Abstract
The SEARCH for Diabetes in Youth Study prospectively identified youth aged <20 years with physician-diagnosed diabetes. Annual type 1 diabetes (T1D) incidence per 100,000 person-years (95% CI) overall, by age-group, and by sex were calculated for at-risk non-Hispanic white (NHW) youth from 2002 through 2009. Joinpoint and Poisson regression models were used to test for temporal trends. The age- and sex-adjusted incidence of T1D increased from 24.4/100,000 (95% CI 23.9–24.8) in 2002 to 27.4/100,000 (26.9–27.9) in 2009 (P for trend = 0.0008). The relative annual increase in T1D incidence was 2.72% (1.18–4.28) per year; 2.84% (1.12–4.58) per year for males and 2.57% (0.68–4.51) per year for females. After adjustment for sex, significant increases were found for youth aged 5–9 years (P = 0.0023), 10–14 years (P = 0.0008), and 15–19 years (P = 0.004) but not among 0–4-year-olds (P = 0.1862). Mean age at diagnosis did not change. The SEARCH study demonstrated a significant increase in the incidence of T1D among NHW youth from 2002 through 2009 overall and in all but the youngest age-group. Continued surveillance of T1D in U.S. youth to identify future trends in T1D incidence and to plan for health care delivery is warranted.
Introduction
Type 1 diabetes (T1D) remains the predominant form of diabetes in childhood (1,2). An increase in incidence over the past three decades worldwide, with significant geographic variation, has been reported (3–11), with some studies showing a downward shift in the age at diagnosis (6–8,12). Although several U.S. registries have demonstrated increases in T1D incidence over the past four decades (13–16), these studies individually represent limited geographic areas. The objectives of the current study were to examine the trends in T1D incidence among non-Hispanic white (NHW) youth from 2002 through 2009 and to determine whether the age at diagnosis changed over the study period.
Research Design and Methods
Study Design and Data Collection
Since 2002, SEARCH for Diabetes in Youth has been a multicenter epidemiologic study conducting population-based case ascertainment of youth diagnosed with diabetes before age 20 years. Youth were identified from centers in California (health plan enrollees from one plan in 7 counties), Colorado (64 counties), Ohio (8 counties), South Carolina (46 counties), and Washington State (5 counties). All centers included endocrinologists in their surveillance networks, with additional cases identified through other health care providers, hospitals, community health centers, clinical and administrative data systems, and diabetes registries. Case reports were validated according to physician diagnosis. Eligibility based on age, county or area of residence, nonmilitary and noninstitutionalized status, and health plan membership at diagnosis (California only) was confirmed, and cases were registered centrally. The ascertainment window was 30 months after 31 December of each incident year. The study was approved by the institutional review board at each center, with case ascertainment and registration performed under a waiver of written informed consent.
All registered youth were invited to complete a survey that included questions about their race/ethnicity. For incident years 2002–2006 and 2008, all youth with nonsecondary diabetes were invited to a research visit. Written informed consent and assent, when appropriate, were obtained (2,17). Blood samples collected during this visit were analyzed for two diabetes autoantibodies (DAAs), GAD65 antibody and IA-2 antibodies, by standardized protocol (18).
Statistical Analysis
Based on the 2002–2003 T1D incidence rate among NHW youth from SEARCH (2), power calculations assuming a significance level of 5% demonstrated 90% power to detect a relative 2% change [consistent with observations in other parts of the world (9)] and a 0.48 absolute change in incidence rate through 2009. The annual numerators included all NHW incident T1D patients (types 1, 1a, and 1b) who were <20 years old on 31 December of the index year. Race/ethnicity was based on self-report (83.5%), medical records (12.7%), or imputation (3.8%). The annual denominators included NHW youth <20 years old on 31 December of the index year who were civilian noninstitutionalized residents of the geographic study areas or members of Kaiser Permanente Southern California (2).
The annual T1D incidence was expressed per 100,000 youth by using data pooled across the five centers. Ninety-five percent CIs were calculated using the skew-corrected inverted score test assuming a binomial distribution (19). Rates were calculated for all youth by four age categories and by sex, with final estimates presented overall, adjusted by age and sex (independently and combined) and by sex, adjusted by age. We presented rates for youth <15 years old for comparison with European studies. Age- and sex-adjusted rates were calculated using direct adjustment with the 2002–2009 U.S. intercensal population estimates. Trends in incidence were tested with Poisson regression models that included age category, sex, and diagnosis year as covariates and then repeated in age-specific models to determine statistically significant trends in incidence within each of the four age categories. Joinpoint Regression Program version 4.04 (http://surveillance.cancer.gov/joinpoint) was used to determine specific inflection points in trends or whether trends were linear.
Completeness of case ascertainment for the four geographically based centers was assessed by using the capture-recapture method (20) and a two-mode ascertainment model (inpatient vs. outpatient sources) (2). Approximately 2% were inpatient only, 33% from outpatient only, and 65% from both sources. The membership-based center did not have the independent data sources required for this method.
The mean age at T1D diagnosis was calculated overall and by sex for each year. We fit a general linear regression model and examined whether significant differences existed in age at diagnosis across the 8-year study period. To identify potential changes in the diagnosis of T1D that could affect trends, we compared the prevalence of the two DAAs among youth with a research visit for 3 incident years from the beginning (2002), middle (2005), and nearest to the end (2008) by using general linear models (continuous variables) and the χ2 and Cochran-Armitage tests for trend (categorical variables).
Results
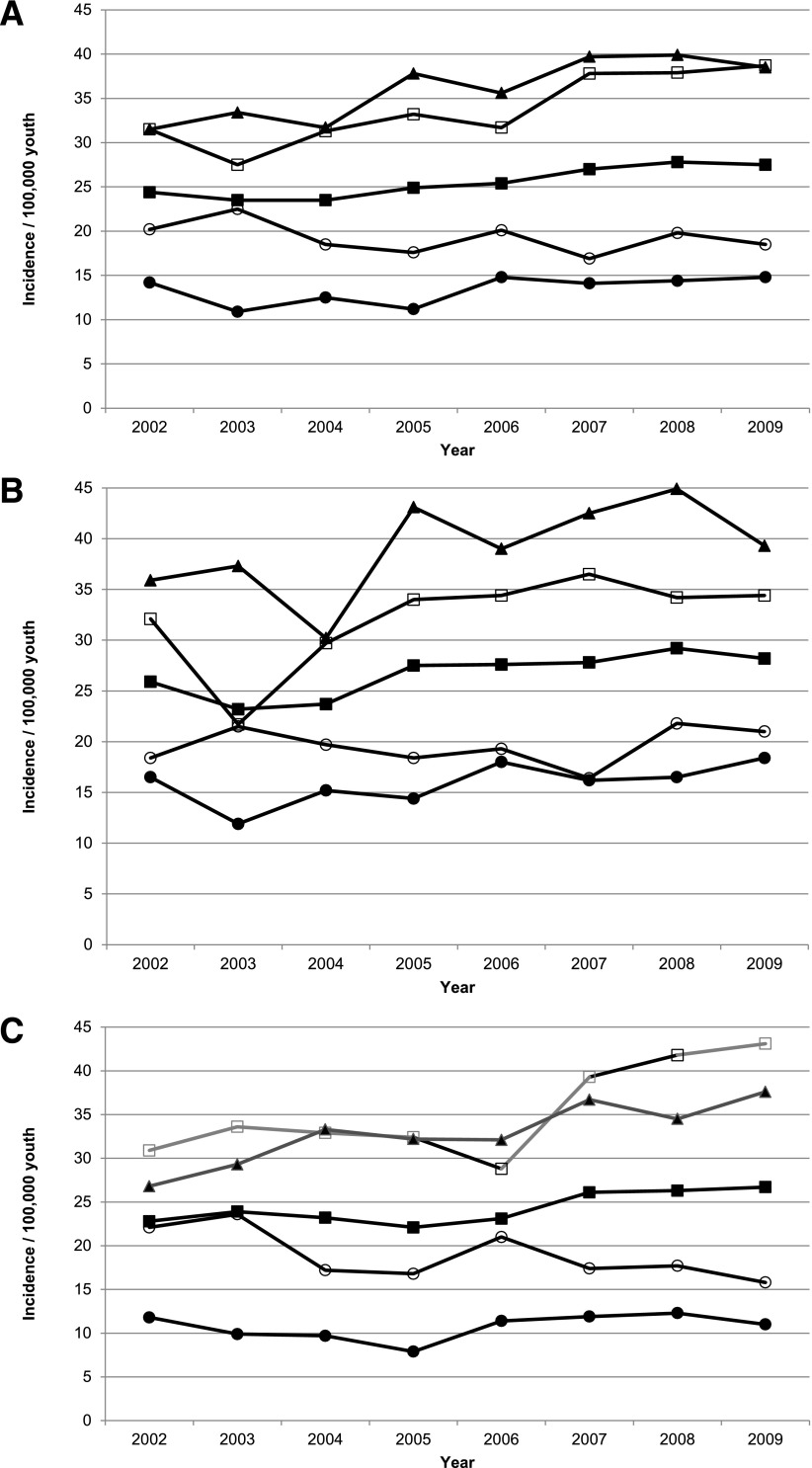
From 2002 through 2009, 5,842 NHW youth with T1D were ascertained from a population of 22,927,337 person-years. Ascertainment completeness overall and by sex was very high, averaging 98.9% (95% CI 98.6–99.1) across the 8-year study period and exceeding 99% for all age-groups except 15–19-year-olds (95.3% [95% CI 95.0–95.6]). The incidence of T1D in NHW youth increased from an age- and sex-adjusted rate of 24.4/100,000 (95% CI 23.9–24.8) in 2002 to 27.4/100,000 (95% CI 26.9–27.9) in 2009 (Table 1). Poisson regression models demonstrated a significant linear increase in incidence (P = 0.0008). The relative annual increase in incidence was estimated to be 2.72% (95% CI 1.18–4.28) per year. Joinpoint analysis showed no evidence of inflection points (data not shown). After adjustment for sex in age-group–stratified models, we found significant linear increases in incidence among youth ages 5–9 years (P = 0.002), 10–14 years (P < 0.001), and 15–19 years (P = 0.004) but not among 0–4-year-olds (P = 0.19) (Fig. 1A).
Table 1.
Incidence of T1D in NHW youth <20 years of age at diagnosis by year, age category, and sex and mean age at diagnosis, overall, and by sex: the SEARCH for Diabetes in Youth Study, 2002–2009
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|
| All youth | ||||||||
| n | 712 | 681 | 672 | 710 | 727 | 768 | 792 | 782 |
| Population (denominator) | 2,917,577 | 2,892,275 | 2,864,217 | 2,853,332 | 2,855,636 | 2,847,022 | 2,849,514 | 2,847,765 |
| Age at diagnosis (years) | 9.68 (4.58) | 9.44 (4.62) | 9.83 (4.54) | 9.90 (4.45) | 9.95 (4.67) | 9.99 (4.33) | 9.80 (4.50) | 9.88 (4.42) |
| Incidence by age category* | ||||||||
| 0–4 years | 20.2 (17.1–23.9) | 22.5 (19.2–26.4) | 18.5 (15.5–22.1) | 17.6 (14.7–21.1) | 20.1 (17.0–23.8) | 16.9 (14.0–20.3) | 19.8 (16.7–23.5) | 18.5 (15.5–22.0) |
| 5–9 years | 31.5 (27.6–35.9) | 27.5 (23.8–31.7) | 31.3 (27.4–35.8) | 33.2 (29.1–37.8) | 31.7 (27.7–36.2) | 37.8 (33.5–42.8) | 37.9 (33.5–42.7) | 38.7 (34.3–43.6) |
| 10–14 years | 31.5 (27.8–35.7) | 33.4 (29.5–37.7) | 31.7 (28.0–36.0) | 37.8 (33.6–42.5) | 35.6 (31.6–40.2) | 39.7 (35.4–44.5) | 39.9 (35.5–44.8) | 38.5 (34.2–43.3) |
| 15–19 years | 14.2 (11.8–17.2) | 10.9 (8.8–13.5) | 12.5 (10.2–15.3) | 11.2 (9.1–13.8) | 14.8 (12.3–17.7) | 14.1 (11.7–17.0) | 14.4 (12.0–17.4) | 14.8 (12.3–17.8) |
| Total | 24.4 (22.7–26.3) | 23.5 (21.8–25.4) | 23.5 (21.7–25.3) | 24.9 (23.1–26.8) | 25.4 (23.7–27.4) | 27.0 (25.1–28.9) | 27.8 (25.9–29.8) | 27.5 (25.6–29.4) |
| Total, age-adjusted | 24.4 (23.9–24.8) | 23.5 (23.1–23.9) | 23.4 (23.0–23.8) | 24.8 (24.4–25.3) | 25.4 (24.9–25.8) | 26.9 (26.4–27.4) | 27.7 (27.2–28.2) | 27.4 (26.9–27.9) |
| Total, sex-adjusted | 24.4 (24.0–24.8) | 23.5 (23.1–24.0) | 23.5 (23.0–23.9) | 24.9 (24.4–25.3) | 25.4 (25.0–25.9) | 27.0 (26.5–27.4) | 27.8 (27.3–28.3) | 27.5 (27.0–27.9) |
| Total, age- and sex-adjusted | 24.4 (23.9–24.8) | 23.5 (23.1–23.9) | 23.4 (23.0–23.8) | 24.8 (24.4–25.3) | 25.4 (25.0–25.9) | 26.9 (26.4–27.4) | 27.7 (27.3–28.2) | 27.4 (26.9–27.9) |
| Females | ||||||||
| n | 324 | 336 | 324 | 307 | 322 | 362 | 365 | 370 |
| Age at diagnosis (years) | 9.19 (4.62) | 8.95 (4.41) | 9.72 (4.18) | 9.48 (4.30) | 9.60 (4.57) | 9.58 (4.30) | 9.45 (4.39) | 9.69 (4.12) |
| Incidence by age category | ||||||||
| 0–4 years | 22.1 (17.6–27.8) | 23.6 (18.9–29.5) | 17.2 (13.3–22.4) | 16.8 (12.9–21.9) | 21.0 (16.6–26.6) | 17.4 (13.4–22.6) | 17.7 (13.7–22.9) | 15.8 (12–20.7) |
| 5–9 years | 30.9 (25.5–37.3) | 33.6 (27.9–40.4) | 32.9 (27.3–39.7) | 32.4 (26.8–39.1) | 28.8 (23.6–35.2) | 39.3 (33.1–46.6) | 41.8 (35.4–49.3) | 43.1 (36.7–50.7) |
| 10–14 years | 26.8 (22.1–32.5) | 29.3 (24.3–35.3) | 33.3 (27.9–39.8) | 32.2 (26.9–38.6) | 32.1 (26.7–38.5) | 36.7 (30.9–43.6) | 34.5 (28.9–41.3) | 37.6 (31.7–44.6) |
| 15–19 years | 11.8 (8.8–15.9) | 9.9 (7.1–13.6) | 9.7 (7.0–13.4) | 7.9 (5.5–11.2) | 11.4 (8.5–15.4) | 11.9 (8.9–15.9) | 12.3 (9.2–16.3) | 11.0 (8.1–15.0) |
| Total | 22.8 (20.5–25.4) | 23.9 (21.5–26.6) | 23.2 (20.8–25.9) | 22.1 (19.8–24.7) | 23.1 (20.7–25.8) | 26.1 (23.5–28.9) | 26.3 (23.7–29.1) | 26.7 (24.1–29.5) |
| Total, age-adjusted | 22.8 (22.2–23.4) | 23.8 (23.2–24.5) | 23.2 (22.6–23.8) | 22.1 (21.5–22.7) | 23.1 (22.5–23.7) | 26.0 (25.3–26.7) | 26.2 (25.6–26.9) | 26.6 (25.9–27.3) |
| Males | ||||||||
| n | 388 | 345 | 348 | 402 | 405 | 406 | 426 | 412 |
| Age at diagnosis (years) | 10.09 (4.51) | 9.93 (4.78) | 9.93 (4.84) | 10.22 (4.55) | 10.23 (4.73) | 10.35 (4.33) | 10.11 (4.57) | 10.04 (4.67) |
| Incidence by age category | ||||||||
| 0–4 years | 18.4 (14.4–23.6) | 21.5 (17.1–27.0) | 19.7 (15.5–25.0) | 18.4 (14.4–23.6) | 19.3 (15.1–24.5) | 16.4 (12.6–21.3) | 21.8 (17.4–27.3) | 21.0 (16.7–26.5) |
| 5–9 years | 32.1 (26.8–38.5) | 21.7 (17.4–27.1) | 29.7 (24.6–36.0) | 34.0 (28.4–40.7) | 34.4 (28.8–41.1) | 36.5 (30.7–43.4) | 34.2 (28.6–40.8) | 34.4 (28.9–41.1) |
| 10–14 years | 35.9 (30.5–42.3) | 37.3 (31.7–43.8) | 30.2 (25.2–36.1) | 43.1 (37.0–50.2) | 39.0 (33.2–45.8) | 42.5 (36.4–49.6) | 44.9 (38.6–52.3) | 39.3 (33.4–46.3) |
| 15–19 years | 16.5 (12.9–21.1) | 11.9 (8.9–15.8) | 15.2 (11.8–19.6) | 14.4 (11.1–18.7) | 18.0 (14.3–22.7) | 16.2 (12.7–20.7) | 16.5 (13.0–21.0) | 18.4 (14.6–23.2) |
| Total | 25.9 (23.5–28.6) | 23.2 (20.9–25.8) | 23.7 (21.3–26.3) | 27.5 (24.9–30.3) | 27.6 (25.1–30.5) | 27.8 (25.2–30.7) | 29.2 (26.6–32.1) | 28.2 (25.6–31.1) |
| Total, age-adjusted | 25.9 (25.3–26.5) | 23.2 (22.6–23.8) | 23.6 (23.0–24.2) | 27.4 (26.8–28.1) | 27.6 (27.0–28.3) | 27.8 (27.1–28.4) | 29.2 (28.5–29.8) | 28.2 (27.5–28.9) |
Data are incidence per 100,000 person-years (95% CI) or mean (SD) unless otherwise indicated. All age- and sex-adjusted rates were calculated by using direct adjustment, with the estimated 2002–2009 U.S. intercensal population as the standard population.
*Age at diagnosis.
Figure 1.
Incidence of T1D among NHW youth aged <20 years at diagnosis overall and by sex and age category: the SEARCH for Diabetes in Youth Study, 2002–2009. A: Total sample. B: Males. C: Females. ■, all youth; ○, 0–4-year-olds; □, 5–9-year-olds; ▲, 10–14-year-olds; ●, 15–19-year-olds.
The incidence increased from 25.9/100,000 in 2002 to 28.2/100,000 in 2009 in males (Fig. 1B) and from 22.8 to 26.7/100,000 in females (Fig. 1C), with mean annual increases of 2.84% (95% CI 1.12–4.58) and 2.57% (0.68–4.51), respectively. Among males, there was a significant linear increase in incidence in 5–9-year-olds (P = 0.006) and 10–14-year-olds (P = 0.02) but not among 0–4-year-olds (P = 0.75) or 15–19-year-olds (P = 0.08). Among females, there was a significant linear increase in 5–9-year-olds (P = 0.001) and 10–14-year-olds (P = 0.004), a decrease among 0–4-year-olds (P = 0.03), and no change among 15–19-year-olds (P = 0.43).
The incidence of T1D in NHW youth <15 years of age increased from an age- and a sex-adjusted rate of 28.0/100,000 in 2002 to 32.2/100,000 in 2009 (Table 2). The estimated relative annual increase for youth <15 years of age was 2.72% (95% CI 1.09–4.36) to 2.68% (0.51–4.88) for females and 2.75% (0.76–4.78) for males.
Table 2.
Incidence of T1D in NHW youth <15 years of age at diagnosis by year and sex: the SEARCH for Diabetes in Youth Study, 2002–2009
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|
| n | 604 | 598 | 577 | 624 | 612 | 659 | 680 | 668 |
| Population (denominator) | 2,159,677 | 2,131,682 | 2,104,425 | 2,086,173 | 2,082,434 | 2,074,901 | 2,077,805 | 2,081,993 |
| Total | ||||||||
| Unadjusted | 28.0 (25.8–30.3) | 28.1 (25.9–30.4) | 27.4 (25.3–29.7) | 29.9 (27.6–32.3) | 29.4 (27.2–31.8) | 31.8 (29.4–34.3) | 32.7 (30.4–35.3) | 32.1 (29.8–34.6) |
| Age-adjusted | 28.0 (27.4–28.5) | 28.1 (27.5–28.6) | 27.4 (26.9–28.0) | 29.9 (29.3–30.5) | 29.4 (28.9–30.0) | 31.8 (31.2–32.4) | 32.8 (32.2–33.4) | 32.2 (31.6–32.8) |
| Sex-adjusted | 28.0 (27.4–28.5) | 28.1 (27.5–28.6) | 27.4 (26.9–27.9) | 29.9 (29.3–30.5) | 29.4 (28.8–30.0) | 31.8 (31.2–32.4) | 32.7 (32.1–33.4) | 32.1 (31.5–32.7) |
| Age- and sex-adjusted | 28.0 (27.4–28.5) | 28.1 (27.5–28.6) | 27.4 (26.9–28.0) | 29.9 (29.3–30.5) | 29.4 (28.9–30.0) | 31.8 (31.2–32.4) | 32.8 (32.2–33.4) | 32.2 (31.6–32.8) |
| Females | ||||||||
| n | 280 | 299 | 288 | 278 | 279 | 317 | 319 | 329 |
| Unadjusted | 26.7 (23.7–30.0) | 28.9 (25.8–32.3) | 28.1 (25.0–31.6) | 27.4 (24.3–30.8) | 27.5 (24.4–30.9) | 31.4 (28.1–35.0) | 31.5 (28.2–35.2) | 32.4 (29.1–36.1) |
| Age-adjusted | 26.7 (25.9–27.4) | 28.9 (28.1–29.7) | 28.1 (27.3–28.9) | 27.4 (26.6–28.2) | 27.5 (26.7–28.3) | 31.4 (30.5–32.2) | 31.5 (30.7–32.4) | 32.5 (31.6–33.4) |
| Males | ||||||||
| n | 324 | 299 | 289 | 346 | 333 | 342 | 361 | 339 |
| Unadjusted | 29.2 (26.2–32.6) | 27.3 (24.4–30.6) | 26.7 (23.8–30.0) | 32.3 (29.1–35.9) | 31.2 (28.0–34.8) | 32.1 (28.9–35.7) | 33.9 (30.6–37.6) | 31.8 (28.6–35.4) |
| Age-adjusted | 29.2 (28.5–30.0) | 27.3 (26.5–28.0) | 26.7 (26–27.5) | 32.3 (31.5–33.1) | 31.2 (30.4–32.1) | 32.2 (31.4–33.0) | 34.0 (33.1–34.9) | 31.9 (31.1–32.7) |
Data are incidence per 100,000 person-years (95% CI) unless otherwise indicated. All age- and sex-adjusted rates were calculated by using direct adjustment with the estimated 2002–2009 U.S. intercensal population as the standard population.
The mean ± SD age at diagnosis ranged from 9.44 ± 4.62 years in 2003 to 9.99 ± 4.33 years in 2007 (Table 1) and did not differ significantly across the study period for the total sample (P for trend = 0.08) or by sex. However, the mean age at diagnosis for all years combined was lower for females (9.46 ± 4.36 years) than for males (10.12 ± 4.62 years, P < 0.001). There was no significant difference in the proportion of youth who were DAA positive for incident years 2002, 2005, and 2008 overall or by age-group (Table 3).
Table 3.
Presence of selected DAAs among SEARCH participants with physician-diagnosed T1D from the 2002, 2005, and 2008 incident cohorts who completed a SEARCH baseline study visit and had DAAs measured: the SEARCH for Diabetes in Youth Study
| Incidence Year |
|||||
|---|---|---|---|---|---|
| 2002 | 2005 | 2008 | P value* | P value for trend† | |
| n | 316 | 324 | 469 | — | — |
| Age at visit (years) | 11.1 (4.0) | 11.1 (4.0) | 10.5 (44) | 0.05 | 0.02 |
| T1D duration (months) | 13.0 (7.0) | 7.8 (5.2) | 8.2 (5.5) | <0.0001 | <0.0001 |
| DAA+ status | |||||
| GAD65+ | 186 (58.9) | 184 (56.8) | 288 (61.4) | 0.42 | 0.53 |
| IA-2A+ | 224 (70.9) | 230 (71.0) | 351 (74.8) | 0.36 | 0.25 |
| Either DAA+ | 267 (84.5) | 278 (85.8) | 413 (88.1) | 0.34 | 0.16 |
| DAA+ by age category (years)‡ | |||||
| 0–4 years | 20/22 (90.9) | 21/28 (75.0) | 52/61 (85.2) | 0.29 | 0.62 |
| 5–9 years | 89/102 (87.3) | 85/98 (86.7) | 134/152 (88.2) | 0.94 | 0.85 |
| 10–14 years | 108/131 (82.4) | 122/141 (86.5) | 152/174 (87.4) | 0.45 | 0.21 |
| ≥15 years | 50/61 (82.0) | 50/57 (87.7) | 75/82 (91.5) | 0.24 | 0.09 |
| DAA+ by sex‡ | |||||
| Female | 121/139 (87.1) | 128/145 (88.3) | 183/204 (89.7) | 0.75 | 0.46 |
| Male | 146/177 (82.5) | 150/179 (83.8) | 230/265 (86.8) | 0.43 | 0.23 |
Data are mean (SD) or n (%) unless otherwise indicated. IA-2A, IA-2 antibodies.
*P values for continuous variables were fit by using a general linear model with time period treated as a class variable. P values for categorical variables are based on χ2 test.
†P values for continuous variables were fit using a general linear model with time period treated as a continuous variable. P values for categorical variables were tested using the Cochran-Armitage test for trend.
‡Data are number positive/total tested (%).
Discussion
The SEARCH study, the largest and most contemporary registry of incident diabetes in North America, observed a significant increasing trend in T1D incidence among NHW youth from 2002 through 2009. On average, T1D incidence increased by 2.72% annually. Incidence increased in all age-groups except 0–4 years. Age at diagnosis did not change significantly. DAA positivity remained consistent, suggesting that using a physician’s diagnosis of T1D as the case definition resulted in similar cases over time.
Compared with SEARCH incidence rates, the Philadelphia Pediatric Diabetes Registry did not find an increase in T1D incidence for NHW youth <15 years old from 1985 to 2004 (P = 0.69) (14). This registry’s 5-year adjusted T1D incidence for 2000–2004 based on 99 cases was 19.2/100,000, which is much lower than the SEARCH rates of 27.4–28.1/100,000 youth <15 years of age in 2002–2004 based on 1,779 cases. Similarly, the Chicago Childhood Diabetes Registry found no linear trend in T1D incidence among NHW youth <18 years of age from 1994 to 2003 (13). It reported T1D incidence rates for 1999–2003 of 15.9 and 14.7/100,000 for males and females, respectively, based on 94 cases, which are also much lower than the SEARCH incidence rates for males and females <19 years of age (23.2 and 23.9/100,000, respectively) in 2003. The lack of significant trends in T1D rates in the Philadelphia and Chicago registries (13,14) may have been a result of their limited power to detect changes in trends. Combining data from two registries, Colorado reported an annual increase of 2.7% (95% CI 1.9–3.6) per year for NHW youth <20 years of age from 1978 to 1988 and 2002 to 2004 (15,16), an estimate identical to the current study’s estimate of 2.72%. A study in Newfoundland and Labrador (Canada) reported that T1D incidence in youth <15 years of age rose from 29.9/100,000 in 1987 to 49.9/100,000 in 2010 based on 931 cases, with increases observed across three age-groups (21).
The median annual change in T1D incidence for youth <15 years of age from the 23 EURODIAB centers was 3.3% per year from 1999 to 2008 (9). Finland observed an annual increase of 3.6% from 1988 until 2005, peaking at 64.9/100,000 in 2006 followed by a plateau through 2011 (22,23). Similarly, Sweden reported significant increases from 1978 to 1984 and then a plateau from 2005 to 2007 (43.9/100,000) (7), with similar rates reported from 2007 to 2011 based on drug registry data (24). In comparison, SEARCH observed a slightly smaller increase (2.7% per year) but continued to see a rise after 2005 when the Northern European centers noted a plateau. The SEARCH 2009 T1D incidence rate for NHW youth <15 years of age of 32.1/100,000 is well below that observed in these Scandinavian countries.
Although the Swedish registry reported a downward shift in age at diagnosis from 1978 to 2000 and a reversal from 2000 to 2007 (7), a recent reanalysis of several sources of data from Sweden suggested that the previously published rates of T1D during adolescence and young adulthood (ages 15–34 years) may have been significantly underestimated, calling into question the magnitude of the shift to younger ages at onset (24). Because these revised analyses did not include cumulative incidence rates for ages 0–34 years, it is not possible to determine whether overall cumulative T1D incidence rates changed or remained stable, as previously suggested (12). The SEARCH study did not observe a downward shift in the age at diagnosis, and the duration of the SEARCH study was not long enough to assess potential shifts in age of onset by birth cohort as was done in Sweden.
The data suggest that the peak age of T1D onset may be younger in females than in males. Data from the Finnish cohort suggested a similar sex difference in age of onset (22), whereas a recent review of 31 studies on T1D incidence found no sex differences in the 0–14-year age-group (25). Overall, differences between the results of SEARCH and other large population-based studies may be due to differences in genetic and environmental risk factors associated with the risk for T1D or for age of onset.
Although the present capture-recapture estimates suggest that case ascertainment completeness is lower for 15–19-year-olds, potentially underestimating rates by up to 5%, this would not affect the estimates of trends and average annual increases because they were observed across the study period. We observed a 2.5% decline in the denominator over the period, which was relatively consistent across the surveillance areas. Future reports will include additional years of incidence data and assess trends for other racial/ethnic groups. This study also has multiple strengths. The size and geographic variation of the populations under surveillance by SEARCH are much larger than all other U.S.-based childhood diabetes registries. Analyses of the distribution of income and education among adults in the regions comprising the SEARCH study suggest that SEARCH is representative of the U.S. population (www.searchfordiabetes.org/public/dspPubs.cfm) (1). Methodological strengths include multiple approaches to case ascertainment, a uniform case definition of diabetes applied across the study period, a high case ascertainment rate, and phenotypic information demonstrating the consistent case definition over time. SEARCH previously reported a good concordance between physician-diagnosed and etiologic diabetes type (26).
In conclusion, SEARCH demonstrated that T1D incidence has been increasing among NHW youth in the U.S. Particularly given the recent plateauing of T1D incidence in Europe, continuing childhood diabetes surveillance in the U.S. is essential to identify future trends and potential causes of these increases.
Supplementary Material
Article Information
Acknowledgments. The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families and health care providers whose participation made this study possible.
Funding. SEARCH for Diabetes in Youth Study is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Contract numbers: Kaiser Permanente Southern California (U48/CCU919219, U01-DP-000246, and U18-DP-002714), University of Colorado Denver (U48/CCU819241-3, U01-DP-000247, and U18-DP-000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01-DP-000248, and 1U18-DP-002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01-DP-000254, and U18-DP-002708-01), University of Washington School of Medicine (U58/CCU019235-4, U01-DP-000244, and U18-DP-002710-01), and Wake Forest School of Medicine (U48/CCU919219, U01-DP-000250, and 200-2010-35171). The authors wish to acknowledge the involvement of General Clinical Research Centers at the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina (National Institutes of Health/National Center for Research Resources grant number UL1RR029882); Seattle Children's Hospital (National Institutes of Health Clinical and Translational Science Awards grant UL1 TR00423 of the University of Washington); University of Colorado Pediatric Clinical and Translational Research Center (grant number UL1 TR000154); the Barbara Davis Center at the University of Colorado Denver (DERC NIH P30 DK57516); the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8 UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.L. contributed to the data research; discussion; and writing, review, and editing of the manuscript. G.I. contributed to the discussion and review and editing of the manuscript. D.D., E.J.M.-D., C.P., and J.T. contributed to the data research, discussion, and review and editing of the manuscript. B.L., S.S., G.J.K., L.D., D.A.S., D.J.P., and R.A.B. contributed to the discussion and review and editing of the manuscript. J.W.T. and R.B.D. contributed to the data analysis, discussion, and review and editing of the manuscript. R.B.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 72nd Annual Scientific Sessions of the American Diabetes Association, Chicago, IL, 8–12 June 2012.
Footnotes
A complete list of the SEARCH for Diabetes in Youth Study Group can be found in the Supplementary Data online.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 3.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes—the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–1403 [DOI] [PubMed] [Google Scholar]
- 4.DIAMOND Project Group . Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 5.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 6.Dahlquist GG, Nyström L, Patterson CC, Swedish Childhood Diabetes Study Group. Diabetes Incidence in Sweden Study Group . Incidence of type 1 diabetes in Sweden among individuals aged 0-34 years, 1983-2007: an analysis of time trends. Diabetes Care 2011;34:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G, Swedish Childhood Diabetes Study Group . Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes 2011;60:577–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno G, Maule M, Biggeri A, et al. Sardinian Group for Diabetes Epidemiology . More than 20 years of registration of type 1 diabetes in Sardinian children: temporal variations of incidence with age, period of diagnosis, and year of birth. Diabetes 2013;62:3542–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist G, Mustonen L, Swedish Childhood Diabetes Study Group . Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 11.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–1526 [DOI] [PubMed] [Google Scholar]
- 12.Pundziute-Lyckå A, Dahlquist G, Nyström L, et al. Swedish Childhood Diabetes Study Group . The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 13.Smith TL, Drum ML, Lipton RB. Incidence of childhood type I and non-type 1 diabetes mellitus in a diverse population: the Chicago Childhood Diabetes Registry, 1994 to 2003. J Pediatr Endocrinol Metab 2007;20:1093–1107 [DOI] [PubMed] [Google Scholar]
- 14.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care 2013;36:1597–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007;30:503–509 [DOI] [PubMed] [Google Scholar]
- 16.Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr 2012;161:652–657 [DOI] [PubMed]
- 17.SEARCH Study Group . SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 18.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998;52:119–126 [Google Scholar]
- 20.Verlato G, Muggeo M. Capture-recapture method in the epidemiology of type 2 diabetes: a contribution from the Verona Diabetes Study. Diabetes Care 2000;23:759–764 [DOI] [PubMed] [Google Scholar]
- 21.Newhook LA, Penney S, Fiander J, Dowden J. Recent incidence of type 1 diabetes mellitus in children 0-14 years in Newfoundland and Labrador, Canada climbs to over 45/100,000: a retrospective time trend study. BMC Res Notes 2012;5:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 23.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA 2013;310:427–428 [DOI] [PubMed] [Google Scholar]
- 24.Rawshani A, Landin-Olsson M, Svensson A, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia 2014;57:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wändell PE, Carlsson AC. Time trends and gender differences in incidence and prevalence of type 1 diabetes in Sweden. Curr Diabetes Rev 2013;9:342–349 [DOI] [PubMed] [Google Scholar]
- 26.Dabelea D, Pihoker C, Talton JW, et al. SEARCH for Diabetes in Youth Study . Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care 2011;34:1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.