Abstract
Technological advances in neuroimaging have enabled researchers to examine, in vivo, the relationship between psychotherapeutic interventions and markers of brain activity. This review focuses on two kinds of neuroimaging studies in psychotherapy: those that examine the patterns of brain activity associated with response to treatments and those that examine the changes that occur in brain activity during treatment. A general, hypothetical neural model of psychotherapy is presented, and support for the model is evaluated across anxiety disorders and major depression. Neuroimaging studies are broadly consistent in observing associations between response to psychotherapy and baseline activity in several key regions within the prefrontal cortex, basal ganglia, and limbic areas. These regions are involved in the generation and regulation of emotion, fear responding, and response to reward. Pre-post examinations of change following psychotherapy also typically observe that psychological treatments for anxiety and depression can affect neural activity in these regions. Despite general consensus that activity in these regions is associated with psychotherapy, substantial discrepancy persists regarding the precise direction of the observed relationships. Methodological challenges of the existing literature are considered, and future directions are discussed.
Psychotherapy's efficacy is well established for a wide range of emotional disorders. Despite this, no psychotherapeutic intervention works equally well for all patients, and the mechanisms through which psychotherapy reduces symptoms and enhances functioning remain difficult to specify. With the advent of neuroimaging technologies, researchers have new tools with which to identify clinically meaningful markers of brain function that are associated with treatment response. Two kinds of associations have been examined. Treatment outcome prediction studies seek to identify those patterns of brain function that confer a higher likelihood that a treatment will work. Treatment mechanism studies examine changes in the brain as a result of the intervention in question, in order to help understand how treatments are exerting their effects. Both types of study hold promise for developing a more complete understanding of the neural mechanisms involved in successful therapy, and both may guide future treatment refinement, novel mechanistic treatment development, and personalized treatment prescriptions tailored to individual patients.
Below, a brief overview of the general neural architecture believed to be relevant for anxiety, depression, and psychotherapeutic interventions for these disorders, is provided. Anxiety and depression are the focus of this review because these are the two disorders for which the most evidence has accrued, and also because there is good reason to believe that anxiety and depression share at least some underlying neural mechanisms. Functional neuroimaging studies of psychotherapy are reviewed, and recent advances towards improving the methodology and clinical relevance of research in this area are highlighted. Ideally, work will continue to progress towards greater relevance and import for the practicing clinician.
Neural Circuitry of Anxiety and Depression
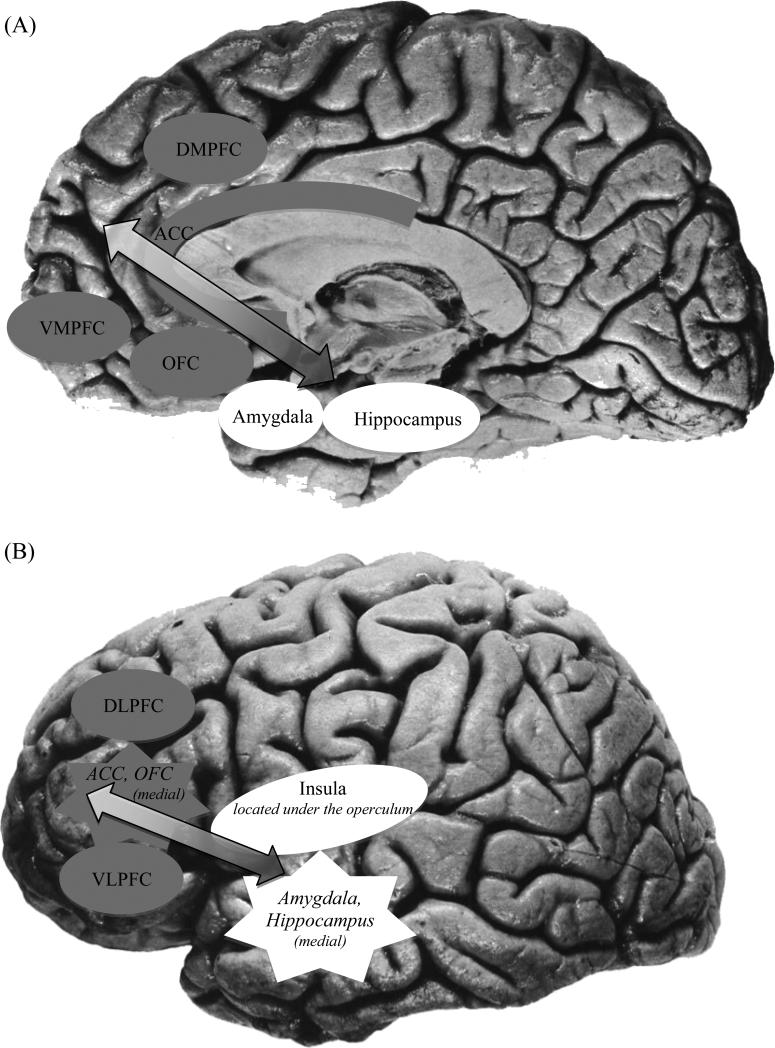
Contemporary neurobiological models of anxiety and depression include both distinct (anxiety- and depression-specific) and overlapping networks of brain regions. As displayed in Figure 1, the neural circuitry of general emotion dysregulation and high negative affect, which is implicated in both types of disorders, includes an interconnected set of brain regions involved in the generation and regulation of emotion (1-3). Limbic structures (such as the amygdala, hippocampus, and insula) react to emotional information. Activity from these regions feeds forward through the anterior cingulate cortex (ACC; involved in the appraisal and encoding of emotion), orbitofrontal cortex (OFC, involved in the integration of affective and sensory information and reward processing) and finally to the dorsomedial and ventromedial prefrontal cortices (DMPFC, VMPFC; involved in self-referential processing and in moderating emotional reactions). The initial activity in limbic regions can be regulated through top-down regions of the prefrontal cortex (PFC). Lateral prefrontal regions, including the dorsolateral and ventrolateral prefrontal cortex (DLPFC; VLPFC; both of which subserve higher-order cognitive functions), interact with the other frontal systems noted above, including the DMPFC, VMPFC, and ACC. These frontal systems are functionally interconnected with the amygdala and other limbic regions (3) and can modulate limbic activity during controlled processing of emotional stimuli (4).
Figure 1.
Neural circuitry of general emotion dysregulation and high negative affect as seen from medial (A; center of brain) and lateral (B; outside of brain) views. Limbic regions (white) such as the amygdala, hippocampus, and insula react to emotional information. Emotional information feeds forward from limbic regions (white) to cortical regions (gray), including the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and finally to the dorsomedial and ventromedial prefrontal cortices (DMPFC, VMPFC). Prefrontal cortical regions (gray) including the ACC, DMPFC, VMPFC, as well as dorsolateral and ventrolateral prefrontal cortices (DLPFC, VLPFC), can provide top-down regulation over limbic regions (white), modulating activity upward or downward depending on the context and goals.
The functioning of disorder specific networks is also key to understanding the relationship between psychotherapy and brain function. In addition to the general emotion processing and emotion regulation networks described above, there is a partially overlapping set of regions that shows increased activation to fear-related stimuli. This system forms a ‘fear network’ and is particularly relevant to the anxious arousal and exaggerated fear responses that characterize anxiety disorders. This fear-responsive circuitry includes limbic regions such as the amygdala, hippocampus, and parahippocampal gyrus, as well as the insula, periaqueductal gray, and medial portions of the PFC (mPFC) including VMPFC, OFC, and ACC (for a more detailed review, see (5)). Finally, the functioning of an additional network, the reward circuit, is particularly relevant in the treatment of major depression (2), as it may play a role in anhedonia. This network of regions includes the ventral striatum, portions of the thalamus, amygdala, OFC and mPFC (for a more detailed review, see (6)).
Hypothesized Neural Circuitry of Psychotherapy
One potential cause for many of the core symptoms of depression and anxiety, particularly those associated with negative emotional experiences, could be an inefficiency of top-down cortical control over regions that respond to emotional stimuli (e.g., limbic and fear-network related regions). Psychotherapy has broadly been hypothesized to remediate these neural abnormalities, and reduce symptoms, via a strengthening of the cortical, top-down emotion regulatory processes (Figure 1; see 7, 8). In this theory, improved PFC and cortical function would lead to enhanced regulation over limbic regions, thereby attenuating emotional reactions to negative inputs. Basic research in healthy control subjects suggests that psychotherapy skills, such as problem-solving, cognitive reappraisal, extinction learning (the critical learning mechanism in exposure therapy for anxiety), and modification of clients’ self-representations, each rely on the function of PFC structures (e.g., DLPFC, VLPFC, VMPFC, ACC; reviewed in (9)). This model therefore suggests that psychotherapy's effects should involve improved regulation in PFC regions and corresponding modulation of regions responding to negative or threatening stimuli.
Whereas the neural models described above permit hypotheses regarding which regions of the brain are likely to be relevant in psychotherapy research, it is considerably more difficult to generate hypotheses regarding the expected direction of effects, e.g., increases vs. decreases in activity. Following review of the existing research findings, a discussion of the complexity in making directional hypotheses is provided.
Neuroimaging and Treatment Outcome Prediction
Anxiety Disorders
The most consistent finding in treatment outcome prediction of anxiety disorders is that better response to therapy is associated with increased baseline hyper-reactivity in limbic or visual processing regions during provocation with threat-related visual stimuli. This pattern may indicate that a willingness or ability to adequately process threatening information is necessary to engage effectively with key psychotherapy strategies (e.g., exposure and habituation to feared stimuli). For instance, in a clinically anxious pediatric sample, better treatment response to either cognitive behavioral therapy or fluoxetine was associated with greater amygdalar activation while attending to threatening social stimuli (10). In a relatively large sample of social anxiety disorder patients who completed cognitive behavioral therapy, better response was predicted by increased activation to fearful faces in higher-order visual processing areas (occipitotemporal cortices, 11). An important strength of this last study was the use of a sophisticated nested cross-validation analysis to ensure that prediction estimates based on brain data were not inflated, as this is a common pitfall in neuroimaging research (12). Using this approach, occipitotemporal activations, in conjunction with behavioral variables, accounted for 41% of treatment outcome variance. This represented a 3-fold increase in comparison to the variance explained by behavioral variables alone, suggesting the added value of neuroimaging techniques from a personalized medicine perspective. Similar findings were reported in a smaller sample of patients with social anxiety disorder, in which increased activation to threatening faces in higher-order visual processing areas (e.g., angular gyrus) predicted better response to cognitive behavioral therapy (13).
With regard to PFC activation, one plausible hypothesis is that successful psychotherapy capitalizes on a patient's existing strengths. Therefore, if psychotherapy indeed relies on PFC regulation of limbic regions, increased PFC function at baseline may confer an improved chance of response. Consistent with this hypothesis, increased response to threatening faces in the DMPFC, ACC, and OFC has been found to predict better response to cognitive behavioral therapy in patients with social anxiety disorder (13), and increased prefrontal (VMPFC/OFC) metabolism at rest has been found to predict better treatment response to behavior therapy in obsessive-compulsive disorder patients (14). Similarly, in a combined sample of adults with generalized anxiety disorder and panic disorder, better response to cognitive behavioral therapy was associated with increased activation of frontal regions during cognitive reappraisal, including DLPFC and precentral gyrus, as well as by increased limbic activations in the insula and a parahippocampal region (15). This latter study represents an important advance towards clinical applicability of neuroimaging findings, as the authors employed a random forest classification procedure to identify neuroimaging variables (i.e., regional brain activations) that would allow individual patients to be robustly classified as therapy responders or non-responders. The combination of regions identified through this analysis yielded accurate classification for 79% of patients.
Of course, the opposing hypothesis is also plausible—that patients with the largest impairments in PFC function (i.e., decreased PFC function at baseline) may be in greatest need of the top-down regulatory skills provided during psychotherapy, and therefore may benefit most from this approach. Support for this contrasting hypothesis was found in two small studies, one examining obsessive compulsive disorder (16), and the other focusing on post-traumatic stress disorder (17). Across these studies, better response to cognitive behavioral therapy was predicted by decreased activity in regulatory regions including the DLPFC (16), VMPFC (16), and ACC (16, 17).
Major Depression
The most common finding in the prediction of treatment outcome for depression involves activity in portions of the ventral ACC, including the pregenual/rostral ACC [portions of Brodmann's Area (BA) 24 and 32] and the subgenual ACC (BA 25). Over 20 studies have converged to indicate that activity in the ventral ACC is associated with response to medications, psychotherapy, and their combination (see 18, 19-21). However, the precise direction and the exact location of predictive effects within the ventral ACC remain controversial. Several studies have observed that increased activity in the rostral ACC is associated with positive response to antidepressant medications (18). By contrast, Siegle and colleagues (19, 20) have demonstrated in three separate samples that lower sustained activity in the subgenual region of the ACC predicts good response to cognitive behavioral therapy for depression. Similar patterns in the ACC following cognitive behavioral therapy have been observed by two independent research groups using different task designs (22, 23).
The picture that emerges from the studies reviewed above seems to indicate that increased activity in ventral ACC should predict superior response to medications whereas decreased activity should predict superior response to cognitive behavioral therapy. There are, however, findings inconsistent with this account. In two separate samples, Mayberg and colleagues (24) (21) examined predictors of general non-response to cognitive behavioral therapy and medications. In both studies, pretreatment hypermetabolism in the ventral ACC was associated with poor response across treatments. Moreover, using some of the same data, Mayberg and colleagues failed to find that activity in the ventral ACC was associated with response to cognitive behavioral therapy (21). Part of the reason for the discrepant findings could stem from differences in imaging technology (i.e., measures of metabolism vs bloodflow) and methods (i.e., task-related activity vs resting activity). In addition, the ACC has been implicated both in the bottom-up generation of emotional experience and in the top-down regulation of limbic areas. As such, directional hypotheses about the nature of activity in this region are particularly difficult (20). It may be that relative increases or decreases in activity during a task are dependent on the resting activity prior to the start of the task (7). Moreover, the components of the ventral ACC, the rostral and subgenual ACC, are distinct subregions, with different cellular properties and separate patterns of connectivity to other regions (see 18). Specifying the precise role of each of these regions in emotion generation and regulation, as well as understanding how the functioning of these regions impacts different types of treatment, is an area of ongoing and active research.
To date, direct comparisons between brain-based predictors of psychotherapy and other active treatments have been rare. In an early, nonrandomized study, the connectivity between nodes of a fronto-limbic-thalamic circuit was examined (25). The authors observed that differences in the connectivity between the hippocampus and lateral PFC and between OFC and medial PFC differentiated responders to cognitive behavioral therapy versus medications. More recently, and in the first study of its kind, McGrath and colleagues (26) examined prescriptive brain-based predictors of differential response to cognitive behavioral therapy versus medication in the context of a randomized clinical trial. Focusing only on those subjects with the clearest clinical outcomes, i.e., those who met full remission criteria versus those who completed the trial without demonstrating much change, the authors identified six regions in which baseline metabolic activity predicted differential response: right anterior insula, right inferior temporal cortex, left amygdala, left premotor cortex, right motor cortex, and the precuneus. Of these, the authors note that activity in the anterior insula constituted the strongest finding. Individuals with reduced metabolism in the anterior insula at baseline were more likely to remit following cognitive behavioral therapy (and less likely following medications), whereas those with increased metabolism were more likely to remit to medications (and less likely following cognitive behavioral therapy).
Other regions have been shown to predict response to psychotherapy for depression in single treatment-modality studies, however, the following relationships have not yet been replicated and should be treated as preliminary. In addition to the ACC findings reviewed above, Siegle and colleagues found evidence that sustained activity in the right amygdala (19) and DLPFC (20) was associated with favorable response to cognitive behavioral therapy. The paracingulate gyrus (27) and vmPFC (28) may also be related to response to cognitive behavioral therapy. Finally, striatal reactivity to rewarding outcomes may predict favorable outcome to cognitive behavioral therapy among depressed adolescents (29).
Neuroimaging and Treatment Mechanisms
Several comprehensive reviews have examined brain-related changes following psychotherapy for anxiety and depression (see, e.g., 8, 30, 31). The review below focuses only on those findings that speak directly to the hypothesized model of the effects of psychotherapy – and, where possible, only on those findings that have been replicated.
Anxiety Disorders
In studies of psychotherapy for anxiety disorders, changes in activity in portions of the PFC and limbic systems are typically observed; however, the direction of changes varies based on the nature of the anxiety disorder and/or the study design. In the PFC, psychotherapy for posttraumatic stress disorder, for example, is associated with increased activation, across multiple different task conditions, following both Eye-movement Desensitization and Reprocessing therapy (e.g., 32, 33) and cognitive behavioral therapy (e.g., 34, 35). Within the PFC, ACC increases following cognitive behavioral therapy are emerging as a particularly well-replicated finding linked to posttraumatic stress disorder symptom decreases (34). In obsessive-compulsive disorder, by contrast, several studies have reported decreased activity in multiple regions of the PFC, including the OFC, (e.g., 36), DLPFC (36, 37), mPFC (37), and ACC (e.g., 36) across different tasks. It is important to keep in mind that in both obsessive-compulsive disorder and posttraumatic stress disorder, single studies have reported contradictory findings (see 8, 30, 31).
Less consistent evidence of changes in PFC activity has been observed in social anxiety disorder. In one study of cognitive behavioral therapy completers (13), decreases in VMPFC and DMPFC during symptom provocation were observed following treatment. However, in a large and well controlled psychotherapy trial, patients with social anxiety disorder engaged in a cognitive reappraisal task following exposure to personalized negative self-beliefs (38). Cognitive behavioral therapy was associated with increased DLPFC and DMPFC activity, earlier temporal onset of DMPFC activity, and increased DMPFC-amygdala functional connectivity, consistent with the hypothesized top-down cortical substrates of this specific therapy skill. This study is particularly notable in that it utilized a more ecologically valid, therapy-relevant cognitive task, allowing for a specific test of hypothesized neural mechanisms during the application of a particular skill.
Prefrontal effects following psychotherapy for panic disorder and specific phobia tend to involve those regions involved in the ‘fear network.’ Specifically, two small, uncontrolled PET studies of panic disorder found increased mPFC and decreased ACC metabolism at rest following cognitive behavioral therapy (39, 40); and a large fMRI study found reductions following cognitive behavioral therapy in left DLPFC during fear acquisition, which was correlated with reduction in agoraphobic symptoms (41). For specific phobia, during symptom provocation, three small studies were consistent in finding decreased medial PFC/ACC activity following cognitive behavioral therapy (42-44), while increased activation in the OFC was found in a distinct sample (e.g., 45).
Changes in limbic activity also appear to depend on the nature of the anxiety disorder in question. Reductions in amygdala activity following psychotherapy have been replicated only in studies of posttraumatic stress disorder (34, 35), whereas reductions in insula activity have been observed more generally. Following cognitive behavioral therapy, decreased insula activity has been reported during presentation of social threat cues in social anxiety disorder (13), during fear learning/acquisition in panic disorder (41), and during symptom provocation in specific phobia (e.g., 43, 44). In the hippocampus, the direction of pre-post changes appears to differ depending on the disorder. Posttraumatic stress disorder has been associated with increases in hippocampal activity following therapy (e.g., 34, 35), whereas reductions in this region have been observed following cognitive behavioral therapy for social anxiety disorder (46) and following psychodynamic psychotherapy for panic disorder (47). Changes in limbic, and other relevant regions, following treatment of obsessive-compulsive disorder have been relatively inconsistent across trials (see 8, 30, 31).
Major Depression
To date, at least two studies have been conducted to examine brain-related changes in response to each of the major schools of psychotherapy for depression: interpersonal psychotherapy, cognitive behavioral therapy, behavioral activation, and psychodynamic psychotherapy. The majority of these studies have examined change following psychotherapy on its own, or in comparison to changes observed over the same period of time in healthy controls. Very few studies have compared changes following psychotherapy with changes following a different active treatment, and to date, only one study (48) has done so in the context of a fully randomized clinical trial.
Across psychotherapies for depression, reductions in activity or metabolism have been observed in several PFC regions, including DLPFC, VLPFC, and/or medial prefrontal regions (e.g., dorsal and ventral mPFC, subgenual ACC, and OFC). Although the precise regions in the PFC differ somewhat across studies and treatments, reductions in one or more of these regions have been observed following interpersonal therapy (49), cognitive behavioral therapy (48, 50), behavioral activation (27), and psychodynamic psychotherapy (51). One of the most well-replicated findings from this literature is that treatment with cognitive behavioral therapy is associated with a reduction in emotional biases towards negative stimuli in the PFC, as evidenced by relative reductions in activity to negative stimuli and increases to positive stimuli (22, 28).
Regarding changes in other neural regions, results to date are more preliminary and mixed. For example, both decreases and increases in activity in the amygdala and hippocampus have been observed in response to cognitive behavioral therapy (23, 28, 50). Furthermore, whereas studies have reported increases in activity in dorsal (23, 50) and ventral (48) areas of the cingulate cortex following cognitive behavioral therapy, Siegle and colleagues (19) observed that cognitive behavioral therapy responders did not show an increase in ventral ACC activity. Rather, the majority of those who responded to treatment had lower sustained activity in the ACC compared to healthy controls both before and after treatment. The precise location within the ACC of these sets of findings differs, and additional work will no doubt be needed to identify sources of these discrepancies.
Preliminary evidence from a single study suggests that treatment with behavioral activation can also affect reward systems in the brain. Behavioral activation was associated with increased activity in the caudate (a component of the striatal reward system) during reward anticipation and with changes to the paracingulate gryus (in mPFC) during reward selection and reward feedback (52). Additional preliminary findings suggest molecular effects of psychodynamic therapy. In two small, preliminary studies, the authors observed an increase in serotonin transporter availability in the midbrain (53) and increases in serotonin receptor density in several frontal regions including OFC, ventral ACC, mPFC, and DLPFC (54) following psychodynamic psychotherapy.
General Discussion
The findings reviewed above are broadly consistent with predictions regarding the neural substrates of psychotherapy. Activity in regions associated with negative emotion, emotion regulation, fear, and reward are associated with response to psychotherapy, and psychotherapy appears to alter the functioning of these regions. Beyond understanding which regions are involved, however, the state of the field has not yet evolved sufficiently to make many specific conclusions regarding the direction of these effects. Conflicting directional observations may, for example, be due to differences in task states (imaging during a resting-state vs. symptom provocation vs. application of a specific therapy skill). Increased PFC function at rest may in fact contribute to decreased capacity for activation of the PFC in response to symptom provocation or skill application, leading to findings in opposing directions depending on the task state that is examined (7). Furthermore, regional increases and decreases observed in neuroimaging are currently subject to multiple interpretations. Increased activation in a given region might be interpreted as reflecting an improvement in the strength of the region's function, or as an impairment in the region's efficiency, reflecting a need for greater activity in order to accomplish the same effect. One goal of future research will be to further clarify the precise nature of associations and to resolve the inconsistencies that have been observed.
The fact that brain function measured pretreatment is associated with the likelihood of response to psychotherapies is important. It suggests that future refinements regarding the precise direction of these effects across multiple kinds of tasks may enable treatments to be selected or individually tailored to the unique needs of the individual. Currently, several studies of psychotherapeutic treatment for anxiety have consistently implicated increased hyperresponsivity of regions reacting to threatening stimuli (e.g., limbic and visual processing areas) as a marker of better response to therapy. Such hyperreactivity may represent a clinically useful biomarker conferring a higher chance of a positive outcome from therapy, perhaps due to increased engagement with anxiety-provoking stimuli at baseline.
The pattern of predictive findings from studies of depression suggests that the functional state of the emotion regulation system, and potentially the reward system, prior to treatment has important consequences for the efficacy of cognitive behavioral therapy. This may not be surprising. Cognitive behavioral therapy is believed to engage and strengthen the patient's ability to regulate and alter their emotional states. Little doubt remains that the ventral portions of the ACC play a key role in determining the likelihood of treatment response for depression, however, more work is needed to specify which specific sub-regions are critical, and what patterns of activity (to which tasks) are predictive of good or bad outcomes.
In order for brain-based predictive findings to become applicable in the clinic, greater clarity is needed regarding the precise task and imaging parameters that will lead to reproducible results at the single-patient level. Future work should aim to build on the strengths of recent studies, which used larger samples (11, 15), randomization of participants to different treatments (26), and more sophisticated analytic approaches that allow researchers to estimate the added benefit of neuroimaging data (11), to examine patterns of communication between brain regions (25), and to draw inferences that are valid at the individual patient level (15). These advances will help to address questions such as which treatment option is best for which patient. Additionally, when multiple brain regions are observed to predict response, as in (26), effort needs to be made to combine these multiple predictors in order to make a single treatment recommendation for the patient (see 55 for a new method for doing so.)
Examining change in brain function over the course of treatment can be a valuable tool with which to understand the mechanisms through which a given treatment is operating. As others have noted (see 30, 56), the best designs for such studies would have at least three groups: the group receiving the treatment in question, a group of similar psychiatric patients who either receive a different treatment or no treatment, and a group of relatively well matched healthy control subjects. With such a design, researchers would be able to determine whether or not the mechanisms observed are unique to the treatment under investigation, and whether the treatment involves normalization of function or the recruitment of compensatory systems. Additionally, researchers must examine and demonstrate that the psychometric properties of neuroimaging-derived markers, e.g., test-retest reliability, are sound (57).
Currently, pre-post psychotherapy neuroimaging studies in anxiety disorders reveal some evidence consistent with hypothesized psychotherapy substrates (e.g., PFC increases, limbic decreases, and/or increased PFC-limbic functional connectivity). Evidence for increased activation in top-down PFC regions has been found most consistently in studies of posttraumatic stress disorder, while the findings in other anxiety disorders tend to suggest limbic decreases, either accompanied by concomitant PFC decreases (e.g., obsessive compulsive disorder), or without consistent evidence of increases or decreases in regulatory regions.
Regarding changes during psychotherapy for depression, the most consistent findings are decreased activity across several regions of the PFC following multiple forms of psychotherapy, and reductions in negatively-biased information processing in the PFC following cognitive behavioral therapy. The fact that psychotherapy is broadly associated with decreased activity in several PFC regions may be surprising, and it appears to run counter to the hypothesis that psychotherapy should strengthen the ability of top-down control regions to modify processing in down-stream systems. There is simply not yet enough information to resolve this issue, which is likely related to the methodological factors discussed above. It is also possible that the hypothesized mechanism of action of psychotherapy for depression is incorrect. More work will be needed to test these possibilities. The most informative studies will be those that test the core hypothesis that psychotherapy exerts effects via a top-down cortical route. If this hypothesis is true, psychotherapies should involve increasing communication between PFC and limbic areas, as evidenced by increased ‘functional connectivity’ across these regions. To date only one treatment study in depression has examined this issue (25), and many more are needed to determine under what conditions psychotherapy can and cannot affect the functioning of the key neural circuits relevant to depression.
Methodological Challenges and Future Research
Although neuroimaging studies have tremendous potential to advance our understanding of psychotherapy process and outcomes, this area of research is relatively new. As such, the clinical relevance and applicability of findings to date are limited. Treatment outcome prediction studies have rarely included more than one treatment condition, and thus cannot differentiate between prescriptive effects (which would allow for the selection of the specific treatment most likely to work for a given patient, relative to other treatments) and prognostic effects (which indicate more generally whether treatment itself is likely to be effective). Similarly, treatment mechanism studies have often used methods that cannot adequately distinguish between mechanisms of change through which the therapy is exerting its specific effects, practice effects associated with repeated testing, and non-specific neural correlates of symptom improvement.
A handful of more recent studies offer a glimpse of the cutting edge within this area of research, through inclusion of larger, well-controlled samples (26, 38, 41, 58), tasks designed to probe specific therapy skills (38, 58), and explicit analysis of the relationship between neural change and treatment efficacy (e.g., symptom reduction, 41, or adherence, 58). Additional research is also needed to examine the effects of specific components of treatment. One notable example is a study utilizing EEG measures of cortical activity to examine the association between brain function and cortical activity before and after a brief, 30 minute, cognitive behavioral therapy analogue training session (59). Approaches like this may aid in identifying the neural mechanisms associated with specific therapeutic interventions. Finally, newer technologies, such as functional near-infrared spectroscopy (fNIRS), may provide researchers with additional tools with which to mitigate some of the methodological challenges noted above (60). During fNIRS, participants wear skullcaps containing devices that emit and detect near-infrared light, with which changes in cortical blood flow can be examined. fNIRS frees participants from the confines of a magnetic scanner, and as such, allows participants to engage in tasks not possible during more standard neuroimaging protocols.
Neuroimaging in psychotherapy is an active and growing area of research. It holds tremendous promise for helping clinicians tailor specific interventions to the needs of individual patients, and it may help scientists to determine how psychotherapeutic treatments work. By continuing to build on recent methodological advances, research in this area has the potential to fulfill its ‘bench-to-bedside’ promise and improve clinical care across the full range of psychological suffering.
4 Multiple Choice CME questions.
Which of the following statements best characterizes neuroimaging findings on the prediction of therapy outcome in anxiety disorders?
Increased activity in limbic and visual regions processing threat stimuli predicts better therapy outcome across anxiety disorders, while the direction of PFC effects is less consistent.
Decreased activity in PFC regions predicts better therapy outcome across anxiety disorders, while the direction of limbic region findings is less consistent.
Increased activity in limbic and visual regions processing threat stimuli predicts better therapy outcome for specific disorders including PTSD, while decreased activity in these regions predicts better therapy outcome for other disorders including OCD.
Decreased activity in limbic and visual regions processing threat, and increased activity in PFC regions, predicts better therapy outcome for social anxiety.
Activity in which region is most consistently implicated in the prediction of response to psychotherapeutic treatments for depression?
Dorsolateral prefrontal cortex (DLPFC)
Ventrolateral prefrontal cortex (VLPFC)
Amygdala
Ventral anterior cingulate cortex (ACC)
Which of the following statements best characterizes pre-post therapy neuroimaging findings in anxiety disorders?
Activity in PFC regions increases while activity in limbic regions decreases across anxiety disorders.
Activity in both PFC and limbic regions decreases across anxiety disorders.
Activity in PFC regions tends to increase for specific disorders including PTSD, while activity in PFC regions tends to decrease for other disorders including OCD.
There are no replicated findings indicating a consistent direction of change in PFC or limbic regions for any specific anxiety disorder.
Which of the following statements best reflects changes in prefrontal cortical activation/metabolism following different psychotherapies for depression?
Decreases in activity/metabolism have been observed following interpersonal and psychodynamic psychotherapy, whereas increases have been observed following cognitive behavioral therapy and behavioral activation.
Increases in activity/metabolism have been observed following interpersonal and psychodynamic psychotherapy, whereas decreases have been observed following cognitive behavioral therapy and behavioral activation.
All four major models of psychotherapy for depression have been associated with increases in prefrontal cortical activity/metabolism.
All four major models of psychotherapy for depression have been associated with decreases in prefrontal cortical activity/metabolism.
Table.
Abbreviations used in this Manuscript
| Brain regions | |
| ACC | Anterior Cingulate Cortex |
| BA | Brodmann's Area |
| DLPFC | Dorsolateral Prefrontal Cortex |
| DMPFC | Dorsomedial Prefrontal Cortex |
| OFC | Orbitofrontal Cortex |
| PFC | Prefrontal Cortex |
| VLPFC | Ventrolateral Prefrontal Cortex |
| VMPFC | Ventromedial Prefrontal Cortex |
| Brain imaging techniques | |
| fMRI | Functional Magnetic Resonance Imaging |
| fNIRS | Functional Near-Infrared Spectroscopy |
| PET | Positron Emission Tomography |
Acknowledgements
JCF is supported by K23MH097889 and RBP is supported by K23MH100259, both awarded by the National Institute of Mental Health.
Footnotes
Authors’ Statement: JCF and RBP report no competing interests.
References
- 1.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. The Lancet. 2012;379(9820):1045–55. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychologist. 2000;55(11):1196–214. doi: 10.1037//0003-066x.55.11.1196. Epub 2001/04/03. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. Epub 2001/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Carvalho MR, Dias GP, Cosci F, de-Melo-Neto VL, Bevilaqua MC, Gardino PF, Nardi AE. Current findings of fMRI in panic disorder: contributions for the fear neurocircuitry and CBT effects. Expert Review of Neurotherapeutics. 2010;10(2):291–303. doi: 10.1586/ern.09.161. Epub 2010/02/09. [DOI] [PubMed] [Google Scholar]
- 6.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68(2):118–24. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 7.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depressions: Treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9(10):788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weingarten CP, Strauman TJ. Neuroimaging for psychotherapy research: Current trends. Psychotherapy Research. 2014:1–29. doi: 10.1080/10503307.2014.883088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frewen P, Dozois DJ, Lanius R. Neuroimaging studies of psychological interventions for mood and anxiety disorders: empirical and methodological review. Clinical psychology review. 2008;28:228–46. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191(1):97–105. doi: 10.1007/s00213-006-0542-9. Epub 2006/09/15. [DOI] [PubMed] [Google Scholar]
- 11.Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG, Pollack M, Gabrieli JD. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70(1):87–97. doi: 10.1001/2013.jamapsychiatry.5. Epub 2012/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biological Psychiatry. 2013:1–3. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brody AL, Saxena S, Schwartz JM, Stoessel PW, Maidment K, Phelps ME, Baxter LR., Jr. FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Research. 1998;84(1):1–6. doi: 10.1016/s0925-4927(98)00041-9. Epub 1998/12/31. [DOI] [PubMed] [Google Scholar]
- 15.Ball TM, Stein MB, Ramsawh HJ, Campbell-Sills L, Paulus MP. Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology. 2013 Epub 2013/11/26. [Google Scholar]
- 16.Olatunji BO, Ferreira-Garcia R, Caseras X, Fullana MA, Wooderson S, Speckens A, Lawrence N, Giampietro V, Brammer MJ, Phillips ML, Fontenelle LF, Mataix-Cols D. Predicting response to cognitive behavioral therapy in contamination-based obsessive-compulsive disorder from functional magnetic resonance imaging. Psychological Medicine. 2013:1–13. doi: 10.1017/S0033291713002766. Epub 2013/11/16. [DOI] [PubMed] [Google Scholar]
- 17.Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychological Methods. 2008;38(4):555–61. doi: 10.1017/S0033291707002231. Epub 2007/11/17. [DOI] [PubMed] [Google Scholar]
- 18.Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. A J Psychiatry. 2006;163(4):735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 20.Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69(9):913–24. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer Iii PE, Craighead WE, Mayberg HS. Pretreatment Brain States Identify Likely Nonresponse to Standard Treatments for Depression. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yoshino A, Ueda K, Suzuki Si, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural Responses to Sad Facial Expressions in Major Depression Following Cognitive Behavioral Therapy. Biol Psychiatry. 2008;64(6):505–12. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. Journal of psychiatry & neuroscience : JPN. 2009;34(3):175. [PMC free article] [PubMed] [Google Scholar]
- 25.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic–frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22(1):409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 26.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA psychiatry (Chicago, Ill) 2013;70(8):821–9. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dichter GS, Felder JN, Smoski MJ. The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: An fMRI investigation. J Affect Disord. 2010;126(1-2):236–44. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45(5):577–87. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):107–18. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: A systematic and critical review. Prog Neurobiol. 2013:1–14. doi: 10.1016/j.pneurobio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Quide Y, Witteveen AB, El-Hage W, Veltman DJ, Olff M. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neuroscience and Biobehavioral Reviews. 2012;36(1):626–44. doi: 10.1016/j.neubiorev.2011.09.004. Epub 2011/10/04. [DOI] [PubMed] [Google Scholar]
- 32.Lansing K, Amen DG, Hanks C, Rudy L. High-resolution brain SPECT imaging and eye movement desensitization and reprocessing in police officers with PTSD. Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17(4):526–32. doi: 10.1176/jnp.17.4.526. Epub 2006/01/03. [DOI] [PubMed] [Google Scholar]
- 33.Pagani M, Hogberg G, Salmaso D, Nardo D, Sundin O, Jonsson C, Soares J, Aberg-Wistedt A, Jacobsson H, Larsson SA, Hallstrom T. Effects of EMDR psychotherapy on 99mTc-HMPAO distribution in occupation-related post-traumatic stress disorder. Nuclear Medicine Communication. 2007;28(10):757–65. doi: 10.1097/MNM.0b013e3282742035. Epub 2007/08/31. [DOI] [PubMed] [Google Scholar]
- 34.Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science. 2007;18:127–9. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 35.Peres JF, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJ, Nasello AG. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychological Medicine. 2007;37(10):1481–91. doi: 10.1017/S003329170700997X. Epub 2007/02/10. [DOI] [PubMed] [Google Scholar]
- 36.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, Togao O, Kanba S. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(8):901–10. doi: 10.1016/j.biopsych.2004.12.039. Epub 2005/04/12. [DOI] [PubMed] [Google Scholar]
- 37.Yamanishi T, Nakaaki S, Omori IM, Hashimoto N, Shinagawa Y, Hongo J, Horikoshi M, Tohyama J, Akechi T, Soma T, Iidaka T, Furukawa TA. Changes after behavior therapy among responsive and nonresponsive patients with obsessive-compulsive disorder. Psychiatry Research. 2009;172(3):242–50. doi: 10.1016/j.pscychresns.2008.07.004. Epub 2009/04/07. [DOI] [PubMed] [Google Scholar]
- 38.Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70(10):1048–56. doi: 10.1001/jamapsychiatry.2013.234. Epub 2013/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasko J, Horacek J, Zalesky R, Kopecek M, Novak T, Paskova B, Skrdlantova L, Belohlavek O, Hoschl C. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinology Letters. 2004;25(5):340–8. Epub 2004/12/08. [PubMed] [Google Scholar]
- 40.Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A, Diksic M, Kuboki T. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33(1):218–26. doi: 10.1016/j.neuroimage.2006.06.017. Epub 2006/08/08. [DOI] [PubMed] [Google Scholar]
- 41.Kircher T, Arolt V, Jansen A, Pyka M, Reinhardt I, Kellermann T, Konrad C, Lueken U, Gloster AT, Gerlach AL, Strohle A, Wittmann A, Pfleiderer B, Wittchen HU, Straube B. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biological Psychiatry. 2013;73(1):93–101. doi: 10.1016/j.biopsych.2012.07.026. Epub 2012/08/28. [DOI] [PubMed] [Google Scholar]
- 42.Paquette V, Lévesque J, Mensour B, Leroux J-M, Beaudoin G, Bourgouin P, Beauregard M. “Change the mind and you change the brain”: effects of cognitive-behavioral therapy on the neural correlates of spider phobia. NeuroImage. 2003;18:401–9. doi: 10.1016/s1053-8119(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 43.Straube T, Glauer M, Dilger S, Mentzel H- J, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage. 2006;29:125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Hauner KK, Mineka S, Voss JL, Paller KA. Exposure therapy triggers lasting reorganization of neural fear processing. Proceedings of the National Academy of Sciences. 2012;109(23):9203–8. doi: 10.1073/pnas.1205242109. Epub 2012/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schienle A, Schafer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia: an fMRI study on exposure therapy. European Archives of Psychiatry and Clinical Neuroscience. 2007;257(8):486–93. doi: 10.1007/s00406-007-0754-y. Epub 2007/09/29. [DOI] [PubMed] [Google Scholar]
- 46.Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of general psychiatry. 2002;59:425–33. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 47.Beutel ME, Stark R, Pan H, Silbersweig D, Dietrich S. Changes of brain activation pre- post short-term psychodynamic inpatient psychotherapy: an fMRI study of panic disorder patients. Psychiatry Research. 2010;184(2):96–104. doi: 10.1016/j.pscychresns.2010.06.005. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. A J Psychiatry. 2007;164(5):778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 49.Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang S- C, Wu H- M, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR. Regional Brain Metabolic Changes in Patients With Major Depression Treated With Either Paroxetine or Interpersonal Therapy: Preliminary Findings. Arch Gen Psychiatry. 2001;58(7):631–40. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 50.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of Cortical-Limbic Pathways in Major Depression: Treatment-Specific Effects of Cognitive Behavior Therapy. Arch Gen Psychiatry. 2004;61(1):34. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 51.Buchheim A, Viviani R, Kessler H, Kächele H, Cierpka M, Roth G, George C, Kernberg OF, Bruns G, Taubner S. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PLoS ONE. 2012;7(3):e33745. doi: 10.1371/journal.pone.0033745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression. BPS. 2009;66(9):886–97. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehto SM, Tolmunen T, Joensuu M, Saarinen PI, Valkonen-Korhonen M, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Changes in midbrain serotonin transporter availability in atypically depressed subjects after oneyear of psychotherapy. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(1):229–37. doi: 10.1016/j.pnpbp.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Karlsson H, Hirvonen J, Salminen J, Hietala J. Increased Serotonin Receptor 1A Binding in Major Depressive Disorder after Psychotherapy, but Not after SSRI Pharmacotherapy, Is Related to Improved Social Functioning Capacity. Psychother Psychosom. 2013;82(4):260–1. doi: 10.1159/000346143. [DOI] [PubMed] [Google Scholar]
- 55.DeRubeis RJ, Cohen ZD, Forand NR, Fournier JC, Gelfand LA, Lorenzo-Luaces L. The Personalized Advantage Index: Translating Research on Prediction into Individualized Treatment Recommendations. A Demonstration. PLoS ONE. 2014;9(1):e83875. doi: 10.1371/journal.pone.0083875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linden DEJ. How psychotherapy changes the brain--the contribution of functional neuroimaging. Mol Psychiatry. 2006;11(6):528–38. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- 57.Barch DM, Mathalon DH. Using brain imaging measures in studies of procognitive pharmacologic agents in schizophrenia: psychometric and quality assurance considerations. Biol Psychiatry. 2011;70(1):13–8. doi: 10.1016/j.biopsych.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldin P, Ziv M, Jazaieri H, Hahn K, Gross JJ. MBSR vs aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Social Cognitive and Affective Neuroscience. 2013;8(1):65–72. doi: 10.1093/scan/nss054. Epub 2012/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biol Psychol. 2005;70(3):141–51. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Roffman JL, Marci CD, Glick DM, Dougherty DD, Rauch SL. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med. 2005;35(10):1385–98. doi: 10.1017/S0033291705005064. [DOI] [PubMed] [Google Scholar]
List of Influential Citations
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA psychiatry (Chicago, Ill) 2013;70(8):821–9. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Archives of General Psychiatry. 2012;69(9):913–924. doi: 10.1001/archgenpsychiatry.2012.65. doi:10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yoshino A, Ueda K, Suzuki Si, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten CP, Strauman TJ. Neuroimaging for psychotherapy research: Current trends. Psychotherapy Research : Journal of the Society for Psychotherapy Research. 2014 doi: 10.1080/10503307.2014.883088. doi:10.1080/10503307.2014.883088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. The Lancet. 2012;379(9820):1045–1055. doi: 10.1016/S0140-6736(11)60602-8. doi:10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P, Dozois DJ, Lanius R. Neuroimaging studies of psychological interventions for mood and anxiety disorders: empirical and methodological review. Clinical psychology review. 2008;28:228–46. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: A systematic and critical review. Prog Neurobiol. 2013 Nov;20:1–14. doi: 10.1016/j.pneurobio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Quide Y, Witteveen AB, El-Hage W, Veltman DJ, Olff M. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neuroscience and Biobehavioral Reviews. 2012 Jan;36(1):626–44. doi: 10.1016/j.neubiorev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013 Oct;70(10):1048–56. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball TM, Stein MB, Ramsawh HJ, Campbell-Sills L, Paulus MP. Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology. 2013 Nov 25; [Google Scholar]