Abstract
Feedback pathways are a common circuit motif in vertebrate brains. Reciprocal interconnectivity is seen between the cerebral cortex and thalamus as well as between basal ganglia structures, for example. Here we review the literature on the nucleocortical pathway, a feedback pathway from the cerebellar nuclei to the cerebellar cortex, which has been studied anatomically but has remained somewhat obscure. This review covers work examining this pathway on a number of levels, ranging from its existence in numerous species, its organization within cerebellar circuits, its cellular composition, and a discussion of its potential roles in motor control. Recent interest in cerebellar modular organization raises the profile of this neglected cerebellar pathway, and it is hoped that this review will consolidate knowledge gained over several decades of research into a useful format, spurring new investigations into this evolutionarily conserved pathway.
Keywords: Purkinje, cerebellar nuclei, interpositus, nucleocortical, feedback, topography, cerebellar anatomy, reciprocal
Initial discovery and observations
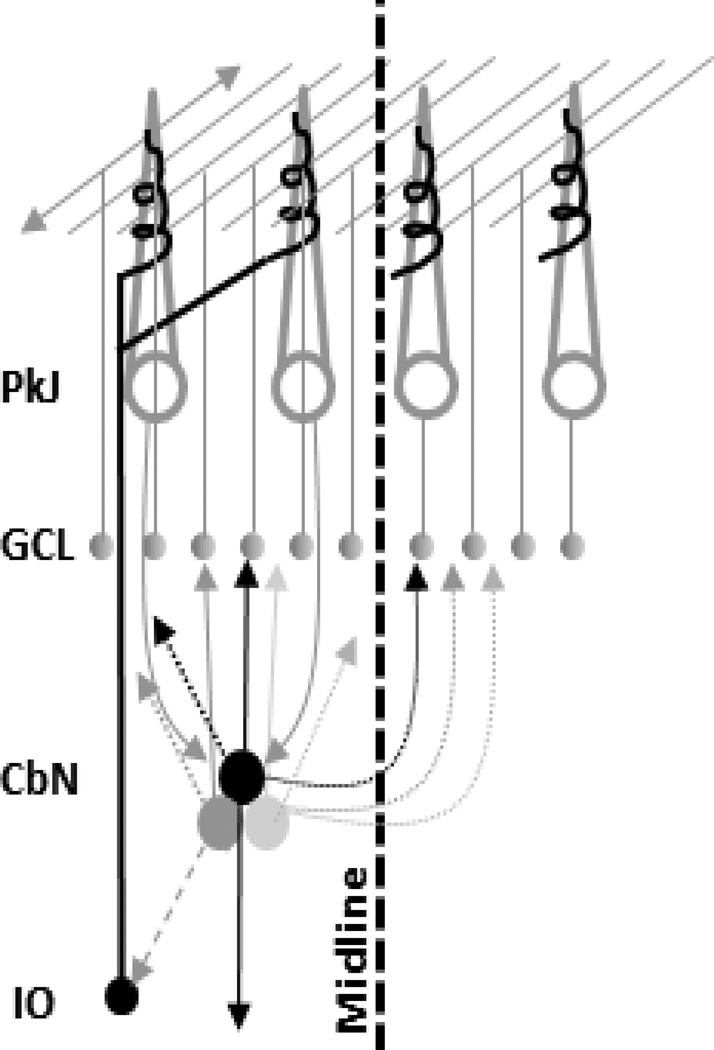
The nucleocortical pathway was first definitively identified in 1976 in two independent studies published within a month of each other. The rapid and widespread replacement of axon degeneration techniques with use of horse radish peroxidase (HRP) transport and tritiated leucine tracing contributed to this nearly simultaneous co-discovery of a nucleocortical pathway by Tolbert et al. (1976) and Gould and Graybiel (1976) in cats [1,2]. Shortly afterward, a similar independent observation of retrogradely labeled neurons in the cerebellar nuclei following HRP injections into the cerebellar cortex was made in pigeons [3]. Tolbert et al. then showed a nucleocortical connection in macaques using a combination of retrograde HRP transport, anterograde tritiated leucine injections and electrical stimulation [4]. A flurry of activity followed. All told, the existence of a nucleocortical pathway was confirmed in rats [5, 6, 7, 8, 9], tree shrew [10], bush babies [11], mouse [12], cats [1,2], pigeons [3] and macaques [4], bringing the number of species in which it was confirmed to seven, suggesting that it is a conserved pathway. The history of these initial discoveries was recently reviewed in personal accounts by Daniel Tolbert, Barbara Brown (Gould), and Ann Graybiel [13–14]. Its basic organization is schematized in Figure 1.
Figure 1.
Organization of the nucleocortical pathway. The primary nucleocortical pathway is reciprocally connected with Purkinje areas that target it (solid arrows). Minor pathways are depicted with dotted lines. The dashed projection from the CbN to the IO is speculative in the sense that it is unknown if it is the IO projecting nuclear neurons that collateralize to the cerebellar cortex to produce the GABAergic mossy fibers. Abbreviations: Pkj: Purkinje layer; GCL: granule cell layer; CbN: cerebellar nuclei; IO: inferior olive.
Early experiments describing the pathway took three main tacks: Retrograde tracing from HRP injections into the cerebellar cortex; orthograde tracing from tritiated leucine injections into the cerebellar nuclei; and electrophysiological recordings coupled with stimulation of the cerebellar cortex to elicit antidromic spikes, all in cats. The key observations from these methods included retrograde label in the CbN following HRP injection into the cerebellar granule cell layer; axons and mossy fiber terminals in the granule cell layer following tritiated leucine injections into the nuclei (see below for discussion of mossy fiber identification); and short latency antidromic spikes in cells recorded in the cerebellar nuclei following cerebellar surface stimulation. Indeed, the observation of antidromic spikes following cerebellar cortex stimulation was first made by Ito and colleagues who later interpreted the findings as resulting from current spread to the nuclei from the stimulation site [15]. Coupled with the anatomical data, however, activation of nucleocortical cells by cerebellar cortex stimulation could be confidently interpreted as representing antidromic propagation. Using axonal degeneration following axotomy, Hámori and Takács estimated that the nucleocortical pathway comprises approximately 5% of the cerebellar mossy fiber population, since extracerebellar mossy fibers degenerated after BC transection [16].
Gross organization: topography and reciprocality
From the earliest anatomical experiments examining the nucleocortical pathway, there was a strong indication that the nucleocortical pathway was organized roughly into sagittal zones: tritiated leucine injections into fastigial and posterior interposed nuclei in cats labeled fibers that terminated in vermal areas while injections into the dentate nuclei labeled fibers terminating in more lateral lobules such as Crus I and II. Interposed nucleus injections, likewise, labeled fibers terminating in intermediate areas [1,2]. Similarly, Gould and Graybiel found an orderly arrangement of retrogradely labeled nuclei following cortical HRP injections, corresponding to injection site.
Subsequent to these initial observations, the topic of topographical organization of the nucleocortical pathway was investigated in a variety of species and with increasing refinement, with both sagittal and rostrocaudal topography observed [17,7]. In a series of studies in cats, Dietrichs and colleagues injected HRP into numerous cerebellar lobules including the paramedian, Simple, Crus I and II, anterior, posterior, paraflocculus, vermis and finally the floccular-nodular lobes [17–21]. While the primary focus of these papers was exploring the patterns of corticonuclear connections, they noted that, in general, retrogradely labeled neurons comprising the nucleocortical pathway followed the same patterns as corticonuclear connections. Thus, zonal, topographical organization was consistent between both the corticonuclear and nucleocortical pathways. For example, the paramedian lobule can be divided into a series of sagittal subzones A through D based on white matter organization [22–23]. The lateral most folia (D2 zone) projects to the ventral and middle lateral nucleus (NL). Slightly more medially, the D1 zone projects to the NL and anterior interposed nucleus transition area (NIA). Middle folia (C2) projects to dorsal posterior interposed nucleus (NIP) and the medial most area (C1) projects to dorsomedial NIP. Since nucleocortical projections followed the same patterns but in reverse, the theme of reciprocal connections between Purkinje neurons and subjacent granule cell layers was supported.
The fact that the nuclei project back to the cerebellar cortical region that provides Purkinje input to it begs the question of whether closed loops form with corticonuclear projecting Purkinje neurons. Gould was among the first to examine reciprocality and found retrogradely labeled nucleocortical neurons overlapping with corticonuclear terminal fields [24]. Indeed, many of the studies examining the topographical organization of the nucleocortical pathway noted that nuclear neurons retrogradely labeled following HRP injections into the cerebellar cortex fell within zones of anterogradely colabeled Purkinje axonal plexes. Numerous studies support the idea that reciprocal corticonuclear and nucleocortical pathways form closed loops, as defined as retrogradely labeled nucleocortical neurons coinciding with anterogradely labeled Purkinje terminal fields [2, 7,9, 10, 17–21; 24–31].
These studies also note retrogradely labeled nucleocortical neurons that occur outside the Purkinje terminal field, suggesting an additional open-loop arrangement. For example, deviations from reciprocality are seen following vermal injections in monkeys which label Purkinje terminals ramifying in the fastigial and interpositus nucleus but label nucleocortical neurons in the dentate nucleus [33,25]. Similarly, HRP injections into anterior lobe intermediate areas III and IV labeled Purkinje axons ramifying in medial NIA, but did not label retrograde neurons in that area. Rather, retrogradely labeled cells appeared in rostromedial NM [19]. Trott et al. quantified the degree to which retrograde label fell within Purkinje terminal zones and found heterogeneity between areas [30,31]. Injections into the paramedian lobule C1 zone revealed the closed-loop arrangement of approximately 67% of nucleocortical neurons, of which approximately 17% appeared on the “fringe” of the Purkinje terminal fields within NIA. The remaining retrograde label fell outside the zone formed by Purkinje axons [30]. Injections into the paramedian lobule C2 pars anterior zone revealed a predominantly reciprocal arrangement (92% overlap with corticonuclear terminals) with pars posterior injections showing similarly high (88% overlap) [31]. Thus, the nucleocortical pathway is seemingly part of both closed and open loops with its cortical afferents, and the degree of reciprocality differs between cortical areas. Of course, it has also been noted that dendrites of nucleocortical neurons that reside outside the reciprocal Purkinje terminal zone could extend dendrites to close the loop, potentially increasing the fraction of nucleocortical neurons that participate in closed loops [10].
Nucleocortical neurons that fell outside of the Purkinje terminal zone were sometimes noted to be less intensely labeled with HRP than those that fell within the terminal fields [27]. It has been speculated that this staining pattern could result from limited tracer uptake by diffusely branching terminal arbors from nucleocortical cells. For example, if the main branch of a nucleocortical neuron primarily arborizes in zones with reciprocal Purkinje neurons but less densely collateralizes in other zones, then tracer injection into the cortex would be taken up sparsely by these sparse collaterals and appear in non-reciprocal areas [27]. Indeed branching of nucleocortical axons is supported by several studies. Tolbert et al., using tritiated leucine as an anterograde tracer, also describe “prolific” branching of nucleocortical fibers [25]. Experiments that involve injections of fluorescent tracers into multiple areas of the cerebellar cortex also support the idea of branching fibers: After injecting somatotopically related but distant patches of cerebellar cortex, Provini et al. report double labeled nucleocortical cells [32]. Similarly, dual injections of fast blue and nuclear yellow into the paramedian lobule and intermediate cortex labeled neurons in NIA and NIP. Although most were singly labeled cell, approximately 20% were double labeled, supporting the idea of branching nucleocortical fibers [11].
Deviations from this scheme of reciprocality were noted in several studies owing to the patchy absence of nucleocortical projections. Specifically, some corticonuclear pathways appear devoid of nuclear projections. Examining this issue directly, Buisseret-Delmas and Angaut found that when focusing on the sagittal arrangement of nucleocortical projections, injections of HRP into the B and C3 zones of rats labeled no nucleocortical fibers [27,28]. Additionally, some patches of C1 and D zones are devoid of nucleocortical fibers. These data are thematically similar to observations made in cats where HRP injections of the C2 zone of the paravermis labeled many more retrograde nucleocortical neurons in the posterior IP than injections into the C1 or C3 zones, which labeled none or just a few cells. Trott et al. also note that C1 injections of Lobule V seem devoid of nucleocortical projections [30]. Nucleocortical fibers may be additionally subdivided against longitudinal and lobular arrangements: While the C2 zone of the paravermal cortex of lobules Vb/c in cats has many nucleocortical fibers [31], the C2 zone of the paraflocculus has none [18,19].
While a majority of the open-loop, non-reciprocal nucleocortical neurons were found in ipsilateral nuclei, non-reciprocal connections were also found contralaterally. Following HRP injections into the granule cell layers of primates, cats, rats, and tree shrews, retrogradely labeled cells in the contralateral cerebellar nuclei have been observed, suggesting that the nucleocortical pathway includes a contralateral projection [7–11,31]. These retrogradely labeled cell populations did not intermingle with Purkinje axon arbors, which are commonly recognized to strictly target ipsilateral nuclei. The fraction of ipsilateral to contralateral cell label was always reported to be low. In cases when label was quantified, ratios between 6:1 and 12:1 ipsi-to-contralateral cells were reported in tree shrews and 10:1 reported in cats [11; 30,31]. On the surface, these data suggest a minor role for the contralateral nucleocortical pathways, although information on the axonal branching characteristics and electrophysiological properties of this population would resolve whether contralaterally projecting nuclear cells exert widespread effects on the cortical circuit.
Similar to the observation of heterogeneity in the existence of nucleocortical projections ipsilateral to HRP injections, not all cortical zones appear to receive contralateral input from the nucleocortical pathway. Injections into the paramedian lobule (PML pars anterior C1 zone) in the cat labeled no contralateral cells in cats. Similarly, HRP injections in longitudinal B zones in rat did not result in contralateral label while injections into the A anc C1 zones did [28]. The neuronal population that makes up the contralateral nucleocortical pathway includes cells immunopositive for both glutamate and GABA, at ratios similar to their ipsilateral partners [8], suggesting that there is not an obvious difference in the neurotransmitter phenotypes employed by ipsi- and contralaterally projecting nucleocortical neurons. Finally, it is unclear whether contralaterally projecting nucleocortical neurons branch to innervate the ipsilateral cortex as well, since bilateral dual fluorescent tracer injections into cerebellar cortical zones did not reveal double labeled neurons in the cerebellar nuclei [11].
Cellular identity
At least three cell types based on neurotransmitter phenotype comprise the cerebellar nuclei: glutamatergic, GABAergic, and glycinergic neurons [34]. Each of these cell types have been suggested to extend feedback projections to the cerebellar cortex. Contemporary studies examining the diversity of cell types in the cerebellar nuclei have included information on electrophysiological properties, projection targets, neurotransmitter expression and most recently transcription factor expression patterns as features differentiating cell types [35–38]. Furthermore, use of transgenic mouse lines that express fluorescent reporters in a subset of neurons aid in identifying populations of nuclear neurons [35–37, 39]. The general conclusion of these studies is 3-fold. First, large premotor projection neurons are primarily glutamatergic, but include a small population of glycinergic fastigial output neurons. Second, GABAergic neurons are small and primarily project to the inferior olive, although it is unclear if all GABAergic neurons project to the IO. Third, two classes of small glycinergic neurons have been defined based on electrophysiological properties that differentiate between spontaneously active or silent neurons. Only silent glycinergic neurons have been shown to project to the cerebellar cortex [35]. (See below). Notably, electrophysiological differences among a majority of nuclear neurons appear to be very subtle, with spontaneous firing the norm, excluding a small population of silent glycinergic neurons [35,36]. Differences among the cell types primarily cluster around small differences in maximal firing rates and inhibitory current kinetics [37]. These data suggest that integrative properties of different neuronal cell types within the cerebellar nuclei may be fairly uniform.
The literature on the nucleocortical pathway has, with few exceptions, relied on cell size as a proxy determinant of neuronal “type”, of which 6 were postulated by Chan-Palay based on soma size and dendritic arborization [34]. Subsequent studies established that a population of small cells included neurons positive for glutamic acid decarboxylase (GAD), suggesting that they are GABAergic, and that these small neurons in part project to the IO [34]. Several studies measured soma size of retrogradely labeled neurons following HRP injections into the cerebellar cortex. Histograms of soma size following HRP injections into the cerebellar cortex in cats revealed a broad distribution of somatic diameters, ranging from 5 – 35 µm [9, 43]. Comparing the distribution of nucleocortical neuron soma size to distributions of labeled somata following either IO or ventrolateral thalamus (VL) injections revealed broad overlap, leaving the identity of nucleocortical neurons inconclusive. VL projecting neurons had somatic diameters ranging from 6–40 µm while those projecting to the IO were on average smaller, ranging from 9–15 µm. Investigations in other species such as rat show similar cell diameter distributions that vary between glutamatergic and IO targets: reticular formation targeting cells ranged in diameter from 12–27 µm, averaging around 20 µm and IO-projecting cells ranged from 5–16 µm, averaging around 10 µm [9, 43].
Similar conclusions were made by Gould who measured a maximum diameter range of retrogradely labeled nucleocortical neurons ranging from 15–60 µm, with the greatest number of somata spanning approximately 30 µm [24]. She also noted that injections into the vestibulocerebellum, specifically the flocculus and nodulus, primarily labeled small to mediumsized neurons, with a diameter range of 10–50 µm and predominant diameters of hovering around 20–25 µm. Whether these size differences reflect different neurotransmitter phenotypes comprising the nucleocortical pathway remains unknown.
Cell diameter measurements of retrogradely labeled nucleocortical neurons were later coupled with immunohistochemical identification of cell type. Retrograde label from cerebellar cortex injections coupled with immunostaining revealed that 56% of nucleocortical afferents were positive for glutamate only, 7.8% positive for GABA only, 23% were positive for both glutamate and GABA, and 11% were neither, and could reflect the glycinergic population [8]. At the EM level, retrogradely labeled nucleocortical neurons included both GABA-immunonegative and immunopositive neurons that were postsynaptic to Purkinje boutons [29]. These authors also correlated immunostaining with measurements of cell diameter to help bridge their experiments with previous studies. Consistent with the idea that glutamatergic output neurons that target the thalamus are larger, on average, than other cell types, they found that glutamate-immunopositive neurons had somatic diameters of 10–35 µm and GABAergic, putative IO-projecting cells, had diameters of 5–22 µm [8].
These data suggested that at least part of the nucleocortical projection is GABAergic, based on colabel of retrogradely labeled nucleocortical neurons with markers for GABAergic phenotypes. Several lines of evidence both support and call into question these conclusions. HRP injections into the C2 zone of the posterior cat cerebellum retrogradely labeled a few nucleocortical neurons, none of which were found to be GABA-positive, calling into question the extent of any GABAergic nucleocortical pathway [41]. On the other hand, Chan-Palay noted uptake of a GAD antigen complex by terminals in the cerebellar cortex which was retrogradely transported to the nuclei. In the opposite direction, she showed GAD-antigen labeled mossy fibers in the cerebellar cortex. Together, these data raised the intriguing possibility of GABAergic mossy fibers [5]. The idea of GABAergic mossy fibers was investigated at the EM level as well, with immunogold labeling against GABA localized to a subset of mossy fibers that were immune to die-back after pedunculectomy, suggesting that they were intrinsic mossy fibers of putative nuclear origin [16]. These investigators also found small GABA immunopositive axon varicosities located at the periphery of glomeruli typical of Golgi cell axons. A second, larger mossy fiber-like ending also strongly labeled for GABA and contained large spheroid and pleomorphic vesicles. These terminals were located centrally within a glomerulus in contrast to the peripheral location of putative Golgi cell axon terminals. Indeed, the small, peripheral profiles that were consistent with terminals of Golgi cells did not degenerate after folial transections whereas the GABAergic mossy fibers degenerated under this manipulation, consistent with their classification as nucleocortical fibers.
Overall, the evidence suggests that a minority of nucleocortical neurons are GABAergic and glycinergic; most are glutamatergic. Clearly modern techniques will help illuminate this issue further and provide insight into the functional role of these overlooked inhibitory projection neurons within the cerebellum.
An example of a recent use of transgenic mice that has advanced our understanding of the chemical identity of a subset of nucleocortical projection neurons is seen in work aimed at characterizing the physiological and morphological properties of glycinergic neurons in the lateral cerebellar nuclei [35]. Uusisaari and Knöpfel used a transgenic mouse line that expresses GFP under the GlyT2 promoter [42] to target whole-cell recordings in an acute brain slice preparation. By filling GlyT2-GFP+ cells with biocytin, they were able to visualize the projection patterns of recorded neurons whose axons were not severed during the acute slicing procedure. In several cases, they identified axons coursing into the cerebellar cortex from glycinergic neurons, indicating that the glycinergic neuronal population of the cerebellar nuclei also provides a nucleocortical projection [35].
Differential connectivity patterns of nucleocortical neurons have also been used as a classification scheme. In studies examining the morphology and electrophysiology of nuclear neurons it has been suggested that nucleocortical projections form as collaterals from cerebellarfugal cells. The early work by Tolbert and colleagues among others suggested that nucleocortical fibers may emanate as collaterals of cerebellar efferents because they observed antidromic spike collision following closely spaced brachium conjuctivum (BC) and cerebellar cortex stimulation [1,25,40, 43]. Similarly, intracellular recordings made in vivo coupled with biocytin fills allowed McCrea and colleagues to reconstruct nuclear neurons. They found two cells with axons extending into both the cerebellar cortex the BC [40]. More extensive antidromic mapping work suggested that nuclear neurons may branch to target the VL, IO and cerebellar cortex [25]. However, in the contemporary literature it is accepted that VL and IO-projecting neurons are non-overlapping populations, calling into question some of these conclusions. One study that provided more definitive proof of collateralization of nucleocortical neurons was a short anatomical study in rats showing double labeled neurons in the cerebellar nuclei following dual fluorescent tracer injections into the VL and cerebellar cortex [44]. Collateralization to the IO was not investigated in this study. It has also been proposed that different subsets of nucleocortical neurons could be defined based on their connection profile with the cortex. Four types of nucleocortical cells were proposed based on intracerebellar projection patterns: ipsilateral reciprocal nucleocortical cells; ipsilateral non-reciprocal cells; contralateral nucleocortical cells and dedicated nucleocortical cells, which project to cortex but not to other areas [26,45].
In summary, the literature suggests that the nucleocortical pathway is primarily composed of glutamatergic neurons, some of which possess collateral branches that innervate extracerebellar targets and the cerebellar cortex. In addition, a number of GABAergic and glycinergic neurons also project to the cortex. Whether these inhibitory neurons also target extracerebellar targets is unclear.
Nucleocortical terminals & targets
Before the nucleocortical pathway was definitively identified in the 1970’s [1,2], reports had surfaced that the cerebellar nuclei could be a source of climbing fibers to Purkinje neurons [46,47]. These studies relied on axonal degeneration techniques and were very soon overturned by work using the more sensitive anatomical tracers such as HRP and tritiated thymidine. Subsequent studies showed that injections restricted to the cerebellar granule cell layer were most successful at yielding retrogradely labeled cells in the cerebellar nuclei, suggesting that nucleocortical projections targeted the GCL. Indeed, no nucleocortical retrograde label was reported following HRP injections confined to the molecular layer. Rather, injections had to include the GCL to label nuclear neurons [17]. Consistent with these data, anterograde tracing from the cerebellar nuclei labeled axons and putative terminals that were restricted to the white matter and GCL. Although the light microscopic-level resolution was not excellent compared to contemporary standards, investigators consistently reached the conclusion that the nucleocortical pathway terminated as mossy fibers [2, 48].
EM work coupled with autoradiography confirmed that at least some nucleocortical axons terminate as mossy fibers in both cats and rats [49,50]. Nucleocortical synapses could form en passant, simple or complex mossy fiber rosettes, which were associated with granule cell dendrites. Non-glomerular contacts were also formed with putative Golgi cells. Putative nucleocortical synapses, identified by their resistance to degeneration following pedunculectomy, occupied normal glomerular orientations. Although degeneration resistant mossy fibers were reportedly “smoother” with fewer irregularities than most normal rosettes, they were centrally located within glomeruli, contacting granule cell dendrites and more peripherally Golgi cell axon terminals [49]. Taken together, these data indicate that the nucleocortical pathway primarily targets the GCL with mossy fibers, regardless of whether terminals are glutamatergic or GABAergic. The morphology of glycinergic nucleocortical terminals is unknown.
Functional importance of the nucleocortical pathway
The role of the cerebellum in movement remains somewhat enigmatic. Among the most common descriptions of computation carried out by cerebellar circuitry include calibration of reflexes as exemplified in its role in the vestibular-ocular reflex (VOR) adaptation. In addition, the oscillatory nature of movements following cerebellar damage and disease has pointed to the idea that the cerebellum computes internal models of body dynamics. Both of these processes benefit from internal feedback relaying outgoing motor commands to sensory areas which use it as a reference to evaluate sensory inflow. Although speculative, nuclear output neurons that collateralize to project back to the cerebellar cortex could be a source of this type of efference copy or corollary discharge information, aiding the cerebellar cortex in monitoring ongoing cerebellar/motor output [51].
The topographical organization of the pathway has inspired other speculations on its function. Reciprocal connections between the nuclei and Purkinje neurons could serve a role in interconnecting internal paths between microzones [48]. This proposed function would be well served by both reciprocal and non-reciprocally organized pathways, with non-overlapping CN-NC fields providing an anatomical substrate for an intercompartmental coordinating system [30,31]. Nucleocortical projections that branch to innervate functionally related but spatially distinct regions of cerebellar cortex could serve to bind these areas [32]. It has also been speculated that lateral “neocerebellar” cortex and the dentate are involved with preprogramming movement while medial “paleocerebellar” cortex are more involved in updating ongoing movements. Lateral zones were found to have more reliable reciprocal connections than medial areas, thus this internal circuitry may suggest differential computations occurring across the medio-lateral extent of the cerebellum [33].
In other brain regions, internal excitatory feedback loops such as those provided by the nucleocortical pathway have been speculated to support waves of re-excitation, amplifying initial excitatory events [52]. If analogous with recurrent excitatory collaterals within the cerebral cortex, the nucleocortical pathway may provide timing signals that underlie motor sequence generation and thereby support the coordination of movement. Furthermore, internal inhibitory feedback loops between Purkinje neurons and GABAergic nucleocortical neurons are somewhat reminiscent of GABAergic networks within the basal ganglia and may similarly be important for refinement of movement. Similarly, corticothalamic and thalamocortical loops are thought to modify thalamic relay and enhance signal-to-noise ratios of sensory input [53]. Thus, if these analogies hold, nucleocortical feedback projections could serve a wide range of possible functions within the cerebellar circuit.
Summary
The nucleocortical pathway consists of a projection from the cerebellar nuclei back to the cerebellar cortex and is conserved amongst diverse vertebrate species. It primarily targets cerebellar cortical areas that project to it, but deviates from strict closed loop reciprocality with open loop ipsi- and contralateral projections. It includes all cell types of the cerebellar nuclei, but data suggest that it is primarily composed of glutamatergic neurons. The nucleocortical pathway appears to terminate as mossy fibers in the granule cell layer, although the morphological characteristics of GABA and glycinergic components of the pathway are less well established. These loops may help support normal motor behavior mediated by the cerebellum, a broad hypothesis that may now be tested with contemporary experimental tools. Indeed, it is likely because cell-type specific manipulations were not feasible that this pathway has suffered a dearth of physiological investigations since its discovery.
Acknowledgements
We thank C. Beitzel for useful feedback on the manuscript. Supported by NIH R01 NS084996-01, the Klingenstein Award in Neuroscience, and the Sloan Research Fellowship (ALP).
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Tolbert DL, Bantli H, Bloedel JR. Anatomical and physiological evidence for a cerebellar nucleocortical projection in the cat. Neuroscience. 1976;1:205–217. doi: 10.1016/0306-4522(76)90078-6. [DOI] [PubMed] [Google Scholar]
- 2.Gould BB, Graybiel AM. Afferents to the cerebellar cortex in the cat: evidence for an intrinsic pathway leading from the deep nuclei to the cortex. Brain Res. 1976;110:601–611. doi: 10.1016/0006-8993(76)90869-6. [DOI] [PubMed] [Google Scholar]
- 3.Clarke PGH. Some visual and other connections to the cerebellum of the pigeon. JCN. 1977;174:535–552. doi: 10.1002/cne.901740307. [DOI] [PubMed] [Google Scholar]
- 4.Tolbert DL, Bantli H, Bloedel JR. The intracerebellar nucleocortical projection in a primate. Exp Br Res. 1977;30:425–434. doi: 10.1007/BF00237266. [DOI] [PubMed] [Google Scholar]
- 5.Chan-Palay V, Palay SL, Wu JY. Gamma-aminobutyric acid pathways in the cerebellum studied by retrograde and anterograde transport of Glutamic acid decarboxylase antibody after in vivo injections. Anat Embryol. 1979;157:1–14. doi: 10.1007/BF00315638. [DOI] [PubMed] [Google Scholar]
- 6.Umetani T. Topographic organization of the cerebellar nucleocortical projection in the albino rat: an autoradiographic orthograde study. Brain Res. 1990;507:216–224. doi: 10.1016/0006-8993(90)90275-g. [DOI] [PubMed] [Google Scholar]
- 7.Päälysaho J, Sugita S, Noda H. Cerebellar corticonuclear and nucleocortical projections in the vermis of posterior lobe of the rat as studied with anterograde and retrograde transport of WGA-HRP. Neuroscience Res. 1990;8:158–178. doi: 10.1016/0168-0102(90)90018-a. [DOI] [PubMed] [Google Scholar]
- 8.Batini C, Compoint C, Buissert-Delmas C, Daniel H, Guegan M. Cerebellar nuclei and the nucleocortical projection in the rat: retrograde tracing coupled to GABA and Glutamate immunohistochemistry. J Comp Neurol. 1992;315:74–84. doi: 10.1002/cne.903150106. [DOI] [PubMed] [Google Scholar]
- 9.Yatim N, Buissert-Delmas C, Buissert P, Compoit C, Angaut P. Nucleus medialis-nucleus interpositus interface: its Olivary and cerebellocortical projections in the rat. J Comp Neurol. 1995;363:1–14. doi: 10.1002/cne.903630102. [DOI] [PubMed] [Google Scholar]
- 10.Haines DE, Pearson JC. Cerebellar corticonuclear-nucleocortical topography: A study of the tree shrew (Tupaia) paraflocculus. JCN. 1979;187:745–758. doi: 10.1002/cne.901870407. [DOI] [PubMed] [Google Scholar]
- 11.Haines DE. Evidence of intracerebellar collateralization of nucleocortical cell processes in a Prosimian Primate (Galago): A fluorescence retrograde study. J Comp Neurol. 1988;275:441–451. doi: 10.1002/cne.902750308. [DOI] [PubMed] [Google Scholar]
- 12.Hendelman WJ, Marshall KC. Axonal projection patterns visualized with horseradish peroxidase in organized cultures of cerebellum. Neuroscience. 1980;5:1833–1846. doi: 10.1016/0306-4522(80)90033-0. [DOI] [PubMed] [Google Scholar]
- 13.Haines DE, Manto MU. The discovery and definitive proof of cerebellar nucleocortical projections 1976. Cerebellum. 2009;8:1–18. doi: 10.1007/s12311-008-0054-8. [DOI] [PubMed] [Google Scholar]
- 14.Haines De, Manto MU. The discovery and definitive proof of cerebellar nucleocortical projections: part 2, or the story continued and confirmed. Cerebellum. 2010;9:1–16. doi: 10.1007/s12311-008-0054-8. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Hongo T, Yoshida M, Okada Y, Obata K. Intracellularly recorded antidromic responses of Deiters’ neurons. Experientia. 1964;20:295–296. doi: 10.1007/BF02151821. [DOI] [PubMed] [Google Scholar]
- 16.Hámori J, Takács J. Two types of GABA-containing axon terminals in the cerebellar glomeruli of cat: an immunogold-EM study. Exp Br Res. 1989;74:471–479. doi: 10.1007/BF00247349. [DOI] [PubMed] [Google Scholar]
- 17.Dietrichs E, Walberg F. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of HRP. I: The Paramedian Lobule. Anat Embryol. 1979;158:13–39. doi: 10.1007/BF00315949. [DOI] [PubMed] [Google Scholar]
- 18.Dietrichs E. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of HRP. III: The anterior lobe. Anat Embryol. 1981;162:223–247. doi: 10.1007/BF00306494. [DOI] [PubMed] [Google Scholar]
- 19.Dietrichs E. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of HRP IV: The paraflocculus. Exp Br Res. 1981;44:235–242. doi: 10.1007/BF00236560. [DOI] [PubMed] [Google Scholar]
- 20.Dietrichs E. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of HRP. V: The posterior lobe vermis and the floccular-nodular lobe. Anat Embryol. 1983;167:449–462. doi: 10.1007/BF00315681. [DOI] [PubMed] [Google Scholar]
- 21.Dietrichs E, Walberg F. The cerebellar corticonuclear and nucleocortical projections in the cat as studied with anterograde and retrograde transport of HRP. II: Lobulus simplex, Crus I and II. Anat Embryol. 1980;161:83–103. doi: 10.1007/BF00304670. [DOI] [PubMed] [Google Scholar]
- 22.Voogd J. Comparative aspects of the structure and fibre connexions of the mammalian cerebellum. Prog Brain Res. 1967;25:94–134. doi: 10.1016/S0079-6123(08)60963-2. [DOI] [PubMed] [Google Scholar]
- 23.Voogd J. The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinás RR, editor. Neurobiology of Cerebellar Evolution and Development. Am Med Assoc.; 1969. [Google Scholar]
- 24.Gould BB. The organization of afferents to the cerebellar cortex in the cat: Projection from the deep cerebellar nuclei. JCN. 1979;184:27–42. doi: 10.1002/cne.901840103. [DOI] [PubMed] [Google Scholar]
- 25.Tolbert DL, Bantli H, Bloedel JR. Organizational features of the cat and monkey cerebellar nucleocortical projection. JCN. 1978;182:39–56. doi: 10.1002/cne.901820104. [DOI] [PubMed] [Google Scholar]
- 26.Haines DE. HRP study of cerebellar corticonuclear-nucleocortical topography of the dorsal culminate lobule-lobule V- in a prosimian primate (Galago): with comments on nucleocortical cell types. JCN. 1989;282:274–292. doi: 10.1002/cne.902820209. [DOI] [PubMed] [Google Scholar]
- 27.Buissert-Delmas C, Angaut P. The cerebellar nucleocortical projections in the rat: A retrograde labeling study using HRP combined to a lectin. Neurosci Letters. 1988;84:255–260. doi: 10.1016/0304-3940(88)90516-2. [DOI] [PubMed] [Google Scholar]
- 28.Buissert-Delmas C, Angaut P. Anatomical mapping of the cerebellar nucleocortical projections in the rat: a retrograde labeling study. JCN. 1989;288:297–310. doi: 10.1002/cne.902880208. [DOI] [PubMed] [Google Scholar]
- 29.Angaut P, Compoint C, Buissert-Delmas C, Batini C. Synaptic connections of Purkinje cell axons with nucleocortical neurons in the cerebellar medial nucleus of the rat. Neurosci Res. 1996;26:345–398. doi: 10.1016/s0168-0102(96)01116-9. [DOI] [PubMed] [Google Scholar]
- 30.Trott JR, Apps R, Armstrong DM. Zonal organization of corticonuclear and nucleocortical projections of the paramedian lobule of the cat cerebellum 1. The C1 zone. Exp Br Res. 1998;118:298–315. doi: 10.1007/s002210050285. [DOI] [PubMed] [Google Scholar]
- 31.Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum 2: The C2 zone. Exp Br Res. 1998;118:316–330. doi: 10.1007/s002210050286. [DOI] [PubMed] [Google Scholar]
- 32.Provini L, Marcotti W, Morara S, Rosina A. Somatotopic nucleocortical projections to the multiple somatosensory cerebellar maps. Neuroscience. 1998;83:1085–1104. doi: 10.1016/s0306-4522(97)00477-6. [DOI] [PubMed] [Google Scholar]
- 33.Tolbert DL, Bantli H. An HRP and autoradiographic study of cerebellar corticonuclear-nucleocortical reciprocity in the monkey. Exp Br Res. 1979;36:563–571. doi: 10.1007/BF00238523. [DOI] [PubMed] [Google Scholar]
- 34.Chan-Palay V, editor. Cerebellar dentate nucleus: Organization, Cytology and Transmitters. Springer-Verlag; 1977. [Google Scholar]
- 35.Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55. doi: 10.1007/s12311-009-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. 2007;97:901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 37.Uusisaari M, Knöpfel T. GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience. 2008;156:537–549. doi: 10.1016/j.neuroscience.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 38.Kodama T, Guerrero S, Shin M, Moghadam S, Faulstich M, du Lac S. Neuronal classification and marker gene identification via single-cell expression profiling of brainstem vestibular neurons subserving cerebellar learning. J Neurosci. 2012;32:7819–7831. doi: 10.1523/JNEUROSCI.0543-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci. 2009;29:10104–10110. doi: 10.1523/JNEUROSCI.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCrea RA, Bishop GA, Kitai ST. Morphological and electrophysiological characteristics of projection neurons in the nucleus interpositus of cat cerebellum. J Comp Neurol. 1978;181:397–420. doi: 10.1002/cne.901810210. [DOI] [PubMed] [Google Scholar]
- 41.Kolston J, Apps R, Trott JR. A combined retrograde tracer and GABA-immunocytochemical study of the projection from nucleus interpositus posterior to the posterior lobe C2 zone of the cat cerebellum. Eur J Neurosci. 1995;7:926–933. doi: 10.1111/j.1460-9568.1995.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 42.Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 43.Tolbert DL, Bantli H, Bloedel JR. Multiple branching of cerebellar afferent projections in cats. Exp Br Res. 1978;31:305–316. doi: 10.1007/BF00237291. [DOI] [PubMed] [Google Scholar]
- 44.Payne JN. The cerebellar nucleocortical projection in the rat studied by the retrograde fluorescent double labeling method. Brain Res. 1983;271:141–144. doi: 10.1016/0006-8993(83)91374-4. [DOI] [PubMed] [Google Scholar]
- 45.Carrea RME, Reissig Mg, Mettler FA. The climbing fibers of the simian and feline cerebellum Experimental inquiry into their origin by lesions of the inferior olives and deep cerebellar nuclei. J Comp Neurol. 1947;87:321–365. doi: 10.1002/cne.900870304. [DOI] [PubMed] [Google Scholar]
- 46.Cohen D, Chambers WW, Sprague JM. Experimental study of the efferent projections from the cerebellar nuclei to the brainstem of the cat. J Comp Neurol. 1958;109:233–259. doi: 10.1002/cne.901090207. [DOI] [PubMed] [Google Scholar]
- 47.Haines DE, Dietrichs E. The Cerebellum – structure and connections. In: Subramony SM, Dürr A, editors. Handbook of Clinical Neurology Volume 103. Ataxic Disorders Elseiver; 2012. pp. 3–36. [DOI] [PubMed] [Google Scholar]
- 48.Legendre A, Courville J. Cerebellar nucleocortical projection with a survey of factors affecting the transport of radioactive tracers. J Comp Neurol. 1986;252:392–403. doi: 10.1002/cne.902520308. [DOI] [PubMed] [Google Scholar]
- 49.Tolbert DL, Kultas-Ilinsky K, Ilinsky I. EM-autoradiography of cerebellar nucleocortical terminals in the cat. Anat Embryol. 1980;161:215–222. doi: 10.1007/BF00305345. [DOI] [PubMed] [Google Scholar]
- 50.Hamori J, Mezey E, Szentagothai J. Electron microscopic identification of cerebellar nucleo-cortical mossy terminals in the rat. 1981;44:97–100. doi: 10.1007/BF00238753. [DOI] [PubMed] [Google Scholar]
- 51.Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- 52.Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 53.Sherman SM, Guillery RW. Thalamus. In: Shepherd GM, editor. The Synaptic Organization of the Brain, Fifth Edition. New York, New York: Oxford Univ Press; 2004. pp. 311–359. [Google Scholar]