Abstract
Selective serotonin reuptake inhibitors (SSRIs) are frequently prescribed to pregnant women. Therefore, research on in utero exposure to SSRIs can be helpful in informing patients and clinicians. The aim of this retrospective two-cohort study was to determine whether there is a statistically significant increase in Chiari I malformations (CIM) in children exposed to SSRIs during pregnancy. A total of 33 children whose mothers received a diagnosis of depression and took SSRIs during pregnancy (SSRI-exposed cohort) were matched to 66 children with no history of maternal depression and no SSRI exposure. In addition, 30 children whose mothers received a diagnosis of depression, but did not receive antidepressants during pregnancy (history of maternal depression cohort), were matched to 60 children with no history of maternal depression and no SSRI exposure. Main outcome was presence/absence of CIM on MRI scans at 1 and/or 2 years of age. Scans were reviewed by two independent neuroradiologists who were blind to exposure status. The SSRI-exposed children were significantly more likely to be classified as CIM than comparison children with no history of maternal depression and no SSRI exposure (18% vs 2%, p=0.003, OR estimate 10.32, 95% Wald confidence limits 2.04–102.46). Duration of SSRI exposure, SSRI exposure at conception, and family history of depression increased the risk. The history of maternal depression cohort did not differ from comparison children with no history of maternal depression and no SSRI exposure in occurrence of CIM (7% vs 5%, p=0.75, OR estimate 1.44, 95% Wald confidence limits 0.23–7.85). Replication is needed, as is additional research to clarify whether SSRIs directly impact risk for CIM or whether this relationship is mediated by severity of depressive symptoms during pregnancy. We would discourage clinicians from altering their prescribing practices until such research is available.
INTRODUCTION
Approximately 6–12% of pregnant women suffer from depressive disorders (Bennett et al, 2004; Gavin et al, 2005; O'Hara and Wisner, 2014). Untreated depression during pregnancy is associated with risks for mothers and babies. Women with antenatal depression are at risk for interpersonal isolation and suicide, are less likely to receive adequate nutrition and obstetrical care, and are more likely to engage in smoking and substance abuse (Marcus and Heringhausen, 2009; Zuckerman et al, 1989). In infants, active depressive symptoms during pregnancy are associated with shorter length of gestation, restricted fetal growth, and lower birth weight (Davalos et al, 2012; Fransson et al, 2011; Grote et al, 2010). Gestational depression is one of the strongest predictors of postpartum depression (Dietz et al, 2007; Gaynes et al, 2005; Milgrom et al, 2008), which is associated with decreased maternal sensitivity and attachment (Campbell et al, 2004; Lindahl et al, 2005; McLearn et al, 2006; Paulson et al, 2006). Thus, antidepressants are frequently prescribed to pregnant women. Recent estimates suggest that >10% of pregnant women fill prescriptions for selective serotonin reuptake inhibitors (SSRIs) (Cooper et al, 2007). Research on prenatal SSRI effects on child health has yielded conflicting results (Byatt et al, 2013; Hanley and Oberlander, 2014; Ross et al, 2013; Yonkers et al, 2009), and is scarce regarding brain development (Malm et al, 2012; Nulman et al, 2012; Oberlander, 2012). To address this gap, our group is performing a prospective longitudinal neuroimaging study of children exposed to SSRIs during pregnancy.
In November 2011, we performed a preliminary analysis of SSRI effects on neonatal brain volume. We combined our data with data from two large neuroimaging studies at the University of North Carolina at Chapel Hill (UNC), one of which focuses primarily on typically developing singletons and the other focuses on twins. All three studies collect MRI scans at 2 weeks, 1 year, and 2 years of age and all scans are reviewed by a neuroradiologist for incidental findings after acquisition. During review of these neuroradiology reports, we noticed that 3 of 15 children (20%) with prenatal SSRI exposure who had received a follow-up scan at 1 and/or 2 years of age were identified as having Chiari I malformation (CIM), a condition in which the cerebellar tonsils extend significantly below the foramen magnum.
CIM is thought to result from underdevelopment of the posterior cranial fossa and overcrowding of the normally developing hindbrain (Aquilina et al, 2009). It is not present at birth, but emerges postnatally (Milhorat et al, 1999). In the first decade of life, a tonsillar herniation of −6 mm exceeds the normal range. The cerebellar tonsils ascend with age such that −5 mm is considered abnormal in the second and third decade and −4 mm is abnormal thereafter (Mikulis et al, 1992). In the past, it was estimated that CIM occurred in ∼1 in every 1000 individuals (Meadows et al, 2000). However, the increased use of diagnostic imaging suggests CIM is much more common. A retrospective review of 14 116 children undergoing MR imaging of the brain or cervical spine for any indication reported a 3.6% rate of CIM (Strahle et al, 2011a). Another study by the same group found a slightly higher prevalence for the same age group (∼5%) (Smith et al, 2013). Although a majority of CIM are asymptomatic, CIM increases risk for multiple neurological phenomena including headaches, ocular and otoneurological disturbances, lower cranial nerve signs, hydrocephalus, and spinal cord syrinx (Di Rocco et al, 2011; Milhorat et al, 1999). In symptomatic cases, surgical intervention may be required. The proportion of CIM cases that are symptomatic is unknown, but the retrospective review described above reported that 32% of CIM cases were considered symptomatic by the treating physician, 23% had spinal cord syrinx, and 35% were treated surgically (Strahle et al, 2011a). Strahle et al (2011a) do not report the extent of overlap between these outcomes, but it is likely to be high. Spontaneous resolution is rare, but has been reported (Miller et al, 2008; Novegno et al, 2008).
To determine whether there is a statistically significant increase in CIM in children with prenatal SSRI exposure, we identified all children in the three UNC studies with scans at 1 and/or 2 years of age whose mothers took SSRIs during pregnancy and who had received a diagnosis of depression before or during pregnancy. This included the 15 children with prenatal SSRI exposure evaluated in our preliminary study. We then performed the following analyses. Serotonin plays an important role in craniofacial development (Moiseiwitsch, 2000). Therefore, we predicted that CIM would be elevated in exposed children.
MATERIALS AND METHODS
Recruitment
Subjects for the current study were drawn from three ongoing neuroimaging studies at UNC, one of which focuses on children exposed to SSRIs during pregnancy (parent study 1), the second focuses primarily on typically developing singletons (parent study 2), and the third focuses on typically developing twins (parent study 3). All three studies use volunteer samples. Parent study 1 is the only study to specifically target mothers with depression and SSRI use during pregnancy. Recruitment takes place through community physicians and relevant clinics at UNC including the perinatal psychiatry clinic and general obstetrics clinics, and mass emails to the UNC community. Parent studies 2 and 3 are open to women with depression with or without SSRI treatment and women with no psychiatric history. Recruitment also takes place through general obstetrics clinics at UNC and other local hospitals, and mass emails to the UNC community. Mothers join during pregnancy or shortly after giving birth. Exclusion criteria in the mother were major medical illness or substance abuse during pregnancy. Use of a psychiatric drug other than an SSRI was an exclusion criterion for the current analysis with the exception of trazodone, low-dose benzodiazepines, and psychostimulants. Exclusion criteria for children were gestational age at birth <32 weeks, major postnatal complications, major congenital anomalies, and metal in the body. Written informed consent was obtained from the subjects' parent(s). Study protocols were approved by the Institutional Review Board of the UNC School of Medicine. Full details on inclusion/exclusion criteria for the parent studies and a flowchart showing how subjects were selected for the current analysis are found in Supplementary Figure S1.
Primary Analyses Cohort 1 (SSRI Exposed)
We identified 33 children (20 male; 13 singletons, 10 twin pairs) with scans at 1 and/or 2 years of age whose mothers took SSRIs during pregnancy (confirmed by maternal self-report and medical record review) and who had received a diagnosis of depression (self-report or medical record review) before or during pregnancy. Self-report was in response to an oral interview. Documents used in the oral interview are available as Supplementary Materials (Maternal Psych History V1, V2, and V3). Medical records included prenatal records and labor and delivery records. Twenty mothers reported that they received a diagnosis of depression before study entry; medical records provided corroborating information in all 20 mothers. Of these women, 15 reported active depression at entry. The other five women did not report active depression at entry. However, the parent studies did not collect direct measures of depressive symptoms and medical records did not include sufficient information to determine whether any of these women developed symptoms after the initial interview. Eight women did not report a diagnosis of depression at entry, but review of medical records indicated such a diagnosis was made before or during pregnancy. Medical records were not sufficiently detailed to determine the exact date of diagnosis. See Supplementary Figure S2 for a graphic breakdown of these categories. The most common diagnoses were major depression disorder (MDD) and depressive disorder not otherwise specified; 1 woman had bipolar disorder (BPD). Sertraline was the most commonly used SSRI (25 children) followed by fluoxetine (4 children), citalopram (3 children), and paroxetine (2 children, one of whom was also exposed to fluoxetine). Reported doses ranged from 25 to 200 mg for sertraline, 20 to 60 mg for fluoxetine, and 20 to 40 mg for citalopram. For paroxetine, 20 mg was the only reported dose. Seven children were exposed to trazodone, 4 to benzodiazepines, and 1 to Adderall, in addition to an SSRI. Reported duration of treatment ranged from 3 weeks to throughout pregnancy. Eleven children had longitudinal scans; 17 only had scans at 1 year, and 5 had scans only at 2 years. Each SSRI-exposed child was matched to two comparison children using propensity scores on potential confounding variables including date of birth, gender, twin status, maternal ethnicity, maternal age, maternal education, and household income. Comparison children came from parent study 2 or 3 (Supplementary Figure S1). The SSRI-exposed children and comparison children were also matched on number of scans and year at scan (1 year or 2 years). Mothers of comparison children did not take SSRIs during pregnancy (confirmed by self-report and medical record review). One comparison mother had a history of anxiety. Other comparison mothers had no psychiatric history (self-report).
Primary Analyses Cohort 2 (History of Maternal Depression)
To test the possibility that history of maternal depression increases risk for CIM in the absence of prenatal SSRI exposure, we identified 30 children (18 male; 12 singletons, 8 twin pairs, 2 unpaired twins) whose mothers did not receive antidepressants during pregnancy (self-report and medical record review), but who received a diagnosis of depression before or during pregnancy. This cohort may potentially share with the SSRI-exposed cohort a generally higher risk of unidentified behaviors (eg, unreported smoking, substance misuse, exposure to other teratogens) as well as genetic factors that could increase risk for CIM. Fourteen mothers reported that they received a diagnosis of depression before study entry; medical records provided corroborating information in 12 mothers. Two of these women reported active depression at entry. The other 10 women did not report active depression at entry. Eight women did not report a diagnosis of depression at study entry, but review of medical records indicated such a diagnosis was made before or during pregnancy. Medical records were not sufficiently detailed to determine the exact date of diagnosis. See Supplementary Figure S2 for a graphic breakdown of these categories. The most common diagnoses were MDD and depressive disorder not otherwise specified. One woman had a past diagnosis of postpartum depression with no subsequent issues. Fifteen children had longitudinal scans; 9 had scans only at 1 year and 6 had scans only at 2 years. One woman took Adderall during her pregnancy. Matching was carried out as before. We allowed overlap between the two comparison groups to ensure the best possible matches were made for each individual; 17 comparison children overlapped.
Descriptive Data
Descriptive data included demographic variables (maternal age, maternal education, total household income, maternal ethnicity, infant sex) and clinical characteristics (gestational age at birth, birth length, duration of stay in neonatal intensive care unit, hypertension during pregnancy, infection during pregnancy, gestational diabetes, and prepregnancy BMI). Clinical characteristics were collected from prenatal records, labor and delivery records, and pediatric records with the exception of infection during pregnancy that was collected via maternal report. Data on nonpsychiatric medication usage, as well as smoking and alcohol intake during pregnancy, were collected via maternal report and review of medical records. Documents used in oral interviews are available as Supplementary Materials (Maternal Medication History and Infection History). See Table 1 for demographic and clinical data. See Supplementary Tables S1 and S2 for nonpsychiatric medication usage, smoking, and alcohol intake. No mothers reported use of illicit substances during pregnancy and medical records did not report signs of prenatal exposure to illicit substances in any participating infants.
Table 1. Comparison of Demographic and Medical History Data between Exposed Children and Matched Comparisons with No History of Maternal Depression and No SSRI Exposure.
| Variable |
Matched comparisons |
SSRI exposed |
P-value | Test statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | N | Mean | SD | Range | N | |||
| Maternal age (years) | 30 | 4 | 19–39 | 66 | 30 | 6 | 20–40 | 33 | 0.99 | 1648.0 |
| Maternal education (years) | 15 | 3 | 6–22 | 66 | 16 | 3 | 12–24 | 33 | 0.35 | 1526.0 |
| Total household income ($) | 88 476 | 56 267 | 214–250 000 | 63 | 73 617 | 59 070 | 3120–260 000 | 32 | 0.15 | 1354.0 |
| Gestational age at birth (days) | 273 | 12 | 239–295 | 66 | 260 | 15 | 229–287 | 33 | <0.001 | 1160.5 |
| Birth length (cm) | 50.4 | 2.4 | 44.0–56.0 | 58 | 47.5 | 3.9 | 34.5–54.0 | 33 | <0.001 | 1080.5 |
| Duration of stay in NICU (days) | <1 | 2 | 0–12 | 66 | 3 | 7 | 0–26 | 33 | 0.003 | 1849.5 |
| Prepregnancy BMI | 26.5 | 7.5 | 18.1–46.9 | 60 | 28.6 | 7.0 | 20.4–48.7 | 27 | 0.05 | 1399.0 |
| No. | % | No. | % | |||||||
| Sex | Male | 19 | 29% | 66 | Male | 13 | 40% | 33 | 0.36 | |
| Female | 47 | 71% | Female | 20 | 60% | |||||
| Maternal ethnicity | Black | 12 | 18% | 66 | Black | 7 | 21% | 33 | 0.79 | |
| White | 54 | 82% | White | 26 | 79% | |||||
| Hypertension in pregnancy | Yes | 3 | 5% | 66 | Yes | 7 | 21% | 33 | 0.01 | |
| No | 63 | 95% | No | 26 | 79% | |||||
| Infection in pregnancy | Yes | 24 | 40% | 60 | Yes | 16 | 48% | 33 | 0.51 | |
| No | 36 | 60% | No | 17 | 52% | |||||
| Gestational diabetes | Yes | 4 | 6% | 66 | Yes | 2 | 6% | 33 | 1.00 | |
| |
No |
62 |
94% |
|
No |
31 |
94% |
|
|
|
|
Matched comparisons |
History of maternal depression No treatment in pregnancy |
|||||||||
| |
Mean |
SD |
Range |
N |
Mean |
SD |
Range |
N |
|
|
| Maternal age (years) | 29 | 4 | 19–42 | 60 | 29 | 5 | 18–37 | 30 | 0.32 | 1482.0 |
| Maternal education (years) | 15 | 4 | 3–22 | 60 | 15 | 4 | 8–24 | 30 | 0.76 | 1401.0 |
| Total household income ($) | 59 544 | 40 476 | 214–195 000 | 56 | 78 513 | 57 887 | 0–250 000 | 30 | 0.21 | 1443.0 |
| Gestational age at birth (days) | 263 | 17 | 225–287 | 60 | 257 | 21 | 211–288 | 30 | 0.11 | 1179.5 |
| Birth length (cm) | 48.3 | 3.0 | 40.0–53.5 | 52 | 47.7 | 3.4 | 40.0–52.5 | 30 | 0.47 | 1169.0 |
| Duration of stay in NICU (days) | 2 | 5 | 0–20 | 60 | 4 | 9 | 0–33 | 30 | 0.12 | 1486.0 |
| Prepregnancy BMI | 27.3 | 7.6 | 18.9–65.5 | 56 | 29.4 | 9.8 | 19.0–57.2 | 26 | 0.62 | 1129.5 |
| No. | % | No. | % | |||||||
| Sex | Male | 32 | 53% | 60 | Male | 18 | 60% | 30 | 0.65 | |
| Female | 28 | 47% | Female | 12 | 40% | |||||
| Maternal ethnicity | Black | 15 | 25% | 60 | Black | 3 | 10% | 30 | 0.10 | |
| White | 42 | 70% | White | 27 | 90% | |||||
| Asian | 3 | 5% | Asian | 0 | 0% | |||||
| Hypertension in pregnancy | Yes | 4 | 7% | 60 | Yes | 8 | 27% | 30 | 0.02 | |
| No | 56 | 93% | No | 22 | 73% | |||||
| Infection in pregnancy | Yes | 19 | 34% | 56 | Yes | 16 | 57% | 28 | 0.06 | |
| No | 37 | 66% | No | 12 | 42% | |||||
| Gestational diabetes | Yes | 3 | 5% | 60 | Yes | 7 | 23% | 30 | 0.01 | |
| No | 57 | 95% | No | 23 | 77% | |||||
Outcome Data
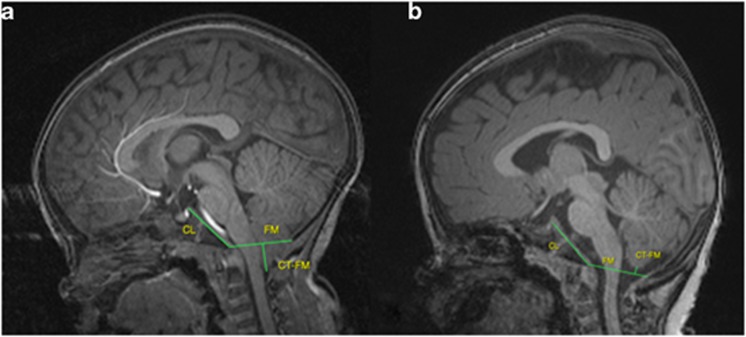
Children received structural MRI scans on a Siemens head-only 3T scanner with MP-RAGE T1-weighted, and TSE, dual-echo (proton density and T2 weighted) sequences. Scans were reviewed by two independent neuroradiologists who were blind to exposure status. They measured the distance between the most inferior part of the cerebellar tonsils and the foramen magnum (CT_FM_Distance, negative values indicate tonsillar herniation), clival length, and foramen magnum anteroposterior diameter (FM_AP_Diameter) (Figure 1). Shorter clival length and larger FM_AP_Diameter are found in individuals with CIM (Aquilina et al, 2009; Bliesener and Schmidt, 1980). Interrater reliability was 0.86/0.87 for CT_FM_Distance, 0.84/0.79 for clival length, and 0.54/0.69 for FM_AP_Diameter (1yr/2yr). Individuals were classified as CIM if tonsillar herniation was ≥−6 mm. In four subjects, classification as CIM differed by rater. These subjects were re-reviewed and a consensus CT_FM_Distance and diagnosis agreed upon. One subject with longitudinal data met criteria for CIM at year 2, but not at year 1; this subject was classified as CIM based on the more recent scan. In two subjects (each with only a single scan), motion prevented quantitative measures, but scans were sufficient to rule out CIM. For two other subjects (both with longitudinal scans), scans at 2 years were not of sufficient quality to rule out CIM; 1-year scans were used to classify these subjects. Note that for quantitative outcomes (CT_FM_Distance, clival length, and FM_AP_Diameter), both longitudinal scans were used unless motion prevented measurement.
Figure 1.
Measurement of CT_FM_Distance, clival length, and FM_AP_Diameter. (a) Measures on a case with tonsillar herniation who would be classified as CIM. (b) Measures on a case without tonsillar herniation.
Although this study focused on CIM, the neuroradiologists noted when other abnormalities were present. Two children had an arachnoid cyst. One child had a large superior cerebellar cistern. Two children had a pineal cyst. One child had a dilated left occipital horn. All children with non-CIM anomalies were comparison children with no SSRI exposure or maternal history of depression. None of the children with CIM had an additional non-CIM abnormality.
Secondary Analyses
We performed secondary analyses in cohort 1 to examine effects of duration and timing of exposure as well as family history of depression. For duration, we subdivided the group of SSRI-exposed children into those who were exposed to an SSRI in three trimesters (N=19) and those who were exposed to an SSRI in <3 trimesters (N=13). In one child, duration of exposure could not be determined. For timing, we subdivided the group of SSRI-exposed children into those who were exposed to an SSRI at conception (N=15) and those who were not exposed to an SSRI at conception (N=15). In three children we could not determine whether the mother was taking an SSRI at conception. Two children (a set of twins) were conceived via in vitro fertilization (IVF). Their mother was taking an SSRI on the date of IVF transfer and these children are included in the exposed at conception subgroup. For family history we subdivided the group of SSRI-exposed children into those whose mothers reported a history of MDD or BPD in her first-degree relatives (N=11) and those whose mothers reported no history of MDD or BPD in her first-degree relatives (N=22). We did not test for differences in quantitative variables in these analyses because of small sample sizes when data are split by age.
Statistical Methods
Statistical analyses were performed using SAS, version 9.2. Cochran–Mantel–Haenzel statistics were used to test for group differences in occurrence of CIM. Logistic regression with Firth's penalized maximum likelihood estimation method was used to estimate odd ratios (ORs). No covariates were included as children had already been matched on potential confounding variables using propensity scores. Two-sided nonparametric Mann–Whitney–Wilcoxon tests were used to compare groups on continuous outcomes, stratified by age at scan. For descriptive data, two-sided nonparametric Mann–Whitney–Wilcoxon tests were used to compare groups on continuous variables and two-sided Fisher's exact tests were used to compare groups on categorical variables.
RESULTS
Primary Analyses Cohort 1 (SSRI Exposed)
Children exposed to SSRIs during pregnancy were significantly more likely to meet criteria for CIM than matched comparison children (Table 2, Cochran–Mantel–Haenzel test statistic=8.64, p=0.003, logistic regression OR estimate 10.32, 95% Wald confidence limits 2.04–102.46). As the cutoff of −6 mm is widely, but not universally, accepted, we tested whether this relationship remained significant when the cutoff was moved to −5 mm or −7 mm; it did. We did not observe a significant difference in average CT_FM_Distance, clival length, or FM_AP_Diameter. The group difference in CT_FM_Distance as a percent of the mean was striking: 87% at 1 year and 125% at 2 years; this equates to an absolute difference of 1.3 mm at 1 year and 1.5 mm at 2 years. Comparisons for clival length were marginally nonsignificant at 1 year (Mann–Whitney–Wilcoxon test statistic=988.5, p=0.07) and 2 years (Mann–Whitney–Wilcoxon test statistic=319.0, p=0.09). All three measures were in the predicted direction of increased risk for CIM in children with prenatal SSRI exposure (Table 3).
Table 2. Proportion of CIM in Exposed Children and Matched Comparisons with No History of Maternal Depression and No SSRI Exposure.
|
SSRI exposed |
Matched comparisonsa |
Statistics |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Test statistic | P-value | OR | Confidence limits | |
| CIM | 6 | 18% | 1 | 2% | 8.64 | 0.003 | 10.32 | 2.04–102.46 |
| No CIM |
27 |
82% |
65 |
98% |
|
|
|
|
|
History of maternal depression No treatment in pregnancy |
Matched comparisons |
Statistics |
||||||
| |
No. |
% |
No. |
% |
Test statistic |
P-value |
OR |
Confidence limits |
| CIM | 2 | 7% | 3 | 5% | 0.10 | 0.75 | 1.44 | 0.23–7.85 |
| No CIM | 28 | 93% | 57 | 95% | ||||
Taking account of overlap, there were 109 children with no history of maternal depression and no SSRI exposure who underwent blinded neuroradiology review, with three classified as CIM for a 3% rate of CIM in unexposed children that is similar to the rate of CIM in children undergoing MR imaging of the brain or cervical spine for any indication (Strahle et al, 2011a, 2011b).
Note that comparison children were allowed to overlap in the two primary analyses; this included one child classified as CIM.
Table 3. Comparison of Continuous Outcomes between Exposed Children and Matched Comparisons with No History of Maternal Depression and No SSRI Exposure.
| Variable |
Matched comparisons |
SSRI exposed |
Difference as percent of control mean |
P-value | Test statistic | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||||
| CT_FM_Distancea Year 1 | 0.15 | 0.32 | 55 | 0.02 | 0.46 | 28 | 87% | 0.23 | 1050 |
| CT_FM_Distance Year 2 | 0.12 | 0.39 | 33 | −0.03 | 0.51 | 16 | 125% | 0.44 | 363.0 |
| Clival length Year 1 | 2.93 | 0.21 | 55 | 2.85 | 0.24 | 28 | 3% | 0.07 | 988.5 |
| Clival length Year 2 | 3.18 | 0.20 | 33 | 3.06 | 0.28 | 16 | 4% | 0.09 | 319.0 |
| FM AP Diameter Year 1 | 2.96 | 0.30 | 55 | 3.03 | 0.33 | 28 | 2% | 0.57 | 1236 |
| FM AP diameter Year 2 | 3.13 | 0.33 | 33 | 3.16 | 0.25 | 16 | <1% | 0.80 | 412.5 |
| Variable |
Matched comparisons |
History of maternal depression No treatment in pregnancy |
Difference as percent of control mean |
P-value | Test statistic | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||||
| CT_FM_Distance Year 1 | 0.16 | 0.39 | 47 | 0.16 | 0.34 | 24 | 0% | 0.80 | 843.0 |
| CT_FM_Distance Year 2 | 0.14 | 0.42 | 39 | 0.22 | 0.47 | 21 | 57% | 0.32 | 704.5 |
| Clival length Year 1 | 3.05 | 0.22 | 47 | 2.97 | 0.28 | 24 | 3% | 0.50 | 808.0 |
| Clival length Year 2 | 3.24 | 0.27 | 39 | 3.17 | 0.24 | 21 | 2% | 0.59 | 605.5 |
| FM AP diameter Year 1 | 2.88 | 0.28 | 47 | 2.97 | 0.18 | 24 | 3% | 0.15 | 982.0 |
| FM AP diameter Year 2 | 3.10 | 0.30 | 39 | 3.07 | 0.23 | 21 | <1% | 0.69 | 614.5 |
CT_FM_Distance is the distance from the most inferior portion of the cerebellar tonsils to the foramen magnum. Negative numbers denote tonsillar herniation. A shorter clival length and larger FM_AP_Diameter have been reported in individuals with CIM (Aquilina et al, 2009; Bliesener and Schmidt, 1980). Thus, all three measures are in the predicted direction of increased risk for CIM in children with prenatal SSRI exposure. In contrast, there is not a consistent relationship between history of maternal depression (no treatment in pregnancy) and continuous measures related to CIM.
All measurements are in cm.
The SSRI-exposed cohort differed from matched comparison children in gestational age at birth (earlier in SSRI-exposed group), birth length (shorter in SSRI-exposed group), duration of stay in the NICU (longer in SSRI-exposed group), and hypertension during pregnancy (more common in SSRI-exposed group) (Mann–Whitney–Wilcoxon test statistic=1160.5, p<0.001; Mann–Whitney–Wilcoxon test statistic=1080.5, p<0.001; Mann–Whitney–Wilcoxon test statistic=1849.5, p=0.003, and two-sided Fisher's exact test p=0.01 respectively, Table 1). Mothers in the SSRI-exposed cohort had marginally higher prepregnancy BMI (NS). To explore whether these differences might account for group differences in occurrence of CIM, we assessed these variables in all unexposed children (no SSRI, no maternal history of depression) identified as having CIM on their 1- and/or 2-year scan in the parent studies during standard review for incidental findings and compared them with all unexposed children with a 1- and/or 2-year scan who were not identified as having CIM (N=8 and N=427 respectively, Supplementary Table 3). There were no statistically significant differences in gestational age at birth, birth length, duration of stay in NICU, or hypertension during pregnancy between unexposed children with or without CIM. In fact, unexposed children with CIM were born later, had greater birth length, and shorter duration of NICU stay than unexposed children without CIM. None of the unexposed children with CIM were exposed to maternal hypertension during pregnancy. Prepregnancy BMI was significantly lower in unexposed children with CIM (Mann–Whitney–Wilcoxon test statistic=681.5, p=0.005). As a sensitivity analysis, we also reran the logistic regression including gestational age at birth, birth length, duration of stay in the NICU, hypertension during pregnancy, and prepregnancy BMI as covariates. Only SSRI exposure was associated with occurrence of CIM (logistic regression OR estimate 26.22, 95% Wald confidence limits 2.64–>999.99, p=0.02). The SSRI-exposed cohort also differed from matched comparison children in prenatal exposure to antihistamines and thyroid medications (see Supplementary Table S1). Two of the eight SSRI-exposed children with antihistamine exposure were classified as CIM. None of the SSRI-exposed children with thyroid medication exposure were classified as CIM. None of the children exposed to benzodiazepines, trazadone, or Adderall in addition to an SSRI were classified as CIM. Non-CIM abnormalities were not associated with SSRI use (Cochran–Mantel–Haenzel test statistic=2.22, p=0.14, logistic regression OR estimate 0.21, 95% Wald Confidence Limits 0.002–2.03).
Primary Analyses Cohort 2 (History of Maternal Depression)
Children of mothers who had received a diagnosis of depression, but who did not receive antidepressants during pregnancy, did not differ from matched comparison children in occurrence of CIM (Cochran–Mantel–Haenzel test statistic=0.10, p=0.75, logistic regression OR estimate 1.44, 95% Wald confidence limits 0.23–7.85, Table 1), nor did they differ in average CT_FM_Distance, clival length, or FM_AP_Diameter. There was not a consistent relationship between history of maternal depression (no treatment in pregnancy) and continuous measures related to CIM (Table 3).
Secondary Analyses
Duration of exposure
Children exposed to SSRIs in all three trimesters were significantly more likely to meet criteria for CIM than matched comparison children (Table 4, Cochran–Mantel–Haenzel test statistic=10.0, p=0.002, logistic regression OR estimate 29.21, 95% Wald confidence limits 3.00–>999.99). Children exposed to SSRIs in less than three trimesters were not significantly more likely to meet criteria for CIM than matched comparison children (Table 4, Cochran–Mantel–Haenzel test statistic=2.00, p=0.16, logistic regression OR estimate 6.36, 95% Wald confidence limits 0.32–955.72).
Table 4. Proportion of CIM in SSRI-Exposed Children and Matched Comparisons with No History of Maternal Depression and No SSRI Exposure (Secondary Analyses).
|
SSRI exposure in 3 trimesters |
Matched comparisons |
Statistics |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Test statistic | p-value | OR | Confidence interval | |
| CIM | 5 | 26% | 0 | 0% | 10.0 | 0.002 | 29.21 | 3–>999.99 |
| No CIM | 14 | 74% | 38 | 100% | ||||
| SSRI Exposure in less than 3 Trimesters | Matched Comparisons | Statistics | ||||||
| No. | % | No. | % | Test Statistic | p-value | OR | Confidence Interval | |
| CIM | 1 | 8% | 0 | 0% | 2.00 | 0.16 | 6.36 | 0.32–955.72 |
| No CIM | 12 | 92% | 26 | 100% | ||||
| SSRI exposure at conception | Matched Comparisons | Statistics | ||||||
| No. | % | No. | % | Test Statistic | p-value | OR | Confidence Interval | |
| CIM | 3 | 20% | 0 | 0% | 6.00 | 0.01 | 17.08 | 1.50–>999.99 |
| No CIM | 12 | 80% | 30 | 100% | ||||
| SSRI exposure post-conception | Matched Comparisons | Statistics | ||||||
| No. | % | No. | % | Test Statistic | p-value | OR | Confidence Interval | |
| CIM | 2 | 13% | 0 | 0% | 4.00 | 0.046 | 11.30 | 0.85–>999.99 |
| No CIM | 13 | 87% | 30 | 100% | ||||
| SSRI+Family History MDD/BPD | Matched Comparisons | Statistics | ||||||
| No. | % | No. | % | Test Statistic | p-value | OR | Confidence Interval | |
| CIM | 5 | 45% | 0 | 0% | 10.0 | 0.002 | 38.08 | 3.57–>999.99 |
| No CIM | 6 | 55% | 22 | 100% | ||||
| SSRI No Family History MDD/BPD | Matched Comparisons | Statistics | ||||||
| No. | % | No. | % | Test Statistic | p-value | OR | Confidence Interval | |
| CIM | 1 | 5% | 1 | 2% | 0.25 | 0.62 | 6.36 | 0.32–955.7 |
| No CIM | 21 | 95% | 43 | 98% | ||||
Timing of exposure
Children who had been exposed to an SSRI at conception were more likely to meet criteria for CIM than matched comparison children (Table 4, Cochran–Mantel–Haenzel test statistic=6.00, p=0.01, logistic regression OR estimate 17.08, 95% Wald confidence limits 1.50–>999.99). Children who had been exposed to an SSRI after conception were more likely to meet criteria for CIM than matched comparison children based on Cochran–Mantel–Haenzel statistics (Table 4, test statistic=4.00, p=0.046), but not based on logistic regression (OR estimate 11.30, 95% Wald confidence limits 0.85–>999.99).
Family history of MDD/BPD
The SSRI-exposed children whose mothers reported a family history of MDD/BPD were more likely to meet criteria for CIM than matched comparison children (Table 4, Cochran–Mantel–Haenzel test statistic=10.0, p=0.002, logistic regression OR estimate 38.08, 95% Wald confidence limits 3.57–>999.99). In contrast, SSRI-exposed children whose mothers reported no family history of MDD/BPD were not significantly more likely to meet criteria for CIM than matched comparison children (Table 4, Cochran–Mantel–Haenzel test statistic=0.25, p=0.62, logistic regression OR estimate 6.36, 95% Wald confidence limits 0.32–955.72). This was particularly interesting as the percentage of children whose mothers reported a family history of MDD/BPD was similar in cohort 1 (SSRI exposed) as compared with cohort 2 (history of maternal depression) (33% in cohort 1 and 40% in cohort 2, Fisher's exact test p=0.61).
DISCUSSION
This study found a striking increase of CIM in children with prenatal SSRI exposure. This finding appeared to be unrelated to differences in gestational age at birth, birth length, duration of stay in NICU, exposure to maternal hypertension, prepregnancy BMI, or exposure to other medications, licit, or illicit substances. However, the parent studies did not perform drug testing and substance use in pregnancy is often underreported. CIM was not elevated in non-SSRI-exposed children of mothers who received a diagnosis of depression before or during pregnancy. The data support one of two interpretations: SSRIs increase risk for CIM, or other factors that differentiate women treated with SSRIs from untreated women with a history of depression increase risk for CIM. Two factors that may be of particular importance are severity of depressive symptoms during pregnancy and genetic risk for depression.
Regarding genetic risk, the percentage of children whose mothers reported a family history of MDD/BPD was similar in the SSRI-exposed cohort and the history of depression cohort. This suggests that the increased rate of CIM in the SSRI-exposed cohort is not simply the result of shared genetic risk for CIM and depression. However, secondary analyses within the SSRI-exposed cohort suggested CIM was more common in children whose mothers had a family history of MDD/BPD. This is compatible with a ‘double hit' model in which increased risk for CIM is the result of an interaction between genetic risk and SSRI exposure (or other factors associated with SSRI exposure). However, subgroup analyses must be treated with caution because of small sample size.
Regarding severity of depressive symptoms, the parent studies did not collect direct measures of symptom severity during pregnancy. In addition, medical records did not provide sufficient information to determine how many mothers reporting nonactive depression at study entry developed symptoms after the initial interview. Thus, we cannot distinguish between the possibility that SSRIs directly impact risk for CIM and the possibility that this relationship is mediated by severity of depressive symptoms during pregnancy.
When interpreting data on medication exposure, one must consider duration and timing of exposure and their relationship to windows of developmental sensitivity. Secondary analyses suggested CIM was more common in children with longer duration of SSRI exposure. In keeping with Hill's criteria for causation, greater exposure generally leads to greater incidence of the effect (Howick et al, 2009). Secondary analyses also suggested CIM was more common in children exposed to SSRIs at conception. Exposure at conception is a proxy for exposure during organogenesis. Although tonsillar herniation is not present at birth, most theories on the pathophysiology of CIM posit an ultimate origin in embryonic or early fetal development (Shoja et al, 2013). However, the true cause(s) of CIM are unknown and any discussion of windows of developmental specificity is speculative. It may seem counterintuitive that duration of SSRI exposure and SSRI exposure at conception were both associated with increased risk for CIM; this is probably the result of overlap between groups. Whereas 68% of children exposed to SSRIs during three trimesters were also exposed at conception, only 15% of children exposed for less than three trimesters were also exposed at conception. It is also possible that both duration of SSRI exposure and SSRI exposure at conception act as proxy measures for severity of depression.
As discussed previously, CIM is thought to result from overcrowding of the posterior cranial fossa (Aquilina et al, 2009). Multiple theories have been proposed to account for this overcrowding, including the modified hydrodynamic theory of Gardner and the occipital dysplasia theory, but none explain all the anomalies seen in CIM (Shoja et al, 2013). Because the pathophysiology of CIM is unclear, it is difficult to formulate specific mechanistic hypotheses linking SSRI exposure to CIM. Nevertheless, serotonin plays a critical role in craniofacial development (Moiseiwitsch, 2000), making a causal pathway from SSRI exposure to CIM theoretically plausible. Serotonin regulates mouse cranial neural crest migration, mesenchymal differentiation, and expression of growth factors and extracellular matrix molecules, including those of the cartilage matrix (Levin et al, 2006). Exposure of cultured mouse embryos to sertraline or fluoxetine disrupts epithelial–mesenchymal interactions important for normal craniofacial morphogenesis (Shuey et al, 1992). In addition, serotonin promotes proliferation of osteoblast precursors, suggesting an important role in ossification (Westbroek et al, 2001). SSRIs inhibit osteoblast formation and function, reduce cell viability, and induce apoptosis in vitro (Hodge et al, 2013). However, it is unclear why bony defects would be subtle (potentially limited to posterior fossa) rather than manifesting as major craniofacial malformations. Redundancy within the serotonergic signaling system may allow most regions to develop normally in the presence of SSRIs. Serotonin-binding proteins could also help maintain appropriate tissue concentrations. Animal studies suggest mandibular and dental development is especially sensitive to variation in serotonin concentration (Moiseiwitsch, 2000). The current results suggest animal studies examining SSRI effects on the posterior fossa are warranted.
It is well established that prenatal stress can cause increased vulnerability to physical and behavioral problems in offspring (Glover, 2014). In a similar manner, depressive symptoms could impact craniofacial development through multiple mechanisms including metabolic disturbance, neuroendocrine disturbance, and poor nutrition (Gonzalez et al, 2011; Henriquez et al, 2013; Pirinen, 1995). Unfortunately, there are no studies exploring these factors and risk for CIM per se. CIM has been reported in association with congenital conditions including craniosynostosis, achondroplasia, acromegaly, growth hormone deficiency, hyperostosis, familial vitamin D-resistant rickets, neurofibromatosis type 1, and a variety of spinal defects (Loukas et al, 2011). A genetic contribution to at least a subset of nonsyndromic cases is strongly suspected (Speer et al, 2003). There is one report of CIM in association with familial vitamin B12 deficiency (Welsch et al, 2013).
Detailed studies of craniofacial development in children exposed to maternal depression and/or SSRIs are not currently available. Published studies on fetal and infant growth in relation to in utero exposure to maternal depression and/or SSRIs have produced inconsistent results. Wisner et al (2009) reported no effect of SSRI exposure or untreated depression on birth length or head circumference at birth. A follow-up study showed no impact of untreated depression or prenatal SSRI exposure on length or head circumference change from birth to 12 months (Wisner et al, 2013). In contrast, Lewis et al (2010) reported reduced birth length in antidepressant-exposed infants that was not correlated with symptom severity. Dubnov-Raz et al (2012) reported reduced head circumference (significant) and birth length (ns) in newborns with prenatal SSRI exposure, but found no effect on bone density as measured by tibial bone speed of sound. In the largest study carried out to date, SSRI exposure was associated with smaller head circumference at birth, whereas untreated depression was not. In addition, prenatal SSRI exposure was associated with reduced growth of the fetal head, with sparing of the body, whereas untreated depression was associated with reduction in total body growth, based on fetal ultrasonography (El Marroun et al, 2012).
The clinical importance of our findings is difficult to assess without data on long-term functional outcomes in this particular group. The natural history of incidental CIM is benign in the majority of cases, but some children develop severe complications. Massimi et al (2011) reported follow-up data for 16 children classified as CIM based on MRI. Thirteen children were asymptomatic at follow-up. Three displayed symptoms (19%), two of whom underwent endoscopic third ventriculostomy for hydrocephalus. Aitken et al (2009) reported follow-up data for 19 patients with incidental CIM. Four children (21%) developed new neurological problems. None required surgery. Intriguingly, two received a physician's diagnosis of depression. Novegna et al (2008) reported on 22 CIM patients recommended for nonsurgical management. Five patients (23%) showed worsening symptoms, three of whom required surgery. Benglis et al (2011) reported on 43 cases with asymptomatic CIM. None (0%) developed symptoms. Finally, Strahle et al (2011b) reported on 147 patients recommended for nonsurgical management. Nine patients developed new symptoms attributed to CIM (6%) and 14 eventually underwent surgical treatment.
It has been our practice to notify parents when CIM is identified on a research scan. This approach is not without controversy as reporting incidental findings can create anxiety and lead to unnecessary and costly medical follow-up. We would not recommend radiological screening for CIM in asymptomatic children with prenatal SSRI exposure. However, given that a diagnosis of CIM is often delayed because of the nonspecific nature of the symptoms; it may be valuable for families in this situation to be aware of our findings.
In conclusion, we identified a marked increase of CIM in children of depressed mothers treated with SSRIs during pregnancy. The strengths of this study are the inclusion of both an SSRI-exposed group of children and a group of non-SSRI-exposed children of mothers with a history of depression, detailed exposure data reflecting both maternal report and medical record review, blinded assessment of CIM, and use of propensity matching to minimize differences between exposed children and comparison children. Weaknesses include relatively small sample sizes, the absence of direct measures of symptom severity, and the fact that the hypothesis being tested was formulated during data acquisition. Replication and additional research are urgently needed. In particular, the rate of CIM in women with active, untreated depression during pregnancy must be determined. We would discourage clinicians from altering prescribing practices until research clarifies whether SSRIs directly impact risk for CIM or whether this relationship is mediated by severity of depressive symptoms during pregnancy. In addition, data on long-term functional outcomes is needed as many individuals with CIM are asymptomatic whereas others experience severe complications requiring surgical intervention. There is no definitive answer to the optimal treatment of women with depression during pregnancy. SSRIs do not appear to be major teratogens, in contrast to mood stabilizers such as valproic acid and carbamazepine that are associated with an increased risk of neural tube defects (Cott and Wisner, 2003). Potential risks of pharmacotherapy must be weighed against the potential risks of active, untreated depression.
FUNDING AND DISCLOSURE
Dr Knickmeyer is a collaborator on two investigator-initiated grants from Pfizer. Dr Meltzer-Brody has received compensation for professional services from UNC. In addition, Dr Meltzer-Brody has received honoraria for presentations given for Johns Hopkins, Children's Hospital of the King's Daughters (Eastern Virginia Medical School), Virginia Postpartum Progress (Annual Meeting), North Carolina Pediatric Society, Academy of Breastfeeding Medicine (Annual Meeting), and Tulane University. Dr Meltzer-Brody has received research grant funding from Astra Zeneca (investigator-initiated study). Ms Woolson has received compensation for professional services from UNC, Rho, CeNeRx, and Janssen Pharmaceuticals. Dr Hamer has received compensation from UNC and has served on an advisory board, or consulted on a clinical trial, or served on a DSMB, for Abbott, Allergan, Alkermes, Cenerx, Columbia University, Endo, Lilly, Novartis, Pfizer, Roche, and Wyeth, and has served as an expert witness in cases involving Forest, Lundbeck, Sun, Caraco, Teva, Barr, Mylan, Eurand, Cephalon, Anesta, and Marial. Dr Knickmeyer, Dr Smith, and Dr Lury have received compensation for professional services from UNC only. Dr Gilmore has received compensation for professional services from UNC. In addition, he has received honoraria for presentations given at the annual meeting of the Neurobehavioral Teratology Society, University of Texas at Houston, National Institutes of Mental Health, the Human Brain and its Development Throughout the Lifespan (University of Utrecht), Wisconsin Symposium on Emotion, 8th International Imaging Genetics Conference, Edmonton Schizophrenia Conference, Institute of Medicine, and Merrill Advanced Studies Center. Dr Smith, Dr Lury, and Dr Gilmore declare no conflict of interest.
Acknowledgments
We thank the participating families who made this project possible as well as the staff of the UNC MRI Research Center, the UNC Neuro Image Research and Analysis Laboratories, and the UNC Early Brain Development Program. This work was supported by the National Institutes of Health (MH064065, MH070890, and HD053000 to Dr Gilmore and MH083045 to Dr Knickmeyer) and the Foundation of Hope for the Research and Treatment of Mental Illness.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aitken LA, Lindan CE, Sidney S, Gupta N, Barkovich AJ, Sorel M, et al. Chiari type I malformation in a pediatric population. Pediatr Neurol. 2009;40:449–454. doi: 10.1016/j.pediatrneurol.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilina K, Merchant TE, Boop FA, Sanford RA. Chiari I malformation after cranial radiation therapy in childhood: a dynamic process associated with changes in clival growth. Childs Nerv Syst. 2009;25:1429–1436. doi: 10.1007/s00381-009-0982-8. [DOI] [PubMed] [Google Scholar]
- Benglis JrD, Covington D, Bhatia R, Bhatia S, Elhammady MS, Ragheb J, et al. Outcomes in pediatric patients with Chiari malformation type I followed up without surgery. J Neurosurg Pediatr. 2011;7:375–379. doi: 10.3171/2011.1.PEDS10341. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bliesener JA, Schmidt LR. Normal and pathological growth of the foramen occipitale magnum shown in the plain radiography. Pediatr Radiol. 1980;10:65–69. doi: 10.1007/BF01001741. [DOI] [PubMed] [Google Scholar]
- Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127:94–114. doi: 10.1111/acps.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SB, Brownell CA, Hungerford A, Spieker SI, Mohan R, Blessing JS. The course of maternal depressive symptoms and maternal sensitivity as predictors of attachment security at 36 months. Dev Psychopathol. 2004;16:231–252. doi: 10.1017/s0954579404044499. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544 e541–544 e545. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Cott AD, Wisner KL. Psychiatric disorders during pregnancy. Int Rev Psychiatry. 2003;15:217–230. doi: 10.1080/0954026031000136848. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15:1–14. doi: 10.1007/s00737-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Di Rocco C, Frassanito P, Massimi L, Peraio S. Hydrocephalus and Chiari type I malformation. Childs Nerv Syst. 2011;27:1653–1664. doi: 10.1007/s00381-011-1545-3. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164:1515–1520. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- Dubnov-Raz G, Hemila H, Vurembrand Y, Kuint J, Maayan-Metzger A. Maternal use of selective serotonin reuptake inhibitors during pregnancy and neonatal bone density. Early Hum Dev. 2012;88:191–194. doi: 10.1016/j.earlhumdev.2011.08.005. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry. 2012;69:706–714. doi: 10.1001/archgenpsychiatry.2011.2333. [DOI] [PubMed] [Google Scholar]
- Fransson E, Ortenstrand A, Hjelmstedt A. Antenatal depressive symptoms and preterm birth: a prospective study of a Swedish national sample. Birth. 2011;38:10–16. doi: 10.1111/j.1523-536X.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106 (5 Pt 1:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28:25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Gonzalez PN, Hallgrimsson B, Oyhenart EE. Developmental plasticity in covariance structure of the skull: effects of prenatal stress. J Anat. 2011;218:243–257. doi: 10.1111/j.1469-7580.2010.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley GE, Oberlander TF. The effect of perinatal exposures on the infant: antidepressants and depression. Best Pract Res Clin Obstet Gynaecol. 2014;28:37–48. doi: 10.1016/j.bpobgyn.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Henriquez R, Olivares R, Caro G, Guevara V, Lizana P. Prenatal stress caused by movement restriction induces changes in the development of skull bone in CF-1 mice progeny. Int J Morphol. 2013;31:1034–1040. [Google Scholar]
- Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74:32–39. doi: 10.1016/j.biopsych.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: what can Bradford Hill's ‘guidelines for causation' contribute. J R Soc Med. 2009;102:186–194. doi: 10.1258/jrsm.2009.090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Galbally M, Opie G, Buist A. Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust N Z J Psychiatry. 2010;44:482–487. doi: 10.3109/00048670903559593. [DOI] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8:77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Loukas M, Shayota BJ, Oelhafen K, Miller JH, Chern JJ, Tubbs RS, et al. Associated disorders of Chiari Type I malformations: a review. Neurosurg Focus. 2011;31:E3. doi: 10.3171/2011.6.FOCUS11112. [DOI] [PubMed] [Google Scholar]
- Malm H, Artama M, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomaki S, et al. Infant and childhood neurodevelopmental outcomes following prenatal exposure to selective serotonin reuptake inhibitors: overview and design of a Finnish Register-Based Study (FinESSI) BMC Psychiatry. 2012;12:217. doi: 10.1186/1471-244X-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Heringhausen JE.2009Depression in childbearing women: when depression complicates pregnancy Prim Care 36151–165.ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi L, Caldarelli M, Frassanito P, Di Rocco C. Natural history of Chiari type I malformation in children. Neurol Sci. 2011;32 (Suppl 3:S275–S277. doi: 10.1007/s10072-011-0684-3. [DOI] [PubMed] [Google Scholar]
- McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118:e174–e182. doi: 10.1542/peds.2005-1551. [DOI] [PubMed] [Google Scholar]
- Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92:920–926. doi: 10.3171/jns.2000.92.6.0920. [DOI] [PubMed] [Google Scholar]
- Mikulis DJ, Diaz O, Egglin TK, Sanchez R. Variance of the position of the cerebellar tonsils with age: preliminary report. Radiology. 1992;183:725–728. doi: 10.1148/radiology.183.3.1584927. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–1017. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- Miller JH, Limbrick DD, Callen M, Smyth MD. Spontaneous resolution of Chiari malformation Type I in monozygotic twins. J Neurosurg Pediatr. 2008;2:317–319. doi: 10.3171/PED.2008.2.11.317. [DOI] [PubMed] [Google Scholar]
- Moiseiwitsch JR. The role of serotonin and neurotransmitters during craniofacial development. Crit Rev Oral Biol Med. 2000;11:230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- Novegno F, Caldarelli M, Massa A, Chieffo D, Massimi L, Pettorini B, et al. The natural history of the Chiari type I anomaly. J Neurosurg Pediatr. 2008;2:179–187. doi: 10.3171/PED/2008/2/9/179. [DOI] [PubMed] [Google Scholar]
- Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, et al. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry. 2012;169:1165–1174. doi: 10.1176/appi.ajp.2012.11111721. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF. Fetal serotonin signaling: setting pathways for early childhood development and behavior. J Adolesc Health. 2012;51 (2 Suppl:S9–16. doi: 10.1016/j.jadohealth.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- Pirinen S. Endocrine regulation of craniofacial growth. Acta Odontol Scand. 1995;53:179–185. doi: 10.3109/00016359509005969. [DOI] [PubMed] [Google Scholar]
- Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, Rehm J, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70:436–443. doi: 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- Shoja MM, Tubbs RS, Oakes WJ.2013Embryology and Pathophysiology of the Chiari I and II MalformationsIn: Tubbs RS, Oakes WJ (eds)The Chiari Malformations Springer Science + Business Media: New York; 55–72. [Google Scholar]
- Shuey DL, Sadler TW, Lauder JM. Serotonin as a regulator of craniofacial morphogenesis: site specific malformations following exposure to serotonin uptake inhibitors. Teratology. 1992;46:367–378. doi: 10.1002/tera.1420460407. [DOI] [PubMed] [Google Scholar]
- Smith BW, Strahle J, Bapuraj JR, Muraszko KM, Garton HJ, Maher CO. Distribution of cerebellar tonsil position: implications for understanding Chiari malformation. J Neurosurg. 2013;119:812–819. doi: 10.3171/2013.5.JNS121825. [DOI] [PubMed] [Google Scholar]
- Speer MC, Enterline DS, Mehltretter L, Hammock PH, Joseph J, Dickerson M, et al. Chiari type I malformation with or without syringomyelia: prevalence and genetics. J Genet Counsel. 2003;12:297–311. doi: 10.1023/A:1023948921381. [DOI] [PubMed] [Google Scholar]
- Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging. J Neurosurg Pediatr. 2011;8:205–213. doi: 10.3171/2011.5.PEDS1121. [DOI] [PubMed] [Google Scholar]
- Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Natural history of Chiari malformation type I following decision for conservative treatment. J Neurosurg Pediatr. 2011;8:214–221. doi: 10.3171/2011.5.PEDS1122. [DOI] [PubMed] [Google Scholar]
- Welsch M, Antes S, Kiefer M, Meyer S, Eymann R. Association of Chiari malformation and vitamin B12 deficit in a family. Childs Nerv Syst. 2013;29:1193–1198. doi: 10.1007/s00381-013-2056-1. [DOI] [PubMed] [Google Scholar]
- Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Bogen DL, Sit D, McShea M, Hughes C, Rizzo D, et al. Does fetal exposure to SSRIs or maternal depression impact infant growth. Am J Psychiatry. 2013;170:485–493. doi: 10.1176/appi.ajp.2012.11121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160 (5pt1:1107–1111. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.