Abstract
Background
Rad (Ras associated with diabetes) GTPase is the prototypic member of a subfamily of Ras-related small G proteins. The aim of the present study was to define whether Rad plays an important role in mediating cardiac hypertrophy.
Methods and Results
We document for the first time that levels of Rad mRNA and protein were decreased significantly in human failing hearts (n=10) compared with normal hearts (n=3; P<0.01). Similarly, Rad expression was decreased significantly in cardiac hypertrophy induced by pressure overload and in cultured cardiomyocytes with hypertrophy induced by 10 μmol/L phenylephrine. Gain and loss of Rad function in cardiomyocytes significantly inhibited and increased phenylephrine-induced hypertrophy, respectively. In addition, activation of calcium-calmodulin–dependent kinase II (CaMKII), a strong inducer of cardiac hypertrophy, was significantly inhibited by Rad overexpression. Conversely, downregulation of CaMKIIδ by RNA interference technology attenuated the phenylephrine-induced hypertrophic response in cardiomyocytes in which Rad was also knocked down. To further elucidate the potential role of Rad in vivo, we generated Rad-deficient mice and demonstrated that they were more susceptible to cardiac hypertrophy associated with increased CaMKII phosphorylation than wild-type littermate controls.
Conclusions
The present data document for the first time that Rad is a novel mediator that inhibits cardiac hypertrophy through the CaMKII pathway. The present study will have significant implications for understanding the mechanisms of cardiac hypertrophy and setting the basis for the development of new strategies for treatment of cardiac hypertrophy.
Keywords: cardiomyopathy, genes, heart diseases, hypertrophy, natriuretic peptides
Cardiac hypertrophy is an important adaptive growth response to facilitate an increase in myocardial contractility. Although sustained hypertrophy is an initial compensatory mechanism to preserve cardiac function, it is an independent major risk factor for cardiac morbidity and mortality.1 Emerging data suggest that pathological structural changes in the heart are induced in part by small G proteins (also called GTPases).2 In the cardiovascular system, small G proteins are implicated in regulation of endothelial function3; vascular smooth muscle cell (VSMC) contraction, proliferation, and migration; and cardiac hypertrophy.4
Rad (Ras associated with diabetes) GTPase, a 33- to 35-kDa protein, was originally identified from skeletal muscle of patients with type 2 diabetes mellitus.5,6 Rad and its closely related GTPases (Gem, Kir, Rem, and Rem2) form an RGK subfamily in the Ras family of small GTPases.2 To date, it has been reported that Rad can inhibit insulin-stimulated glucose uptake in myocyte and adipocyte cell lines.7 In addition, RGK GTPases are able to function as potent inhibitors of voltage-dependent calcium channels by directly binding to their β-subunit.8 Moreover, Rad and Gem can modulate cytoskeleton remodeling through the Rho/Rhokinase pathway.9 Thus, Rad is a key GTPase that has many important biological functions.
In recent studies designed to explore the role of Rad in the cardiovascular system at large, we demonstrated that Rad expression was upregulated in VSMCs during vascular lesion formation. Overexpression of Rad significantly inhibited the attachment and migration of VSMCs and suppressed neointimal formation after balloon injury.10 Interestingly, we and others11 recently found that Rad is most abundantly expressed in the heart, which indicates that Rad may be involved in cardiac physiology and/or pathophysiological processes. In the present study, using gain-of-function and loss-of-function strategies both in vivo and in vitro, we have documented for the first time that Rad is a novel mediator that inhibits cardiac hypertrophy through the calmodulin-dependent kinase II (CaMKII) pathway.
Methods
Cardiomyocyte Cultures
Primary cardiomyocytes were prepared from 2-day-old Sprague-Dawley rats as described previously.12 Cardiomyocytes were plated at a density of 2.5×105 cells/well in 12-well plates and cultured for 16 to 20 hours in DMEM that contained 10% fetal bovine serum. Overexpression of Rad was achieved by infecting the cardiomyocytes with 10 plaque-forming units (pfu) per cell of the recombinant adenovirus containing the human Rad cDNA (Ad-Rad) for 48 hours. The adenovirus carrying green fluorescent protein (Ad-GFP) was used as the negative control for Ad-Rad, as described previously.10 Knockdown of Rad was achieved by infecting the cardiomyocytes with 10 pfu/cell of adenovirus carrying Rad short hairpin RNA (shRNA) oligonucleotides (Ad-Rad-RNAi), which we generated using the Knockout Adenoviral RNAi System 1 from Clontech (Mountain View, Calif). The Rad RNAi oligonucleotide sequence for the knockdown is 5′-CGAGACCTTCAGGC GGCGCTA-3′. The adenovirus carrying scrambled Rad shRNA oligonucleotides (Ad-Rad-RNAi-Control) was used as the negative control for Ad-Rad-RNAi. CaMKIIδ Stealth small interfering RNA oligonucleo-tides (5′-AUAUUCUGCCACUUCCCAUCACGGC-3′ and 5′-GCCGUGAUGGGAAGUGGCAGAAUAU-3′) were purchased from Invitrogen (Carlsbad, Calif). Knockdown of CaMKIIδ in cardiomyocytes was achieved by transfection of 20 nmol/L CaMKIIδ small interfering RNA oligonucleotides (CaMKIIi) with Lipofectamine 2000 (Invitrogen) for 24 hours.
Induction and Characterization of Cardiomyocyte Hypertrophy In Vitro
Cardiomyocyte hypertrophy was induced by treatment of quiescent cardiomyocytes (serum-free DMEM, 24 hours) with 10 μmol/L phenylephrine (PE) for the required times. The effects of PE were confirmed by determining the mRNA levels of cardiomyocyte hyper-trophic markers by use of quantitative real-time polymerase chain reaction (qRT-PCR) normalized to 18S rRNA. Cardiomyocyte protein synthesis was determined by 3H-leucine incorporation. In brief, neonatal rat cardiomyocytes infected with Ad-Rad, Ad-GFP, Ad-Rad-RNAi, or Ad-Rad-RNAi-Control were kept in serum-free DMEM for 24 hours. Next, the cells were treated with 10 μmol/L PE for 24 hours, and [3H]Leu (1 μCi/mL) was added to the wells 18 hours before the cells were harvested. To precipitate proteins, cells were washed 3 times with ice-cold PBS, and subsequently, ice-cold 10% trichloroacetic acid was added to the wells for 2 hours. The trichloroacetic acid was then removed, and wells were washed with 95% ethanol. After they were dried at room temperature, 200 μL of 0.5 mol/L NaOH was added to the wells. Samples were transferred to scintillation vials to measure 3H-Leu incorporation.
Thoracic Transverse Aortic Constriction–Induced Cardiac Hypertrophy
Rad knockout mice (Rad-KO; Data Supplement) and their wild-type littermate control mice (10 weeks old) were anesthetized by intraperitoneal injection of xylazine (5 mg/kg) and ketamine (100 mg/kg).13 A heating pad was used to maintain body temperature. The use of a horizontal incision at the level of the suprasternal notch allows direct visualization of the transverse aorta without entering the pleural space and thus obviates the need for mechanical ventilation. We banded the transverse aorta between the right innominate and left carotid arteries to the diameter of a 27-gauge needle (body weight 25 to ≈27 g) using a 7-0 silk suture. Sham operations on sexand age-matched mice only omitted the actual aortic banding and served as a control for all experimental groups. Fourteen days after surgery, hearts were harvested. Cardiac hypertrophy was determined by heart weight–to–body weight ratio, myocardial cross sections, and expression levels of 2 cardiac hypertrophy markers, atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP).
Western Blotting
Western blot analyses were performed as described previously.10 A rabbit anti-Rad polyclonal antibody (1:2000 dilution) reported previously6 was used in the present study. Rabbit anti-phospho-CaMKII (1:1000 dilution) polyclonal antibodies and rabbit anti-CaMKII (1:1000 dilution) polyclonal antibodies were purchased from Upstate Inc (Charlottesville, Va).
CaMKII Activity Assay
Left ventricles isolated from mice or neonatal rat cardiomyocytes were washed 3 times with ice-cold PBS and then lysed with modified RIPA buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Nonidet-P40, 1% sodium deoxycholate, 1 mmol/L sodium vanadate, 10 mmol/L sodium pyrophosphate, 10 mmol/L NaF, 1% Triton X-100, 0.5% SDS, 0.1% EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mmol/L PMSF). Lysates were cleared by centrifugation, and the total protein concentration was determined by the Bio-Rad protein assay reagent (catalog #500-0006, Hercules, Calif). CaMKII in the cell lysates (200 μg of proteins) was immunoprecipitated by incubation with anti-CaMKII antibody and protein A beads at 4°C overnight. CaMKII kinase activities were examined by an assay kit from Upstate (catalog #17-135) according to the manufacturer's instructions.
Histological Analysis
Hearts were harvested from anesthetized mice after fixation via transcardial perfusion with 4% formaldehyde (pH 8.0) and fixed overnight. After dehydration, samples were embedded in paraffin wax according to standard laboratory procedures. Sections of 5 μm were stained with hematoxylin and eosin for routine histopathological examination with light microscopy. For determination of myocyte cross-sectional areas, 70 individual cells per slide were determined by digitization of the images and computerized pixel counting. Only nucleated cardiac myocytes from areas of transversely cut muscle fibers were included in the analysis.14
Patient Samples
Left ventricular myocardium was obtained from patients undergoing heart transplantation owing to end-stage heart failure (n=10). Mean age was 49±18 years, and all patients were in New York Heart Association class IV with ejection fractions <20%. All patients were taking ACE inhibitors and β-blockers. For comparison, we obtained left ventricular tissue samples from donor hearts of accident victims without known cardiac trauma that could not be transplanted for technical reasons (n=3). Mean age of the normal heart donors was 47±11 years. The study was approved by the Institutional Review Board for human subject research at the Baylor College of Medicine and the University of Michigan.
Statistical Analysis
Results are reported as mean±SD. Differences between mean values were evaluated by Student t test or ANOVA, with values of P<0.05 indicating a significant difference. Two independent samples from Figure 1C and 1D were used for a 2-tailed, unpaired Student t test. Data taken at different points in time, as shown in Figure 2A and 2B, were analyzed by repeated-measures ANOVA and 1-way ANOVA followed by Newman-Keuls multiple comparison procedure to account for multiple testing. Two-way ANOVA was used to analyze the data in Figures 3 and 4, with small samples in each comparison group. One-way ANOVA followed by Newman-Keuls multiple comparison was used in Figure 5B, 5C, and 5D. Figure 6B and 6C were analyzed by repeated-measures ANOVA and 1-way ANOVA followed by Newman-Keuls multiple comparison procedure to account for multiple testing.
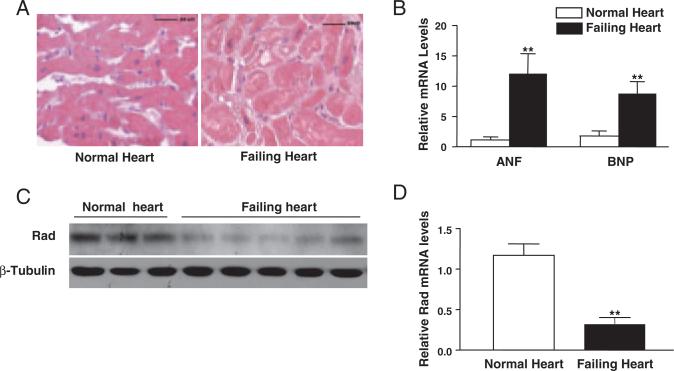
Figure 1.
Expression levels of Rad were downregulated in human failing hearts. A, Representative hematoxylin and eosin stain of human heart cross sections of normal and failing human hearts (scale bar=50 μm). B, qRT-PCR was used to measure expression levels of 2 cardiac hypertrophy markers, ANF and BNP. Values for the specific mRNA levels normalized by 18S rRNA levels are expressed as mean±SD (n=3 for normal human hearts and n=10 for failing human hearts, **P<0.01 versus normal human hearts). C and D, Expression levels of Rad were downregulated in human failing hearts as judged by Western blot analysis (C) and qRT-PCR (D). β-Tubulin and 18S rRNA were used as internal controls, respectively. Data are shown as mean±SD (**P<0.01 versus normal human hearts).
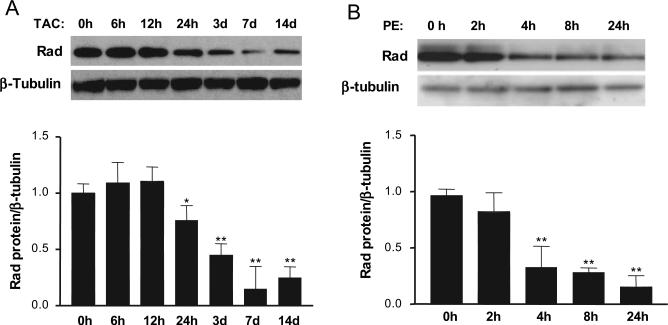
Figure 2.
Rad is downregulated in cardiomyocyte hypertrophy models in vivo and in vitro. Representative Western blots show expression levels of Rad protein. A, Samples from TAC-induced cardiac hypertrophy. B, Samples from PE (10 μmol/L)-induced neonatal rat cardiomyocyte hypertrophy. Average values normalized by β-tubulin from 3 independent experiments are shown in the bottom panel (*P<0.05 and **P<0.01 compared with 0 hours).
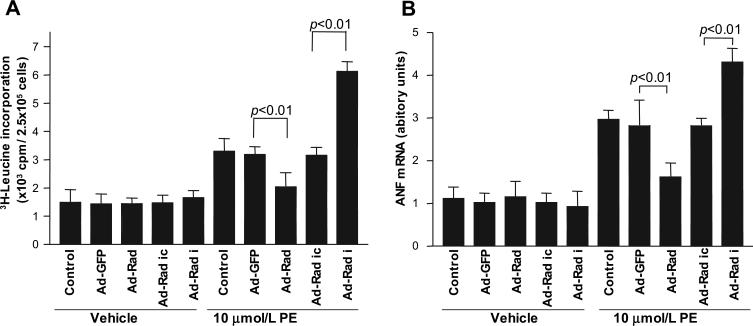
Figure 3.
Rad inhibits cardiomyocyte hypertrophy. Rat neonatal cardiomyocytes were infected by 10 pfu/cell of Ad-Rad or Ad-Rad-RNAi (Ad-Rad i) and used in 10 μmol/L PE-induced 3H-Leu incorporation assay (A) and to determine expression of ANF, a hypertrophy marker in cardiomyocytes, by qRT-PCR analyses (B). Ad-GFP and Ad-Rad RNAi-Control (Ad-Rad ic) were used as controls for Ad-Rad and Ad-Rad-RNAi, respectively. Control was cardiomyocytes without adenoviral infection. Values are expressed as mean±SD (n=6 in each group). Vehicle: PBS. ANF mRNA levels were normalized by 18S rRNA levels.
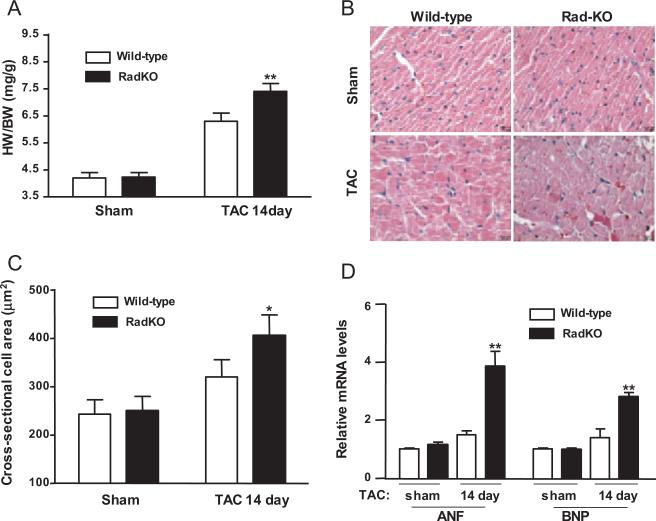
Figure 4.
Rad-deficient mice are more susceptible to pressure overload–induced cardiac hypertrophy. A, Heart weight to body weight ratio (HW/BW). Hearts were harvested at day 14 after TAC procedure. n=6. B, Representative images of hematoxylin-and-eosin–stained left ventricular cross sections (bars=20 μm) of wild-type sham, Rad-KO sham, wild-type TAC, and Rad-KO TAC mice. C, Cross-sectional cell area in wild-type sham, Rad-KO sham, wild-type TAC, and Rad-KO TAC mice. *P<0.05, Rad-KO TAC vs wild-type TAC. D, qRT-PCR was used to measure expression levels of 2 cardiac hypertrophy markers, ANF and BNP, in these mice at 14 days after TAC. Values for mRNA levels normalized by 18S rRNA levels are expressed as mean±SD (n=6; **P<0.01 versus wild-type TAC).
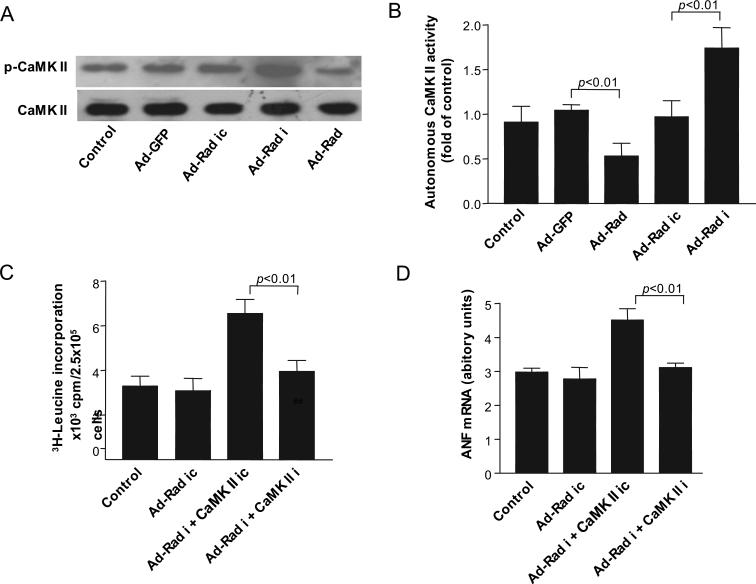
Figure 5.
Rad inhibits CaMKII phosphorylation and autonomous CaMKII activity. A, Representative Western blots showing phosphorylated CaMKII and total CaMKII levels in rat neonatal cardiomyocytes infected with 10 pfu/cell of Ad-GFP, Ad-Rad, Ad-Rad-RNAi (Ad-Rad i), or Ad-Rad-RNAi-Control (Ad-Rad ic). B, Cell lysates of neonatal rat cardiomyocytes infected with 10 pfu/cell Ad-GFP, Ad-Rad, Ad-Rad I, or Ad-Rad ic were used to measure autonomous CaMKII activity as described in Methods. C and D, 3H-Leu incorporation (C) and ANF mRNA (D) levels in the presence of 10 μmol/L of PE-treated rat neonatal cardiomyocytes were determined for each group. Ad-Rad ic was used as the control for Ad-Rad-RNAi. Scrambled CaMKIIδ RNAi oligonucleotide (CaMK II ic) was used as the control for CaMKIIδ RNAi (CaMK II i). Control was rat neonatal cardiomyocytes without adenoviral infection. Values are expressed as mean±SD (n=6 in each group). ANF mRNA levels were normalized by 18S rRNA levels.
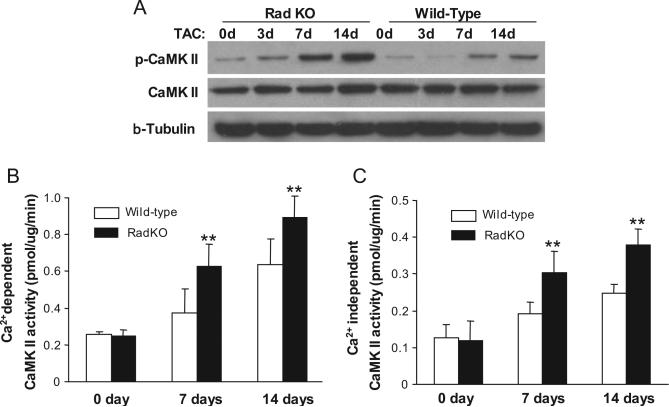
Figure 6.
Rad deficiency increases CaMKII phosphorylation in the heart. A, Representative images of Western blots showing increased CaMKII phosphorylation in Rad-KO mice compared with wild-type littermate controls after TAC. B and C, CaMKII activity in left ventricles after TAC. Extracts from left ventricles of TAC mice were assayed in vitro for Ca2+- dependent CaMKII activity (B) in the presence of Ca2+/CaM or for autonomous CaMKII activity (C) in the presence of 5 mmol/L EGTA. Values are expressed as mean±SD (n=6, **P<0.01 versus wild-type littermate controls).
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Rad Expression Is Significantly Decreased in Human Failing Hearts
Rad is highly expressed in the human heart and in skeletal muscle (Data Supplement Figure I), in agreement with a recent report.11 The abundant expression of Rad in the heart suggests that Rad may play an important role in cardiac diseases. As the first step in exploring the potential role of Rad in cardiac hypertrophy, we collected left ventricular myocardium samples from patients undergoing heart transplantations because of end-stage heart failure. These failing hearts displayed severe hypertrophic morphology, as shown in Figure 1A. Using qRT-PCR, we demonstrated that 2 markers of cardiac hypertrophy, ANF and BNP, were increased significantly by 11-fold and 6.8-fold, respectively, in these human failing hearts (Figure 1B; P<0.01 versus normal hearts). Intriguingly, Western blotting showed that Rad protein levels were decreased significantly in all human failing hearts (n=5) compared with normal hearts (n=3; Figure 1C). Similar data were also observed in samples from 5 other human failing hearts (data not shown). In addition, relative Rad mRNA levels were decreased in human failing hearts compared with normal hearts according to qRT-PCR (Figure 1D; 0.42±0.21 versus 1.12±0.38, P<0.01). Thus, the present data strengthen the notion that Rad plays an important role in the mediation of cardiac remodeling.
Rad Expression Is Decreased in Experimental Hypertrophic Cardiomyocytes In Vivo and In Vitro
To explore the potential function of Rad in the heart, we used the well-established cardiac hypertrophy model induced by thoracic transverse aortic constriction (TAC). We documented that Rad protein levels were decreased significantly in a time-dependent manner from 24 hours to day 14 after TAC during the progression of cardiac hypertrophy (Figure 2A). Furthermore, we established a PE-induced cardiomyocyte hypertrophy model in vitro using primary cultures of neonatal rat cardiomyocytes. In this model, we found that levels of Rad protein were downregulated significantly 4 hours after PE treatment (10 μmol/L) and remained low for at least 24 hours after PE treatment (Figure 2B). Taken together, these results demonstrate for the first time that decreased Rad expression is associated with cardiac hypertrophy, which suggests a potentially important role of Rad in this process.
Rad Inhibits 3H-Leu Incorporation in Cardiomyocytes Induced by PE
To explore whether Rad has a protective role in cardiac remodeling, we performed gain-of-function and loss-of-function studies in cultured cardiomyocytes using adenovirus containing human Rad cDNA10 and Rad shRNA, as described in Methods. Ad-Rad infection (10 pfu/cell) led to a substantial increase in the level of Rad protein of ≈4-fold, and Ad-Rad-RNAi (10 pfu/cell) effectively decreased Rad protein expression by >90% in neonatal rat cardiomyocytes, respectively (Data Supplement Figure IIA). Using 3H-Leu incorporation, we found that Rad overexpression significantly reduced PE-induced protein synthesis (2031±506 versus 3186±273 cpm, P<0.01), whereas Rad knockdown dramatically increased protein synthesis by ≈2-fold compared with the corresponding Ad-Rad-RNAi control (6122±836 versus 3154±694 cpm, P<0.01; Figure 3A). In addition, increased Rad levels inhibited expression of the PE-induced hypertrophy marker ANF by 47% in cardiomyocytes treated with Ad-Rad (1.9±0.3 versus 2.8±0.5, P<0.01), whereas Rad knockdown led to a significant increase of ANF expression (4.3±0.3 versus 2.5±0.2, P<0.01; Figure 3B). Taken together, these findings suggest that Rad may play an important protective role during cardiac remodeling through a mechanism that involves inhibition of cardiomyocyte hypertrophy.
Rad-KO Mice Are More Susceptible to TAC-Induced Cardiac Hypertrophy
To uncover the potential role of Rad in vivo, we generated Rad-deficient mice (Rad-KO) for the present study (Data Supplement Figure IIIA). Rad expression was absent in the heart of Rad-KO mice (Data Supplement Figure IIIB and IIIC). Rad-KO mice showed normal heart weight, body weight, and heart-weight–to–body-weight ratio compared with wild-type littermate controls in the present study (Figure 4A). The morphology of myocardial cross sections was also similar, as judged by hematoxylin-and-eosin staining (Figure 4B, Sham). Hemodynamic analysis by in vivo cardiac catheterization revealed that basal left ventricular contractility (assessed by maximal +dP/dt) and diastolic function (assessed by maximal −dP/dt) were similar in Rad-KO and control mice (Data Supplement Figure IV). Dobutamine stimulation increased left ventricular contractility and diastolic function in a dose-dependent manner in both Rad-KO and control mice; however, Rad-KO mice showed weaker left ventricular contractility than control mice at higher doses of dobutamine (2.5 and 5.0 μg/kg body weight). Taken together, these results suggest that Rad deficiency affects cardiac systolic function in response to increased cardiac stress.
To further define the role of endogenous Rad in cardiac remodeling, we performed TAC in Rad-KO and wild-type littermate control mice. We documented that heart-weight– to-body-weight ratio (Figure 4A) and myocardial cross sections (Figure 4B and 4C; Data Supplement Figure V) were increased significantly in Rad-KO mice compared with wild-type littermates 14 days after TAC. In addition, we examined the expression of several cardiac hypertrophy markers in Rad-KO mice and wild-type littermate controls after TAC using qRT-PCR analyses. At 10 weeks of age, Rad-KO and control mice had similar basal expression levels of the cardiac hypertrophy markers ANF and BNP. After the TAC procedure, both ANF and BNP expression levels were upregulated significantly by 2.4-fold (3.77±0.46 versus 1.56±0.16, P<0.01) and 1.9-fold (2.76±0.2 versus 1.44±0.32, P<0.01), respectively, in Rad-KO mice compared with wild-type littermate controls (Figure 4D). Thus, these data provide evidence that downregulation of Rad in vivo can promote the progression of cardiac hypertrophy.
Rad Interacts With the Ca2+ -Calmodulin-CaMKII Pathway in Cardiomyocytes
It has been well documented that activated (phosphorylated) CaMKII is a strong inducer of cardiac hypertrophy.15 Our previous studies demonstrated that Rad can interact with calmodulin (CaM) and CaMKII in skeletal muscle cells.16 To determine whether the Ca2−-CaM-CaMKII pathway mediates the protective effects of Rad in cardiac hypertrophy, we performed a coimmunoprecipitation assay in cultured neonatal rat cardiomyocytes. Using this method, we found that there is a strong interaction between Rad and CaM, as well as between Rad and CaMKII, in cardiomyocytes (Data Supplement Figure VI), which is similar to our previous findings in skeletal muscle cells.16 Furthermore, overexpression of Rad significantly inhibited CaMKII phosphorylation, whereas Rad knockdown increased CaMKII phosphorylation in cardiomyocytes (Figure 5A). We also demonstrated that CaMKII activity was decreased in Rad-overexpressing cells but increased in Rad knockdown cells (Figure 5B). To date, emerging data suggest that CaMKIIδ plays a critical role in cardiac hypertrophy.17 To further address whether CaMKIIδ is the critical mediator of Rad-inhibited cardiac hypertrophy, we reduced CaMKIIδ expression by RNAi technology in neonatal rat cardiomyocytes in combination with Rad knockdown. Interestingly, double knockdown of CaMKIIδ and Rad in cardiomyocytes significantly decreased PE-induced 3H-Leu incorporation (Figure 5C; 3957±492 versus 6622±786 cpm, P<0.01) and ANF mRNA expression (Figure 5D; 3.1±0.2 versus 4.5±0.4, P<0.01) compared with only Rad knockdown. In agreement with this observation, we further documented that in the present in vivo model, TAC-induced CaMKII phosphorylation in the heart was significantly increased in Rad-KO mice compared with wild-type littermate controls (Figure 6A).
To further define the effects of Rad on CaMKII activity in the heart, we measured the Ca2−-dependent CaMK activity of ventricular extracts from wild-type and Rad-KO mouse hearts using a specific peptide (KKALRRQETVDAL) as the substrate provided with the CaMKII kinase assay kit from Upstate Inc. As shown in Figure 6B, basal levels of Ca2+-dependent CaMK activity in the hearts of Rad-KO mice and wild-type control littermates are similar when a reaction system with 1 mmol/L CaCl2 is used. Although Ca2+-dependent CaMK activity is significantly increased in both wild-type and Rad-KO mice at 7 and 14 days after the TAC procedure, the Rad-KO mice showed significantly higher Ca2+-dependent CaMK activity (32% increase, n=6, P<0.01). Because CaMKII becomes independent of Ca2+/CaM when activated, and its Ca2+-independent kinase activity can be assayed in the presence of EGTA,18 we measured Ca2+-independent CaMKII activity (autonomous activity of CaMKII). Rad-KO hearts showed significantly greater autonomous CaMKII activity (33% increase, n=6, P<0.01) than wild-type littermate controls at 7 and 14 days after the TAC procedure, although the basal levels (day 0) of autonomous CaMK activity were similar (Figure 6C). Taken together, the present data imply that Rad is a novel cardioprotective mediator that prevents cardiac hypertrophy through the inhibition of CaMKII activity.
Discussion
Cardiac hypertrophy is an important adaptive growth response that facilitates an increase in myocardial contractility. It is postulated that pathological structural changes in the heart are induced in part by small G proteins that govern a wide spectrum of functions, including regulation of cell proliferation, migration, apoptosis, and cytoskeletal rearrangement.2,4 Rad was originally identified by subtraction cloning and found to be overexpressed in skeletal muscle of patients with type 2 diabetes mellitus.6 To date, the function of Rad remains largely unknown in the cardiovascular system. In the present study, we have shown that although Rad is highly expressed in the normal myocardium, it is dramatically decreased in human failing hearts. In addition, the present data demonstrated for the first time that Rad is a novel and critical mediator that prevents and inhibits cardiac hypertrophy through inhibition of the CaMKII pathway.
To date, >100 identified small GTPases constitute a superfamily that comprises at least 6 subfamilies: Ras, Rho (Rho, Rac, and Cdc42), Arf (ADP ribosylation factor), Rab, Ran, and RGK (Rad/Gem/Kir).2,19 In the cardiovascular system, small GTPases are implicated in regulation of endothelial function3; VSMC contraction, proliferation, and migration10; and cardiac hypertrophy.4,20–22 We have reported that inhibition of the Rho/ROK (Rho-kinase) signaling pathway reduces VSMC migration and decreases neointimal formation.10 The inhibition of Ras attenuates atherosclerosis in apolipoprotein E–deficient mice and neointimal formation in porcine coronary balloon angioplasty.23 Furthermore, inhibition of Rac1 induces regression of coronary atherosclerosis in a pig model treated with interleukin-1β.24 Activation of RhoA is a critical component of hypertension, whereas oxyhemoglobin-induced Rho/ROK activation is a major causative component of cerebral vasospasm.25,26 Thus, the targeting of small GTPases and their downstream signaling could provide novel therapeutic approaches for the treatment of cardiovascular disorders. Actually, the importance of small GTPases as targets for therapeutic purposes in the cardiovascular system is underscored by experimental and clinical studies with statins that protect against cardiac hypertrophy and atherosclerosis by targeting GTPases beyond their cholesterol-lowering effects.27,28
Rad, a 33- to 35-kDa protein (with 61% amino acid sequence identity to Gem and Kir)5,29 is the prototypic member of a new subfamily of Ras-related GTPases that lacks the typical prenylation motifs at the C terminus and was originally identified from skeletal muscle of patients with type 2 diabetes mellitus.6 Genetic studies linking a trinucleotide-repeat polymorphism in Rad and type 2 diabetes mellitus are controversial, with 2 studies in favor30,31 and 1 larger population study against.32 Although an association between Rad upregulation and type 2 diabetes mellitus could not be confirmed, a correlation with obesity was reported.33 It was also reported that Rad inhibits insulin-stimulated glucose uptake in myocyte and adipocyte cell lines.7 A recent report revealed that Rad is highly expressed in a breast cancer cell line with high tumorigenic and metastatic potential, promoting growth and accelerating cell cycle transition.34 However, during our previous studies of Rad in VSMCs, we found that overexpression of Rad in VSMCs had no effect on cell proliferation,10 which suggests that its role is cell-specific and contextual. Interestingly, a previous study demonstrated that Gem and Rad can negatively regulate the function of the Rho/ROK signaling pathway through association with the Rho effector, ROK, in neuroblastoma.35 Indeed, our published data showed that Rad also physically interacts with ROK but not with RhoA in VSMCs.10 Compared with other tissues, Rad is highly expressed in the heart; however, the functions of Rad in heart remain largely unknown. Intriguingly, a recent study demonstrated that overexpression of a Rad dominant-negative mutant in cardiomyocytes leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart.36
Multifunctional CaMKs are transducers of Ca2+ signals. Several transgenic mouse models have confirmed that CaMKs play an important role in the development of cardiac hypertrophy.37 CaMKII is a proarrhythmic signaling molecule in cardiac hypertrophy in vivo.38 CaMKII proteins are encoded by 4 genes, α, β, γ, and δ. Whereas the α- and β-genes are neuron specific, the γ- and δ- genes are expressed in most somatic cells.39 To date, emerging data suggest that CaMKIIδ plays a critical role in cardiac hypertrophy.17 Indeed, it was reported that genetic mouse model of cardiac CaMKII inhibition, which genetically targeted a conserved region of the CaMKII regulatory domain with cDNA encoding an inhibitory peptide, substantially prevented maladaptive remodeling from excessive β-adrenergic receptor stimulation and myocardial infarction.40 In the present study, we demonstrated a direct physical interaction between Rad and CaMKII in cardiomyocytes by coimmunoprecipitation. Also, we documented that overexpression of Rad in cardiomyocytes inhibits CaMKII phosphorylation and autonomous activity, whereas CaMKII phosphorylation and autonomous activity are dramatically increased in cardiomyocytes with Rad knockdown. In the present in vitro model, cardiomyocytes with Rad knockdown were more susceptible to PE-induced cardiomyocyte hypertrophy, as inferred from 3H-Leu incorporation and ANF expression; however, cells with both CaMKIIδ and Rad knockdown were resistant to PE-induced cardiomyocyte hypertrophy. Using TAC, a procedure that mimics the pressure overload–induced cardiac hypertrophy model, we further documented that Rad expression was decreased significantly in hypertrophic hearts and that Rad deficiency led to significantly exacerbated cardiac hypertrophy in association with increased CaMKII phosphorylation and activation in the heart. Thus, the present results strongly suggest that Rad is a novel mediator of cardiac hypertrophy via inhibition of CaMKII activity.
In summary, the present data document for the first time that Rad, through its ability to strongly decrease CaMKII phosphorylation and activation, is an endogenous inhibitor of cardiac hypertrophy. Our studies provide new insights into understanding the mechanisms of cardiac hypertrophy and may have new, significant implications for the development of novel strategies for treatment of cardiac hypertrophy through specific targeting of the Rad signaling pathway within this family of small GTPases.
Supplementary Material
CLINICAL PERSPECTIVE.
This study identifies a novel role for the small G protein-Rad GTPase in the process of cardiac hypertrophy induction, which is notable because it appears to be relevant to human cardiovascular disease. Recently, Rad GTPase has emerged as an important protein in cardiac function since, as it is most abundantly expressed in the heart. The data shown in the present study strongly support the idea that Rad GTPase serves as a cardiac hypertrophy inhibitor. First, human samples displaying clinical heart failure showed decreased Rad GTPase protein and mRNA expression levels. Secondly, a murine model of Rad GTPase deficiency was more susceptible to cardiac hypertrophy than were wild-type littermates. Finally, an association between Rad GTPase and inhibition of calmoudulin kinase II phosphorylation and activity was documented, thereby inhibiting the progression toward cardiac hypertrophy. Viewed this way, therapeutic activation of Rad GTPase signaling offers a theoretical but testable strategy for counteracting hypertrophic stimuli at the cardiomyocyte level.
Acknowledgments
Sources of Funding
Dr Chen was supported by National Institutes of Health grants HL068878 and HL075397. Drs Chang and Xie were supported by an American Heart Association Postdoctoral Fellowship from the Greater Midwest Affiliate (0625705Z) and a Southeast Affiliate (0525510B) grant, respectively. Dr Zhu was supported by the Natural Science Foundation of China (30671027).
Footnotes
Disclosures
None.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intra-cellular signalling pathways. Nat Rev. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Kelly K. The RGK family: a regulatory tail of small GTP-binding proteins. Trends Cell Biol. 2005;15:640–643. doi: 10.1016/j.tcb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Fryer BH, Field J. Rho, Rac, Pak and angiogenesis: old roles and newly identified responsibilities in endothelial cells. Cancer Lett. 2005;229:13–23. doi: 10.1016/j.canlet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 6.Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 7.Moyers JS, Bilan PJ, Reynet C, Kahn CR. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- 8.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci U S A. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward Y, Kelly K. Gem protein signaling and regulation. Methods Enzymol. 2005;407:468–483. doi: 10.1016/S0076-6879(05)07038-2. [DOI] [PubMed] [Google Scholar]
- 10.Fu M, Zhang J, Tseng YH, Cui T, Zhu X, Xiao Y, Mou Y, De Leon H, Chang MM, Hamamori Y, Kahn CR, Chen YE. Rad GTPase attenuates vascular lesion formation by inhibition of vascular smooth muscle cell migration. Circulation. 2005;111:1071–1077. doi: 10.1161/01.CIR.0000156439.55349.AD. [DOI] [PubMed] [Google Scholar]
- 11.Hawke TJ, Kanatous SB, Martin CM, Goetsch SC, Garry DJ. Rad is temporally regulated within myogenic progenitor cells during skeletal muscle regeneration. Am J Physiol Cell Physiol. 2006;290:C379–C387. doi: 10.1152/ajpcell.00270.2005. [DOI] [PubMed] [Google Scholar]
- 12.Kaburagi S, Hasegawa K, Morimoto T, Araki M, Sawamura T, Masaki T, Sasayama S. The role of endothelin-converting enzyme-1 in the devel opment of alpha1-adrenergic-stimulated hypertrophy in cultured neonatal rat cardiac myocytes. Circulation. 1999;99:292–298. doi: 10.1161/01.cir.99.2.292. [DOI] [PubMed] [Google Scholar]
- 13.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, Ivashchenko Y, Lohse MJ, Engelhardt S. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med. 2005;11:837–844. doi: 10.1038/nm1272. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 16.Moyers JS, Bilan PJ, Zhu J, Kahn CR. Rad and Rad-related GTPases interact with calmodulin and calmodulin-dependent protein kinase II. J Biol Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardio Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 19.Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–4946. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 20.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 21.Grounds HR, Ng DC, Bogoyevitch MA. Small G-protein Rho is involved in the maintenance of cardiac myocyte morphology. J Biol Chem. 2005;95:529–542. doi: 10.1002/jcb.20441. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Hikoso S, Kashiwase K, Takeda T, Watanabe T, Mano T, Matsumura Y, Ueno H, Hori M. The small GTP-binding protein Rac1 induces cardiac myocyte hypertrophy through the activation of apoptosis signal-regulating kinase 1 and nuclear factor-kappa B. J Biol Chem. 2003;278:20770–20777. doi: 10.1074/jbc.M213203200. [DOI] [PubMed] [Google Scholar]
- 23.Work LM, McPhaden AR, Pyne NJ, Pyne S, Wadsworth RM, Wainwright CL. Short-term local delivery of an inhibitor of Ras farnesyltransferase prevents neointima formation in vivo after porcine coronary balloon angioplasty. Circulation. 2001;104:1538–1543. doi: 10.1161/hc3801.095661. [DOI] [PubMed] [Google Scholar]
- 24.Morishige K, Shimokawa H, Eto Y, Kandabashi T, Miyata K, Matsumoto Y, Hoshijima M, Kaibuchi K, Takeshita A. Adenovirus-mediated transfer of dominant-negative rho-kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol. 2001;21:548–554. doi: 10.1161/01.atv.21.4.548. [DOI] [PubMed] [Google Scholar]
- 25.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 26.Wickman G, Lan C, Vollrath B. Functional roles of the rho/rho kinase pathway and protein kinase C in the regulation of cerebrovascular con striction mediated by hemoglobin: relevance to subarachnoid hemorrhage and vasospasm. Circ Res. 2003;92:809–816. doi: 10.1161/01.RES.0000066663.12256.B2. [DOI] [PubMed] [Google Scholar]
- 27.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorin D, Cohen L, Del Villar K, Poullet P, Mohr R, Whiteway M, Witte O, Tamanoi F. Kir, a novel Ras-family G-protein, induces invasive pseudohyphal growth in Saccharomyces cerevisiae. Oncogene. 1995;11:2267–2271. [PubMed] [Google Scholar]
- 30.Wang GY, Niu TH, Chen CZ, Li QF, Xu XP. A novel Rad gene polymorphism combined with obesity increases risk for type 2 diabetes mellitus. Chin Med J (Engl) 2004;117:770–771. [PubMed] [Google Scholar]
- 31.Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K. Analysis of trinucleotide-repeat combination polymorphism at the rad gene in patients with type 2 diabetes mellitus. Metabolism. 1999;48:173–175. doi: 10.1016/s0026-0495(99)90029-x. [DOI] [PubMed] [Google Scholar]
- 32.Orho M, Carlsson M, Kanninen T, Groop LC. Polymorphism at the rad gene is not associated with NIDDM in Finns. Diabetes. 1996;45:429–433. doi: 10.2337/diab.45.4.429. [DOI] [PubMed] [Google Scholar]
- 33.Paulik MA, Hamacher LL, Yarnall DP, Simmons CJ, Maianu L, Pratley RE, Garvey WT, Burns DK, Lenhard JM. Identification of Rad's effector-binding domain, intracellular localization, and analysis of expression in Pima Indians. J Cell Biochem. 1997;65:527–541. [PubMed] [Google Scholar]
- 34.Tseng YH, Vicent D, Zhu J, Niu Y, Adeyinka A, Moyers JS, Watson PH, Kahn CR. Regulation of growth and tumorigenicity of breast cancer cells by the low molecular weight GTPase Rad and nm23. Cancer Res. 2001;61:2071–2079. [PubMed] [Google Scholar]
- 35.Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Murata M, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 37.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson ME. Calmodulin kinase and L-type calcium channels: a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–161. doi: 10.1016/j.tcm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- 40.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.