Abstract
We previously demonstrated that micro-RNAs (miRNAs) 132 and 212 are differentially upregulated in response to obesity in two mouse strains that differ in their susceptibility to obesity-induced diabetes. Here we show the overexpression of miRNAs 132 and 212 enhances insulin secretion (IS) in response to glucose and other secretagogues including nonfuel stimuli. We determined that carnitine acyl-carnitine translocase (CACT; Slc25a20) is a direct target of these miRNAs. CACT is responsible for transporting long-chain acyl-carnitines into the mitochondria for β-oxidation. Small interfering RNA–mediated knockdown of CACT in β-cells led to the accumulation of fatty acyl-carnitines and enhanced IS. The addition of long-chain fatty acyl-carnitines promoted IS from rat insulinoma β-cells (INS-1) as well as primary mouse islets. The effect on INS-1 cells was augmented in response to suppression of CACT. A nonhydrolyzable ether analog of palmitoyl-carnitine stimulated IS, showing that β-oxidation of palmitoyl-carnitine is not required for its stimulation of IS. These studies establish a link between miRNA-dependent regulation of CACT and fatty acyl-carnitine–mediated regulation of IS.
Introduction
Cells have evolved mechanisms to regulate fuel utilization in response to changes in substrate availability. Glucose oxidation leads to inhibition of β-oxidation through the production of malonyl-CoA, a potent inhibitor of carnitine palmitoyl transferase-1 (CPT-1) (1), a gateway into mitochondrial β-oxidation. Conversely, acetyl-CoA, a product of β-oxidation, inhibits pyruvate dehydrogenase (1), a critical enzyme in the glycolytic pathway. Fatty acids potentiate glucose-stimulated insulin secretion (IS) (2), and insulin suppresses adipose tissue triglyceride hydrolysis. The inhibitory effect of insulin on adipose tissue lipolysis leads to a decrease in circulating fatty acids and thus comprises a negative feedback loop (3).
The mechanism by which fatty acids regulate IS is not fully elucidated. Acute exposure to fatty acids stimulates IS, whereas chronic exposure to fatty acids suppresses IS (2). Chronic fatty acid treatment of β-cells in the presence of high glucose leads to a decrease in the expression of Pdx1, a transcription factor (4). Pdx1 is required for pancreatic development (4). Palmitate is incorporated into ceramide, an inhibitor of phosphatidylinositol-3-kinase and Akt, both of which are involved in insulin signaling (5). Loss of activity of these kinases leads to blunted insulin signaling (1,6), which has been hypothesized to decrease Pdx1 translocation into the nucleus.
Multiple pathways have been proposed to explain the acute stimulatory effects of fatty acids on IS. Several conventional protein kinase C (PKC) isozymes are activated by fatty acids, which in turn leads to increased IS (7). Conversely, fatty acids suppress several novel PKC isozymes that inhibit IS, leading to enhanced IS (8). Fatty acids acutely regulate cellular Ca2+ levels through activation of GPR40, leading to enhanced IS (9).
We previously showed that genetically imposed obesity (Lepob/ob) induces the expression of micro-RNAs (miRNAs) 132 and 212 in pancreatic islets (10). In diabetes-resistant C57BL/6J (B6) mice, the induction was ∼13-fold, whereas in diabetes-susceptible BTBR T (+) tf/J (BTBR) mice, the induction was reduced to approximately threefold. In this study we show that overexpression of these miRNAs enhances IS in response to a variety of secretagogues, suggesting that the strain difference in their regulation may contribute to diabetes susceptibility. We identify the mitochondrial carnitine acyl-carnitine translocase (CACT; Slc25a20) as a direct target of the miRNAs that mediates their effect on IS. The downregulation of CACT causes an accumulation of cellular acyl-carnitine molecules and enhances their effect on IS.
Research Design and Methods
Reagents
Insulin from INS-1 cells and mouse islets was measured with an in-house ELISA using an anti-insulin antibody from Fitzgerald Industries (Acton, MA). RPMI growth media, Hanks’ balanced salt solution, and Lipofectamine 2000 were bought from Life Technologies. Palmitic acid (PA), BSA, palmitoyl-L-carnitine (PC), collagenase type XI, diazoxide (DZX), 8-Br-cAMP, L-arginine, and small interfering RNAs (siRNAs) against CACT (custom siRNA-duplex 5′-CAAAGAAGCUGUAUCAGGA[dT][dT] 5′-UCCUGAUACAGCUUCUUUG[dT][dT])–negative control scrambled siRNAs (cat. no. SIC001) all were obtained from Sigma-Aldrich (St. Louis, MO). Chemically modified PremiR miRNA precursors and negative control #1 were purchased from Ambion (Foster City, CA). RNA and miRNA isolation kits were purchased from QIAGEN (Valencia, CA). Antibody against CACT was obtained from Abcam (Cambridge, MA), and antibody against Vdac was a generous gift from Dave Pagliarini, University of Wisconsin-Madison. Goat antirabbit secondary antibodies were purchased from Cell Signaling Technology (Boston, MA). The 14C-PA, 14C-PC, and 14C-U-glucose all were purchased from Perkin Elmer, Inc. POC-16 (palmitoyl carnitine ether), an analog of PC in which the ester linkage between the fatty acid and carnitine is replaced by an ether linkage with C16, was a generous gift from the Bronfman laboratory, Pontificia Universidad Católica de Chile. The pmirGLO dual luciferase construct was a generous gift from the Sugden laboratory at University of Wisconsin-Madison.
Cell Lines and Mouse Islet Treatments
INS-1–derived rat insulinoma cell lines, 832/3 and 832/13, were used in this study. The cells were cultured in RPMI 1640 with 10% FBS and 11 mmol/L glucose, as described by Bhatnagar et al. (11). Pancreatic islets of Langerhans were isolated from C57BL/6J mice by collagenase digestion and a Ficoll gradient separation as previously described (11).
Taqman Quantitative PCR Analysis of Selective miRNAs
Fluorogenic Taqman probes for miRNAs 132, 212, and 375 were purchased from Applied Biosystems. Relative expression levels of miRNAs of interest were determined by real-time quantitative PCR using the ABI PRISM 7900 Sequence Detection System from Applied Biosystems. The mRNA level of the INS-1 gene (the predominant isoform in INS-1 cells) was detected by Taqman quantitative PCR using a specific probe from Applied Biosystems and normalized to β-actin mRNA levels.
Transfection of INS-1 Cells with siRNA/miRNA Oligonucleotides
siCACT and negative control oligonucleotides were transfected into INS-1 832/13 or 832/3 cells (∼80 nmol oligonucleotides/500,000 cells) using Lipofectamine 2000, as described previously (11). Experiments illustrated in Fig. 2 and Supplementary Figs. 1, 2, and 3 were performed using the INS-1 832/3 subclone, whereas the remaining experiments used the 832/13 subclone. In our experience, 832/13 cells are generally more adherent and, therefore, more amenable to our studies. The effect of PC or the miRNAs on IS was observed equally in both subclones of the INS-1 cells (data not shown).
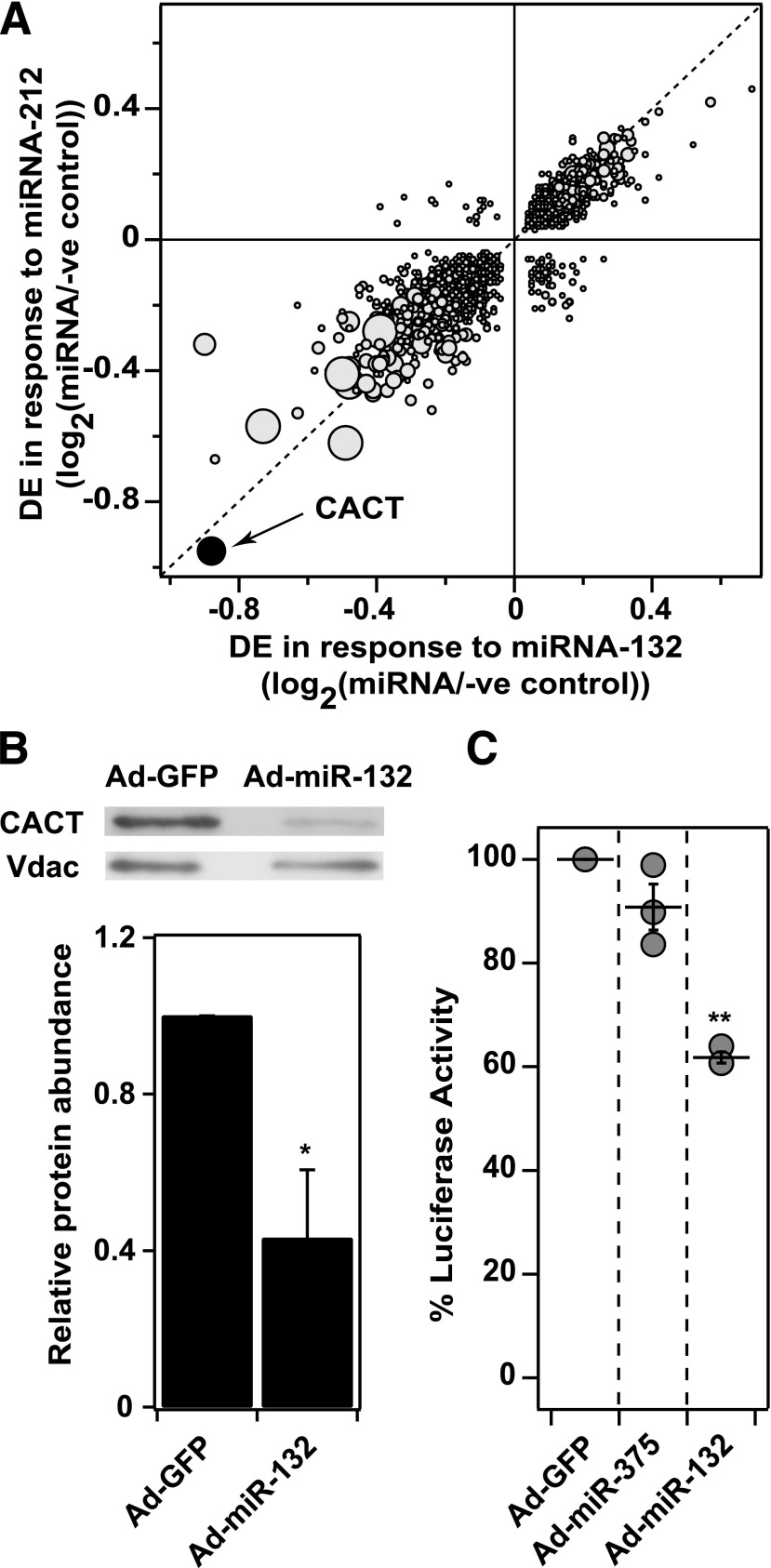
Figure 2.
miRNA-132 directly targets CACT for downregulation. A: Gene expression profiling in INS-1 832/3 cells following miRNA overexpression. Differential expression (DE) resulting from miRNA-132 is plotted against DE for miRNA-212 24 h after overexpression of the miRNAs using oligonucleotides (P < 0.05). Larger circles indicate greater statistical significance of DE. Axes show the log10 of the fold-change (miRNA over negative control). Negative values indicate downregulation of expression. CACT is highlighted as the most downregulated gene in response to both miRNAs. B: miRNA-132 decreases CACT protein level. Western blot analysis was used to evaluate the effect of miRNA-132 on CACT protein levels in INS-1 832/3 cells. Ads were used to overexpress either miRNA-132 or GFP. Cells were harvested 48 h after infection, and 25 µg of protein was loaded in each lane; Vdac protein was used to normalize loading. Blot is representative of three independent experiments. C: CACT is the direct target of miRNA-132. INS-1 832/3 β-cells were transfected with a dual luciferase reporter construct that contained the 3′-UTR for the rat CACT gene, and one of three viruses (MOI 10): Ad-GFP, Ad-miR-375, or Ad-miR-132 (n = 3 experiments for each condition). After 48 h, luminescence in the cells was measured. *P < 0.05; **P < 0.01.
Generation of Adenovirus Overexpressing miRNA Oligonucleotides
Adenoviruses (Ads) overexpressing miRNAs 132, 212, and 375 were generated in the Duke University core Ad laboratory, as described in Supplementary Table 1. The forward and reverse oligonucleotides then were cloned into a Gateway entry plasmid (pENTR1A; Invitrogen), driven by the human 1H promoter, and a green fluorescent protein (GFP) expression cassette driven by a cytomegalovirus promoter.
Infection of INS-1 Cells With Ad Overexpressing miRNA Oligonucleotides
After seeding (24 h), cells were treated with the Ads at an multiplicity of infection (MOI) of 10 in OPTIMEM transfection media for 2 h; 48 h later, cells were used for the IS and luciferase reporter studies or harvested for Western blot analysis.
IS Assay
The IS assay in INS-1 cells and mouse islets was performed as previously described (11). The mouse islet IS experiment evoked by PC-BSA included a digitonin pretreatment (20 µg/mL, 20 min at 37°C) to achieve improved penetration of PC, as described previously (12).
Microarray Expression Profiling
miRNAs 132 and 212 were overexpressed using oligonucleotides in INS-1 832/3 cells. Cells were harvested at both 10 and 24 h. RNA was extracted from the lysate using the QIAGEN (RNeasy) kit. RNA array hybridizations were performed at Rosetta Inpharmatics (Seattle, WA). Profiling was performed as previously described (13). mRNAs were considered to be differentially expressed (DE) if they were in the top 5% of genes altered by miRNA upregulation.
CACT Protein Quantification
Cells were harvested by a Western lysis buffer (20 mmol/L Tris-HCl [pH 7.5], 150 mmol/L sodium chloride, 1 mmol/L Na2EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L sodium orthovanadate, 1 µg/mL leupeptin, 0.5 mM sodium fluoride, phenylmethylsulfonyl fluoride, and 1 protease inhibitor cocktail tablet) 48 h after infection/transfection. The cell lysates were sonicated and centrifuged at 13,000 × g for 10 min. The supernatant was discarded and the pellet was resuspended and sonicated in the same Western lysis buffer with an additional 1% SDS. CACT protein was detected using a rabbit anti-Slc25a20 polyclonal antibody (Abcam) at a dilution of 1:1,000. The CACT protein intensity was normalized to Vdac, an abundant mitochondrial protein, which was detected using a rabbit anti-Vdac polyclonal antibody at a dilution of 1:2,000. Secondary goat antirabbit antibody was used at 1:5,000 dilution. Immunoblotting was performed using a standard protocol (11).
Luciferase Assay
The 3′ untranslated region (UTR) of the rat CACT gene was fused behind the firefly gene in the pmirGLO expression construct (Promega). This construct also contained a Renilla luciferase gene, which was used to normalize for transfection efficiency, according to the manufacturer’s directions. INS-1 832/3 cells were transfected with pmirGLO with or without cotransfection of either Ad-GFP, Ad-miR-132, or Ad-miR-375 (MOI 10) 24 h after seeding. We measured luminescence in INS-1 cells 48 h later using a Tecan M1000 microplate reader.
PA and PC Conjugation to BSA
PA was conjugated to BSA by mixing 67 µL of 25 mg/mL palmitate solution (heated to 70°C) with 933 µL of 70 mg/mL BSA solution at 70°C to yield a final concentration of 6 mmol/L palmitate. PC (and POC-16) was conjugated to BSA using the protocol described by Brockenbrough and Korc (14). Briefly, PC (100 mmol/L) was added to a 3.3% BSA-containing Krebs-Ringers solution and heated to 45°C for 5 min to obtain a 1 mmol/L stock solution of PC-BSA.
β-Oxidation Assay
After transfection/infection (48 h), the cells were maintained in 1.5 mmol/L glucose for 2 h, followed by a 2-h treatment with PA (300 μmol/L with 0.5 μCi/ml 14C-PA), PC (50 μmol/L with 0.1 μCi/ml 14C-PC), 1.5 mmol/L glucose, and 1 mmol/L L-carnitine. Following the 2-h incubation period, cellular media were transferred into sealed scintillation vials with a small Eppendorf tube containing 0.3 mL of 1 N sodium hydroxide. To liberate gaseous 14CO2 from the media, 0.1 mL of 70% perchloric acid was added; the vials were resealed and maintained at room temperature overnight, resulting in carbon dioxide (CO2) accumulating in the base. The amount of 14CO2 produced was determined by liquid scintillation counting of 0.3 mL of the base, and the amounts of acid soluble metabolites (15) were determined by measuring the amount of 14C-labeled metabolite remaining in the media after perchloric acid precipitation.
Glucose Oxidation Assay
Glucose oxidation was measured using the same protocol described above for β-oxidation. Cells were treated with 15 mmol/L glucose containing 0.5 μCi/mL 14C-glucose for CO2 production.
Acyl-Carnitine Profiling in INS-1 Cells
After transfection, cells were cultured for 48 h in RPMI media supplemented with 1 mmol/L L-carnitine. Following this, cells were maintained in 1.5 mmol/L glucose for 2 h, followed by a 2-h treatment with or without 50 μmol/L (16) in Krebs-Ringer buffer (11) containing 1.5 mmol/L glucose. Cells then were harvested and acyl-carnitine esters were determined by MS/MS spectrometry, as previously described (17).
GPR40 Assay
The GPR40 assay was performed as described by Tan et al. (18).
Expression of Data and Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was determined using a Student t test, which compared the two conditions. A two-way ANOVA was used in cases where more than two conditions were compared. A P value <0.05 was considered statistically significant.
Results
miRNAs 132 and 212 Enhance IS from Pancreatic β-Cells
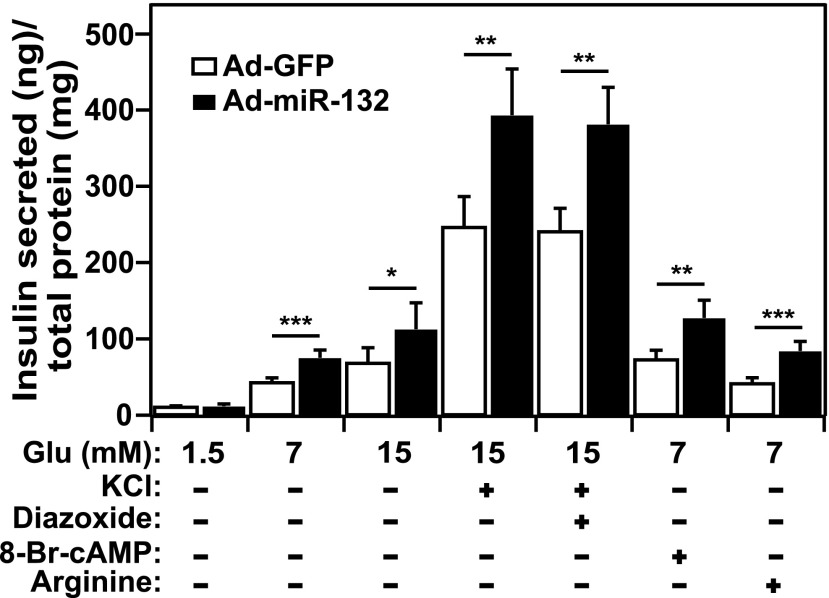
To evaluate the effects of miRNA 132 on β-cell function, we used an Ad to overexpress the miRNA in INS-1 832/13 cells while monitoring IS. Under these conditions, IS was significantly augmented in response to multiple secretagogues; glucose, potassium chloride (KCl), DZX with high glucose, cAMP, and L-arginine (Fig. 1). In contrast, miRNA-132 did not stimulate IS at basal glucose (1.5 mmol/L). miRNA-212 overexpression also stimulated IS in the presence of glucose and KCl (Supplementary Fig. 1A). miRNAs 132 and 212 did not alter insulin mRNA or protein expression (Supplementary Fig. 1B and C), indicating that the miRNAs stimulate IS by modulating the insulin secretory pathway. The effects of miRNA-132 on IS are consistent with those described in a recent report (20). Our positive control, miRNA-375, suppressed IS (Supplementary Fig. 1A) without altering insulin mRNA or protein (Supplementary Fig. 1B and C), as previously reported (21). Our negative control, Ad-GFP, did not affect IS compared with nontreated cells (Supplementary Fig. 2).
Figure 1.
miRNA-132 enhances IS from pancreatic β-cells. The rat-derived β-cell line, INS-1 832/13, was infected with Ad overexpressing Ad-miRNA-132 or Ad-GFP (negative control). IS in response to increasing glucose concentrations or various combinations of glucose and KCl (40 mmol/L), DZX (250 μmol/L), cAMP (3 mmol/L), or L-arginine (15 mmol/L), was measured 48 h after infection. IS is normalized to total cellular protein and expressed as IS (nanograms) normalized for total protein (19). Each condition was performed six times. *P < 0.05; **P < 0.01; ***P < 0.001.
Mitochondrial CACT Is a Direct Target of miRNAs 132 and 212
While we and another group (20) showed that miRNAs 132 and 212 stimulate IS, the molecular targets of the miRNAs are unknown. To identify putative targets that mediate the effects of miRNAs 132 and 212 on IS, we overexpressed the miRNAs in INS-1 832/3 cells, followed by whole-genome microarray profiling at 10 h (Supplementary Fig. 3A) and 24 h (Fig. 2A). Approximately 3,000 genes were DE in response to the miRNAs at both time points (Supplementary Data 2). Of these two sets of DE genes (assessed at 10 and 24 h), ∼60% were common. Interestingly, only ∼14% of the DE genes contained a seed region for the miRNAs, suggesting that the majority of the DE genes may be indirect targets of the miRNAs. At 10 and 24 h, the mitochondrial CACT was the most downregulated gene (reduced by ∼80%) in response to the overexpression of miRNA 132 or 212. In addition, miRNA-132 caused a ∼60% decrease in the abundance of the CACT protein (Fig. 2B).
To demonstrate that CACT mRNA is a direct target of miRNA-132, we cloned the 3′ UTR of CACT into a vector encoding firefly luciferase and Renilla. The overexpression of miRNA-132 resulted in a significant loss (∼40%; P < 0.001) of luciferase activity, whereas miRNA-375 had no effect (Fig. 2C). Taken together, these data provide strong evidence that miRNA-132 directly targets CACT expression, leading to a reduction in CACT protein.
Downregulation of CACT Enhances IS from Pancreatic β-Cells
The mRNA targets of siRNA oligonucleotides are more specific than those of miRNAs. To determine whether CACT is a target gene responsible for the effect of miRNAs 132 and 212 on IS, we asked whether siRNAs that were designed to selectively suppress CACT mimic the effect of the miRNAs on IS. siRNA-mediated suppression of CACT (∼60% mRNA and ∼40% protein; Supplementary Fig. 4A and B) resulted in an increase in IS in response to basal glucose (Fig. 3A), and, in addition, to all the secretagogues shown to be affected by miRNA 132 (Fig. 1).
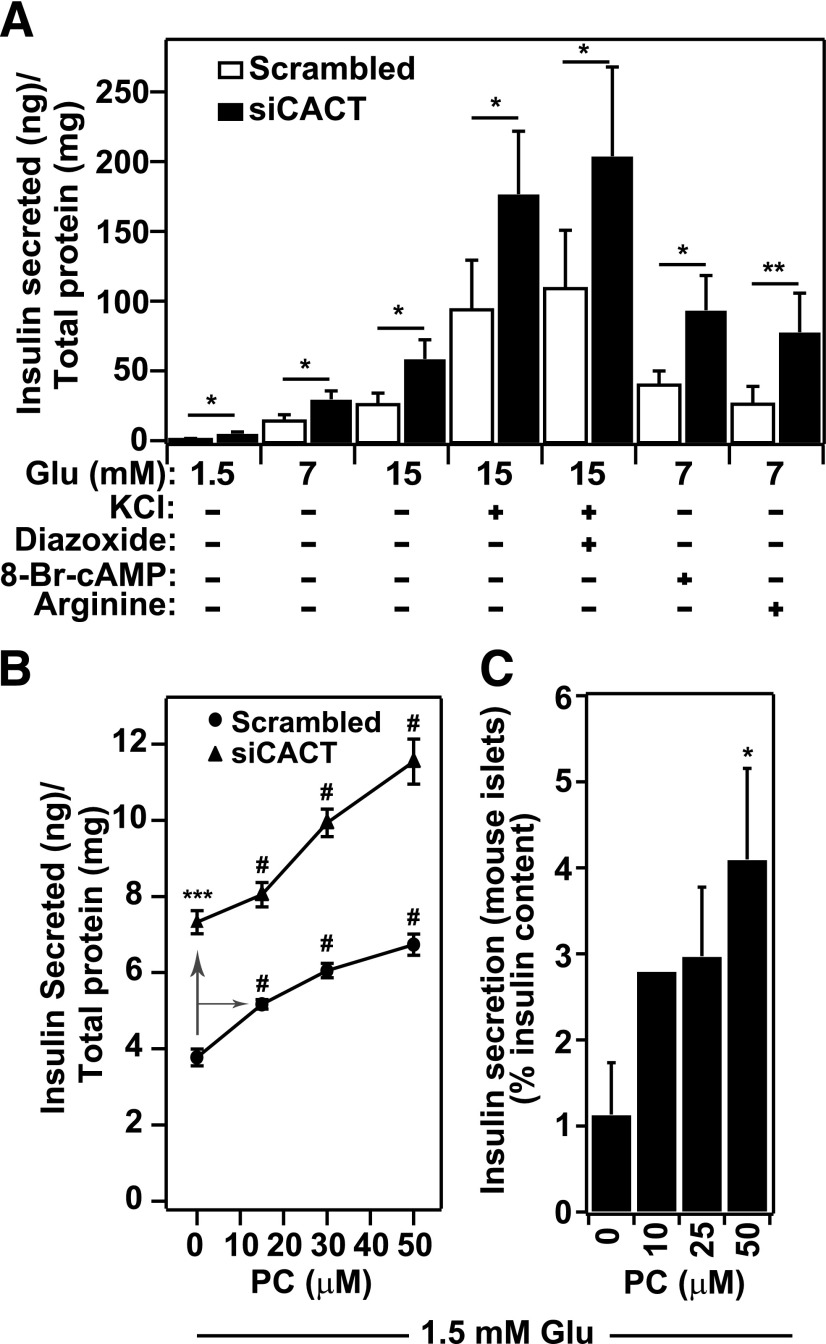
Figure 3.
Long-chain carnitine esters are potent insulin secretagogues. A: CACT knockdown enhances IS. IS from INS-1 832/13 β-cells was measured in response to siRNA-mediated knockdown of CACT. Cells were transfected with siRNA against CACT or a scrambled oligonucleotide while plating cells (reverse transfection) and incubated for 48 h before measuring IS. IS in response to increasing glucose concentrations (1.5, 7, and 15 mmol/L); elevated KCl (40 mmol/L) at 15 mmol/L glucose; a combination of elevated KCl, 15 mmol/L glucose, and 250 μmol/L DZX; or 3 mmol/L cAMP or 15 mmol/L L-arginine at 7 mmol/L glucose was measured. IS is normalized to total cellular protein and expressed as IS (nanograms) normalized for total protein (19). Each condition was performed five or more times. *P < 0.05; **P < 0.01. B: PC mimics the stimulatory effect of CACT knockdown on IS. IS in INS-1 832/13 cells in response to exogenous PC-BSA (15, 30, and 50 μmol/L), alone or in combination with CACT suppression by siRNA oligonucleotides, was measured. Cells were transfected with siRNAs CACT or negative control oligonucleotides (Scr-siRNA). After 48 h, IS was stimulated with 1.5 mmol/L glucose alone or in the presence of PC-BSA. IS is normalized to total cellular protein. Experiments were done a total three times. ANOVA calculation showed that P < 0.01 (#) for dose-dependent effect of PC-BSA compared with no addition of PC on either scrambled or siCACT transfected cells. ***P < 0.001 for effect of CACT knockdown. C: PC potentiates IS from primary mouse islets. Islets were isolated from B6 mice and used for ex vivo IS assays. After purification, islets were maintained in Krebs-Ringer buffer media at 1.7 mmol/L glucose for 20 min in the presence of digitonin (20 µg/mL) and then for 45 min without digitonin (preincubation), followed by a 45-min incubation with 1.7 mmol/L glucose in the presence of 10, 25, and 50 μmol/L PC to stimulate IS. IS was quantified using an insulin ELISA and normalized to total insulin content in islets. All experiments were performed three times (except the effect at 10 μmol/L PC-BSA, which was observed twice). *P < 0.05.
CACT is located in the inner mitochondrial membrane and mediates the influx of fatty acyl-carnitine esters into the mitochondria in preparation for β-oxidation (22). We hypothesized that the miRNA-dependent downregulation of CACT leads to reduced β-oxidation and elevates cellular levels of acyl-carnitine molecules, which in turn promote IS.
To test this hypothesis, we first asked whether a long-chain fatty acyl-carnitine stimulates IS when directly added to pancreatic β-cells. At 15–50 μmol/L, PC (16) resulted in a dose-dependent increase in IS in the presence of 1.5 mmol/L glucose (Fig. 3B). Knockdown of CACT by siRNAs further increased the effect of PC on IS. In addition to INS-1 832/13 cells, PC promoted a dose-dependent increase in IS from cultured mouse islets (Fig. 3C).
In addition to PC, 50 μmol/L stearoyl-carnitine also promoted IS from INS-1 832/3 β-cells (Supplementary Fig. 5). Finally, 600 μmol/L nonesterified palmitate augmented IS when acutely added to INS-1 832/3 cells (Supplementary Fig. 5), as has been previously reported (2).
CACT Knockdown Reduces β-Oxidation
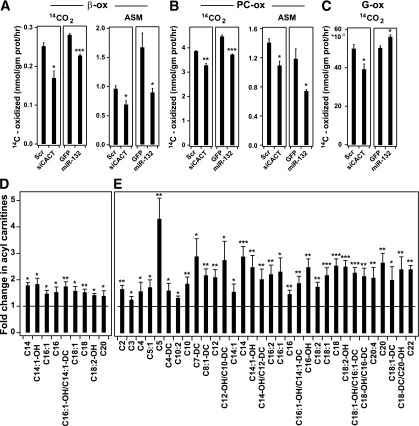
A reduction in CACT protein may lead to reduced β-oxidation because of diminished mitochondrial import of acyl-carnitine molecules. By decreasing the production of acetyl-CoA, glucose oxidation may increase because of the derepression of pyruvate dehydrogenase, as predicted by the Randle hypothesis (1). To discriminate between reduced β-oxidation and enhanced glycolysis in response to knockdown of CACT, we measured rates of palmitate, PC, and glucose oxidation in INS-1 832/13 cells. Palmitate and PC oxidation were significantly reduced by siRNA against CACT and by miRNA 132 (Fig. 4A and B). Glucose oxidation was significantly suppressed by siRNA against CACT, whereas miRNA 132 caused a small increase in glucose oxidation (Fig. 4C).
Figure 4.
CACT knockdown reduces β-oxidation and leads to acyl-carnitine accumulation. A–C: siCACT and miRNA 132 both blunt β-oxidation. INS-1 832/13 cells were transfected with CACT siRNAs or scrambled (Scr) oligonucleotides as negative control or were infected with Ad-GFP or Ad-miRNA-132. Palmitate oxidation (14CO2 released and ASM) (A), palmitoyl-carnitine oxidation (14CO2 released and ASM) (B), and glucose oxidation (C) were measured 48 h after transfection/infection, as described in Research Design and Methods. Units for all the graphs are nanomoles 14C per milligram protein per hour. D, E: Metabolic profile demonstrates an accumulation of acyl-carnitines in response to CACT downregulation. INS-1 832/13 cells were transfected with CACT siRNAs or control oligonucleotides (Scr-siRNA) and maintained for 48 h in RPMI supplemented with 1 mmol/L L-carnitine. The culture medium was refreshed without (D) or with (E) PC (50 μmol/L) and 1.5 mmol/L glucose 2 h before harvesting the cells for metabolic profiling. All metabolite measurements in response to CACT siRNA are normalized to control measurements. All bars represent statistically significant upregulation in metabolites. All experiments were performed three times.*P < 0.05; **P < 0.01; ***P < 0.001.
CACT Knockdown Increases Cellular Acyl-Carnitine Concentrations
We used mass spectrometry–based metabolic profiling to survey the pool of acyl-carnitines in INS-1 832/13 cells in the presence and absence of exogenous acyl-carnitine (50 μmol/L PC). In response to siRNA-mediated knockdown of CACT, we found that long-chain acyl-carnitine esters, ranging from C-14 to C-20, were significantly increased (1.5- to 2-fold; P < 0.05) (Fig. 4D). PC resulted in a significant elevation in carnitine esters with short- and long-chain lengths in response to CACT knockdown (Fig. 4E). This experiment was performed while maintaining a chronic exogenous level of L-carnitine (1 mmol/L for 48 h) to ensure that free carnitine concentrations were not limiting for CPT-1 activity. These studies were repeated in the absence of exogenous L-carnitine (Supplementary Fig. 6A and B), revealing that under these conditions CACT knockdown led to an increase in fewer long-chain acyl-carnitines. Absolute concentrations for all acyl-carnitine molecules profiled in both studies are provided in Supplementary Data 3.
A Nonhydrolyzable Palmitoyl-Carnitine Analog (POC-16) Enhances IS
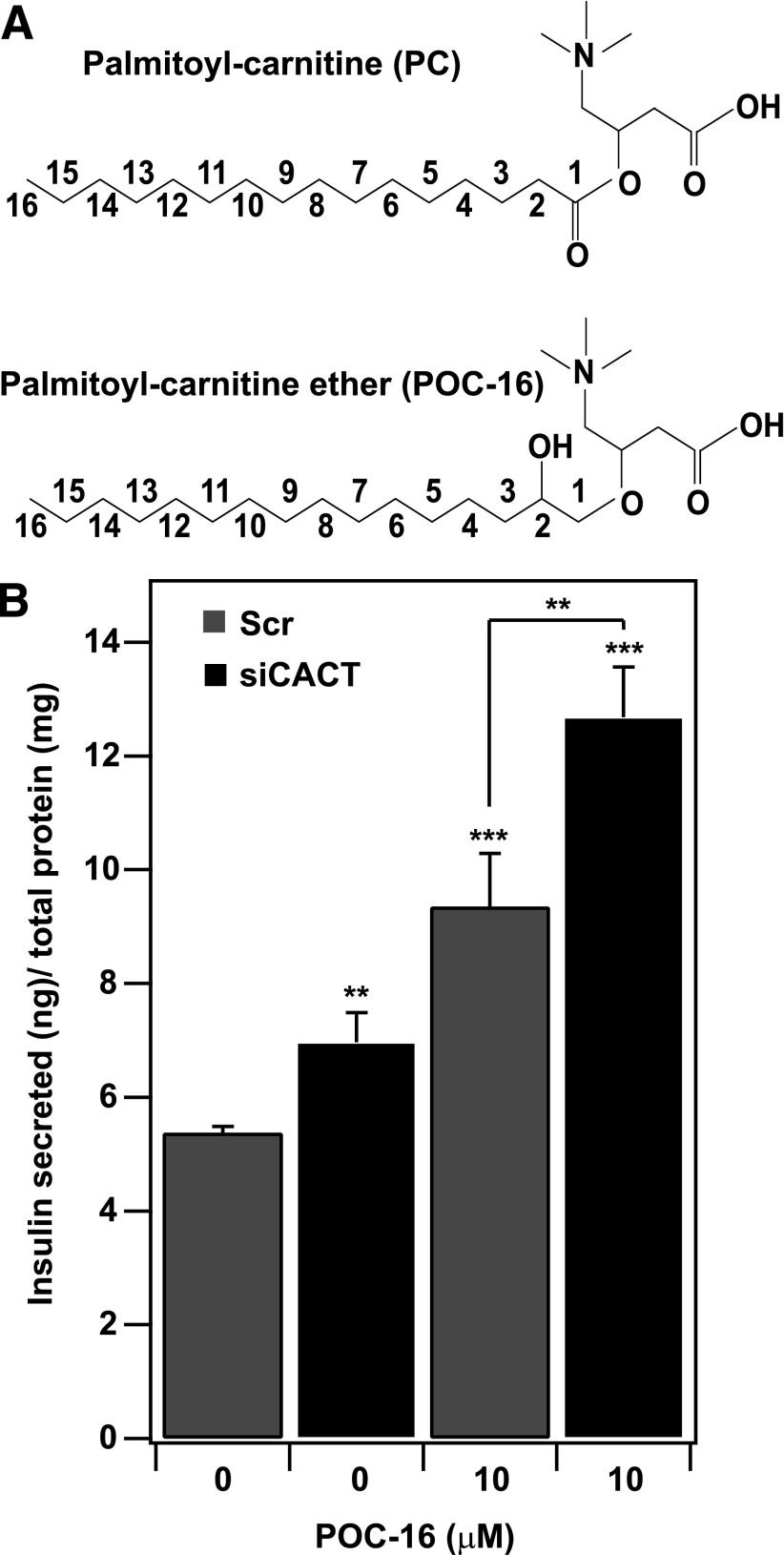
To determine whether long-chain carnitine esters are direct insulin segretagogues, we asked whether a nonhydrolyzable analog of PC promotes IS. In contrast to PC, the POC-16 analog lacks the C1 carbonyl group and contains an alcohol attached to C2, yielding an ether linkage between the fatty acyl group and carnitine (Fig. 5A). This ether linkage renders POC-16 nonhydrolyzable (23). We hypothesized that if PC is a direct signaling molecule that affects IS, then POC-16 should mimic the effect of PC. POC-16 was as effective as PC in promoting IS (Figs. 3B and 5B). POC-16 enhanced IS at basal glucose by ∼90%, and the effect was augmented ∼2.5-fold when coupled with knockdown of CACT. This supports our hypothesis that cytosolic PC acts directly to promote IS.
Figure 5.
A nonhydrolyzable analog of PC enhances glucose-stimulated IS. A: Molecular structure of PC and POC-16, an analog of PC in which the ester linkage between the fatty acid and carnitine is replaced by an ether linkage with C16. B: POC-16 stimulates IS from INS-1 832/13 cells. IS was monitored 48 h after cells were treated with either CACT siRNAs or negative control siRNAs (Scr) in the presence or absence of POC-16 (10 μmol/L, conjugated to BSA) and 1.5 mmol/L glucose. Insulin secreted was normalized to total cellular protein. The experiment was performed four times. **P < 0.01; ***P < 0.001.
Discussion
In this study we show that fatty acyl-carnitines are potent stimulators of IS. Upregulation of miRNAs 132 and 212 in pancreatic β-cells results in CACT suppression. CACT inhibition leads to inhibition of β-oxidation and accumulation of cellular long-chain fatty acyl-carnitine esters. In addition to carnitine esters, we also show that POC-16, a nonhydrolyzable analog of PC (23), is capable of enhancing IS (Fig. 5B), suggesting that the bond between the hydrocarbon and carnitine does not need to be hydrolyzed to stimulate IS.
The role of fatty acids in IS has been extensively studied. Herrero et al. (24) showed that overexpression of a mutant form of CPT-1 that is insensitive to malonyl-CoA leads to enhanced β-oxidation and impaired IS. Furthermore, islets from mice deficient in peroxisome proliferator–activated receptor-α decreased β-oxidation and increased IS (25,26). However, Mulder et al. (27) and Boucher et al. (28) showed that overexpression of malonyl-CoA decarboxylase leads to increased β-oxidation without affecting IS. However, these studies did not measure acyl-carnitines during manipulations of β-oxidation. Our data support an inverse relationship between β-oxidation and IS: reduced oxidation leads to increased secretion in parallel with increases in long-chain acyl-carnitines. In a recent review, Prentki et al. (2) also suggest that CPT-1 is a negative modulator of IS, which, when highly active, channels free fatty acids for β-oxidation, decreasing the production of lipid amplification signals of IS. A recent study by Klett et al. (29) also showed the importance of both acyl-CoAs and acyl-CoA synthetase for IS. These studies collectively demonstrate the importance of fatty acids and their analogs in IS.
miRNAs 132 and 212 are located within ∼200 base pairs of each other in the rat, mouse, and human genomes, suggesting that they are likely generated from a common primary miRNA precursor molecule (30). These miRNAs have a ∼75% sequence similarity and a ∼97% similar list of putative target genes (31). A genome-wide screen identified miRNAs 132 and 212 as targets of the transcription factor cAMP-response element binding protein (30). Accordingly, expression of these miRNAs is increased in response to an elevation in cellular cAMP, a signaling molecule that is critical for IS and β-cell survival (32,33). Recent publications also showed that, along with obesity and diabetes, high-fat feeding leads to an increase in the levels of miRNA 132 (20). In our mouse models, miRNAs 132 and 212 both were upregulated in islets in response to obesity, but this occurred to a lesser extent in diabetic mice. Moreover, our data show that CACT, a direct target of the miRNAs, is highly upregulated in diabetic obese mice compared with nondiabetic obese mice (34). These results suggest that the miRNA-dependent downregulation of CACT may be a mechanism to enhance the insulin secretory response.
In humans, CACT deficiency is a rare autosomal recessive disease and is characterized by a diminished capacity for β-oxidation, which can lead to a variety of severe metabolic disorders, including hypoketotic hypoglycemia, hyperammonemia, cardiac and hepatic dysfunction, skeletal muscle weakness, and encephalopathy (22,35). These phenotypes primarily reflect defects in muscle and hepatic β-oxidation. An early diagnostic criterion for CACT deficiency in humans is accumulation of plasma long-chain acyl-carnitines (22). Deletion of steroid receptor coactivator-3 in mice, which regulates CACT, leads to metabolic consequences similar to those that occur in CACT deficiency (36). Our results demonstrate that these same lipid classes significantly increase in β-cells in response to CACT knockdown (Fig. 4D and E). Long-chain fatty acyl-carnitines showed a stronger correlation with IS (Fig. 3B) than did short-chain fatty acyl-carnitines, with the exception of C5 (Supplementary Table 1). This agrees with previous findings indicating that long-chain fatty acids are more potent insulin secretagogues (7). C5 carnitine inhibits β-oxidation by antagonizing butyryl-CoA and octanoyl-CoA dehydrogenase (37). However, others have argued that acyl-carnitines are not associated with the regulation of IS. Glasgow et al. (38) measured steady-state insulin in response to chronically elevated long-chain acyl-carnitines, whereas we focused our studies on acute regulation of IS. Pepin et al. (39) showed that acute exposure to L-3-hydroxybutyrate or L-3-hydroxyglutarate did not change IS. However, our studies used long-chain fatty acyl-carnitines.
Surprisingly, we observed a slight decrease in glucose oxidation in response to CACT knockdown in addition to reduced β-oxidation. Because CACT is responsible for a bidirectional flux of acyl-carnitines, it is possible that CACT knockdown leads to an accumulation of acetyl-CoA in the mitochondrial matrix, leading to inhibition of glycolysis.
Our results demonstrate that long-chain acyl-carnitine esters are insulin secretagogues. The role of fatty acyl-CoA molecules in promoting IS has been widely studied (40); however, the mechanism is not well understood. Palmitoyl-CoA accumulation is known to increase protein acylation, which also leads to increased IS (41). Studies involving a neuroblastoma cell line show that, in addition to palmitoyl-CoA, PC could also be involved in protein palmitoylation (42). However, POC-16 is not a substrate for protein palmitoylation, suggesting that PC is unlikely to regulate IS through palmitoylation. Fatty acids also have been shown to regulate cellular Ca2+ levels through activation of GPR40 (41), leading to enhanced IS. However, we did not observe an effect of PC on GPR40 activation (Supplementary Fig. 7).
PC negatively regulates conventional and atypical PKCs (43,44). In addition, POC-16 suppresses conventional PKCs (17). Phosphatidylserine (PS) rescues the inhibitory effect of POC-16 on conventional PKC activity, suggesting that POC-16 may bind to the PS-binding domain on PKCs (43). PS binds to both the C1 and C2 domains of PKCs (45), indicating that PC could act as a ligand for either or both of these domains. PKCs have been shown to be both positive (23,43) and negative regulators of IS (45), indicating that PC could act as a ligand for either or both of these domains. PKCs have been shown to be positive (46,47) as well as negative regulators of IS (48,49).
siCACT enhanced IS at low glucose, suggesting that acyl-carnitines do not require glucose metabolism to influence IS. Several key components of the insulin secretory machinery, for example, synaptotagmins and Munc13 homologs, contain C1 and/or C2 domains (50), raising the possibility that fatty acyl-carnitines may directly interact with these proteins to regulate their function. Cytosolic accumulation of fatty acyl-CoA caused by reduced β-oxidation can lead to increased synthesis of diacylglycerol, monoacylglycerol, and phospholipids (2,7). A recent study by Zhao et al. (51) suggests that monoacylglycerols can bind the C1 domain of Munc13-1, a critical protein involved in vesicle exocytosis, and thus mediate enhanced insulin exocytosis.
In addition to stimulating secretion by binding exocytosis-mediating proteins, PC and other acyl-carnitines activate voltage-dependent calcium channels (52). PC can suppress or activate L-type calcium channels (53,54), depending on experimental conditions. PC activates the ryanodine receptor responsible for intracellular calcium–induced calcium response (55). PC also has been shown to inhibit K-ATP channels in ventricular myocytes (56), suggesting a mechanism by which PC could promote Ca2+ influx, leading to enhanced IS.
Acyl-carnitines have recently become a biomarker in metabolic syndrome (57,58). Our studies show that fatty acyl-carnitines are potent insulin secretagogues. These molecules are elevated in the sera of patients with insulin resistance (57). Elevated acyl-carnitine molecules, as observed with CACT suppression or in humans with CACT mutations (22), could be associated with increased IS that occurs in subjects with metabolic syndromes. The resulting hyperinsulinemia could lead to insulin resistance.
Supplementary Material
Article Information
Acknowledgments. The authors are grateful for the generous support and advice of Dr. Christopher Newgard (Duke University). The authors thank Miguel Bronfman (Catholic University, Chile) for generously donating the POC-16 compound and Alberto Guerra and Aron Haun for their technical assistance.
Funding. Statistical support was provided by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) (grant UL1TR000427). A.D.A. was supported by grants from the NIH (DK56593 and DK66369). R.M. has received grants from COBRE (NIH-8-P20-GM103528), NORC (NIH-2P30-DK072476), ADA-1-10-BS-129, NIH-R01DK089641. D.M.M. has received a grant from the NIH (R01-DK089312).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S.S. performed experiments and wrote the manuscript. M.E.R., J.S., O.I., R.M., and Y.-P.Z. performed experiments. S.B. provided technical advice and support. E.E.S. and N.A.T. designed the study. D.M.M. supervised the metabolomics analysis and interpreted results. M.P.K. comanaged the project. A.D.A. supervised the project, the interpretation of the data, and the preparation of the manuscript. A.D.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1677/-/DC1.
References
- 1.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 2013;18:162–185 [DOI] [PubMed] [Google Scholar]
- 3.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009;50:3–21 [DOI] [PubMed] [Google Scholar]
- 4.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem 1997;272:30261–30269 [DOI] [PubMed] [Google Scholar]
- 5.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 2003;278:30015–30021 [DOI] [PubMed] [Google Scholar]
- 6.Littman ED, Pitchumoni S, Garfinkel MR, Opara EC. Role of protein kinase C isoenzymes in fatty acid stimulation of insulin secretion. Pancreas 2000;20:256–263 [DOI] [PubMed] [Google Scholar]
- 7.Yaney GC, Korchak HM, Corkey BE. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal beta-cells. Endocrinology 2000;141:1989–1998 [DOI] [PubMed] [Google Scholar]
- 8.Majumdar S, Rossi MW, Fujiki T, et al. Protein kinase C isotypes and signaling in neutrophils. Differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem 1991;266:9285–9294 [PubMed] [Google Scholar]
- 9.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003;422:173–176 [DOI] [PubMed] [Google Scholar]
- 10.Zhao E, Keller MP, Rabaglia ME, et al. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome 2009;20:476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatnagar S, Oler AT, Rabaglia ME, et al. Positional cloning of a type 2 diabetes quantitative trait locus; tomosyn-2, a negative regulator of insulin secretion. PLoS Genet 2011;7:e1002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colca JR, Wolf BA, Comens PG, McDaniel ML. Protein phosphorylation in permeabilized pancreatic islet cells. Biochem J 1985;228:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su WL, Kleinhanz RR, Schadt EE. Characterizing the role of miRNAs within gene regulatory networks using integrative genomics techniques. Mol Syst Biol 2011;7:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockenbrough JS, Korc M. Inhibition of epidermal growth factor binding in rat pancreatic acini by palmitoyl carnitine: evidence for Ca2+ and protein kinase C independent regulation. Cancer Res 1987;47:1805–1810 [PubMed] [Google Scholar]
- 15.Ramachandran P, Fitzwater SP, Aneja S, et al. Prospective multi-centre sentinel surveillance for Haemophilus influenzae type b & other bacterial meningitis in Indian children. Indian J Med Res 2013;137:712–720 [PMC free article] [PubMed] [Google Scholar]
- 16.Jakupciak JP, Wang W, Markowitz ME, et al. Mitochondrial DNA as a cancer biomarker. J Mol Diagn 2005;7:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muoio DM, Noland RC, Kovalik JP, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 2012;15:764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan CP, Feng Y, Zhou YP, et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes 2008;57:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velasco R, Zharkikh A, Affourtit J, et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 2010;42:833–839 [DOI] [PubMed] [Google Scholar]
- 20.Nesca V, Guay C, Jacovetti C, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia 2013;56:2203–2212 [DOI] [PubMed] [Google Scholar]
- 21.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004;432:226–230 [DOI] [PubMed] [Google Scholar]
- 22.Wang GL, Wang J, Douglas G, et al. Expanded molecular features of carnitine acyl-carnitine translocase (CACT) deficiency by comprehensive molecular analysis. Mol Genet Metab 2011;103:349–357 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Huidobro T, Valenzuela E, Leisewitz AV, Valderrama J, Bronfman M. Anti-proliferative effect of two novel palmitoyl-carnitine analogs, selective inhibitors of protein kinase C conventional isoenzymes. Eur J Biochem 1999;266:855–864 [DOI] [PubMed] [Google Scholar]
- 24.Herrero L, Rubí B, Sebastián D, et al. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the beta-cell impairs glucose-induced insulin secretion. Diabetes 2005;54:462–471 [DOI] [PubMed] [Google Scholar]
- 25.Gremlich S, Nolan C, Roduit R, et al. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology 2005;146:375–382 [DOI] [PubMed] [Google Scholar]
- 26.Tordjman K, Standley KN, Bernal-Mizrachi C, et al. PPARalpha suppresses insulin secretion and induces UCP2 in insulinoma cells. J Lipid Res 2002;43:936–943 [PubMed] [Google Scholar]
- 27.Mulder H, Lu D, Finley J, 4th, et al. Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) beta-cells. J Biol Chem 2001;276:6479–6484 [DOI] [PubMed] [Google Scholar]
- 28.Boucher A, Lu D, Burgess SC, et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 2004;279:27263–27271 [DOI] [PubMed] [Google Scholar]
- 29.Klett EL, Chen S, Edin ML, et al. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem 2013;288:21618–21629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vo N, Klein ME, Varlamova O, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A 2005;102:16426–16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 2010;11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 1987;67:1185–1248 [DOI] [PubMed] [Google Scholar]
- 33.Keller DM, Clark EA, Goodman RH. Regulation of microRNA-375 by cAMP in pancreatic β-cells. Mol Endocrinol 2012;26:989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller MP, Choi Y, Wang P, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 2008;18:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobazzi V, Invernizzi F, Baratta S, et al. Molecular and functional analysis of SLC25A20 mutations causing carnitine-acylcarnitine translocase deficiency. Hum Mutat 2004;24:312–320 [DOI] [PubMed] [Google Scholar]
- 36.York B, Reineke EL, Sagen JV, et al. Ablation of steroid receptor coactivator-3 resembles the human CACT metabolic myopathy. Cell Metab 2012;15:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland PC, Sherratt HS. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Effects of the free acids and their carnitine esters on coenzyme A-dependent oxidations in rat liver mitochondria. Biochem J 1973;136:157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasgow AM, Engel AG, Bier DM, et al. Hypoglycemia, hepatic dysfunction, muscle weakness, cardiomyopathy, free carnitine deficiency and long-chain acylcarnitine excess responsive to medium chain triglyceride diet. Pediatr Res 1983;17:319–326 [DOI] [PubMed] [Google Scholar]
- 39.Pepin E, Guay C, Delghingaro-Augusto V, Joly E, Madiraju SR, Prentki M. Short-chain 3-hydroxyacyl-CoA dehydrogenase is a negative regulator of insulin secretion in response to fuel and non-fuel stimuli in INS832/13 β-cells. J Diabetes 2010;2:157–167 [DOI] [PubMed] [Google Scholar]
- 40.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996;97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 2004;53:1007–1019 [DOI] [PubMed] [Google Scholar]
- 42.Nałecz KA, Szczepankowska D, Czeredys M, Kulikova N, Grześkiewicz S. Palmitoylcarnitine regulates estrification of lipids and promotes palmitoylation of GAP-43. FEBS Lett 2007;581:3950–3954 [DOI] [PubMed] [Google Scholar]
- 43.Sobiesiak-Mirska J, Nałecz MJ, Nałecz KA. Interaction of palmitoylcarnitine with protein kinase C in neuroblastoma NB-2a cells. Neurochem Int 2003;42:45–55 [DOI] [PubMed] [Google Scholar]
- 44.Katoh N, Wrenn RW, Wise BC, Shoji M, Kuo JF. Substrate proteins for calmodulin-sensitive and phospholipid-sensitive Ca2+-dependent protein kinases in heart, and inhibition of their phosphorylation by palmitoylcarnitine. Proc Natl Acad Sci U S A 1981;78:4813–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JE, Giorgione J, Newton AC. The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry 2000;39:11360–11369 [DOI] [PubMed] [Google Scholar]
- 46.Harris TE, Persaud SJ, Jones PM. Atypical isoforms of pKc and insulin secretion from pancreatic beta-cells: evidence using Gö 6976 and Ro 31-8220 as Pkc inhibitors. Biochem Biophys Res Commun 1996;227:672–676 [DOI] [PubMed] [Google Scholar]
- 47.Uchida T, Iwashita N, Ohara-Imaizumi M, et al. Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J Biol Chem 2007;282:2707–2716 [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Nagasawa M, Yamada S, Mogami H, Suzuki Y, Kojima I. Bimodal role of conventional protein kinase C in insulin secretion from rat pancreatic beta cells. J Physiol 2004;561:133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, et al. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab 2007;6:320–328 [DOI] [PubMed] [Google Scholar]
- 50.Sheu L, Pasyk EA, Ji J, et al. Regulation of insulin exocytosis by Munc13-1. J Biol Chem 2003;278:27556–27563 [DOI] [PubMed] [Google Scholar]
- 51.Zhao S, Mugabo Y, Iglesias J, et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab 2014;19:993–1007 [DOI] [PubMed] [Google Scholar]
- 52.Spedding M, Mir AK. Direct activation of Ca2+ channels by palmitoyl carnitine, a putative endogenous ligand. Br J Pharmacol 1987;92:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Corr PB. Influence of long-chain acylcarnitines on voltage-dependent calcium current in adult ventricular myocytes. Am J Physiol 1992;263:H410–H417 [DOI] [PubMed] [Google Scholar]
- 54.Liu QY, Rosenberg RL. Activation and inhibition of reconstituted cardiac L-type calcium channels by palmitoyl-L-carnitine. Biochem Biophys Res Commun 1996;228:252–258 [DOI] [PubMed] [Google Scholar]
- 55.Dumonteil E, Barré H, Meissner G. Effects of palmitoyl carnitine and related metabolites on the avian Ca(2+)-ATPase and Ca2+ release channel. J Physiol 1994;479:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haruna T, Horie M, Takano M, et al. Alteration of the membrane lipid environment by L-palmitoylcarnitine modulates K(ATP) channels in guinea-pig ventricular myocytes. Pflugers Arch 2000;441:200–207 [DOI] [PubMed] [Google Scholar]
- 57.Sampey BP, Freemerman AJ, Zhang J, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS ONE 2012;7:e38812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.