Abstract
Type 1 diabetes (T1D) results from a complex interplay between genetic susceptibility and environmental factors that have been implicated in the pathogenesis of disease both as triggers and potentiators of β-cell destruction. CD8 T cells are the main cell type found in human islets, and they have been shown in vitro to be capable of killing β-cells overexpressing MHC class I. In this study, we report that CD8 T cells infiltrate the exocrine pancreas of diabetic subjects in high numbers and not only endocrine areas. T1D subjects present significantly higher CD8 T cell density in the exocrine tissue without the presence of prominent insulitis. Even T1D donors without remaining insulin-containing islets and long disease duration show elevated levels of CD8 T cells in the exocrine compartment. In addition, higher numbers of CD4+ and CD11c+ cells were found in the exocrine tissue. Preliminary data in type 2 diabetic (T2D) subjects indicate that overall, there might be a spontaneous inflammatory infiltration of the exocrine tissue, common to both T1D and T2D subjects. Our study provides the first information on the precise tissue distribution of CD8 T cells in pancreata from T1D, T2D, autoantibody-positive, and healthy control subjects.
Introduction
Type 1 diabetes (T1D) is a complex chronic disorder in which the pancreatic insulin-producing β-cells are destroyed by the immune system (1). The precise nature of genetic and environmental factors that cause T1D is still not known. Already prior to diagnosis, autoantibodies are present, and thereafter, it is presumed that self-reactive lymphocytes become activated and infiltrate the pancreas, contributing to β-cell destruction. Previous reports from Foulis et al. (2) and our laboratory (3) have shown hyperexpression of MHC class I in β-cells and the presence of autoantigen-specific CD8 T cells in pancreatic islets. Such cytotoxic T cells can kill β-cells overexpressing MHC class I in vitro (4). CD4 T cells, B cells, natural killer cells, and macrophages can also be found in insulitic lesions; their specificity for β-cell antigens in situ is not known, and they are thought to participate in β-cell killing through the release of inflammatory mediators that trigger β-cell apoptosis (5). Cytokines and chemokines from inflammatory cells may also act indirectly to activate or recruit cells to the sites of metabolic stress and inflammation (4,6,7).
Conversely, type 2 diabetes (T2D) has been historically characterized as a nonautoimmune disease linked to obesity, insulin resistance, and inflammation (8–10). However, this notion has recently been challenged, since autoreactive T cells were found in increased numbers in patients diagnosed with T2D and linked to more rapid loss of C-peptide (11–13). Furthermore, it is likely, at least in the U.S., that a significant proportion of T2D patients with low BMIs and insulin dependence in reality have autoimmune T1D (14). Lastly, overlap of insulin resistance and autoimmunity is now more frequently observed in younger diabetic patients and poses significant therapeutic challenges (15). In general, chronic immune activation might be a characteristic of T1D and T2D.
Traditionally, islet inflammation (insulitis) has been the hallmark of T1D. It is frequently present in young patients with a duration of disease between 1 month and 1 year (60–73%), but its detection dramatically decreases in older patients (30%) and over time (16). Even in recent-onset patients, the number of T cells infiltrating the islets is often very limited, which has prompted a recent position article on the definition of insulitis, in which >15 CD45+ cells per islet in a minimum of 3 islets should be evident (17). The surroundings of the islets are often overlooked in regards to cellular infiltration. However, it is known that the reduction in insulin production by β-cells can influence the exocrine pancreas and the secretory response to gut hormones and neurotransmitters (18). Moreover, the pattern of blood flow in the pancreas implies that the exocrine tissue receives part of its supply through the islets, and it is exposed to large concentrations of hormones and antigens (18,19). Some studies have shown that the exocrine pancreas is reduced in size by about one-third in T1D (20,21), which is accompanied by a reduction in function (22). Whether this is a consequence of the lack of insulin secretion or might be related to an autoimmune-mediated inflammation is unknown. The role of the exocrine pancreas in the development or maintenance of diabetes is therefore not well understood.
The aim of the current study was to provide information on the precise tissue distribution of CD8 T cells, the main cell type implicated in β-cell destruction. We have taken advantage of the availability of tissues from the Network for Pancreatic Organ Donors with Diabetes (nPOD), which procures frozen pancreas samples from healthy (control), autoantibody-positive (Ab+), T1D, and T2D individuals in a coordinated effort across the U.S. and all around the world. We present the first systematic study that distinguishes between exocrine and endocrine pancreatic CD8 T cell infiltration and also takes into account the periphery of the islets. Both T1D with short (<5 years) and long (>5 years) duration of disease and T2D subjects presented significantly higher CD8 T cell density in the exocrine pancreas without the presence of prominent insulitis and even in diabetic cases without remaining insulin-containing islets (ICIs). Our findings establish the presence of increased immune cell infiltration in the exocrine compartment of the pancreas in diabetes, for which the pathogenetic significance remains to be determined.
Research Design and Methods
Subjects
Human pancreata were collected from cadaveric organ donors through nPOD. Six-micrometer sections from frozen pancreas samples from T1D (n = 31), T2D (n = 11), diabetes-free with T1D-associated islet autoantibodies (Ab+; n = 14), and healthy control subjects (n = 15) were obtained. Donors with pancreatitis were identified based on information provided by nPOD. Pancreatitis is noted when observed in the histopathology section and rated from mild to severe, focal to multifocal, acute/chronic, or mixed. Clinical history is listed when the information has been provided in the chart. Table 1 shows summarized demographic information for each group. Supplementary Table 1 with detailed demographic and histological information has been included as supplementary online material. All experimental procedures were approved by the La Jolla Institute for Allergy and Immunology Institutional Review Board–approved protocol number DI3-054-1112.
Table 1.
Donor demographic information
| Control | Ab+ | T1D | T2D | Total | |
|---|---|---|---|---|---|
| n | 15 | 14 | 31 | 11 | 71 |
| Age (years) [mean (± SEM)] | 22.57 (± 2.99) | 32.02 (± 3.18) | 23.54 (± 1.6) | 36.32 (± 3.02) | 26.98 |
| Female [n (%)] | 4 (26.67) | 7 (50) | 14 (45.16) | 7 (63.64) | 32 (45.07) |
| Male [n (%)] | 11 (73.33) | 7 (50) | 17 (54.84) | 4 (36.36) | 39 (54.93) |
| Ethnicity [n (%)] | |||||
| African American | 2 (13.33) | 2 (14.29) | 3 (9.68) | 4 (36.36) | 11 (15.49) |
| Caucasian | 9 (60.00) | 10 (71.43) | 25 (80.65) | 2 (18.18) | 46 (64.79) |
| Hispanic | 4 (26.67) | 2 (14.29) | 3 (9.68) | 5 (45.45) | 14 (19.72) |
| BMI [mean (± SEM)] | 24.43 (± 1.81) | 26.35 (± 1.98) | 24.37 (± 0.66) | 34.73 (± 1.86) | 26.37 |
| Disease duration [mean (± SEM)] | 9.99 (± 1.50) | 10.86 (± 2.77) | 10.21 | ||
| C-peptide (ng/mL) [mean (± SEM)] | 5.38 (± 1.41) | 6.37 (± 1.51) | — | 7.78 (± 2.94) | 3.62 |
Demographic information is shown including age, male-to-female ratio, ethnicity, BMI, disease duration, and C-peptide levels.
Immunofluorescence
For characterization of the sections and quantification of cells, sections were subject to a standard immunofluorescence staining protocol (3). Briefly, sections were fixed with 0.4% paraformaldehyde and blocked with goat serum. Stainings for insulin, glucagon, CD8, CD4, or CD11c were performed at room temperature for 1 h using the following antibodies: polyclonal guinea pig anti-insulin (DakoCytomation; 1/140) or polyclonal rabbit anti-glucagon (DakoCytomation; 1/130), monoclonal mouse anti-CD8α (IgG1, clone HIT8a; BD Biosciences, 1/100), monoclonal mouse anti-human CD4 (IgG1, clone 34930; R&D Systems, 1/100), and monoclonal mouse anti-human CD11c (IgG1k, clone 3.9; eBioscience; 1/100). Detection was done at room temperature for 45 min using polyclonal goat anti-guinea pig IgG, Alexa Fluor 488 (Invitrogen; 1/1,000) or polyclonal goat anti-rabbit IgG, Alexa Fluor 488 (Invitrogen; 1/1,000), F(ab’)2 fragment of goat anti-mouse IgG, Alexa Fluor 594 (Invitrogen; 1/1,000), or goat anti-mouse IgG (H+L), Alexa Fluor 555 (Invitrogen; 1/1,000) antibodies. After washing, sections were mounted and analyzed using an Eclipse 80i microscope (Nikon).
Image Acquisition and Analysis
For CD8 quantification, pancreas sections were stained, and an average of 10–15 images (surface area of 1.215 mm2) from each tissue section were acquired using a Nikon digital DXM1200C camera and Nikon ACT-1C Camera Controller Software unless otherwise indicated. To determine the number of CD8 T cells infiltrating the pancreas, image analysis was performed by using a custom macro developed in MATLAB (The Mathworks, Inc., Natick, MA) and ImageJ (National Institutes of Health). Briefly, islet regions were identified as contiguous areas of insulin or glucagon staining at or above a threshold intensity value. The periphery of the islet was defined using a dilation tool and expanding its perimeter by 100 × 100 pixels (15–20 µm). CD8+ cells were identified as areas of CD8 staining using optimized and identical threshold values for intensity and size for all the images. A comparison between manual counts and software-assisted counts was performed in 15 images in order to validate the macro used to quantify CD8+ cells. In addition, all software-processed images were manually checked to identify any possible errors. For CD4 and CD11c counts, five images from each donor were analyzed manually. Average infiltration rates (cells/mm2) were calculated for each donor and used as individual and independent samples in the subsequent statistical analyses.
β- and α-cell areas were determined as the percentage of the total area of the image that was positive for insulin or glucagon staining using a custom macro developed for ImageJ (National Institutes of Health).
Statistical Analysis
Group differences were analyzed using one-way ANOVA follow by a Holm-Sidak multiple comparisons test or Kruskal-Wallis follow by a Dunn multiple comparisons test. Differences between group pairs were analyzed with a Student t test or Mann-Whitney test. Correlations between CD8 T cell density and clinical parameters were analyzed using Pearson correlation with two-tailed significance test. Outliers were identified and removed from the analysis using the Rout method, establishing a maximum false discovery rate (FDR) of 1% (Q = 1). All analyses were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA). Data in bar graphs and tables are presented as mean ± SEM unless otherwise indicated. Findings were assumed statistically significant at P < 0.05.
Results
High Numbers of CD8+, CD4+, and CD11c+ Cells Infiltrate the Exocrine Pancreas of Ab+ and Diabetic Donors
In order to elucidate if there was a high level of infiltration and possible inflammation in the pancreas of diabetic donors, the number of CD8+, CD4+, and CD11c+ cells was assessed in healthy, Ab+, T1D, and T2D donors and compared (Fig. 1 and Supplementary Fig. 1). The highest densities were generally found for CD8+ cells; CD4+ cells were less abundant for all the groups, while CD11c densities were more variable (Fig. 2). As the majority of cells were found in the exocrine tissue, cell densities were further calculated in this compartment only. Interestingly, Ab+ donors presented a high number of CD4+ and CD11c+ cells (Fig. 2A and B), which might indicate that both cell types have a relevant role in the first stages of the disease. In addition, individual density values showed significant differences in CD4 and CD11c infiltration between control subjects and T1D with both short and long disease duration. Since the interaction between CD4+ and CD11c+ cells is essential for the initiation of immune responses, correlation between these cells types was analyzed. A strong positive linear correlation was found (R2 = 0.5992; P < 0.0001), indicating that accumulation of both cells types in the exocrine pancreas might be “directly proportional” and that the presence of a certain amount of one of these cell types might predict the presence of the other (Fig. 2C). Furthermore, analysis of exocrine CD8+ infiltration showed significant differences between healthy control subjects and diabetic donors (T1D and T2D) (Fig. 2D).
Figure 1.
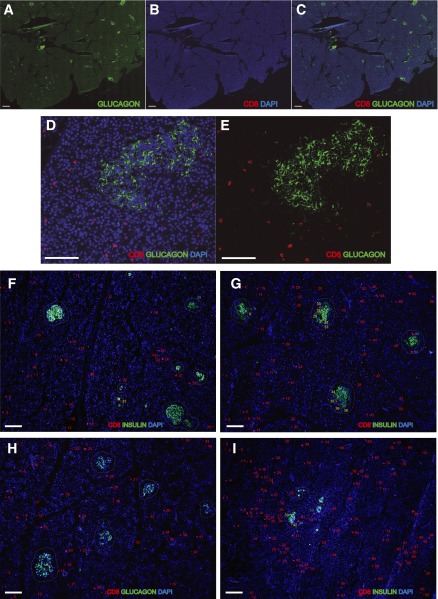
Quantification of CD8+ T cells in pancreatic tissue sections. Representative quantification of CD8 T cells in pancreata is shown (see Research Design and Methods for details). A–E: Frozen pancreas sections from a T1D donor with <5 years of disease duration were stained for glucagon and CD8. Images were acquired using a BIOREVO BZ-9000 slide scanner system (Keyence, Osaka, Japan). Representative images of exocrine and endocrine pancreatic CD8 T cell quantification in healthy (F), Ab+ (G), T1D (H), and T2D donors (I). Numbers represent CD8 T cell quantification in the exocrine pancreas (in red), in the periphery of the islet (in blue), and in the endocrine tissue (in yellow). The contour of the islets is defined by a dashed line. Scale bars, 500 μm in A–C and 100 μm in D–I.
Figure 2.
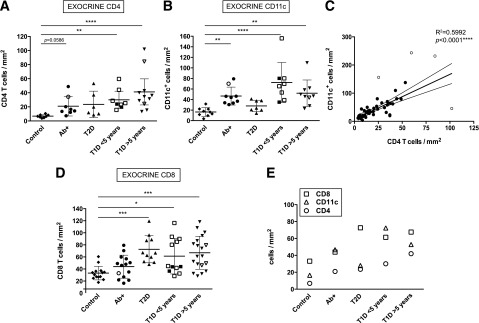
Diabetic donors present high exocrine infiltration. A, B, and D: Mean exocrine density values (cells/mm2) for each donor and group are shown for CD4+, CD11c+, and CD8+. (CD4: n = 10 for control subjects, n = 8 Ab+, n = 6 T2D, n = 8 T1D <5 years, and n = 11 T1D >5 years. CD11c: n = 10 control subjects, n = 9 Ab+, n = 8 T2D, n = 9 T1D <5 years, and n = 10 T1D >5 years. CD8: n = 15 control subjects, n = 14 Ab+, n = 11 T2D, n = 12 T1D <5 years, and n = 19 T1D >5 years.) Open symbols indicate donors in which the nPOD Pathology Core found signs of insulitis. C: Linear regression analysis of CD4 and CD11c density (n = 42). Outliers are shown as open circles. The corresponding R2 and P values are indicated in the graph. E: Mean density value (cells/mm2) for CD8+, CD4+, and CD11c+ cells for the exocrine pancreas is shown. For statistical analysis, nonparametric Kruskal-Wallis test followed by Dunn multiple comparisons test was used to determine significance. Linear regression analysis was used in C. Outliers were identified using the Rout method (FDR of 1% [Q = 1]). Two and one outliers from the control group were identified in A and D, respectively; in B, one control, one T2D, and two T1D donors were identified as outliers. *Significant difference between groups (P ≤ 0.05); **significant difference between groups (P ≤ 0.01); ***significant difference between groups (P ≤ 0.001); ****significant difference between groups (P ≤ 0.0001).
Overall, high numbers of both CD4+ and CD11c+ cells were found in the exocrine pancreas of Ab+ and T1D donors, along with CD8+ cells. CD8 T cells were the predominant cell type in all the groups except in Ab+ and T1D donors with short duration of disease, in which CD11c+ cells were found in similar and higher numbers, respectively (Fig. 2E).
Diabetic Subjects Present High Exocrine CD8 Infiltration in the Pancreas
As CD8+ T cells are the main cell type implicated in the destruction of β-cells and were found in high numbers in diabetic donors, the presence of these cells in the islets and the surrounding tissue was further evaluated. CD8 T cells were quantified using computer-assisted image analysis and manual counts (Fig. 1). Next, CD8 T cell density was calculated based on glucagon and/or insulin staining in order to compare cell counts between different groups. Interestingly, increased CD8 T cell density in the islets was seen in Ab+ and in T1D donors with short duration of disease (<5 years) (Fig. 3A). Infiltration of the endocrine compartment seemed to be transient as CD8 T cell density was similar to control subjects in T1D donors with longer duration of disease (>5 years) (Fig. 3A). Moreover, the level of infiltration in T2D subjects was comparable to that of healthy control subjects. In contrast, infiltration in the periphery of the islets was significantly higher in T2D donors (Fig. 3B). Interestingly, pancreatic CD8 infiltration was strikingly different in diabetic donors compared with control subjects (Fig. 3C). Both T1D and T2D subjects presented significantly higher levels of infiltrating CD8 T cells in the pancreas. Furthermore, mean CD8 density was high in Ab+ donors.
Figure 3.
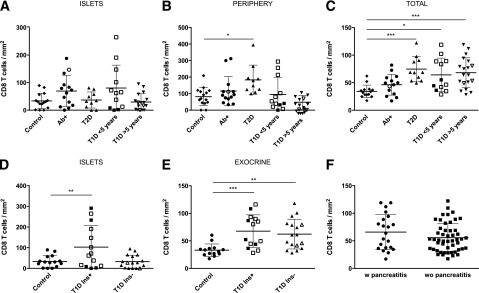
Distribution of CD8 infiltration in the pancreas of healthy, Ab+, T1D, and T2D donors. Mean CD8 density values (cells/mm2) for each donor and group are shown in the islets in A and D, in the periphery in B, in the exocrine pancreas in E, and as total CD8 T cell density in C. Open symbols indicate donors in which the nPOD Pathology Core found signs of insulitis. A–C: n = 15 control subjects, n = 14 Ab+, n = 11 T2D, n = 12 T1D <5 years, and n = 19 T1D >5 years. D and E: T1D Ins−, T1D donors with only IDIs; T1D Ins+, T1D donors with ICIs (n = 15 control subjects, n = 14 T1D Ins+, and n = 17 T1D Ins−). F: Mean CD8 density values (cells/mm2) for each donor are shown for donors with pancreatitis (n = 21) and donors without pancreatitis (n = 50). For statistical analysis, one-way ANOVA with Holm-Sidak multiple comparisons test and Kruskal-Wallis followed by Dunn multiple comparisons test were used to determine significance. A Student t test was applied in F. Mean ± SD is shown in A–F. Outliers were identified using the Rout method (FDR of 1% [Q = 1]). In A and B, one outlier T2D and two outliers T1D >5 years were identified; in D, one outlier control was identified. *Significant difference between groups (P ≤ 0.05); **significant difference between groups (P ≤ 0.01); ***significant difference between groups (P ≤ 0.001). w, with; wo, without.
Further characterization of the relationship between endocrine and exocrine infiltration revealed that endocrine CD8 T cell density was higher than in the rest of the tissue only in Ab+ and T1D donors with short duration of disease (ratio islet density/total density Ab+ = 1.569; T1D <5 years = 1.482 vs. control = 1.013) (data not shown).
Insulin Drives CD8 Infiltration in the Islets but not in the Exocrine Tissue
In order to further elucidate the role of insulin and β-cell presence in the level of infiltration in both exocrine and endocrine compartments, CD8 infiltration was analyzed in T1D with ICIs and donors with only insulin-deficient islets (IDIs). A significant difference in the level of infiltration in the islets between the two groups was seen (Fig. 3D). Thus, insulin seemed to be driving the infiltration of CD8 T cells in the islets. However, CD8 T cell density in the exocrine pancreas was similar in both T1D with ICIs and T1D with only IDIs (Fig. 3E). In addition, accumulation of CD8 T cells in the islets in T1D with ICIs compared with T1D with only IDIs and control subjects was illustrated by a higher ratio between islet and total infiltration (ratio islet density/total density T1D donors with ICIs = 1.548 vs. T1D donors with only IDIs = 0.6193 vs. control subjects = 1.013) (data not shown).
Lastly, as pancreatitis is a common pathological phenomenon in patients with diabetes that might explain the differences between the groups, the level of CD8 infiltration was analyzed in donors with and without pancreatitis. No significant differences were found in terms of CD8 infiltration (Fig. 3F). This suggests that the fact of having a concomitant pancreatitis did not explain the high levels of CD8 infiltration detected in some of these donors.
Diabetic Donors Present Reduced Islet Area and Pancreatic Weight
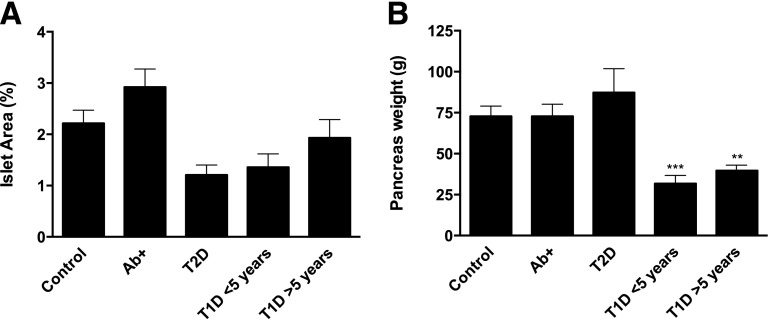
Next, islet area was analyzed to evaluate possible differences in terms of islet destruction in these groups. A paradoxical increase in relative islet area in Ab+ donors was seen, while there was a marked decrease in T1D donors with short duration of disease (Fig. 4A). Differences between the groups were not statistically significant, but a strong tendency to lower area in T1D and T2D donors was seen. Similarly, pancreas weight was greatly reduced in both T1D with <5 years and T1D with >5 years of disease duration (Fig. 4B).
Figure 4.
T1D donors present lower islet area and pancreatic weight compared with healthy control subjects. A: Bars represent mean relative islet area (%) for each group based on insulin and glucagon staining. Differences among the groups were not statistically significant, but a strong tendency to lower area in T1D and T2D donors was seen. B: Bars represent mean pancreatic weight for each group based on information provided by nPOD (n = 14 control subjects, n = 14 Ab+, n = 11 T2D, n = 11 T1D <5 years, and n = 19 T1D >5 years). For statistical analysis, one-way ANOVA with Holm-Sidak multiple comparisons test and Kruskal-Wallis followed by Dunn multiple comparisons test were used to determine significance in A and B, respectively. Mean ± SEM values are shown. **Significant difference between groups (P ≤ 0.01); ***significant difference between groups (P ≤ 0.001).
CD8 T Cells Are Similarly Distributed in the Pancreas of Diabetic Donors and Control Subjects
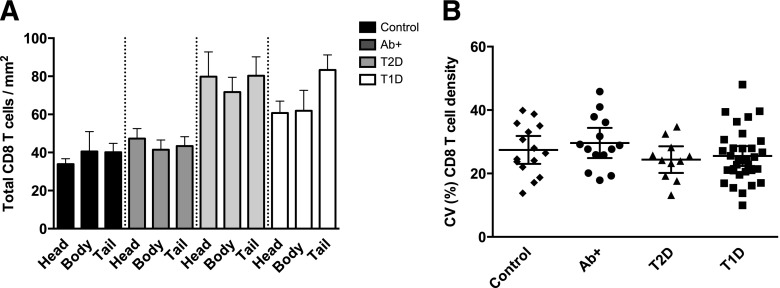
In order to study whether CD8 T cells might be preferentially infiltrating certain parts of the pancreas, total CD8 T cell density was analyzed in head, body, and tail regions and compared. There were no significant differences among these three regions within the groups (Fig. 5A); therefore, CD8 T cells were similarly distributed in the pancreas. Only a small increase in the tail region of T1D donors was noticed. Next, we analyzed if significant variations in CD8 T cell density could be found between different images taken from the same tissue section and donor. A direct comparison of the coefficient of variation (CV) for CD8+ cell numbers between healthy control subjects and T1D donors revealed no major differences (Fig. 5B); the mean CV and 95% CI between the two cohorts were similar and comparable to the other groups (Ab+ and T2D). This lack of difference between the groups suggests that the interimage/area variation was not associated with the disease status of the subject.
Figure 5.
CD8 T cells are similarly distributed in the pancreas of diabetic donors and control subjects. A: Bars represent mean CD8 T cell density (cells/mm2) for each region (head, body, and tail) and group (n = 23 control subjects, n = 32 Ab+, n = 21 T2D, and n = 33 T1D). Mean ± SEM values are shown. B: Population mean and 95% CIs of interimage variation (CV) in total CD8 T cell density are shown for each donor and group (n = 15 control subjects, n = 14 Ab+, n = 11 T2D, and n = 31 T1D). For statistical analysis, one-way ANOVA with Holm-Sidak multiple comparisons test in B and Kruskal-Wallis followed by Dunn multiple comparisons test in A were used to determine significance.
Correlation Between CD8 T Cell Density and Clinical Parameters
The possible correlation between pancreatic CD8 T cell density and clinical parameters was then analyzed. Only a weak (r = 0.2850) but significant (P = 0.0160) correlation with age was found, while there was no correlation with other parameters (Fig. 6). A previous report by In't Veld et al. (23) described an increased inflammatory infiltration throughout the pancreatic parenchyma in organ donors with extended life support. In our cohort, CD8 T cell density did not correlate with the time spent in the intensive care unit (ICU) (P = 0.0778; r = 0.2186) (Fig. 6C). Lastly, no correlation was found between CD8 T cell density and duration of disease in diabetic donors (T1D and T2D) (data not shown).
Figure 6.
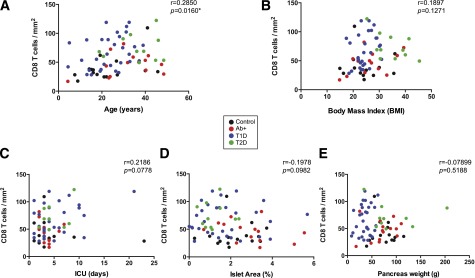
Correlation between CD8 T cell density and clinical parameters. Correlation analysis of CD8 T cell density and clinical parameters, corresponding P and r values are shown for control subjects (black), Ab+ (red), T1D (blue), and T2D (green). A: CD8 T cell density correlates with age (r = 0.2850; P = 0.0160; n = 71). B: CD8 T cell density does not correlate with BMI (r = 0.1897; P = 0.1271; n = 66). C: There was no correlation between CD8 T cell density and time spent in ICU (r = 0.2186; P = 0.0778; n = 66). CD8 T cell density does not correlate with relative islet area (r = −0.1978; P = 0.0982; n = 71) (D) or pancreas weight (r = −0.07899; P = 0.5188; n = 69) (E). Linear regression analysis was used to determine significance. *Significant correlation (P ≤ 0.05).
In order to rule out the possibility of demographic factors influencing our results, an ANOVA test followed by a Bonferroni correction was performed for age and BMI. Significant age differences were only found between control subjects and T2D and between T1D and T2D donors. BMI was different between T2D donors and the rest of the groups. There were no differences between the groups with regards to the time spent in the ICU (a Kruskal-Wallis test with Dunn correction was applied). Finally, duration of disease was similar in both T1D and T2D groups (Mann-Whitney test, P = 0.4671). In conclusion, differences in pancreatic CD8 infiltration between the groups cannot be explained by differences in age, BMI, ICU time, or disease duration. However, although unlikely, differences in age and BMI in the T2D cohort do not allow us to discard that these parameters might be influencing our results in this particular group since these subjects are commonly characterized by both higher age and BMI. Further studies in this cohort should be done separately in order to extend the number of donors analyzed and find suitable control subjects.
Discussion
In this study, we describe for the first time that CD8+, CD4+, and CD11c+ cells are present in high numbers in the exocrine pancreas of Ab+ individuals and patients with T1D. In addition, preliminary data shows that CD8 T cells might be also present in high numbers in the exocrine compartment in T2D donors. Antigen-presenting cells are necessary for antigen-specific activation of T cells. In this regard, dendritic cells (DCs) (CD11c+) provide critical antigenic stimulation to naive CD4+ T cells (24). Our data support the dependency of CD4+ T cells on the host antigen-presenting cells, as a strong correlation was found between frequencies of CD4+ and CD11c+ cells. Both cell populations were elevated in the exocrine pancreas of T1D donors, which indicates a high level of immune activation. In addition, Nikolic et al. (25) proposed that DCs are essential for the retention of lymphocytes in the early pancreatic lesions even before the onset of progressive insulitis, which goes along with our observations, since CD11c+ cells were elevated in the exocrine tissue of both Ab+ and T1D donors with short duration of disease. Moreover, Calderon et al. (26) have shown that islet DCs expressed high levels of cell-derived peptide–MHC complexes with the potential of being presented to T cells. CD11c+ cells might therefore play a role in the level of immune activation and CD4 and CD8 infiltration in the pancreas. As CD8 T cells are the main cell type implicated in the destruction of β-cells, their presence in high numbers in the exocrine tissue might point toward a hitherto unappreciated involvement of the exocrine pancreas in the initiation and propagation of diabetes, which could have important diagnostic and therapeutic consequences. In the oncology field, it has been demonstrated that infiltrating T cells have a major effect on the clinical attributes of human cancer. The analysis of the location, density, and functionality of different immune cell populations has allowed the identification of tumor environment (27,28). As correlation between the level of immune cell infiltration in the pancreas and clinical outcome seems unfeasible because of the difficulty of obtaining biopsied tissue, the number of infiltrating cells could therefore be correlated with circulating numbers in the blood, which could serve as a valuable prognostic biomarker. However, little is known about the correlation of cell numbers in blood and pancreas. Consequently, there is still a lot of work to be done in the diabetes field regarding the identification of all the components of the pancreatic environment, including cellular and cytokine milieus, which could be essential for guiding innovative therapies.
Previous studies in our laboratory have identified antigen-specific CD8 T cells in pancreata from diabetic donors (3). The presence of these cells in the islets was very limited; however, only a few recent-onset donors (1 week to 1 year of disease duration) were studied. Future investigations in biopsy samples from patients at onset of T1D (29) (DiViD study) will provide critical information about the presence of insulitis and autoreactive T cells within weeks from diagnosis. Our current data additionally indicate that CD8 T cells infiltrate the islets in high numbers only in Ab+ and T1D donors with short duration of disease, but these cells remain in the organ even if β-cells have been completely lost. This might indicate that the presence of CD8 T cells in the pancreas is not only antigen-driven, which is in agreement with previous reports (30,31). Recently, it has emerged that tissue-resident memory T cells can permanently reside in peripheral tissues (32,33). Local inflammation is sufficient to attract effector T cells into the tissue, where they can form tissue-resident memory T cells and persist indefinitely (34). However, the pattern of memory T cell trafficking in the pancreas is still not well understood.
Previous reports have suggested that immune cell recruitment to the islets is orchestrated by β-cells themselves, creating a chemoattractant gradient through chemokine interactions (35,36) that disappears once β-cells are lost (37). However, our data show that cells can be found in large numbers in the exocrine tissue of both T1D donors with remaining ICIs and T1D donors with only IDIs, suggesting that the presence of β-cells is not essential for the trafficking of immune cells to the organ. Moreover, Sarkar et al. (36) showed that CXCL10 expression is not restricted to islet lesions in mice and human T1D patients, but it is generally expressed across both endocrine and exocrine tissue. The detection of CCL5/8 and CXCL9, and, to a lesser degree, CX3CL1, in the exocrine pancreas further supports the presence of inflammatory alterations in the acinar tissue of T1D donors, which goes along with our observations regarding cell infiltration. Overall, a high number of diabetic subjects seemed to have an ongoing clinically important inflammatory process within the exocrine pancreas, which might be accompanied by a substantial loss of exocrine function and parenchyma in T1D donors (20,21,38,39). In addition, preliminary data from our laboratory show an increase in exocrine MHC class I expression in both T1D and T2D donors, which might be related to some extent to the level of CD8 T cell infiltration in the exocrine compartment (T.R.-C. and M.G.v.H., unpublished observations). Further comparison between donors with and without pancreatitis show no significant differences in terms of CD8 infiltration. Exocrine inflammation might therefore be the consequence of oxidative stress in acinar cells due to exposure to high circulating levels of glucose or other hormonal factors, which might also lower the threshold for an attack of pancreatitis in patients with diabetes (18,22,40). Further evaluation should be done regarding other cell types in donors with acute or chronic pancreatitis and with or without diabetes to better define the relationship between pancreatitis and diabetes.
In the elusive quest for the origin and cause of the disease, the immunological link between the pancreas and the gut has been an active subject of research over the past few years. Several reports suggest that the gut is structurally and immunologically abnormal in a subset of individuals prone to T1D (41,42). Skog et al. (38) have proposed that gut reflux, increased permeability, and bacterially induced inflammation in certain lobules of the pancreas might contribute to the disease, and therefore, anatomical alterations might reflect a unique and distinct pattern of infiltration in the pancreas. In our study, there were no significant differences between pancreas head, body, and tail regions within the groups, despite a slight increase in the level of CD8+ cells in the tail of T1D donors. The heterogeneity of the disease was therefore not reflected in the level of CD8 T cell infiltration, at least with regards to the three main anatomical parts which comprise the pancreas.
Lastly, our preliminary data show that T2D subjects present high exocrine CD8 infiltration. However, an extensive study with more donors and suitable control subjects with higher BMI and age is needed to fully understand the possible consequences of our findings for T2D pathophysiology. In addition, as our cohort of T2D donors is young (36.3 years old), a higher level of infiltration might be found in older donors with chronic inflammation. In general, inflammation is often characterized by an increase in cytokine levels and infiltrating immune cells in the local sites. Nishimura et al. (43) proposed that CD8 T cells induce the recruitment of macrophages in obesity, thereby mediating the development of insulin resistance. Conversely, it has been shown that adipocytes and macrophages secrete chemoattractants for inflammatory cells, as well as large amounts of tumor necrosis factor-α and other cytokines such as interleukin-1β (44,45) that might recruit CD8 T cells. Both cytokines and other inflammatory cells might play a role in the high level of pancreatic exocrine infiltration observed in our cohort of T2D donors. We are currently investigating the possible contribution of interleukin-1β as well as macrophage and CD8 infiltration to the pathogenesis of T2D in a larger cohort of subjects.
In conclusion, the data presented in this study favor the view that T1D is a dynamic disease that changes over time with an autoimmune and a major inflammatory component. Traditionally, the disease has been characterized by the presence of insulitis and β-cell loss. In this study, we claim a possible involvement of the exocrine pancreas in the development and maintenance of disease. Important questions regarding antigen specificities arise in light of our findings. Are the infiltrating cells antigen specific or just bystander? Which is the main molecule responsible for the immune attraction and persistence of T cells in the pancreas? The answers to these questions would help us to understand the already complicated pathogenesis of T1D and would allow the development of new therapeutic approaches.
Supplementary Material
Article Information
Acknowledgments. The authors thank Grzegorz Chodaczek of La Jolla Institute for Allergy and Immunology for help with image acquisition and analysis; Ken Coppieters of Novo Nordisk and Charles Thivolet of Institut National de la Santé et de la Recherche Médicale (INSERM) U1060, Lyon-Sud Hospital, for critical review of this manuscript; and Priscilla Colby of La Jolla Institute for Allergy and Immunology for administrative assistance.
Funding. This research was performed with the support of nPOD, a collaborative T1D research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations, partnering with nPOD to provide research resources, are listed at www.jdrfnpod.org/our-partners.php. This study was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant R01-AI0-92453-03 and the Wenner-Gren Foundations.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.R.-C. performed and designed experiments, interpreted data, and wrote the manuscript. O.E. performed CD4 and CD11c+ experiments and analyzed data. N.A. performed experiments. J.Z.-G. designed the macros used for computer-assisted software analysis and helped with statistical analysis. M.G.v.H. designed experiments, interpreted data, and wrote the manuscript. M.G.v.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Preliminary results from this study were presented at the nPOD 5th Annual Scientific Meeting, Atlantic Beach, FL, 10–13 February 2013; the nPOD 2014 Annual Meeting, Atlantic Beach, FL, 23–26 February 2014; and Immunology of Diabetes Society 13th International Congress, Lorne, Australia, 7–11 December 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0549/-/DC1.
See accompanying article, p. 3572.
References
- 1.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 2.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. Int Immunol 2012;24:339–346 [DOI] [PubMed] [Google Scholar]
- 5.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci 2013;1281:16–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med 1998;4:781–785 [DOI] [PubMed] [Google Scholar]
- 7.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 2006;19:80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz) 2013;61:119–125 [DOI] [PubMed] [Google Scholar]
- 9.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 2012;8:709–716 [DOI] [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 11.Brooks-Worrell B, Narla R, Palmer JP. Islet autoimmunity in phenotypic type 2 diabetes patients. Diabetes Obes Metab 2013;15(Suppl. 3):137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol 2013;171:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarikonda G, Pettus J, Phatak S, et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun 2014;50:77–82 [DOI] [PubMed] [Google Scholar]
- 14.Kaminski BM, Klingensmith GJ, Beck RW, et al. Body mass index at the time of diagnosis of autoimmune type 1 diabetes in children. J Pediatr 2013;162:736–740e1 [DOI] [PubMed] [Google Scholar]
- 15.Pozzilli P, Guglielmi C. Double diabetes: a mixture of type 1 and type 2 diabetes in youth. Endocr Dev 2009;14:151–166 [DOI] [PubMed] [Google Scholar]
- 16.In’t Veld P. Insulitis in human type 1 diabetes: The quest for an elusive lesion. Islets 2011;3:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 2013;56:2541–2543 [DOI] [PubMed] [Google Scholar]
- 18.Chen N, Unnikrishnan I R, Anjana RM, Mohan V, Pitchumoni CS. The complex exocrine-endocrine relationship and secondary diabetes in exocrine pancreatic disorders. J Clin Gastroenterol 2011;45:850–861 [DOI] [PubMed] [Google Scholar]
- 19.Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology 1980;79:466–473 [PubMed] [Google Scholar]
- 20.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 21.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 22.Hardt PD, Krauss A, Bretz L, et al. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol 2000;37:105–110 [DOI] [PubMed] [Google Scholar]
- 23.In’t Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes 2010;59:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol 2007;179:5041–5053 [DOI] [PubMed] [Google Scholar]
- 25.Nikolic T, Bouma G, Drexhage HA, Leenen PJ. Diabetes-prone NOD mice show an expanded subpopulation of mature circulating monocytes, which preferentially develop into macrophage-like cells in vitro. J Leukoc Biol 2005;78:70–79 [DOI] [PubMed] [Google Scholar]
- 26.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci U S A 2008;105:6121–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306 [DOI] [PubMed] [Google Scholar]
- 28.Ma C, Zhang Q, Ye J, et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J Immunol 2012;189:5029–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 30.Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010;53:690–698 [DOI] [PubMed] [Google Scholar]
- 31.Bettini M, Vignali DA. T cell-driven initiation and propagation of autoimmune diabetes. Curr Opin Immunol 2011;23:754–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009;10:524–530 [DOI] [PubMed] [Google Scholar]
- 33.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 2013;31:137–161 [DOI] [PubMed] [Google Scholar]
- 34.Mackay LK, Stock AT, Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 2012;109:7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppieters KT, Amirian N, Pagni PP, et al. Functional redundancy of CXCR3/CXCL10 signaling in the recruitment of diabetogenic cytotoxic T lymphocytes to pancreatic islets in a virally induced autoimmune diabetes model. Diabetes 2013;62:2492–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar SA, Lee CE, Victorino F, et al. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 2012;61:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frigerio S, Junt T, Lu B, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med 2002;8:1414–1420 [DOI] [PubMed] [Google Scholar]
- 38.Skog O, Korsgren S, Melhus A, Korsgren O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes 2013;20:118–123 [DOI] [PubMed] [Google Scholar]
- 39.Sato M, Yamamoto K, Mayama H, Yamashiro Y. Exocrine pancreatic function in diabetic children. J Pediatr Gastroenterol Nutr 1984;3:415–420 [DOI] [PubMed] [Google Scholar]
- 40.Solanki NS, Barreto SG, Saccone GT. Acute pancreatitis due to diabetes: the role of hyperglycaemia and insulin resistance. Pancreatology 2012;12:234–239 [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre DE, Powell KL, Strom A, Scott FW. Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu Rev Nutr 2006;26:175–202 [DOI] [PubMed] [Google Scholar]
- 42.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM. Enteropathy precedes type 1 diabetes in the BB rat. Gut 2004;53:1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 44.Lagathu C, Yvan-Charvet L, Bastard JP, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia 2006;49:2162–2173 [DOI] [PubMed] [Google Scholar]
- 45.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 2003;100:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.