Abstract
Lymphadenopathy in autoimmune and other lymphoproliferative diseases is in part characterized by immunoblasts and vascular proliferation. The lymph node vasculature, along with the nonvascular stromal compartment, supports lymphocyte function, and targeting vascular-stromal expansion in inflamed nodes may modulate lymphocyte function in disease. CD11c(+) cells are essential for vascular-stromal proliferation and the upregulation of vascular endothelial growth factor (VEGF) needed for vascular proliferation. However, targetable CD11c(+) cell-derived molecular mediators, the identity of relevant CD11c(+) cells, and whether CD11c(+) cells directly stimulate VEGF-expressing stromal cells are poorly understood. In this study we show that CD11c(+) CD11b(+) CCR2-dependent monocytes and CCR7-dependent dendritic cells express IL-1β. IL-1β blockade, IL-1β deficiency in radiosensitive cells, and CCR2/CCR7 double deficiency but not single deficiency all attenuate immunization-induced vascular-stromal proliferation. gp38(+) stromal fibroblastic reticular cells (FRCs) that express VEGF are enriched for Thy1(+) cells and partially overlap with CCL21-expressing FRCs, and FRC VEGF is attenuated with IL-1β deficiency or blockade. IL-1β localizes to the outer borders of the T zone, where VEGF-expressing cells are also enriched. Ex vivo, CD11b(+) cells enriched for IL-1β(+) cells can directly induce cultured gp38(+)Thy1(+) FRCs to upregulate VEGF. Taken together, these results suggest a mechanism whereby multiple recruited CD11c(+) populations express IL-1β and directly modulate FRC function to help promote the initiation of vascular-stromal growth in stimulated lymph nodes. These data provide new insight into how CD11c(+) cells regulate the lymph node vascular-stromal compartment, add to the evolving understanding of functional stromal subsets, and suggest a possible utility for IL-1β blockade in preventing inflammatory lymph node growth.
Keywords: Spleen and lymph nodes, Stromal cells, Endothelial cells, Dendritic cells, Monocytes/macrophages, Inflammation
Introduction
Lymphocytes in lymphoid tissues interact with a vascular-stromal compartment that can support and modulate T and B cell function. During immune responses, lymph nodes swell, and the vascular-stromal compartment undergoes a concomitant proliferative expansion (1–4). In autoimmune disease such as lupus, the enlarged lymph nodes can show T zone hyperplasia, with proliferating lymphocytes and apparent vascular proliferation in the paracortex and interfollicular regions (1, 5). Targeting vascular-stromal expansion may be a means by which to therapeutically modulate lymphocyte function.
The vascular and stromal elements in lymph nodes serve distinct roles but they are also functionally intertwined. Blood vessels deliver oxygen, micronutrients, and the antigen-specific lymphocytes needed to mount immune responses. The high endothelial venules (HEVs) are the sites of lymphocyte extravasation and are characterized by cuboidal endothelial cells and expression of adhesion molecules such as peripheral node addressin (PNAd) (6). The lymphatic vasculature is comprised of sinuses which bring cells and antigen in from the periphery or deliver cells to efferent lymphatic flow. The vasculature is suspended within a stromal infrastructure that is most apparent in the T zone and consists of collagen-rich fibrils ensheathed by reticular cells. The compartment between the fibrillar core and the reticular cells can act as a conduit system that transports small molecules that can reach the blood vessels even from distal sites. T zone reticular cells have additional functions such as expression of CCL19 and CCL21 to promote T zone compartmentalization, IL-7 to support T cell survival, as well as molecules that modulate T cell tolerance and activation (7, 8). T zone reticular cells are often termed “fibroblastic reticular cells” (“FRCs”) and marked by expression of gp38/podoplanin/T1alpha. However, gp38 is also expressed by reticular cells in other compartments and by a T zone stromal population that expresses lower levels of CCL19 and CCL21 than classic T zone reticular cells (7, 9, 10), and here, we will refer to all gp38+ reticular cells as fibroblastic reticular cells (FRCs). VEGF is required for vascular proliferation at homeostasis and in stimulated nodes, and FRCs adjacent to and near vessels in the T zone and medulla are the main expressors of VEGF mRNA (11).
The proliferative expansion of the vascular-stromal compartment after immunization can be divided into several distinct phases. The initiation phase occurs in the first 2 days and is dependent on CD11c+ cells, independent of T and B cells, and marked by rapid upregulation of endothelial and FRC proliferation with limited expansion in cell numbers (12, 13). This is followed by a T and B cell-dependent expansion phase and subsequent re-establishment of quiescence and stabilization(1). The identity of the CD11c+ cells that mediate the initiation phase has been elusive. CD11c+ MHCIIhi dendritic cells that include mostly skin-derived dendritic cells (14–16) and CD11cmedMHCIImed cells that include monocytes, monocyte-derived cells, and plasmacytoid dendritic cells (17, 18) accumulate in large numbers while CD11chi MHCIImed presumed dendritic cells accumulate less rapidly. Depletion of CD11chi MHCIImed cells led to a small decrease in endothelial cell proliferation, but, surprisingly, selectively depleting or excluding skin-derived dendritic cells from the lymph node was not important (12, 19). These results, then, point to a potential role for CD11cmedMHCIImed cells or for multiple populations working together in initiating vascular-stromal growth.
A key interaction for the upregulation of vascular-stromal proliferation at day 2 may be that of CD11c+ cells with FRCs, as dendritic cells can interact closely with the reticular network (19–22). VEGF-expressing FRCs, HEVs and smaller blood vessels, and CD11c+ cells are all enriched in the T zone, especially between and under follicles (11, 19, 22). In vitro, bone marrow-derived dendritic cells stimulated 3T3 fibroblasts to upregulate VEGF (12), but the relationship between endogenous CD11c+ cells and FRCs and the molecular mediators involved remain to be better understood.
IL-1β is an inflammatory cytokine that can be expressed by dendritic cells, monocytes, and monocyte-derived cells and can stimulate synovial fibroblasts to upregulate VEGF (23–25). This led us to examine the role of IL-1β and IL-1β-expressing cells in lymph node vascular-stromal regulation. Here, we show that IL-1β plays a partial role in the stimulation of vascular-stromal proliferation and FRC VEGF expression and that recruited CCR2-dependent monocytes and CCR7-dependent dendritic cells together are important sources of IL-1β. These data point to a model whereby multiple CD11c+ populations collaborate to modulate FRC activation and the initiation of vascular-stromal growth.
Materials and Methods
Animals
Mice between 6–12 weeks old were used. C57Bl/6 mice were from The Jackson Laboratory (Bar Harbor, ME), Taconic Farms (Hudson, NY), or National Cancer Institute (Frederick, MD) or our own breeding colony. RAG1−/− mice and CCR2−/− mice were from Jackson Laboratory. CCR7−/− mice were as described (26). CCR2−/− mice were bred with CCR7−/− to generate CCR2−/−CCR7−/− mice. IL-1β−/− mice were as described (27). VEGF-lacZ mice were as described (11, 12, 28). All animal procedures were performed in accordance with the regulations of the Institutional Animal Use and Care Committee at the Hospital for Special Surgery (New York, NY).
Mouse treatments and immunizations
Mice were immunized with OVA in CFA (OVA/CFA) by hind footpad injection of 20 μl of 1mg/ml OVA/CFA or 30ul OVA/CFA at 3 areas in the back as described previously (13). Specified mice received IP injections of 150ug rat-anti-mouse IL-1β (R&D Systems, Minneapolis, MN) at 8 hours before immunization.
For bone marrow chimeras, recipients were lethally irradiated using one dose of 875 rads from an X-ray source and then reconstituted with wild-type or IL-1β-deficient bone marrow cells for at least 6 weeks (12).
For the study of BrdU uptake, mice received intraperitoneal injections of 2 mg BrdU (Sigma-Aldrich, St. Louis, MO) at 18 and 1 h before sacrifice and were fed water containing 0.8 mg/ml BrdU in between.
Real time PCR
For whole lymph node IL-1β mRNA analysis, tissues were snap frozen in liquid nitrogen and homogenized prior to RNA extraction using the RNeasy Minikit (Qiagen, Venlo, Netherlands). SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA) was used to generate cDNA. IQ Sybr-Green Supermix kit (Bio-Rad, Hercules, CA) was used for real-time PCR. Message levels were quantified relative to GAPDH expression. The following primers were used:
IL-1β F: 5′-TGAAGTTGACGGACCCCAAA -3′
IL-1β R: 5′-TGATGTGCTGCTGCGAGATT -3′
VEGF (all isoforms) F: 5′-GGAGATCCTTCGAGGAGCACTT -3′
VEGF (all isoforms) R: 5′-GGCGATTTAGCAGCAGATATAAGAA -3′
VEGF 120/164/188 F: 5′-GCC AGC ACA TAG GAG AGA TGA GC -3′
VEGF 120 R: 5′-TTG GCT TGT CAC ATT TTT CTG G -3′
VEGF 164 R: 5′-CAA GGC TCA CAG TGA TTT TCT GG -3′
VEGF 188 R: 5′-AAC AAG GCT CAC AGT GAA CGC T-3′
GAPDH F: 5′-ATGTGTCCGTCGTGGATCTGA -3′
GAPDH R: 5′-TTGAAGTCGCAGGAGACAACCT -3′
ELISA for IL-1β
For whole lymph node IL-1β, draining popliteal nodes were taken 1 day after immunization, sliced open, and incubated at one or two lymph nodes/per well in 250ul serum-free DMEM x 4 hours. Supernatant was taken and IL-1β measured by ELISA (R&D Systems).
For IL-1β expression by monocytes, neutrophils, and MHCIIhi dendritic cells, sorted cells were washed 1x with PBS and cultured at 0.3–1 x 106 cells/ml in DMEM/10%FBS overnight in 96 well plates. Supernatants were taken for ELISA.
Flow cytometric analysis and sorting
For flow cytometric staining and analysis, procedure is essentially as described (12, 13). Lymph nodes were digested with type II collagenase (Worthington, Lakewood, NJ) and stained with antibodies for flow cytometry. Antibodies used were against CD45 (BD Biosciences, San Jose, CA), CD31 (BD Biosciences), peripheral node addressin (PNAd; BD Biosciences), gp38 (BioLegend, San Diego, CA, or Developmental Studies Hybridoma Bank, Iowa City, IA), Thy1 (BD Biosciences), BrdU (Invitrogen, Carlsbad, CA), IL-1β pro-form (eBioscience), MHCII (BioLegend), CD11c, CD11b and GR1 (all from BD Biosciences), VEGF (VEGF Ab-1 from Thermo Fisher Scientific, Waltham, MA), CCL21 (R&D Systems), IL-1 R1 (R&D Systems). For intracellular staining with anti-VEGF, the procedure of Angeli and colleagues (29) was essentially followed using the BD Cytofix/Cytoperm kit. Intracellular staining for IL-1β and CCL21 was also performed using this kit and following manufacturers instructions. Cells were analyzed using a FACSCanto (BD Biosciences) and CellQuest Pro (BD Biosciences) or FlowJo (Tree Star, Ashland, OR) software.
For sorting of monocytes, neutrophils, and MHCIIhi dendritic cells, RAG1-1−/− mice were used, as the absence of lymphocytes allowed for faster sorting. Draining lymph nodes were taken at day 1 after immunization, digested with collagenase, and stained with antibodies. Live gated cell subsets were then sorted using a FACSVantage (BD Biosciences).
Bone marrow derived dendritic cell (BMDC) generation
BMDCs were generated as described (13). In brief, bone marrow cells were cultured at 2.6 × 105 cells/ml in RPMI supplemented with 10% FCS and 7.5% vol/vol of supernatant from J558L cells expressing GM-CSF. LPS (0.5 μg/ml) (Sigma-Aldrich) was added on day 7. BMDCs were harvested on day 8, labeled with 1.7 μM CFSE (Invitrogen), and washed three times prior to injection.
Staining of lymph node sections
For standard immunohistochemistry, 7 um sections were fixed in cold acetone for 10 minutes before applying primary antibody. Secondary antibodies were conjugated to alkaline-phosphatase or horseradish peroxidase and visualized using Naphthol AS-MX phosphate with Fast Blue or Fast Red salt (Sigma-Aldrich) or diaminobenzidine (Pierce Chemical Co.) (12, 13). Anti-laminin was from Sigma-Aldrich and anti-CD11b was from BD Biosciences.
For IL-1β staining of lymph node sections, lymph nodes were fixed in 2% paraformaldehyde at 4 degrees Celsius for 45 minutes, incubated in 20% sucrose in PBS overnight, and then frozen. Seven micron sections were then stained with goat anti-mouse IL-1β (R&D Systems) overnight at 4 degrees Celsius followed by anti-goat-alkaline phosphatase (Jackson Immunoresearch).
Visualization of beta-galactosidase activity in VLZ lymph nodes was as described (11). Lymph nodes were fresh frozen in Tissue-Tek OCT compound. Seven micron sections were fixed with ice-cold 2% paraformaldehyde/0.125% glutaraldehyde and washed with 2 mM MgCl2 and then with 2 mM MgCl2 /0.02% Nonidet P-40/0.01% sodium deoxycholate in PBS. Beta-galactosidase activity was visualized by incubation at 37°C for 18 h in 1 M X-gal solution (X-gal (Sigma-Aldrich) in 2 mM MgCl2/0.02% Nonidet P-40/0.01% sodium deoxycholate/0.03% K3Fe(CN)6/0.03% C6FeK4N6*3H2O in PBS). Sections were then washed in TBS and then stained with additional primary and secondary antibodies.
Co-cultures
FRC cultures were all generated from unimmunized mice. Collagenase digested lymph node cells were plated at 5 x 106 per ml in tissue culture wells in RPMI with 10% FBS. Cells were cultured for 7 days, depleted of CD45+ and CD31+ cells via magnetic selection, and used directly (passage 1) or passaged one time before use (passage 2) for experiments. Cells were plated at 5 x 104 cells per well in 24-well plates or 8,500 cells per well in 96-well plates for 2 days before addition of CD11b+ or CD11b− cells.
For enrichment of IL-1β-expressing cells, RAG1−/− mice were immunized with OVA/CFA for 2 days and lymph nodes were digested with collagenase. Cells were selected via anti-CD11b and magnetic depletion and added at 0.5 x 106 cells per well in 24 well plates or 8.5 x 104 cells per well in 96 well plates. For comparisons of cells from wild-type or IL-1β−/− mice, lymph node cells were first depleted of B and T cells using antibodies to CD3 and B220 and then CD11b+ cells were selected. For real-time PCR, cultures were trypsinized at 6h, the FRCs were separated from the CD45+ cells by magnetic depletion and subject to RNA extraction. For VEGF ELISA, supernatant was taken from 48 hour cultures. VEGF ELISAs were performed using a commercial kit (R&D Systems), and values normalized to allow comparisons across multiple experiments using different sized wells (ie 24 well and 96 well formats).
Results
IL-1β and IL-1β-expressing cells accumulate in lymph nodes by day 1 after immunization
We examined for IL-1β expression in draining lymph nodes after immunization with OVA/CFA. By day 1, IL-1β mRNA was increased by about 6-fold (Figure 1A). RAG1−/− lymph nodes showed a similar phenomenon (Figure 1A), suggesting that IL-1β upregulation was not dependent on T and B cells. We assessed supernatants after incubating lymph nodes overnight, and day 1 nodes yielded about 4 times more IL-1β protein than unstimulated nodes (Figure 1B), corroborating the mRNA data and suggesting the presence of functional IL-1β.
Figure 1. IL-1β is upregulated and expressed by CD11c+CD11b+ cells after immunization.
(A–B) Mice were immunized with OVA/CFA in footpads and draining popliteal lymph nodes were examined at day 1. (A) Quantitative PCR analysis of IL-1β at day 1 in whole lymph nodes in wild-type (WT) and RAG1−/− mice. Bars represent 4 lymph nodes per group for wild-type mice and 3 lymph nodes per group for RAG1−/− mice over 2 experiments each. (B) Relative IL-1β protein in supernatant after incubation of lymph nodes. Bars represent 3 samples each over 3 experiments. (C) Flow cytometric analysis showing upregulation of IL-1β in multiple populations of CD11c+ cells at day 1 and day 2 after immunization. (D and E) Further flow cytometric analysis of indicated IL-1β+ populations at day 1 after immunization: (D) MHCIIhi cells (E) MHCIIlow-med cells. (F) Cytospin preparation of indicated cell population sorted from day 1 lymph nodes. Images representative of 3 independent experiments. (G) IL-1β protein in supernatant of (CD11c med MHCII low-med CD11b+ GR-1hi ) neutrophils, (CD11c med MHCII low-med CD11b+ GR-1med ) monocytes, and CD11c+ MHCII hi dendritic cells sorted from day 1 lymph nodes. Results are expressed as pg of IL-1β /cell; symbols represent samples from each of 3 experiments. (H) Relative distribution of IL-1β+ cells at 1 day or 2 days after immunization. For (A–B, G–H), error bars represent standard deviation, *=p<.05 and **=p<.01 by t-test..
Flow cytometry showed an increased number of IL-1β+ cells by day 1 (Figure 1Ca, 1Cf). The IL-1β-expressing cells at day 1 were comprised of several populations that expressed varying levels of CD11c and MHCII (Figure 1Ca–c, 1Cf–h). IL-1β+ cells in the CD11c+MHCIIhi gate were mostly EpCAM- (Figure 1Ch, 1D), suggesting that they were not Langerhans cells (30). These cells were mostly CD11b+ and 14% were Gr1+ (Figure 1D), suggesting that they could be a mixture of dermal, inflammatory, or monocyte-derived dendritic cells that entered via the afferent lymphatics and, potentially, blood (15, 16, 30, 31).
The majority of IL-1β+ cells at day 1 were CD11clow-medMHCIIlow-med (Figure 1Ch). Some of these cells appeared to “spill over” into the CD11chiMHCIImed gate (Figure 1Ch). The MHCIIlow-med cells were mostly CD11bhi and Gr-1+ (Figure 1Cj). Gr-1 antibody can detect both Ly6G, found specifically on neutrophils, and Ly6C, found on both monocytes and neutrophils (32–34). A small percentage of the MHCIImed-lo IL-1β-expressing cells at day 1 were Gr-1hiLy6G+ neutrophils while the remainder were mostly Gr1medLy6G- (Figure 1E). Cytospin preparations confirmed that Gr-1hiLy6G+ cells were polymorphonuclear in morphology while Gr1medLy6G- cells were mononuclear and had the appearance of monocytes (Figure 1F). The major populations that expressed IL-1β at day 1, then, were monocytes, neutrophils, and MHCIIhi dendritic cells. Upon incubation, all three populations secreted IL-1β into the supernatant, with neutrophils and monocytes secreting more than MHCIIhi cells (Figure 1G). Numerically, monocytes comprised the largest population of IL-1β+ cells (Figure 1H).
By day 2, the profile of IL-1β-expressing cell shifted, with CD11c+MHCIIhi dendritic cells comprising over 40% of IL-1β-expressing cells (Figure 1Ck–m, 1H). Of the MHCIIlow-med IL-1β+ populations, the monocytes remained the dominant population (Figure 1H). There was also a more defined CD11chiMHCIImed population and this population had the same profile of CD11b and Gr1 expression as CD11cmedMHCIImed cells (Figure 1Cm–o), suggesting that some of the monocytes had upregulated CD11c. Together, these results showed that there was a rapid upregulation of IL-1β in the draining nodes, with monocytes, MHCIIhi dendritic cells, and neutrophils being the main sources. However, the dynamics of IL-1β-expressing cells changed quickly, with MHCIIhi dendritic cell contributions rising between days 1 and 2.
IL-1β is important for vascular-stromal proliferation after immunization
To test the importance of IL-1β, we treated mice with anti-IL-1β and examined endothelial cell proliferation, gating as shown in Figure 2A. Anti-IL-1β had no effect on lymph node cellularity (Figure 2B), but there was about a 30% decrease in endothelial cell proliferation in the draining popliteal nodes, suggesting that IL-1β blockade partially prevented the upregulation of endothelial cell proliferation (Figure 2C). Proliferation of total, HEV, and non-HEV mixed endothelial cell populations was attenuated (Figure 2C). Further separation of the non-HEV populations indicated that attenuation of proliferation was attributable to the effects on non-HEV blood endothelial cells, while lymphatic cell proliferation was unaffected (Figure 2C). At day 2, only HEV endothelial cell but not other endothelial cell numbers are modestly increased (12), and endothelial cell numbers were not affected by anti-IL1β (data not shown). FRC proliferation was also attenuated (Figure 2D) at this time point prior to FRC expansion (12).
Figure 2. IL-1β is an important mediator of endothelial cell and FRC proliferation.
(A) Gating for endothelial cells in flow cytometric analysis. Of the CD45−CD31+ cell “total endothelial cell” population, PNAd+ are HEV endothelial cells (HEV EC) and PNAd− cells are mixed lymphatic and blood vascular nonHEV endothelial cells (nonHEV mixed). PNAd-gp38− cells are nonHEV blood vascular endothelial cells (nonHEV BEC), and PNAd-gp38+ are lymphatic endothelial cells (LEC). (B–D) Mice treated with control IgG or anti-IL-1β and draining popliteal (pop) and non-draining brachial (br) nodes were examined at day 2. (B) Lymph node cellularity. (C) Endothelial cell proliferation as indicated by percent of endothelial cell population that is BrdU+. (D) (CD45−CD31−gp38+ ) FRC proliferation rate. (E–G) Draining lymph nodes of WT→WT control or IL-1β−/− →WT chimeras were examined at day 2 after footpad immunization with OVA/CFA. (E) Lymph node cellularity. (F) Endothelial cell proliferation rate. (G) FRC proliferation rate. For B, D, E and G each symbol represents one mouse. For (C) N= 7 mice per condition, (F) N= 8 mice per condition. For (C,D,F,G), *=p<.05 and **p<.01 by t-test when compared to same cell subset in control lymph nodes.
Bone marrow chimeras comprised of wild-type mice reconstituted with IL-1β-deficient bone marrow showed very few IL-1β+ cells (0.14% +/− 0.07 of total cells as compared to 2.68% +/− 1.18 in WT→WT chimeras; p= 3.6E-06 by t-test, n=10), consistent with the idea that radiosensitive hematopoietic cells were the main sources IL-1β and were replaced by donor bone marrow. The IL-1β−/− chimeras were similar to anti-IL1β-treated mice in showing unchanged lymph node cellularity (Figure 2E) and attenuated total, HEV, and non-HEV blood endothelial cell proliferation (Figure 2F). Also, as with anti-IL1β, lymphatic endothelial cell proliferation was unchanged (Figure 2F) and FRC proliferation was reduced (Figure 2G). Together, these data together suggest that IL-1β from radiosensitive hematopoietic cells is required to mediate full upregulation of blood vascular endothelial and FRC proliferation at the initiation of vascular-stromal growth.
Exclusion of IL-1β-expressing cells from lymph nodes reduces vascular proliferation
We sought to understand which IL-1β+ population was important for endothelial cell proliferation. CCR2 mediates the accumulation of monocytes and some monocyte-derived dendritic cells into stimulated lymph nodes (16, 35–37). CCR2-deficient mice showed reduced numbers of CD11cmed-hiMHCIImed IL-1β+ cells (Figure 3A), but had normal levels of endothelial cell proliferation (Figure 3B).
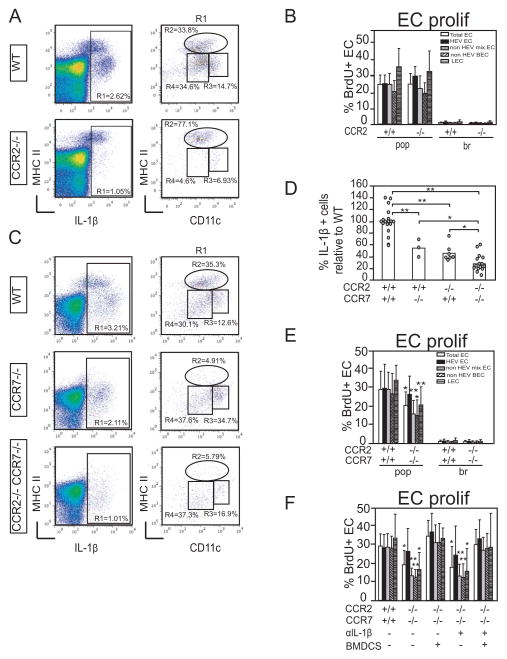
Figure 3. Exclusion of CCR2- and CCR7-dependent IL-1β expressing cells results in reduced endothelial cell proliferation.
Mice were immunized in the footpad with OVA/CFA and draining popliteal and non-draining brachial nodes were examined at day 2. (A) IL-1β expression in draining nodes from wild-type and CCR2−/− mice. (B) Endothelial cell proliferation rate. N=12 mice per condition. (C) IL-1β expression in draining nodes of wild-type, CCR7−/−, and CCR2−/−CCR7−/− mice. (D) Percentage relative to that in wild-type mice of IL-1β+ cells in CCR7 −/−, CCR2 −/− and CCR2 −/− CCR7 −/− mice. Each circle represents one mouse. (E) Endothelial cell proliferation rate in CCR2−/−CCR7−/− mice. N=10 mice per condition. (F) Endothelial cell proliferation in CCR2−/− CCR7−/− mice upon BMDC supplementation. CCR2−/− CCR7−/− mice were injected in the left footpad with 106 BMDCs while the right footpad served as an internal control at 10 hours prior to OVA/CFA immunization in both footpads. The left and right nodes were then analyzed individually at 48 hours after OVA/CFA. Mice also received either 150ug control IgG or anti-IL-1β IP at 8 hours prior to BMDC injection. Bars represent 11 lymph nodes for the wild-type mice and 4–5 lymph node per condition for the CCR2−/− CCR7−/− mice. For (B,D,E,F), error bars represent standard deviation. *=p<.05 and **=p<.01 by t-test when compared to the same subpopulation in wild-type mice.
MHCIIhi cells are likely to enter the lymph node in a CCR7-dependent manner (15, 30, 38), and, indeed, CCR7−/− mice lacked these cells (Figure 3C). We have shown previously that lack of CCR7-dependent cells was not sufficient to attenuate vascular proliferation (12), so we generated CCR2−/−CCR7−/− mice. These mice had fewer IL-1β+ cells than either CCR2 or CCR7 singly-deficient mice (Figure 3C–D). Similar to the effect of IL-1β blockade or deficiency, CCR2−/−CCR7−/− mice showed attenuated total and nonHEV blood endothelial cell proliferation (Figure 3E). In contrast to IL-1β blockade or deficiency, however, HEV proliferation was not affected and lymphatic proliferation was reduced (Figure 3E), suggesting that there were additional roles for CCR2 and CCR7-dependent cells together in modulating proliferation of these endothelial subsets. Footpad injection of bone marrow-derived dendritic cells (BMDCs) prior to OVA/CFA restored endothelial cell proliferation to wild-type levels (Figure 3F), suggesting that CCR2−/−CCR7−/− lymph nodes were capable of fully upregulating endothelial cell proliferation and that the lower response to OVA/CFA alone was not due to developmental issues that limit the vascular response. Only 7% of BMDCs expressed detectable levels IL-1β (data not shown), and the augmented endothelial proliferation was not blocked by anti-IL1β (Figure 3F). This indicated that BMDCs did not augment endothelial cell proliferation by serving as a source of IL-1β and that multiple mechanisms can drive upregulation of endothelial cell proliferation. Of significance, this IL-1β blockade experiment also showed that the limited proliferation in response to OVA/CFA alone was not dependent on IL-1β (Figure 3F), supporting the idea that CCR2−/−CCR7−/− nodes were lacking key sources of IL-1β. CCR2−/−CCR7−/− nodes had normal neutrophil numbers (10,308 +/− 4,076 cells in wild-type mice and 14,363 +/− 5,621 cells in CCR2−/−CCR7−/− nodes; p=.16 t-test, n=7 mice per group), and the lack of IL-1β-dependence also supported the idea that neutrophils were not a key source of IL-1β. Together, our results suggested that CCR2-dependent monocytes and CCR7-dependent dendritic cells together are the main IL-1β-expressing cells that contribute to driving vascular proliferation upon immunization with OVA/CFA.
IL-1β-expressing cells regulate FRC VEGF expression in vivo and in vitro
We asked whether IL-1β modulation of endothelial cell proliferation was associated with modulation of stromal VEGF. As understanding of the different non-endothelial stromal cell subsets in lymph nodes is still emerging, we first performed some additional characterization of these subsets. Thy1 is a marker of activated fibroblasts (39), and FRCs had varying levels of Thy1 expression (Figure 4A). VEGF+ FRCs were enriched for cells that were Thy1+ (Figure 4B). In contrast, cells that expressed CCL21 were enriched for cells that were Thy1neg-low (Figure 4B). Co-staining for VEGF and CCL21 showed that there were CCL21+VEGF+ as well as CCL21-VEGF+ FRCs (Figure 4C). The highest level of VEGF was expressed by CCL21− cells (Figure 4C). For gp38− cells, Thy1 marked the integrin α7+ pericytes identified recently by Turley and colleagues (40) (Figure 4A). Pericytes express lower levels of VEGF mRNA than FRCs (40), and VEGF staining was not detectable in pericytes at homeostasis (Figure 4D). FRCs expressed the activating type I IL-1 receptor, IL-1R1, while pericytes expressed relatively little IL-1R1 (Figure 4E). Blood vascular and lymphatic endothelial cells also expressed IL-1R1(Figure 4E), suggesting the possibility that IL-1β could directly stimulate endothelial cells in vivo, although IL-1β suppresses endothelial proliferation in vitro (41, 42). Upon immunization, FRCs showed a nearly 2-fold upregulation of IL-1R by day 1 while the other cell subsets did not show consistent differences (1.94 +/−0.76 –fold increase in % IL-1R1+ cells for FRCs, p=0.04, n=4; 0.8 +/− 0.25 for blood endothelial cells, p=.35, n=4; 1.18 +/− 1.24 for pericytes, p=.77, n=3; 1.37 +/−.49 for lymphatic endothelial cells, p=.17, n=3). Given that FRCs expressed higher levels of VEGF and IL-1R1 than pericytes, we focused on the FRCs and asked whether VEGF in these cells could be modulated by IL-1β in vivo. Indeed, IL-1β blockade and IL-1β deficiency both reduced FRC VEGF levels (Figure 4F,G), suggesting a scenario whereby IL-1β acts on IL-1R1-expressing FRCs to promote VEGF expression.
Figure 4. FRC characterization and partial dependence of FRC VEGF on IL-1β.
(A–D) Homeostatic brachial lymph nodes were digested with collagenase and stained for flow cytometric analysis of stromal subsets using indicated markers. Each stain representative of at least 3 mice. (A) Flow cytometry plots showing Thy1 expression on FRCs and gp38-integrin α7+ pericytes. (B) Flow cytometry plots showing VEGF and CCL21 staining in FRCs. (C–D) Histograms showing VEGF staining in (C) CCL21+ and CCL21− FRCs and (D) FRCs versus pericytes. (E) IL-1R1 expression on FRCS, pericytes, blood endothelial cells, and lymphatic endothelial cells from homeostatic pooled brachial and inguinal lymph nodes. (F) Draining popliteal lymph nodes of mice treated with control IgG or anti-IL-1β were analyzed by flow cytometry at day 3 after immunization for the percentage of FRCs that was VEGF+. (G) FRC VEGF levels in draining nodes of wild-type and IL-1β −/− mice at day 2 after immunization was analyzed as in (F). Each symbol represents one sample. For (E,F), *=p<.05 and **=p<.01 by t-test for indicated comparisons.
In tissue sections, IL-1β staining was mainly localized to outer T zone areas that were under and between the B follicles (Figure 5A–E). Co-staining for laminin showed that IL-1β and CD11b both localized in the vessel- and reticulum-rich area that delineates the T zone boundary with follicles. Using VEGF-lacZ mice, we also observed VEGF-expressing cells in the vessel- and reticulum-rich areas of the outer T zone, although, in contrast to the IL-1β staining, the interfollicular zones had as much if not more VEGF than the areas under the follicles (Figure 5F–H). These data suggested a partially overlapping distribution of IL-1β and VEGF expression and the possibility of direct VEGF modulation by IL-1β at vessel-rich areas at the outer borders of the T zone.
Figure 5. IL-1β and VEGF are both at the outer T zones.
(A–C) Nearby sections from a day 2 draining popliteal lymph node were stained for indicated markers. (A) CD11b. B cell follicles “F”, the T zone “T”, and medulla “M” are marked. (B) IL-1β. (C) Control IgG for IL-1β stain. IL-1β −/− also showed no staining with IL-1β antibody (data not shown). (D–E) Nearby sections from a day 2 popliteal node stained for (D) IL-1b and laminin and (E) CD11b and laminin. (F–G) Day 2 draining popliteal node from VEGF-lacZ reporter mouse stained for (F) CD11b (G) β-galactosidase activity and laminin. (H) Enlargement of upper aspect of (G). All images are representative of lymph nodes from at least 2 mice. Scale bar =100um.
To test whether IL-1β-expressing cells could directly modulate FRC VEGF expression, we enriched for IL-1β+-expressing cells by magnetic selection for CD11b+ cells and cultured them with FRCs. CD11b rather than CD11c was used for cell enrichment, as IL-1β+ cells were mostly CD11cmed-lo and CD11bhi (Figure 1C). The CD11b+ fraction was enriched for IL-1β+ cells and somewhat for CD11c+ cells when compared to the CD11b− fraction (Figure 6A). FRC cultures were 99% gp38+ with Thy1 levels increasing with passage number (Figure 6B). FRCs were VEGF+ (Figure 6C), and, consistent with the loss of CCL19 and 21 mRNA expression over 7 days in culture (43), we detected little if any CCL21 protein (Figure 6D).
Figure 6. CD11c+CD11b+ cells induce FRCs to upregulate VEGF in an IL-1β-dependent manner in vitro.
(A) Characterization of CD11b+ and CD11b− cells isolated from RAG1−/− lymph nodes at day 2 after immunization. (B) Characterization of cultured FRCs. Upper plot is of FRCs that were passage 1 at time of plating and examined 2 days after plating. Lower graph shows the increase in Thy1hi cells with passage number. (C–D) Histograms showing (C) VEGF and (D) CCL21 expression in cultured FRCS (E) VEGF protein levels in supernatant of FRCs co-cultured with CD11b+ or CD11b− cells. N=10 wells over 4 experiments. (F) VEGF mRNA in FRCs after co-culture. N=3 samples over 3 experiments. (G) VEGF protein levels in supernatant of FRCs co-cultured with wild-type or IL-1β−/− CD11b+ cells. N=9 wells per condition over 3 experiments. (H) VEGF mRNA in FRCs after co-culture with wild-type or IL-1β−/− CD11b+ cells. The specific VEGF isoforms evaluated are as indicated. N=4 samples per condition over 4 experiments. For (E–H), *=p>.05 and **=p<.01 t-test.
Addition of the CD11b+ cell fraction to the FRCs led to a nearly 4-fold increase in supernatant VEGF levels (Figure 6E). The CD11b− fraction was less potent at inducing increased VEGF levels (Figure 6E), and CD11b+ and − cells by themselves did not yield detectable levels of VEGF in the supernatant (Figure 6E). For mRNA assessment, the FRCs were separated from the tightly adherent CD11b+ and − cells by magnetic depletion of CD45+ cells. FRC VEGF mRNA was upregulated after co-culture with CD11b+ but not CD11b− cells (Figure 6F). CD11b+ cells from IL-1β−/− mice were less able than wild-type cells to induce upregulation of VEGF protein when cultured with FRCs (Figure 6G). QPCR for overall VEGF expression in the FRCs also showed that IL-1β−/− CD11b+ cells were less able than wild-type cells to upregulate VEGF mRNA (Figure 6H), although there was no difference on average between wild-type and IL-1β−/− cocultures (Figure 6H). Soluble VEGF may better reflect expression of shorter VEGF isoforms such as VEGF 120 (44), and VEGF120 upregulation was attenuated in the IL-1β−/− co-cultures (Figure 6H). There was also a trend toward attenuation of VEGF164 upregulation, but the comparison between wild-type and IL-1β−/− cocultures did not meet statistical significance (p=0.05). These results are consistent with the idea that endogenous CD11c+CD11b+ cells are able to directly stimulate FRCs to upregulate VEGF in part by their expression of IL-1β.
IL-1β deficiency results in reduced lymph node growth and vascular-stromal expansion over time
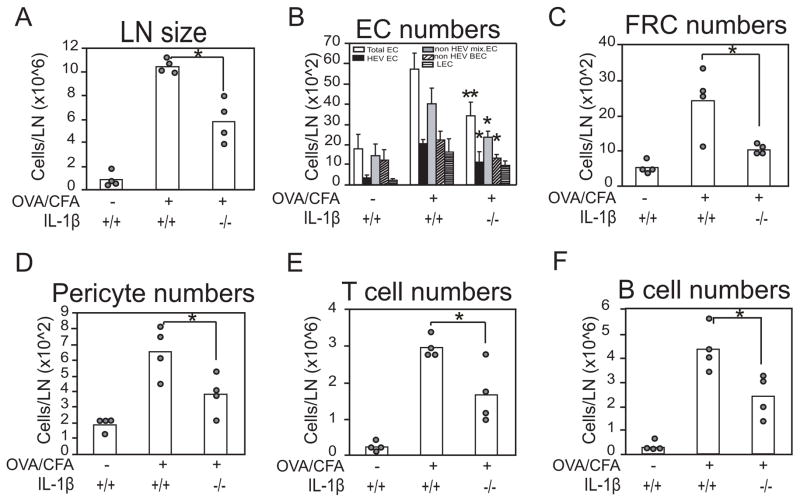
After the initial upregulation of vascular-stromal proliferation, endothelial cells and FRCs expand in number (12, 13). By day 4 after immunization in IL-1β−/− mice, lymph node cellularity and numbers of total, HEV and nonHEV blood endothelial cells, and FRCs were reduced when compared to wild-type mice (Figure 7A–C). Similar to FRCs, pericytes also increased in number by day 4, and IL-1β deficiency resulted in reduced expansion (Figure 7D). T cells and B cells were both reduced in numbers in the IL-1β−/− mice (Figure 7E, F), consistent with the idea of a reduced immune response and with studies showing a role for IL-1β in lymph node lymphocyte priming in some systems (45–47). Taken together, our results suggest that an early accumulation of IL-1β expressing monocytes and dendritic cells after OVA/CFA modulates the initial vascular-stromal activation and proliferation, which then has the potential to modulate the subsequent vascular-stromal expansion during the developing immune response.
Figure 7. IL-1β deficiency attenuates lymph node and lymph node vacular-stromal expansion.
WT or IL-1β−/− mice were immunized with OVA/CFA in footpads and draining popliteal nodes were examined at day 4. (A) Lymph node cellularity. (B) Endothelial cell numbers. (G) FRC numbers. (D) pericyte numbers. (E) T and (F) B cell numbers. For E,F, forward scatterlow and side scatterlow lymphocytes were gated and MHCII-CD11c- cells T cells and MHCI+CD11c- B cells were enumerated. For A, C, D, E, F, each symbol represents one mouse and *=p<.05 for indicated comparisons. For B, bars represent 4 mice over 3 experiments for the total EC and LEC and 3 mice over 2 experiments for HEV EC, non HEV mixed EC and non HEV BEC. *=p<.05; **=p<.01 by t-test relative to OVA/CFA-stimulated wild-type controls.
Discussion
Proliferative growth of the lymph node vascular-stromal compartment is a potential therapeutic target for treating autoimmune and other lymphoproliferative diseases. Our results suggested that vascular growth is initiated in part by recruited IL-1β-expressing monocytes and dendritic cells that can directly stimulate FRCs to upregulate VEGF. In addition to FRC VEGF, IL-1β was required for FRC proliferation, and these results together point to a monocyte/dendritic cell-FRC axis as key in the T and B cell-independent initiation phase of lymph node vascular-stromal growth.
We have identified a collaboration between recruited CCR2-dependent monocytes and CCR7-dependent dendritic cells in initiating lymph node vascular-stromal growth. Out of the CCR7-dependent cells, the relative roles of monocyte-derived, Flt3-dependent inflammatory, and migratory dendritic cells remains to be elucidated (14–16). CD11c+ cells have been implicated in lymphangiogenesis and growth of tertiary lymphoid tissues and also angiogenesis in other inflamed tissues (48–54), and this could reflect in part a similar collaboration between IL-1β-expressing CCR2-dependent monocytes and CCR7-dependent dendritic cells. Of note is that the IL-1β-expressing MHCIIhi dendritic cells in lymph nodes may only require CCR7 to migrate to lymph nodes and may therefore not be CCR7-dependent to localize within peripheral tissues.
Neutrophils also expressed IL-1β at days 1 and 2, and we did not evaluate their importance in this study. Attempts to deplete with Ly6G antibody led to downregulation of Ly6G but not reduction in numbers (Benahmed and Lu, unpublished observations). While Gr-1 depletion could have addressed the roles of neutrophils and monocytes together, Ly6C was expressed on lymph node endothelial cells (34)(Benahmed and Lu, unpublished observations), making any effect on endothelial cell proliferation impossible to interpret. However, indirect evidence from our data suggests that neutrophil-derived IL-1β did not make significant contributions. First, neutrophils comprised only 10–15% of the IL-1β-expressing cells at both days 1 and 2. Second, although neutrophil numbers were at wild-type levels in CCR2−/−CCR7−/− mice, IL-1β blockade in these mice had no effect, suggesting that neutrophils were not an important source of IL-1β. However, we do not preclude an important role for other neutrophil-derived molecules in aspects of vascular-stromal regulation.
Our results together suggest a role for IL-1β in upregulating FRC VEGF, which then can contribute to the upregulation of endothelial cell proliferation. IL-1R1 was also expressed by a proportion of blood endothelial cells, and it is possible that IL-1β can modulate endothelial cell proliferation directly rather than or in addition to effects on FRC VEGF expression. However, IL-1β is a positive regulator of IL-1R1 expression (55, 56), and FRCs but not blood endothelial cells upregulated IL-1R after immunization, raising the possibility that FRCs were the cells being exposed to additional IL-1β. That FRCs but not endothelial cells would directly receiving additional IL-1β signals is possible if, for example, monocytes upregulated IL-1β only after entry into the lymph node. Also, direct exposure of endothelial cell cultures to IL-1β suppresses proliferation (41, 42), supporting a role for indirect regulation by IL-1β in vivo. Direct testing of the relative roles of endothelial and FRC IL-1R1 will require cell-specific deletion and awaits further investigation.
IL-1β and IL-1β-expressing cells played a partial role in the initial stages of vascular-stromal growth, indicating that other mediators remain to be identified. CD11c+ cell depletion has a severe effect on vascular-stromal proliferation (12, 13), and it will be useful in future studies to understand the identity of molecular mediators expressed by other CD11c+ cell population(s). Notably, CD11chi cell depletion before immunization has a partial effect on the initiation phase (19), and, potentially, CD11c hi cells along with IL-1β-expressing monocytes and MHCIIhi dendritic cells together control vascular-stromal proliferation upon lymph node stimulation.
That BMDC-induced augmentation of endothelial cell proliferation in CCR2−/−CCR7−/− mice was IL-1β-independent showed that the relative importance of IL-1β may depend on the particular system. Indeed Luther and colleagues recently published that FRC proliferation at day 5 after immunization with OVA in the adjuvant Montanide ISA-25 was not IL-1β-dependent (57). It will be interesting to understand whether IL-1β plays a role in initiating lymph node vascular-stromal proliferation in any disease state. We have shown that morphologic vascular alterations in an acute autoimmune model resemble those induced by OVA/CFA (58) and that long-term alterations in vascular function in a lupus model are similar to those found during the re-establishment of quiescence and stabilization after model immunization (19, 59). Given these similarities between the model immunizations and disease states, it seems possible that IL-1β plays a similar role in some instances of pathologic lymph node growth and could potentially be targeted at least in the initial stages of lymphoproliferation. Indeed, IL-1β blockade early after disease onset can be quite effective in systemic onset juvenile arthritis, a disease characterized in part by enlarged lymph nodes that have immunoblasts and vascular proliferation (60–62), and we speculate that perhaps part of the effectiveness is attributable to limiting lymph node vascular-stromal growth. IL-1β can also be found in lymphomas (63) and it may be worth considering IL-1β blockade as part of a maintenance regimen to prevent recurrence of lymphoproliferative disease after acute disease treatment.
Acknowledgments
The authors wish to acknowledge Jenn Chia for help with microscopy, Dr. Giorgio Perino for help with cytospin evaluation, other members of the Lu lab for feedback and helping hands, and Kenneth Rock for kind provision of IL-1β−/− breeder mice.
Abbreviations
- FRC
fibroblastic reticular cell
- HEV
high endothelial venule
- VEGF
vascular endothelial growth factor
Footnotes
This work was funded by NIH R01-AI069800, NIH R01 AI079178, Kirkland Center for Lupus Research, and St. Giles Foundation (TL).
References
- 1.Benahmed F, Ely S, Lu TT. Lymph node vascular-stromal growth and function as a potential target for controlling immunity. Clinical Immunology. 2012;144:109–116. doi: 10.1016/j.clim.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph Node Fibroblastic Reticular Cells Construct the Stromal Reticulum via Contact with Lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao S, Ruddle NH. Synchrony of High Endothelial Venules and Lymphatic Vessels Revealed by Immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 5.Ioachim H, Medeiros L. Ioachim’s Lymph Node Pathology. Lippincott, Williams and Wilkins; New York: 2009. [Google Scholar]
- 6.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 7.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-Like Reticular Stromal Cell Layer Common to Adult Secondary Lymphoid Organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 10.Mionnet C, Mondor I, Jorquera A, Loosveld M, Maurizio J, Arcangeli ML, Ruddle NH, Nowak J, Aurrand-Lions M, Luche H, Bajenoff M. Identification of a new stromal cell type involved in the regulation of inflamed B cell follicles. PLoS Biol. 2013;11:e1001672. doi: 10.1371/journal.pbio.1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chyou S, Ekland EH, Carpenter AC, Tzeng T-CJ, Tian S, Michaud M, Madri JA, Lu TT. Fibroblast-Type Reticular Stromal Cells Regulate the Lymph Node Vasculature. J Immunol. 2008;181:3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, Lu TT. Coordinated Regulation of Lymph Node Vascular-Stromal Growth First by CD11c+ Cells and Then by T and B Cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng TC, Chyou S, Tian S, Webster B, Carpenter AC, Guaiquil VH, Lu TT. CD11chi dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J Immunol. 2010;184:4247–4257. doi: 10.4049/jimmunol.0902914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katakai T, Hara T, Lee JH, Gonda H, Sugai M, Shimizu A. A novel reticular stromal structure in lymph node cortex: an immuno-platform for interactions among dendritic cells, T cells and B cells. Int Immunol. 2004;16:1133–1142. doi: 10.1093/intimm/dxh113. [DOI] [PubMed] [Google Scholar]
- 23.Jackson JR, Minton JA, Ho ML, Wei N, Winkler JD. Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta. J Rheumatol. 1997;24:1253–1259. [PubMed] [Google Scholar]
- 24.Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev. 243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazuda DJ, Lee JC, Young PR. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. Journal of Biological Chemistry. 1988;263:8473–8479. [PubMed] [Google Scholar]
- 26.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 27.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 29.Tan KW, Yeo KP, Wong FH, Lim HY, Khoo KL, Abastado JP, Angeli V. Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. J Immunol. 2012;188:4065–4080. doi: 10.4049/jimmunol.1101854. [DOI] [PubMed] [Google Scholar]
- 30.Merad M, Sathe P, Helft J, Miller J, Mortha A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annual Review of Immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotta G, Edwards EW, Sangaletti S, Bennett C, Ronzoni S, Colombo MP, Steinman RM, Randolph GJ, Rescigno M. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–1263. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bruijn MF, van Vianen W, Ploemacher RE, Bakker-Woudenberg IA, Campbell PA, van Ewijk W, Leenen PJ. Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: a flow cytometric alternative to differential counting. J Immunol Methods. 1998;217:27–39. doi: 10.1016/s0022-1759(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 33.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. The Journal of Immunology. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 34.Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA, Berg EL, Butcher EC. Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma. Eur J Immunol. 1988;18:1819–1826. doi: 10.1002/eji.1830181125. [DOI] [PubMed] [Google Scholar]
- 35.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid MA, Takizawa H, Baumjohann DR, Saito Y, Manz MG. Bone marrow dendritic cell progenitors sense pathogens via Toll-like receptors and subsequently migrate to inflamed lymph nodes. Blood. 2011;118:4829–4840. doi: 10.1182/blood-2011-03-344960. [DOI] [PubMed] [Google Scholar]
- 39.Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, Gebhardt C, Averbeck M, Anderegg U, Simon JC. Dermal Fibroblasts Induce Maturation of Dendritic Cells. J Immunol. 2007;178:4966–4974. doi: 10.4049/jimmunol.178.8.4966. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, Brenner MB, Carroll MC, Mooney DJ, Turley SJ, Zhou Y, Shinton SA, Hardy RR, Bezman NA, Sun JC, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Bellemare-Pelletier A, Narayan K, Sylvia K, Kang J, Gazit R, Garrison B, Rossi DJ, Jojic V, Koller D, Jianu R, Laidlaw D, Costello J, Collins J, Cohen N, Brennan P, Shay T, Regev A, Kim F, Rao TN, Wagers A, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Best AJ, Knell J, Goldrath A, Heng T, Kreslavsky T, Painter M, Mathis D, Benoist C. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cozzolino F, Torcia M, Aldinucci D, Ziche M, Almerigogna F, Bani D, Stern DM. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990;87:6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Agarwal S. Mechanical signals activate vascular endothelial growth factor receptor-2 to upregulate endothelial cell proliferation during inflammation. J Immunol. 2010;185:1215–1221. doi: 10.4049/jimmunol.0903660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139:1371–1380. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 45.Nambu A, Nakae S, Iwakura Y. IL-1beta, but not IL-1alpha, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. International Immunology. 2006;18:701–712. doi: 10.1093/intimm/dxl007. [DOI] [PubMed] [Google Scholar]
- 46.Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. The Journal of Experimental Medicine. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L, Nakai K, Pravda E, Hornstein MD, D’Amato RJ, Folkman J. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. Faseb J. 2008;22:522–529. doi: 10.1096/fj.07-9034com. [DOI] [PubMed] [Google Scholar]
- 49.Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, D’Amato R, Ingber DE. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. Faseb J. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 51.Nakai K, Fainaru O, Bazinet L, Pakneshan P, Benny O, Pravda E, Folkman J, D’Amato RJ. Dendritic cells augment choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:3666–3670. doi: 10.1167/iovs.07-1640. [DOI] [PubMed] [Google Scholar]
- 52.Muniz LR, Pacer ME, Lira SA, Furtado GC. A Critical Role for Dendritic Cells in the Formation of Lymphatic Vessels within Tertiary Lymphoid Structures. The Journal of Immunology. 2011;187:828–834. doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, Worbs T, Forster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akahoshi T, Oppenheim JJ, Matsushima K. Interleukin 1 stimulates its own receptor expression on human fibroblasts through the endogenous production of prostaglandin(s) J Clin Invest. 1988;82:1219–1224. doi: 10.1172/JCI113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellehumeur C, Blanchet J, Fontaine JY, Bourcier N, Akoum A. Interleukin 1 regulates its own receptors in human endometrial cells via distinct mechanisms. Hum Reprod. 2009;24:2193–2204. doi: 10.1093/humrep/dep192. [DOI] [PubMed] [Google Scholar]
- 57.Yang C-Y, Vogt TK, Favre Sp, Scarpellino L, Huang H-Y, Tacchini-Cottier F, Luther SA. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proceedings of the National Academy of Sciences. 2014;111:e109–118. doi: 10.1073/pnas.1312585111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar V, Chyou S, Stein JV, Lu TT. Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy. Frontiers in Immunology. 2012;3:282. doi: 10.3389/fimmu.2012.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chyou S, Tian S, Ekland EH, Lu TT. Normalization of the Lymph Node T Cell Stromal Microenvironment in lpr/lpr Mice Is Associated with SU5416-Induced Reduction in Autoantibodies. PLoS ONE. 2012;7:e32828. doi: 10.1371/journal.pone.0032828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nigrovic PA, Mannion M, Prince FHM, Zeft A, Rabinovich CE, van Rossum MAJ, Cortis E, Pardeo M, Miettunen PM, Janow G, Birmingham J, Eggebeen A, Janssen E, Shulman AI, Son MB, Hong S, Jones K, Ilowite NT, Cron RQ, Higgins GC. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: Report of forty-six patients from an international multicenter series. Arthritis & Rheumatism. 2011;63:545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 61.Schneider R, Laxer RM. Systemic onset juvenile rheumatoid arthritis. Baillieres Clin Rheumatol. 1998;12:245–271. doi: 10.1016/s0950-3579(98)80018-6. [DOI] [PubMed] [Google Scholar]
- 62.Valente RM, Banks PM, Conn DL. Characterization of lymph node histology in adult onset Still’s disease. J Rheumatol. 1989;16:349–354. [PubMed] [Google Scholar]
- 63.Ruco LP, Pomponi D, Pigott R, Stoppacciaro A, Monardo F, Uccini S, Boraschi D, Tagliabue A, Santoni A, Dejana E, et al. Cytokine production (IL-1 alpha, IL-1 beta, and TNF alpha) and endothelial cell activation (ELAM-1 and HLA-DR) in reactive lymphadenitis, Hodgkin’s disease, and in non-Hodgkin’s lymphomas. An immunocytochemical study. Am J Pathol. 1990;137:1163–1171. [PMC free article] [PubMed] [Google Scholar]