Abstract
Paradoxically, N-methyl-D-aspartate (NMDA) receptor antagonists are used to model certain aspects of schizophrenia as well as to treat refractory depression. However, the role of different subunits of the NMDA receptor in both conditions is poorly understood. Here we used biochemical and behavioral readouts to examine the in vivo prefrontal efflux of serotonin and glutamate as well as the stereotypical behavior and the antidepressant-like activity in the forced swim test elicited by antagonists selective for the GluN2A (NVP-AAM077) and GluN2B (Ro 25-6981) subunits. The effects of the non-subunit selective antagonist, MK-801; were also studied for comparison. The administration of MK-801 dose dependently increased the prefrontal efflux of serotonin and glutamate and markedly increased the stereotypy scores. NVP-AAM077 also increased the efflux of serotonin and glutamate, but without the induction of stereotypies. In contrast, Ro 25-6981 did not change any of the biochemical and behavioral parameters tested. Interestingly, the administration of NVP-AAM077 and Ro 25-6981 alone elicited antidepressant-like activity in the forced swim test, in contrast to the combination of both compounds that evoked marked stereotypies. Our interpretation of the results is that both GluN2A and GluN2B subunits are needed to induce stereotypies, which might be suggestive of potential psychotomimetic effects in humans, but the antagonism of only one of these subunits is sufficient to evoke an antidepressant response. We also propose that GluN2A receptor antagonists could have potential antidepressant activity in the absence of potential psychotomimetic effects.
INTRODUCTION
It is well established that N-methyl-D-aspartate (NMDA) receptors play a key role in several psychiatric disorders and their pharmacological treatments. For instance, schizophrenia has been associated with NMDA receptor hypofunction insofar as the administration of NMDA receptor antagonists can evoke positive and negative symptoms as well as characteristic cognitive deficits closely resembling those of the illness (Javitt and Zukin, 1991; Krystal et al, 1994). In rodents, NMDA receptor antagonists cause hyperlocomotion and stereotypies (Homayoun et al, 2004; López-Gil et al, 2012), behaviors that have been potentially related to positive symptoms of schizophrenia (Lipska and Weinberger, 2003; Sams-Dodd, 1997), and are thought to result from excessive dopaminergic and serotonergic activities (Jentsch et al, 1998; Lucki, 1998). In addition to these behavioral changes, NMDA receptor antagonists also augment the release of dopamine (Moghaddam et al, 1997), glutamate, and serotonin (5-hydroxytryptamine (5-HT)) in the medial prefrontal cortex (mPFC) of rats (Amargós-Bosch et al, 2006; López-Gil et al 2007, 2012). It is postulated that this increased release of glutamate in the mPFC stimulates prefrontal output to brainstem monoaminergic nuclei, thereby increasing monoaminergic cell firing and prefrontal monoamine efflux (López-Gil et al, 2007). From a molecular viewpoint, NMDA receptors are tetrameric structures assembled from two obligatory GluN1 subunits and two GluN2A (formerly NR2A) or GluN2B (formerly NR2B) subunits. However, triheteromers formed by GluN1/GluN2A/GluN2B subunits are also abundant in the neocortex and hippocampus of the adult rodent brain (Traynelis et al, 2010). Despite the widespread use of acute NMDA receptor antagonism to model schizophrenia (Adell et al, 2012), the contribution of GluN2 subunits to the biochemical and behavioral changes elicited by NMDA receptor antagonists is poorly understood. In this regard, there is one study reporting that the GluN2B, rather than GluN2A, subunit substituted for the disruption of prepulse inhibition (PPI) of the startle response and hyperactivity induced by the NMDA receptor antagonist phencyclidine (Chaperon et al, 2003). In addition, Ro 25-6981 was found to disinhibit hippocampal pyramidal neurons by reducing GABAergic output (Hanson et al, 2013), a feature also known to model psychosis (Lisman et al, 2008). Therefore, it appears that the blockade of the GluN2B subunit is sufficient to elicit psychotomimetic activity.
In the field of mood disorders, NMDA receptor blockers are of particular relevance for treatment-resistant depression (Skolnick et al, 2009). Indeed, there is compelling evidence that the NMDA receptor antagonist, ketamine, evokes rapid (within 2 h) and robust antidepressant effects that can last for a week (Berman et al, 2000; Zarate et al, 2006a). Interestingly, the same doses of ketamine that evoke psychotic symptoms also produce antidepressant effects. It is possible that psychosis is an immediate consequence of NMDA receptor blockade and reflects, in part, the ability of these drugs to stimulate glutamate release (Moghaddam et al, 1997; López-Gil et al, 2007). However, a component of the antidepressant response seems to be a delayed consequence of the glutamate released by ketamine that would stimulate AMPA receptors (Maeng et al, 2008; Koike et al, 2011; Akinfiresoye and Tizabi, 2013). Thus, both effects are mechanistically associated but temporally dissociated.
Because of these adverse side effects of ketamine, much research is focused in improving the pharmacological profile of NMDA receptor antagonists by investigating whether subtype selectivity could achieve a better therapeutic index. In this regard, preclinical studies have shown that the GluN2B receptor antagonist, Ro 25-6981, possesses antidepressant-like effects (Maeng et al, 2008; Li et al, 2010). On the other hand, inactivation of the GluN2A has also been shown to evoke antidepressant-like activity in mice (Boyce-Rustay and Holmes, 2006). Furthermore, in the clinic, several investigational drugs have manifested some efficacy in the treatment of depressive states, such as the GluN2B receptor antagonists CP-101,606 (Preskorn et al, 2008) and MK 0657 (Ibrahim et al, 2012), the NMDA receptor glycine-site partial agonist GLYX-13 (Burgdorf et al, 2013), and the low-trapping nonselective NMDA channel blocker AZD6765 (Sanacora et al, 2014; Zarate et al, 2013). However, the rapid and robust effects of ketamine are clear, whereas in comparison, the effects of MK 0657 and AZD6765 are modest and short-lived. In contrast, memantine, a NMDA channel blocker with similarity to ketamine, appears not to possess antidepressant effects when administered alone (Zarate et al, 2006b), which might be because of its unusual binding properties (Chen and Lipton, 2005), and not be representative of NMDA channel blockers as a whole. In addition, CP-101,606 did produce severe dissociative effects in depressed patients (Preskorn et al, 2008). Therefore, the results of clinical studies are not consistent with the notion that GluN2B antagonists may have a better profile than channel blockers like ketamine.
Overall, GluN2A and GluN2B receptor subunits may be anatomically and functionally segregated and play distinct roles in the regulation of prefrontal synaptic response to different inputs carrying contextual and affective information. Thus, it has been proposed that afferents from ventral hippocampus may preferentially synapse onto postsynaptic sites containing GluN2B subunits, whereas projections from basolateral amygdala may preferentially synapse onto postsynaptic sites containing GluN2A subunits (Flores-Barrera et al, 2014). In the present study, we sought to examine the effects of GluN2A and GluN2B subunits in the acute NMDA receptor antagonist model of schizophrenia as well as in the treatment of depression-like effects in the rat. To this end, we have assessed the biochemical and behavioral changes elicited by NVP-AAM077 (Auberson et al, 2002), a competitive antagonist showing ca. 10-fold selectivity for the rat GluN2A subunit compared with the GluN2B subunit (Neyton and Paoletti, 2006), and Ro 25-6981, which is ∼5000-fold selective for GluN2B over GluN2A (Fischer et al, 1997). The results are compared with the effects of a nonselective NMDA receptor channel blocker such as dizocilpine (MK-801). The antidepressant-like effects were measured in the forced swim test (FST), whereas the possible emergence of psychotomimetic adverse effects was associated with the ability to induce stereotypical behavior.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River Laboratories, Cerdanyola del Vallès, Spain) weighing 280–350 g were used. Food and water were always available. All experimental procedures followed European Union regulations (Official Journal of the European Communities L358/1, 18 December 1986), and were approved by the Institutional Animal Care and Use Committee.
Drugs and Reagents
Serotonin (5-HT), glutamate, MK-801, and o-phthaldialdehyde (OPA) reagent (containing 1 mg OPA per ml solution with 2-mercaptoethanol as the sulfhydryl moiety) were purchased from Sigma-Aldrich (Tres Cantos, Spain). Citalopram hydrobromide and Ro 25-6981 were purchased from Tocris (Bristol, UK). NVP-AAM077 was obtained from Novartis (Basel, Switzerland). MK-801 and Ro 25-6981were dissolved in saline and 50% dimethyl sulfoxide (DMSO), respectively. NVP-AAM077 was dissolved in NaOH 0.1 M, and then the pH was raised to ∼7 with HCl 0.1 M and taken to a final dilution with distilled water. All drugs were injected intraperitoneally (i.p.). Appropriate vehicles were administered as the respective control groups.
Intracerebral Microdialysis
Concentric dialysis probes with a 4-mm membrane length were implanted under sodium pentobarbital anesthesia (60 mg/kg i.p.) in the mPFC (AP +3.2 mm, L ±0.6 mm, DV −6.0 mm; from bregma), according to Paxinos and Watson (2005). A representative histological section showing the location of the concentric dialysis probe in the mPFC is illustrated in Supplementary Figure S1. In all cases, microdialysis experiments were conducted 24 h after surgery in freely moving rats by continuously perfusing probes with aCSF (125 mM NaCl, 2.5 mM KCl, 1.26 mM CaCl2, 1.18 mM MgCl2, and 1 μM citalopram) at a rate of 1.5 μl/min. Dialysate samples of 30 μl were collected every 20 min, and 5-HT and glutamate were determined by HPLC. At the completion of dialysis experiments, rats were given an overdose of sodium pentobarbital and a Fast Green solution was perfused through the dialysis probes to stain the surrounding tissue.
Biochemical Analysis
The concentration of 5-HT in dialysate samples was determined by an HPLC system as described previously (López-Gil et al, 2007). It consisted of a Waters 717plus autosampler (Waters Cromatografia, Cerdanyola, Spain), a Hewlett-Packard series 1050 pump (Agilent Technologies, Las Rozas, Spain), a 3-μm octadecylsilica (ODS) column (7.5 × 0.46 cm; Beckman, San Ramon, CA), and an amperometric detector Hewlett-Packard 1049 (Agilent Technologies) set at an oxidation potential of 0.6 V. The mobile phase consisted of 0.15 M NaH2PO4, 1.8 mM octyl sodium sulfate, 0.2 mM EDTA (pH 2.8, adjusted with phosphoric acid), and 30% methanol and was pumped at 0.7 ml/min. For the determination of glutamate, another HPLC system was used that consisted of a Waters 717plus autosampler, a Waters 600 quaternary gradient pump, and a Nucleosil 5-μm ODS column (10 × 0.4 cm; Teknokroma, Spain). Dialysate samples were precolumn derivatized with OPA reagent and all this process was carried out by the autosampler. Briefly, 90 μl distilled water was added to the 10 μl dialysate sample and this was followed by the addition of 15 μl of the OPA reagent. After 2.5 min of reaction, 80 μl of this mixture was injected into the column. Detection was carried out with a Waters 470 Scanning Fluorescence Detector using excitation and emission wavelengths of 360 and 450 nm, respectively. The mobile phase was pumped at 0.8 ml/min and consisted of two components: Solution A, made up of 0.05 M Na2HPO4, 28% methanol, adjusted to pH 6.4 with 85% H3PO4, and solution B, made up of methanol/H2O (8:2 ratio). After elution of the glutamate peak at 3 min with 100% solution A, a gradient was established going from 100% solution A to 100% solution B in 2 min. After washing out late eluting peaks (3 min), the mobile phase returned to initial conditions (100% solution A) in 2 min. The detection limits for 5-HT and glutamate were 4 fmol and 0.3 pmol, respectively (signal-to-noise ratio 3).
Behavioral Measures
Gross behavioral scores were assessed by direct observation of rats undergoing in vivo microdialysis and were based on a method described previously (Jackson et al 2004). Stereotypies were rated during the last 5 min of each 20-min sample. Animals received a score of 0 (absence), 1 (presence), or 2 (intense >50% of the 5-min block) for each of the following motor stereotypies commonly associated with NMDA antagonism: reciprocal forepaw treading, side-to-side head weaving, and hyperlocomotion (turning). Total scores for each rat were calculated by summing the individual values of each behavior during each 5-min period.
Before FST was conducted, rats were handled daily for 1 week for habituation. On day 1 (pretest), rats were placed in a clear plexyglas cylinder (46 cm height, 20 cm diameter) filled with 24±1 °C water to a height of 30 cm for 15 min. After this pretest, animals were returned to their home cages and dried under a lamp for 30 min. The test was conducted 24 h after the pretest session in the same cylinder for 5 min, and was videotaped (Videotrack, View Point, Lyon, France). The 5-min test session was divided into 5-s epochs. At the end of each epoch, the predominant behavior was rated as immobility, climbing, and swimming by an experimenter blind to the treatment. As these drugs allegedly possess a fast antidepressant action and display similar pharmacokinetic profiles in microdialysis studies (Mabrouk et al, 2013), their administration was carried out 20 min before the test session.
General locomotor activity was measured in independent groups of animals in an open field arena (100 × 100 × 40 cm) with black plastic walls dimly lighted. As for the FST, 20 min after drug administration, ambulation was recorded during 15 min by a video camera connected to a computer (Videotrack) and expressed as the distance traveled (cm) in 15 min.
Statistics
Data are expressed as mean±SEM. One- or two-way analysis of variance (ANOVA) followed by post hoc Newman–Keuls multiple comparisons test was used to analyze differences among three or more independent groups. In all cases, the level of significance was set at p<0.05.
RESULTS
5-HT and Glutamate Efflux in the mPFC
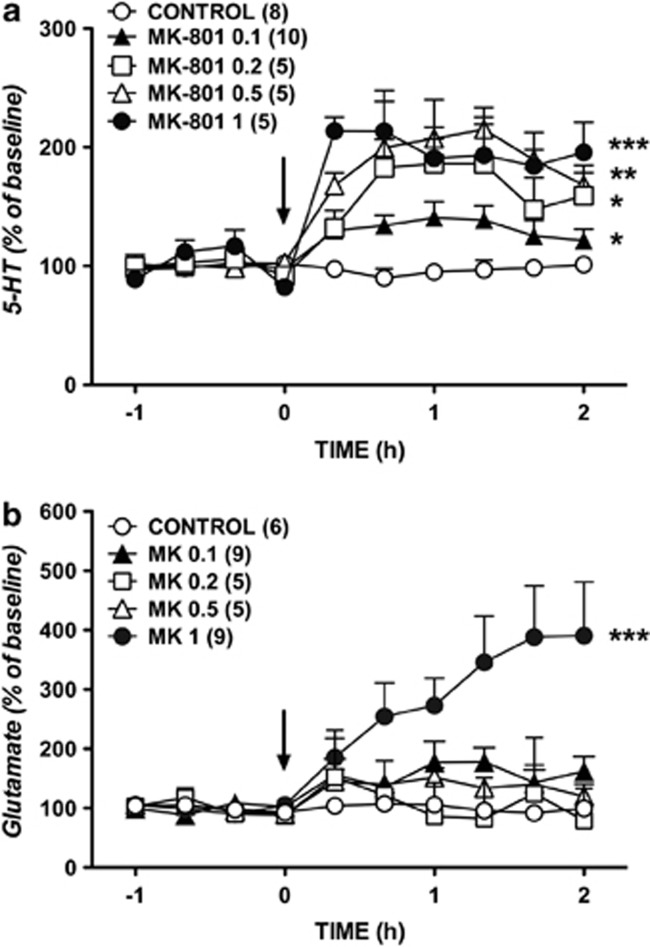
Two-way ANOVA showed that the administration of MK-801 (0.1–1 mg/kg, i.p.) enhanced dose dependently the extracellular concentration of 5-HT (Figure 1a) as demonstrated by the significant effect of treatment (F4, 25=4.83, p=0.005027), time (F9, 225=7.24, p=0.0001), but not treatment × time interaction (F36, 225=1.23, p=0.182942). The post hoc comparisons (Newman–Keuls test) showed each dose of MK-801 elevated extracellular 5-HT (p=0.043335, 0.1 mg/kg; p=0.012458, 0.2 mg/kg; p=0.001491, 0.5 mg/kg; p=0.000153, 1 mg/kg). The increase of prefrontal 5-HT elicited by the dose of 1 mg/kg MK-801 was higher than that of 0.1 and 0.2 mg/kg MK-801 (p=0.003202 and p=0.018113, respectively). The systemic administration of MK-801 also increased extracellular glutamate (Figure 1b), as shown by the significant effect of treatment (F4, 25=10.91, p=0.000029), time (F9, 225=11.86, p=0.00001), and treatment × time interaction (F36, 225=6.55, p=0.00001). However, only the dose of 1.0 mg/kg MK-801 significantly increased dialysate glutamate concentration (p<0.0005 with respect to all other doses; Newman–Keuls test).
Figure 1.
Effects of systemic administration of 0.1, 0.2, 0.5, and 1 mg/kg of MK-801 (arrow) on the efflux of 5-HT (a) and glutamate (b) in the mPFC. Data (mean±SEM) are expressed as percentage changes of the four basal pretreatment values. Number of animals is given in parentheses. The control group received one injection of saline (arrow). *p<0.05, **p<0.005, ***p<0.0005, Newman–Keuls test following ANOVA.
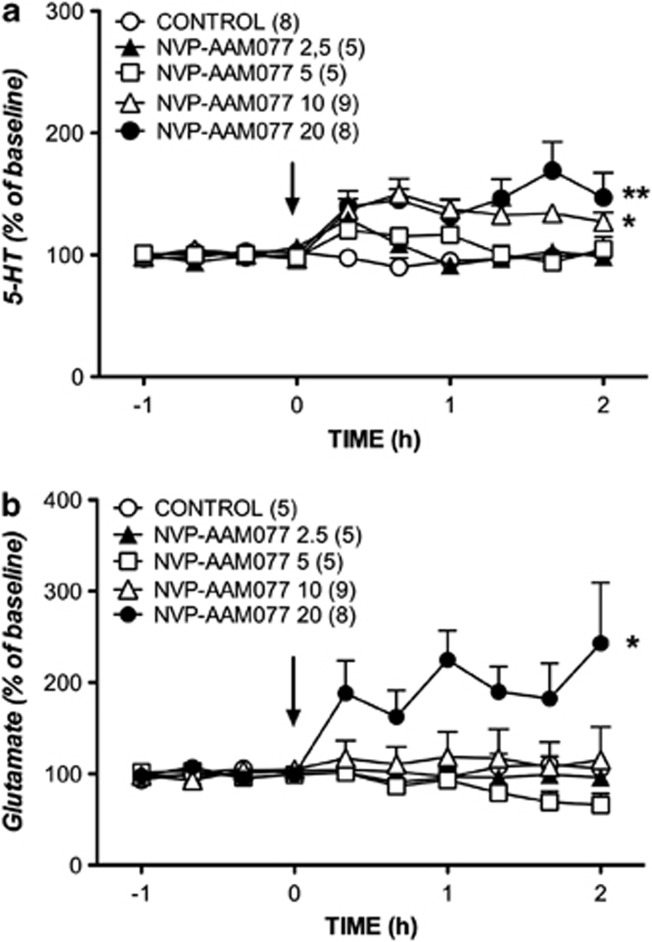
Similar effects were observed with the GluN2A subunit selective NVP-AAM077. Thus, two-way ANOVA showed that the administration of NVP-AAM077 (2.5–20 mg/kg, i.p.) increased the extracellular concentration of 5-HT (Figure 2a) as demonstrated by the significant effect of treatment (F4, 27=6.83, p=0.000620), time (F9, 243=6.68, p=0.00001), and treatment × time interaction (F36, 243=2.46, p=0.000028). The post hoc comparisons showed that the higher doses of NVP-AAM077 (10 and 20 mg/kg) increased 5-HT levels (p=0.014584 and p=0.002951, respectively, Newman–Keuls test). The systemic administration of NVP-AAM077 also increased extracellular glutamate (Figure 2b), as shown by the significant effect of treatment (F4, 27=3.99, p=0.011408). However, the effects of time (F9, 243=1.18, p=0.310293) and the interaction × time (F36, 243=1.37, p=0.088482) for glutamate did not show significant differences. The post hoc comparisons showed that only the highest dose of 20 mg/kg was able to increase the concentration of glutamate (p=0.026992, Newman–Keuls test).
Figure 2.
Effects of systemic administration of 2.5, 5, 10, and 20 mg/kg of NVP-AAM077 (arrow) on the efflux of 5-HT (a) and glutamate (b) in the mPFC. Data (mean±SEM) are expressed as percentage changes of the four basal pretreatment values. Number of animals is given in parentheses. The control group received one injection of vehicle (arrow). *p<0.05, **p<0.005, Newman–Keuls test following ANOVA.
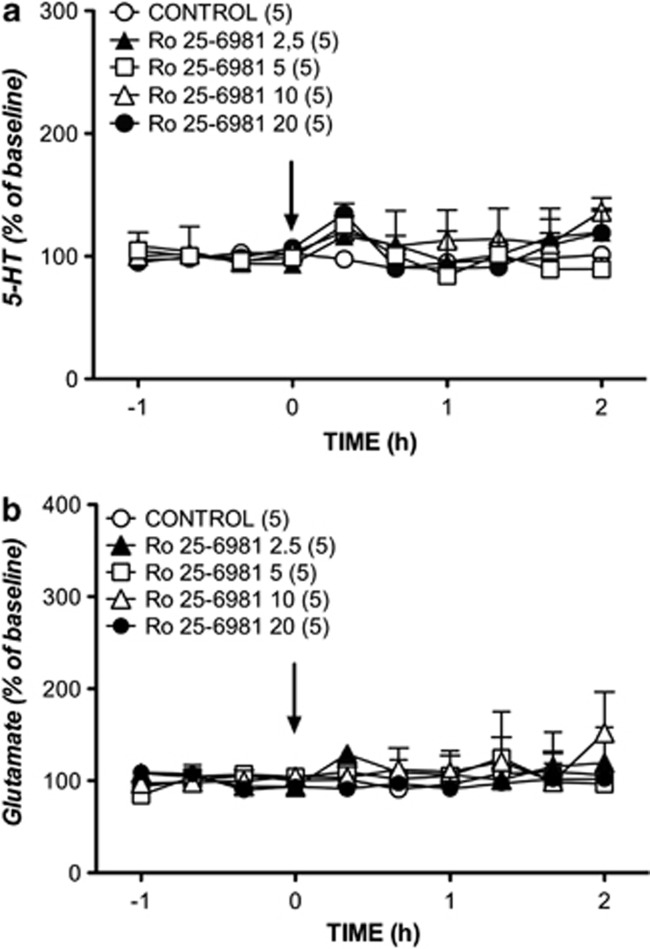
In contrast to nonselective (MK-801) and GluN2A subunit selective (NVP-AAM077) NMDA receptor antagonists, the GluN2B subunit selective antagonist, Ro 25-6981, did not exert any influence on the extracellular concentrations of 5-HT and glutamate at any of the doses tested (Figure 3).
Figure 3.
Effects of systemic administration of 2.5, 5, 10, and 20 mg/kg of Ro 25-6981 (arrow) on the efflux of 5-HT (a) and glutamate (b) in the mPFC. Data (mean±SEM) are expressed as percentage changes of the four basal pretreatment values. Number of animals is given in parentheses. The control group received one injection of vehicle (arrow).
Stereotypy Scores
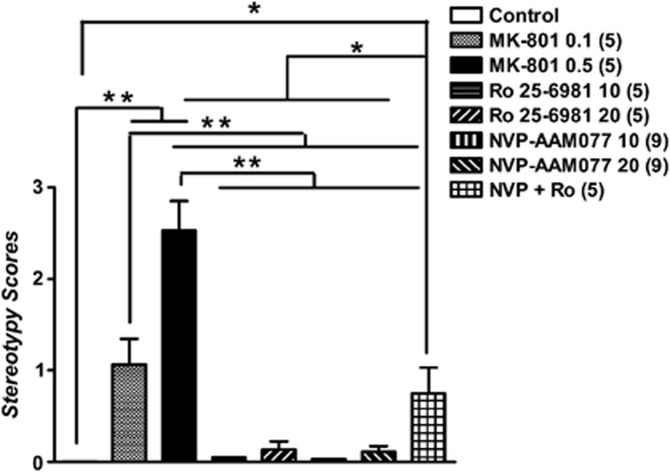
As shown in Figure 4, one-way ANOVA showed that the administration of the three NMDA receptor antagonists had a significant effect on the stereotypy scores (F6, 42=43.02, p=0.0001). The post hoc comparisons, however, indicated that only MK-801 (p<0.001, Newman–Keuls test) and the combination of NVP-AAM077 and Ro 25-6981 (p<0.05, Newman–Keuls test) induced marked stereotypies at the doses tested. In contrast, both NVP-AAM077 and Ro 25-6981 alone produced virtually no stereotypy.
Figure 4.
Effects of systemic administration of MK-801 (0.1 and 0.5 mg/kg), Ro 25-6981 (10 mg/kg), NVP-AAM077 (10 and 20 mg/kg), and the combination (NVP+Ro) of NVP-AAM077 (20 mg/kg) and Ro 25-6981 (20 mg/kg) on stereotypy scores. Both doses of MK-801 and NVP+Ro significantly increased stereotypies (turning, forepaw treading, head weaving) in rats. Systemic administration of Ro 25-6981 and NVP-AAM077 did not induce stereotypies. Behavioral scores are expressed as mean±SEM. *p<0.05, **p<0.001, Newman–Keuls test following ANOVA.
Antidepressant-Like Effects in the FST
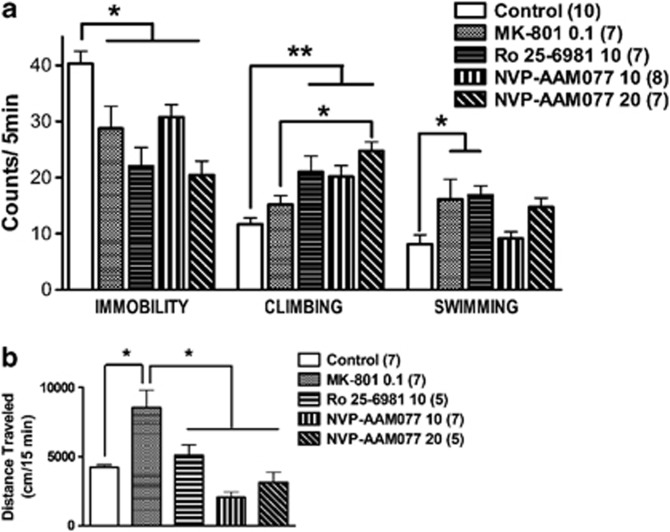
The acute administration of the three NMDA receptor antagonists studied had significant effects on immobility (F4, 32=7.81, p=0.000165), climbing (F4, 32=7.51, p=0.000212), and swimming (F4, 32=4.12, p=0.008372) behaviors measured in the FST (Figure 5a). The post hoc comparisons showed that the the three agents reduced immobility, but only the subunit selective inhibitors (NVP-AAM077 and Ro 25-6981) increased climbing (p<0.05, Newman–Keuls test). At the dose tested (0.1 mg/kg), the MK-801-induced reduction of immobility and increase in swimming was not suggestive of a true antidepressant-like effect, but rather of an enhanced general locomotor activity as depicted in Figure 5b (F4, 29=10.53, p<0.0001). The post hoc comparisons showed that MK-801 evoked hyperlocomotion (p<0.01, Newman–Keuls test), whereas the other agents did not alter locomotor activity.
Figure 5.
Effects of systemic administration of MK-801 (0.1 mg/kg), Ro 25-6981 (10 mg/kg), and NVP-AAM077 (10 and 20 mg/kg) on immobility, climbing, and swimming behaviors in the FST (a) and on locomotor activity in an open field (b). Data are expressed as mean±SEM. The three agents reduced immobility, but only Ro 25–6981 and NVP-AAM077 increased climbing. Swimming was increased only by MK-801 and Ro 25-6981. *p<0.05, **p<0.001, Newman–Keuls test following ANOVA. MK-801 (0.1 mg/kg) induced an increase in general locomotor activity (*p<0.01, Newman–Keuls test following ANOVA).
DISCUSSION
The main findings of this work are: first, both NVP-AAM077 and Ro 25-6981 possess antidepressant-like activity and, second, neither of these compounds alone exhibit psychotomimetic-like activity at the doses tested, but they did so when administered together. This and previous work from our lab demonstrated that the noncompetitive NMDA receptor antagonist, MK-801, induced stereotypies that were accompanied by an increased release of glutamate and 5-HT in the mPFC (López-Gil et al, 2007). MK-801 is a NMDA channel blocker that is not subunit selective, although it inhibits NMDA receptors containing the GluN2A subunit faster than those containing the GluN2B subunit (Gielen et al, 2009). Therefore, the question remained as to whether the MK-801-induced behavioral and biochemical effects were dependent on any specific GluN2 subunit. The present results show that the blockade of the GluN2A subunit is sufficient to evoke an enhanced release of glutamate and 5-HT, but suggests that blockade of both GluN2A and GluN2B subunits are necessary for stereotypical behavior to emerge. Our results are at odds with clinical observations showing that the sole blockade of the GluN2B subunit is sufficient to induce dissociative effects in depressed patients (Preskorn et al, 2008). The lack of effect of Ro 25-6981 could be because of an insufficient dosage or it is also possible that a transient surge in either 5-HT or glutamate in the mPFC could have been missed because of dilution effects in the 20-min dialysate sample. Alternatively, the effects of Ro 25-6981 may be area specific because it has been reported that this compound increases dialysate glutamate in other brain areas such as the globus pallidus (Mabrouk et al, 2013). Furthermore, it is also known that stereotypies produced by MK-801 do not result from changes in prefrontal cortex activity (López-Gil et al, 2012). On the other hand, although NVP-AAM077 failed to evoke stereotypical behavior, it has been reported that it can disrupt spatial learning (Hu et al, 2009), working memory (Smith et al, 2011), and increase aberrant gamma activity (Kocsis, 2012). Thus, it would seem at first sight that the blockade of the GluN2A subunit may contribute to cognitive deficits induced by NMDA receptor antagonists, but dissociative effects may be rather associated with GluN2B antagonists. However, recent studies showed that AZD6765, a nonselective, low-affinity NMDA receptor antagonist, has minimal dissociative effects (Sanacora et al, 2013; Zarate et al, 2014). This would suggest that the mechanisms that mediate the psychotomimetic effects are more complicated than simply resulting from the inhibition of a specific GluN2 subunit, and may rather be related to actions on specific circuits in the brain.
The next step in the present study was to examine whether the selective blockade of GluN2A or GluN2B subunits was equally effective in producing an antidepressant response. MK-801 was shown before to display antidepressant-like effects without altering locomotor activity, but at lower doses (0.05 mg/kg) than those tested in the present study (Maeng et al, 2008). Previous work had already demonstrated that Ro 25-6981 possessed antidepressant-like properties in the FST, the novelty-suppressed feeding test (NFST), and the deficit in sucrose consumption elicited by chronic unpredictable stress (Li et al, 2010, 2011). In rodents, these antidepressant effects are hypothesized to result from glutamate release and AMPA receptor activation (Li et al, 2010; Maeng et al, 2008).
In the clinic, GluN2B receptor antagonists have shown efficacy in treating depression (Preskorn et al, 2008; Ibrahim et al, 2012). Furthermore, a genome-wide association study evidenced alterations in the gene encoding the GluN2B subunit, GRIN2B, in depression (Aragam et al, 2013) and recent work revealed that GRIN2B confers susceptibility to treatment-resistant depression (Zhang et al, 2014). Post-mortem studies, however, have yielded discrepant results with no changes (Duric et al, 2013) or decreased expression of GluN2A and GluN2B in depression (Feyissa et al, 2009). It is possible that differences in the brain region examined or in subunit expression ratio account for such discrepancies. However, to the best of our knowledge, no GluN2A receptor antagonist is under evaluation for this pathological condition. Only one study has reported that the genetic inactivation of the GluN2A subunit induces anxiolytic- and antidepressant-like effects in mice (Boyce-Rustay and Holmes, 2006). Our results confirmed the antidepressant-like action of Ro 25-6981 and preliminarily extended this effect to the blockade of the GluN2A receptor subunit by NVP-AAM077. Furthermore, the acute increase in glutamate induced by NVP-AAM077 and its action on AMPA receptors can contribute to the release of BDNF and subsequent increase in synaptogenesis (Duman and Aghajanian, 2012) and synaptic plasticity (Maeng et al, 2008) that may be suggestive of an effective antidepressant action. Importantly, the lack of stereotypical behavior suggests that both Ro 25-6981 and NVP-AAM077 have the potential to show antidepressant effects without evoking psychotomimetic effects. Thus, independent blockade of either GluN2A or GluN2B subunits may show promise in the treatment of refractory depression, with a similar or better safety profile than nonselective NMDA receptor antagonists such as ketamine. Nevertheless, ketamine exhibits a faster onset of antidepressant response than GluN2B receptor antagonists (2 h vs 2–5 days). In contrast, GluN2A receptor antagonists could have a faster onset of therapeutic action because NMDA receptors containing GluN2A subunits possess a higher probability of channel opening (Gielen et al, 2009) and/or inducing changes in cortical 5-HT and glutamate. The role of GluN2B subunit is not limited to the modulation or prefrontal recurrent excitatory activity that is implicated in negative symptoms (Wang et al, 2013). Both behavioral and electrophysiological studies have recently shown that the GluN2B-mediated transmission is critical for sustaining hippocampal-to-prefrontal cortex function and plasticity (Gilmartin et al, 2013; Flores-Barrera et al, 2014).
In summary, our results show that the blockade of both subunits is necessary to develop psychotic-like effects, whereas the blockade of one of these subunits is sufficient to elicit an antidepressant-like action in the FST. Further research is needed to confirm the potential antidepressant efficay of selective GluN2A receptor antagonists as well as abuse liability and cognitive impairment after chronic exposure.
FUNDING AND DISCLOSURE
Dr Auberson is an employee of Novartis Institutes for Biomedical Research, the provider of NVP-AAM077. The other authors declare no conflict of interest.
Acknowledgments
L Jiménez-Sánchez was the recipient of a predoctoral fellowship from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). We also thank Novartis for the generous gift of NVP-AAM077. This work was supported by the Instituto de Salud Carlos III, Subdirección General del Evaluación y Fomento de la Investigación (FIS Grants PI10-01103 and PI13-00038) that were co-funded by the European Regional Development Fund (‘A way to build Europe'). Funding from the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) and the Generalitat de Catalunya (2009SGR220) is also acknowledged.
The funding agencies had no role in the design and conduct of the study, collection, management, analyses, and interpretation of the data; and preparation, review, or approval of the manuscript and the decision to submit it for publication.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adell A, Jiménez-Sánchez L, López-Gil X, Romón T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia. Schizophrenia Bull. 2012;38:9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl) 2013;230:291–298. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargós-Bosch M, López-Gil X, Artigas F, Adell A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol. 2006;9:565–573. doi: 10.1017/S1461145705005900. [DOI] [PubMed] [Google Scholar]
- Aragam N, Wang KS, Anderson JL, Liu X. TMPRSS9 and GRIN2B are associated with neuroticism: a genome-wide association study in a European sample. J Mol Neurosci. 2013;50:250–256. doi: 10.1007/s12031-012-9931-1. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperon F, Müller W, Auberson YP, Tricklebank MD, Neijt HC. Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav Pharmacol. 2003;14:477–487. doi: 10.1097/01.fbp.0000091471.79060.ed. [DOI] [PubMed] [Google Scholar]
- Chen H-SV, Lipton SA. Pharmacological implications of two distinct mechanisms of interaction of memantine with N-methyl-D-aspartate-gated channels. J Pharmacol Exp Ther. 2005;314:961–971. doi: 10.1124/jpet.105.085142. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75:508–516. doi: 10.1016/j.biopsych.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Weber M, Meilandt WJ, Wu T, Luu T, Deng L, et al. GluN2B antagonism affects interneurons and leads to immediate and persistent changes in synaptic plasticity, oscillations, and behavior. Neuropsychopharmacology. 2013;38:1221–1233. doi: 10.1038/npp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hu M, Sun YJ, Zhou QG, Auberson YP, Chen L, Hu Y, et al. Reduced spatial learning in mice treated with NVP-AAM077 through down-regulating neurogenesis. Eur J Pharmacol. 2009;622:37–44. doi: 10.1016/j.ejphar.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: behavioral and neurochemical evidence. Psychopharmacology (Berl) 1998;138:89–95. doi: 10.1007/s002130050649. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR.2003Animal models of schizophreniaIn: Hirsch SR, Weinberger DR, (eds)Schizophrenia Blackwell Science: Malden; 388–402. [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- López-Gil X, Jiménez-Sánchez L, Romón T, Campa L, Artigas F, Adell A. Importance of inter-hemispheric prefrontal connection in the effects of non-competitive NMDA receptor antagonists. Int J Neuropsychopharmacol. 2012;15:945–956. doi: 10.1017/S1461145711001064. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Mabrouk OS, Mela F, Calcagno M, Budri M, Viaro R, Dekundy A, et al. GluN2A and GluN2B NMDA receptor subunits differentially modulate striatal output pathways and contribute to levodopa-induced abnormal involuntary movements in dyskinetic rats. ACS Chem Neurosci. 2013;4:808–816. doi: 10.1021/cn400016d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: Limitations of the pharmacological approach. J Neurosci. 2006;36:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier/Academic Press: Amsterdam; 2005. [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol. 1997;8:196–215. [PubMed] [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. 2014Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects Mol Psychiatry,doi: 10.1038/mp.2013.130(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, et al. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology (Berl) 2011;205:203–216. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl) 2014;231:685–693. doi: 10.1007/s00213-013-3297-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.