated measures: F(1,62)=46.47; p<0.001). No genotype or treatment effects were found as for this parameter. A different profile was found as for time mice spent inactive (Figure 2f): RTT mice showed longer durations of inactivity compared with wt controls (main effect of genotype: F(1,31)=11.85; p=0.002). Post hoc comparisons on the genotype*repeated measures interaction (F(1,31)=2.45; p=0.095) revealed that such an effect was only evident on the third time interval (p<0.01 after post hoc comparison). A complete restoration of wt-like levels of inactivity on the third time interval in RTT mice was achieved by the LP-211 treatment (wt, veh vs RTT, veh: p<0.01; RTT, veh vs RTT, LP-211: p<0.01, after post hoc comparisons on the genotype*treatment*repeated measures interaction: F(1,31)=3.03; p=0.055; Figure 2f).
A main effect of genotype was also found as for Bar holding (main effect of genotype: F(1,31)=5.26; p=0.029), with RTT mice spending less time engaged in this behavior. The LP-211 treatment did not affect this parameter. No genotype or treatment effects were found as for Rearing and Grooming. None of the mice displayed stereotypic behaviors.
Spontaneous spatial novelty preference task
On the 10th day of the schedule, mice were tested in the spontaneous spatial novelty preference task (Lyon et al, 2011; Sanderson et al, 2007). During the exposure phase, no genotype or treatment effects were found as for latency to leave the start arm, time exploring the ‘other' (to-be-familiar) arm as well as for arm entries (start and other arms combined) (data not shown), thus confirming comparable levels of locomotion and exploration in the four experimental groups. A discrimination ratio was calculated ((novel arm/(novel+other arm)) for time spent in arms during the test phase, and statistical analysis revealed that RTT mice exhibited significantly reduced novelty discrimination than wt controls (p<0.05, after post hoc comparisons on the genotype*treatment interaction: F(1,33)=5.20; p=0.029; Figure 2g). Impaired short-term spatial memory on the exploratory driven, spatial novelty preference task (Sanderson et al, 2007) was thus demonstrated in RTT mice. LP-211 treatment significantly increased novelty preference in both genotypes (main effect of treatment: F(1,33)=11.0; p=0.002; Figure 2g). Such an effect was however much more marked in the RTT genotype, as confirmed by post hoc comparisons on the genotype*treatment interaction (RTT, Veh vs RTT, LP-211: p<0.01; wt, Veh vs wt, LP-211: p>0.10). A wt-like performance in the spatial novelty preference task was thus restored in LP-211-treated RTT mice.
5-HT7 Receptor Expression is Reduced in RTT Mouse Brain
To clarify the relevance of 5-HT7Rs in RTT pathophysiology and to understand the molecular mechanisms underlying LP-211-dependent rescue of RTT symptoms, 5-HT7R localization and density were evaluated in the cortex and hippocampus of RTT mice, two behaviorally relevant brain areas in which the 5-HT7R is highly expressed (Hedlund, 2009).
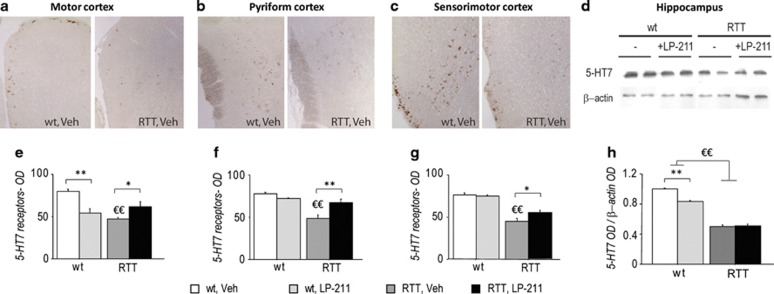
We found that RTT mice were characterized by a lower 5-HT7R density in different cortical regions compared with wt subjects (motor cortex—main effect of genotype: F(1,10)=10.51; p=0.009, Figure 3a and e; pyriform cortex—main effect of genotype: F(1,9)=26.88; p<0.001, Figure 3b and f; sensorimotor cortex—main effect of genotype: F(1,9)=67.24; p<0.001, Figure 3c and g). Reduced 5-HT7 protein expression (∼50% of wt level) was also confirmed by western blot in RTT hippocampus (main effect of genotype: F(1,12)=572.95; p<0.001, Figure 3d and h).
Figure 3.
5-HT7 receptor density is reduced in the brain of RTT mice. (a–c) Representative photomicrographs showing the increase in immunoreactivity in the Motor (a), Pyriform (b), and Sensorimotor (c) cortical brain areas of wild-type (left panels) compared with RTT mice (right panels). All images are magnified equally ( × 20). (d) Examples of western blot analysis (representing a summarized view corresponding to two animals per group) of 5-HT7 and β-actin proteins in hippocampi of RTT and wt mice in control conditions (Veh) or treated with LP-211. (e–g) Histograms illustrate the semiquantitative evaluation of 5 HT7 receptor immunoreactivity in cortical brain areas. Values are expressed as means of optical density (OD) values of the immunoperoxidase labeling. (h) Histogram illustrates the semiquantitative densitometric analysis of western blot analysis in the hippocampus, obtained by OD of protein bands normalized with OD of β-actin bands. OD ratios are expressed as the average fold increase vs wt controls. LP-211 or vehicle were i.p.-administered for 7 consecutive days. Data are mean±SEM. Statistical significance was calculated by two-way ANOVA with Tukey's post hoc test. €, wt; Veh vs RTT, Veh; p<0.05; *, RTT, Veh vs RTT, LP-211, p<0.05, after post hoc comparisons on the genotype*treatment interaction.
Notably, treatment with LP-211 selectively increased 5-HT7 immunoreactivity in RTT cortical regions, thus restoring wt-like levels of receptor density (RTT, Veh vs RTT, LP-211: p<0.05, after post hoc comparisons on the genotype*treatment interactions: motor cortex: F(1,10)=27.58; p<0.001, Figure 3e; pyriform cortex: F(1,9)=13.76; p=0.005, Figure 3f; sensorimotor cortex: F(1,9)=3.18; p=0.108, Figure 3g). Hippocampal levels of the 5-HT7R were not affected by the LP-211 treatment in RTT mice (Figure 3h). By contrast, a significant drug-induced reduction in 5-HT7R density appeared in wt mice in both motor cortex (p<0.01 after post hoc comparison; Figure 3e) and hippocampus (p<0.01 after post hoc comparisons on the genotype*treatment interaction: F(1,12)=27.57; p<0.001; Figure 3h).
LP-211 Treatment Reverses RTT-Related Molecular Alterations in Mouse Brain
We reasoned that basal alterations in 5-HT7R density in RTT brains may result in alterations at the level of Rho GTPases signaling effector molecules and that pharmacological stimulation of the 5-HT7R may recover them.
LP-211 Effects on Molecular Pathways Related to Cytoskeletal Remodeling
Accumulating evidence suggest that two of the leading mechanisms by which Rho GTPases affect actin cytoskeleton dynamics involve phosphorylation and inactivation of both Group I p21-activated kinase family of serine/threonine kinases (PAK1–3) and of cofilin, an actin filament depolymerizing/severing factor (De Filippis et al, in press). In this line, alterations in these signaling pathways have been reported in different disorders associated with intellectual disability (Dolan et al, 2013; Ma et al, 2012).
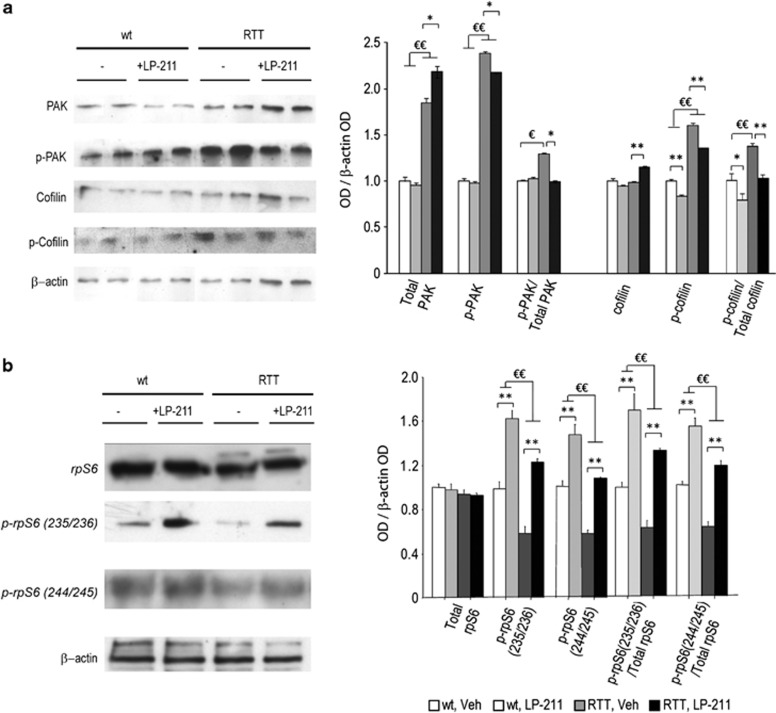
We found that both the amount and the activation of PAK and cofilin were altered in the hippocampus of RTT mice (Figure 4a). In particular, both PAK and its phosphorylated form (p-PAK) were upregulated in RTT mouse brain compared with wt littermates (main effect of genotype: PAK: F(1,12)=121.07; p<0.001; p-PAK: F(1,12)=621.74; p<0.001). Consistently, p-PAK/PAK ratio, which provides an index of the net functionality of the kinase, was shifted toward increased phosphorylation (inactivation) in RTT mouse hippocampus compared with wt controls (p<0.05 after post hoc comparison on the genotype*treatment interaction: F(1,12)=4.64; p=0.052). RTT mouse hippocampi also showed increased cofilin phosphorylation (p-cofilin: main effect of genotype: F (1, 12)=83.77; p<0.001), in the absence of changes in its non-phosphorylated form (cofilin). Increased cofilin inactivation, as demonstrated by increased p-cofilin/total cofilin ratio, was thus evident in the hippocampus of RTT mice (main effect of genotype: F(1,12)=29.56; p<0.001).
Figure 4.
Stimulation of the 5-HT7 receptor reverses RTT-related alterations in PAK, cofilin and rpS6 activity. (a) Left: representative western blot analysis (summarized view corresponding to two animals per group) of PAK, p-PAK, cofilin, p-cofilin and β-actin proteins in hippocampi of RTT and wt mice in control conditions (Veh) or treated with LP-211. Right: semiquantitative densitometric analysis, obtained by optical density (OD) of protein bands normalized with OD of β-actin bands. (b) Left: representative western blot analysis (summarized view corresponding to one animal per group) of rpS6, p-rpS6 Ser 235/236, p-rpS6 Ser 240/244 and β-actin proteins in hippocampi of RTT and wt mice in control conditions (Veh) or treated with LP-211. Right: semiquantitative densitometric analysis, obtained by OD of protein bands normalized with OD of β-actin bands. OD ratios are expressed as the average fold increase vs wt controls. LP-211 or vehicle were i.p.-administered for 7 consecutive days. Data are mean±SEM. Statistical significance was calculated by two-way ANOVA with Tukey's post hoc test. €, wt, Veh vs RTT, Veh; p<0.05; *, RTT, Veh vs RTT, LP-211, p<0.05, after post hoc comparisons on the genotype*treatment interaction.
Restoration of wt-like levels of both PAK and cofilin activity was achieved in LP-211-treated RTT mouse brain. In particular, 5-HT7R activation in RTT mouse brain increased PAK protein levels (RTT, Veh vs RTT, LP-211: p<0.05 after post hoc comparisons on the genotype*treatment interaction: F (1,12)=4.45; p=0.056) and decreased its phosporylated form (p-PAK: p<0.05 after post hoc comparisons on the genotype*treatment interaction: F(1,12)=3.47; p=0.087). These LP-211-induced changes resulted in the normalization of the p-PAK/PAK ratio in RTT mice (RTT, Veh vs RTT, LP-211: p<0.05 after post hoc comparisons on the genotype*treatment interaction: F (1,12)=4.64; p=0.052). A clear-cut normalization of the p-cofilin/total cofilin ratio was also evident in LP-211-treated RTT mouse brain (Figure 4), which showed a slight increase in the non-phosphorylated form and a 25% decrease of p-cofilin compared with vehicle-treated RTT mice (cofilin: RTT, Veh vs RTT, LP-211: p<0.01 after post hoc comparisons on the genotype*treatment interaction: F (1,12)=11.75; p=0.005; p-cofilin: main effect of treatment: F (1,12)=27.77; p<0.001; p-cofilin/total cofilin: main effect of treatment: F(1,12)=24.21; p<0.001).
A different profile was observed in wt mice in which only cofilin phosphorylation was impacted by the LP-211 treatment (Figure 4a). Consistently, the ANOVA yielded a main effect of treatment for both p-cofilin (F(1,12)=27.77; p<0.001) and p-cofilin/total cofilin ratio (F(1,12)=24.21; p<0.001). Present findings are consistent with previous data suggesting that RhoA-cofilin and Rac-PAK constitute two different Rho GTPases-dependent signaling cascades (Rex et al, 2009) and confirm that Rac-PAK signaling pathway is not affected by the 5-HT7R stimulation in wt mice (Kobe et al, 2012; Kvachnina et al, 2005).
LP-211 Effects on Molecular Pathways Related to Protein Synthesis
Based on emerging literature pointing to Rho GTPases as molecular hubs governing both cytoskeletal remodeling and protein synthesis (De Filippis et al, in press), we reasoned that the LP-211-induced activation of Rho GTPases may have restored the well-established defects in AKT/mTOR signaling and protein translation in RTT mouse brain (Ricciardi et al, 2011). To test this hypothesis, we investigated the phosphorylation level of the ribosomal protein (rp) S6, a downstream target of mTOR and S6 kinase, which has a direct role in regulating protein translation (Ricciardi et al, 2011).
Neither genotype nor treatment effects were found as for total rpS6 protein level (Figure 4b). Phosphorylation levels of the rpS6 at both Ser235/236 and Ser240/244 were significantly reduced in the hippocampus of RTT mice (main effect of genotype: p-rpS6(236/238): F(1,12)=41.85; p<0.001; p-rpS6(240/244): F(1,12)=53.07; p<0.001; Figure 4b), thus confirming previous reports (Ricciardi et al, 2011). Consistently, p-rpS6/ total rpS6 ratio, which provides an index of the net functionality of the kinase, was shifted toward decreased phosphorylation (inactivation) in RTT mouse hippocampus compared to wt controls (main effect of genotype: p-rpS6(236/238): F(1,12)=18.20; p<0.001; p-rpS6(240/244): F(1,12)=48.71; p<0.001).
Of note, LP-211 treatment significantly increased the levels of S6 phosphorylation in both genotypes (main effect of treatment: p-rpS6(236/238)/rpS6: F(1,12)=103.89; p<0.001; p-rpS6(240/244) /rpS6: F(1,12)=74.57; p<0.001) and the p-rpS6/ total rpS6 ratio (main effect of treatment: p-rpS6(236/238)/rpS6: F(1,12)=67.71; p<0.001; p-rpS6(240/244)/rpS6: F(1,12)=106.09; p<0.001), thus restoring wt-like levels of S6 phosphorylation in RTT mouse brain (Figure 4b).
DISCUSSION
This study provides the first in vivo evidence that stimulation of the 5-HT7R by systemic administration with LP-211, a novel selective brain-penetrant agonist (Hedlund et al, 2010), does impact Rho GTPases-mediated signaling pathways in the brain and reverses RTT-related behavioral and molecular alterations. The first evidence that 5-HT7 receptor density is consistently reduced in the brain of a RTT mouse model is also provided.
A widespread beneficial effect of the LP-211 treatment on RTT-related symptomatology was evidenced; consistent with previous studies demonstrating that this novel drug acts consistently onto exploration, emotional behaviors and learning and memory (Adriani et al, 2012; Cifariello et al, 2008; Romano et al, 2014; Ruocco et al, 2014a; Ruocco et al, 2014b), we found that systemic treatment with LP-211 restored a wt-like profile in RTT mice in a variety of behavioral paradigms, including the Light/Dark test, a well-established paradigm for the assessment of the anxiety-related profile in rodents, and the Dowel test, which measures motor abilities in mice. Of note, the beneficial effect of LP-211 on motor learning abilities in RTT mice was still evident after a 5-day-long wash-out period, suggesting lasting effects of the treatment. These results are consistent with previous reports demonstrating that a transient increase in Rho GTPases activation by a single administration of CNF1 produces long-lasting behavioral effects (De Filippis et al, 2012; Loizzo et al, 2013). As LP-211-induced improvements in the Dowel test were not paralleled by amelioration in the wire hanging test, present data exclude that improved performance in the Dowel test may be merely due to effects on limb strength.
The performance of RTT mice in the Marble burying test was also restored. Even though the marble burying behavior has been interpreted as a manifestation of stereotypic behavior and/or increased anxiety (Gyertyan, 1995; Thomas et al, 2009), other authors suggested that the number of marbles buried simply provides an indirect measurements of exploratory behavior (ie digging) (Deacon, 2006; Gyertyan, 1995). This latter view is confirmed by our results: decreased marble burying behavior in RTT mice was in fact accompanied by increased anxiety and by the absence of spontaneous stereotypic behavior. Notably, the same behavioral profile shown by RTT mice in this test has been previously reported in 5-HT7 knockout mice (Hedlund and Sutcliffe, 2007), thus providing further support to the role for this receptor in the manifestation of this behavior.
A normalization of spontaneous locomotor activity was also uncovered in LP-211-treated RTT mice exposed to a novel cage. Under these experimental conditions, RTT mice showed a hypoactive phenotype during the third time interval (ie after a 75-min long exposure to the novel cage), which was completely counteracted by the LP-211 treatment. Abnormal habituation to the environment, rather than hypoactivity per se, thus occurred in RTT mice. In addition, as no differences in locomotor activity were uncovered between the four experimental groups during the first two intervals (ie within 40 min of cage exposure), present results exclude that our findings in other behavioral paradigms may have been biased by the effects of the LP-211 treatment on general motor activity.
Of note, the LP-211 treatment also normalized the hyperactive profile RTT mice showed under higher arousal-inducing conditions (ie during the Light/Dark test), thus confirming the anxiolytic properties of the treatment.
An increasing number of studies has demonstrated a role for the 5-HT7R in cognitive processes, particularly on hippocampal-dependent learning and memory (Gasbarri et al, 2008; Roberts and Hedlund, 2012; Sarkisyan and Hedlund, 2009). In this line, we report here that the LP-211 treatment restored the defective performance of RTT mice in the novelty preference task, a hippocampal-dependent test assessing rapidly acquired, short-term spatial memory in a non-aversive experimental context (Lyon et al, 2011; Sanderson et al, 2007). At this stage, however, we cannot completely rule out the possibility that the anxiolytic properties of the drug may have to some extent contributed to the improved performance of RTT mice in the novelty preference test. Further studies are needed to clarify this issue.
As a whole, present findings demonstrate that the LP-211 treatment exerts beneficial effects on different RTT-related behavioral alterations, thus suggesting that targeting this receptor subpopulation may represent a novel therapeutic strategy for RTT. The 7-day long treatment we adopted in our study did not however affect general health parameters such as gait, fur, or kyphosis. Future studies are needed to evaluate whether longer periods of treatment may impact these gross phenotypic abnormalities in RTT mice. Given the role of stereotyped behavior in RTT, we would have been interested in verifying whether the LP-211 treatment may modulate this pervasive symptom (Hedlund and Sutcliffe, 2007). We did not however observe any spontaneous stereotypic behavior in MeCP2–308 mice, thus confirming previous reports (Ricceri et al, 2008). Further studies are thus needed to test this hypothesis.
The LP-211 treatment also restored in RTT mouse hippocampus the alterations in the amount and the activation of PAK and cofilin, two Rho GTPases effector molecules that are essential for both spine formation during early LTP and synaptic potentiation (Kramar et al, 2006; Rex et al, 2009; Wosiski-Kuhn and Stranahan, 2012). Even though further studies are needed to understand how mutations in the MeCP2 gene lead to the observed alterations in 5-HT7 receptor density and PAK and cofilin activity, present results bring into light yet unknown RTT-related molecular alterations in signaling pathways critically involved in activity-dependent spine formation, synaptic potentiation (Rex et al, 2009; Wosiski-Kuhn and Stranahan, 2012) and regulation of learning and memory abilities (Liston et al, 2013; Meng et al, 2003; Rust et al, 2010).
Notably, LP-211 also rescued in RTT mouse hippocampus the hypophosphorylation of the ribosomal protein (rp) S6, a downstream target of mTOR and S6 kinase, whose reduced mTOR-mediated activation in MECP2 mutant neurons has been suggested to be responsible for the altered protein translational control (Ricciardi et al, 2011). Such results are particularly interesting in light of a recent study demonstrating that the normalization of Akt/mTOR signaling, and their downstream target rpS6, in an in vitro RTT human model is sufficient to rescue disease-related cellular impairments caused by MECP2 mutation (Li et al, 2013).
As a whole, present findings indicate that pharmacologically targeting 5-HT7Rs improves specific behavioral and molecular manifestations of RTT. Of note, such beneficial effects were accompanied by a normalization of 5-HT7R receptor density in RTT mouse brain, which was increased to wt-like levels by the LP-211 treatment.
Increased receptor availability following the repeated stimulation of the receptor was indeed an unexpected finding. Such surprising effect was however exerted by the LP-211 treatment only under dysfunctional baseline conditions (ie, in RTT mouse brain), as the chronic stimulation of the receptor with the agonist slightly, but significantly, decreased 5-HT7R in wt mice, as expected. The assays we used to determine the level of the receptor in brain areas do exclude the possibility that changes in internalization or dimerization levels of the receptor may account for the changes we evidenced in its expression, since we always measured the total levels. Based on the results presented here, demonstrating that LP-211 is able to normalize signaling pathways critically involved in protein translation in RTT mouse brain, we are tempted to speculate that improved LP-211-induced protein synthesis may account for the observed increase in 5-HT7R expression in RTT mouse brain. Of note, however, a marked increase in rpS6 phosphorylation was also found as for wt mice, which was accompanied by a decrease in 5-HT7R expression. We cannot therefore exclude that other mechanisms might have contributed to determine such effects on 5-HT7R density (eg, at the level of gene expression, which is known to be disrupted by mutations in the MeCP2 gene). Further studies will be needed to clarify this issue.
As a whole, present data demonstrate that stimulation of the 5-HT7 receptor does impact signaling pathways critically involved in the regulation of activity-dependent cytoskeletal remodeling and protein synthesis, two biological processes that have key roles in several intellectual disability disorders (Ba et al, 2013; D'Antoni et al, 2014; Ramakers, 2002). In this line, accumulating evidence suggest that targeting downstream effectors of Rho GTPases, such as the well-established Group I PAKs (Dolan et al, 2013; Ma et al, 2012) and the emerging mTOR/ S6 kinase (Bhattacharya et al, 2012), may represent a novel therapeutic strategy for different disorders associated with cognitive dysfunction (De Filippis et al, in press). The potential for such a therapeutic strategy is further highlighted by recent studies demonstrating that activation of brain Rho GTPases by CNF1 rescues the neurobehavioral abnormalities of two disorders associated with cognitive dysfunction (De Filippis et al, 2012; Loizzo et al, 2013). Of note, the therapeutic efficacy of these novel strategies extended beyond cognitive deficits and impacted other domains, such as motor coordination and emotional profiles (De Filippis et al, 2012; Dolan et al, 2013; Loizzo et al, 2013).
Our results lie in the same direction and suggest that a therapeutic strategy based on targeting the brain 5-HT7R may prove valid also for other disorders characterized by mental disability (De Filippis et al, in press). In this line, activation of 5-HT7Rs reversed electrophysiological abnormalities in hippocampal slices from a mouse model of Fragile X syndrome (Costa et al, 2013). Whether 5-HT7-induced restoration of PAK activity and/or mTOR/ S6 kinase signaling, which are altered in Fragile X mouse brain (Bhattacharya et al, 2012; Chen et al, 2010; Dolan et al, 2013; Hayashi et al, 2007; Maurin et al, 2014), had a role in this reversal remains to be elucidated.
In conclusion, present results deepen our knowledge on the molecular bases of RTT pathology and provide the first evidence that pharmacologically targeting 5-HT7Rs in the brain by a selective agonist improves specific behavioral and molecular manifestations of RTT. Moreover, LP-211 treatment was shown to activate brain RhoGTPases mechanisms and to rescue the abnormal activation of the Rho GTPases effectors, PAK, cofilin, and rpS6, in RTT mouse brain. Given the involvement of Rho GTPases and their effector molecules in many pathological conditions (De Filippis et al, in press; Ramakers, 2002), and the paucity of drugs targeting these signaling cascades in vivo, present findings represent the first step toward the validation of a innovative systemic treatment that might be extended also to other disorders associated with intellectual disabilities.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Emilia Romano, Sophie Cappuyns, and Mattia Musto for technical assistance and to Luigia Cancemi and Giovanni Dominici for animal care. This research was supported by AIRETT (Italy) and IRE-IFO (RF2008) ‘MECP2 phosphorylation and related kinase in Rett syndrome' to GL. BDF received support also from the Jerome Lejeune Foundation (France).
References
- Adriani W, Travaglini D, Lacivita E, Saso L, Leopoldo M, Laviola G. Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology. 2012;62:833–842. doi: 10.1016/j.neuropharm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Auer M, Hausott B, Klimaschewski L. Rho GTPases as regulators of morphological neuroplasticity. Ann Anat. 2011;193:259–266. doi: 10.1016/j.aanat.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba W, van der Raadt J, Nadif Kasri N. Rho GTPase signaling at the synapse: Implications for intellectual disability. Exp Cell Res. 2013;319:2368–2374. doi: 10.1016/j.yexcr.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76:325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30:10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifariello A, Pompili A, Gasbarri A. 5-HT(7) receptors in the modulation of cognitive processes. Behav Brain Res. 2008;195:171–179. doi: 10.1016/j.bbr.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Costa L, Spatuzza M, D'Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, et al. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry. 2013;72:924–933. doi: 10.1016/j.biopsych.2012.06.008. [DOI] [PubMed] [Google Scholar]
- D'Antoni S, Spatuzza M, Bonaccorso CM, Musumeci SA, Ciranna L, Nicoletti F, et al. 2014Dysregulation of group-I metabotropic glutamat (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism Neurosci Biobehav Rev(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- De Filippis B, Fabbri A, Simone D, Canese R, Ricceri L, Malchiodi-Albedi F, et al. Modulation of RhoGTPases improves the behavioral phenotype and reverses astrocytic deficits in a mouse model of Rett syndrome. Neuropsychopharmacology. 2012;37:1152–1163. doi: 10.1038/npp.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B, Ricceri L, Laviola G. Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 2010;9:213–223. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- De Filippis B, Romano E, Laviola G.Aberrant Rho GTPases signaling and cognitive dysfunction: in vivo evidence for a compelling molecular relationship Neurosci Biobehav Rev(in press). [DOI] [PubMed]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Diana G, Valentini G, Travaglione S, Falzano L, Pieri M, Zona C, et al. Enhancement of learning and memory after activation of cerebral Rho GTPases. Proc Natl Acad Sci USA. 2007;104:636–641. doi: 10.1073/pnas.0610059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci USA. 2013;110:5671–5676. doi: 10.1073/pnas.1219383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Cifariello A, Pompili A, Meneses A. Effect of 5-HT(7) antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav Brain Res. 2008;195:164–170. doi: 10.1016/j.bbr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyan I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995;6:24–31. [PubMed] [Google Scholar]
- Hagberg B. Clinical manifestations and stages of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Leopoldo M, Caccia S, Sarkisyan G, Fracasso C, Martelli G, et al. LP-211 is a brain penetrant selective agonist for the serotonin 5-HT(7) receptor. Neurosci Lett. 2010;481:12–16. doi: 10.1016/j.neulet.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett. 2007;414:247–251. doi: 10.1016/j.neulet.2006.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SM, Vykoukal J, Lechler P, Zeitler K, Gehmert S, Schreml S, et al. Noninvasive in vivo assessment of muscle impairment in the mdx mouse model—a comparison of two common wire hanging methods with two different results. J Neurosci Methods. 2012;203:292–297. doi: 10.1016/j.jneumeth.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kobe F, Guseva D, Jensen TP, Wirth A, Renner U, Hess D, et al. 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J Neurosci. 2012;32:2915–2930. doi: 10.1523/JNEUROSCI.2765-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, et al. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5-HT7 receptor agents: structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther. 2011;129:120–148. doi: 10.1016/j.pharmthera.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, De Giorgio P, Fracasso C, Guzzetti S, Caccia S, et al. Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J Med Chem. 2008;51:5813–5822. doi: 10.1021/jm800615e. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Muffat J, Cheng AW, Orlando DA, Loven J, et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons. Cell Stem Cell. 2013;13:446–458. doi: 10.1016/j.stem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzo S, Rimondini R, Travaglione S, Fabbri A, Guidotti M, Ferri A, et al. CNF1 increases brain energy level, counteracts neuroinflammatory markers and rescues cognitive deficits in a murine model of Alzheimer's disease. PLoS One. 2013;8:e65898. doi: 10.1371/journal.pone.0065898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon L, Burnet PW, Kew JN, Corti C, Rawlins JN, Lane T, et al. Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology. 2011;36:2616–2628. doi: 10.1038/npp.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Frautschy SA, Cole GM. PAK in Alzheimer disease, Huntington disease and X-linked mental retardation. Cell Logist. 2012;2:117–125. doi: 10.4161/cl.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys A, Haegeman G, Van Craenenbroeck K, Vanhoenacker P. Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol Neurobiol. 2011;43:228–253. doi: 10.1007/s12035-011-8175-3. [DOI] [PubMed] [Google Scholar]
- Maurin T, Zongaro S, Bardoni B.2014Fragile X Syndrome: from molecular pathology to therapy Neurosci Biobehav Rev(e-pub ahead of print). [DOI] [PubMed]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Nativio P, Pascale E, Maffei A, Scaccianoce S, Passarelli F. Effect of stress on hippocampal nociceptin expression in the rat. Stress. 2012;15:378–384. doi: 10.3109/10253890.2011.627071. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol. 2008;19:501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. Rett syndrome treatment in mouse models: searching for effective targets and strategies. Neuropharmacology. 2012;68:106–115. doi: 10.1016/j.neuropharm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Ricciardi S, Boggio EM, Grosso S, Lonetti G, Forlani G, Stefanelli G, et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet. 2011;20:1182–1196. doi: 10.1093/hmg/ddq563. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Hedlund PB. The 5-HT(7) receptor in learning and memory. Hippocampus. 2012;22:762–771. doi: 10.1002/hipo.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Ruocco LA, Nativio P, Lacivita E, Ajmone-Cat MA, Boatto G, et al. 2014Modulatory effects following subchronic stimulation of brain 5-HT7-R system in mice and rats Rev Neurosci(e-pub ahead of print). [DOI] [PubMed]
- Ruocco LA, Romano E, Treno C, Lacivita E, Claudio A, Gironi-Carnevale UA, et al. Emotional and risk seeking behavior after prepuberal subchronic or adult acute stimulation of 5-HT7-Rs in naples high excitability rats. Synapse. 2014;68:159–167. doi: 10.1002/syn.21724. [DOI] [PubMed] [Google Scholar]
- Ruocco LA, Treno C, Gironi-Carnevale UA, Arra C, Boatto G, Nieddu M, et al. Prepuberal stimulation of 5-HT7-R by LP-211 in a rat model of hyper-activity and attention-deficit: permanent effects on attention, brain amino acids and synaptic markers in the fronto-striatal interface. PLoS One. 2014;9:e83003. doi: 10.1371/journal.pone.0083003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Gorlich A, et al. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RM, Seeburg PH, et al. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Sarkisyan G, Hedlund PB. The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res. 2009;202:26–31. doi: 10.1016/j.bbr.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen EJ, Ramakers GJ. Rho proteins, mental retardation and the neurobiological basis of intelligence. Prog Brain Res. 2005;147:295–317. doi: 10.1016/S0079-6123(04)47022-8. [DOI] [PubMed] [Google Scholar]
- Wosiski-Kuhn M, Stranahan AM. Transient increases in dendritic spine density contribute to dentate gyrus long-term potentiation. Synapse. 2012;66:661–664. doi: 10.1002/syn.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]