Abstract
In rodents, brown adipose tissue (BAT) regulates cold- and diet-induced thermogenesis (CIT; DIT). Whether BAT recruitment is reversible and how it impacts on energy metabolism have not been investigated in humans. We examined the effects of temperature acclimation on BAT, energy balance, and substrate metabolism in a prospective crossover study of 4-month duration, consisting of four consecutive blocks of 1-month overnight temperature acclimation (24°C [month 1] → 19°C [month 2] → 24°C [month 3] → 27°C [month 4]) of five healthy men in a temperature-controlled research facility. Sequential monthly acclimation modulated BAT reversibly, boosting and suppressing its abundance and activity in mild cold and warm conditions (P < 0.05), respectively, independent of seasonal fluctuations (P < 0.01). BAT acclimation did not alter CIT but was accompanied by DIT (P < 0.05) and postprandial insulin sensitivity enhancement (P < 0.05), evident only after cold acclimation. Circulating and adipose tissue, but not skeletal muscle, expression levels of leptin and adiponectin displayed reciprocal changes concordant with cold-acclimated insulin sensitization. These results suggest regulatory links between BAT thermal plasticity and glucose metabolism in humans, opening avenues to harnessing BAT for metabolic benefits.
Introduction
Unhealthy diet and physical inactivity are the major culprits to the obesity crisis, although other environmental factors may also contribute (1). An overlooked component in energy balance is adaptive thermogenesis, which comprises diet-induced thermogenesis (DIT) and cold-induced thermogenesis (CIT). DIT is the portion of energy expended after food ingestion, beyond the energy cost of digestion/absorption (2). The CIT response defends core temperature during cold exposure (3). In rodents, both processes are chiefly regulated by brown adipose tissue (BAT). Through the action of uncoupling protein 1 (UCP1), energy is converted into heat and represents a form of energy expenditure (EE) as energy is dissipated to the environment. BAT stimulation protects animals against diet-induced obesity and glucose intolerance (4).
In addition to “classic BAT” in the interscapuar region, cold exposure also induces the emergence of brown adipocyte-like cells (beige/brite adipocytes) within white adipose tissue (WAT) in animals (5,6). Brown/beige fat generates heat from glucose/lipids, and their high substrate utilization underlies protection against diet-induced insulin resistance in genetic, pharmacological, and/or transplantation models of invigorated brown/beige fat status (7–9). In humans, histological examination had demonstrated the presence of BAT in adult in the 1970–1980s (10–12), although BAT whole-body abundance was not fully appreciated until its visualization was made possible by positron emission tomography (PET)/computed tomography (CT) (13–17). BAT is not only inducible in humans (18,19) but also exhibits oxidative capacity (20) and classic BAT/beige fat features (21,22), thus forming the basis for the quest of BAT/beige fat-enhancing strategies as antiobesity treatments (23).
Acute cold exposure (hours) increases BAT activity (13,15–17,24), while longer-term exposure (days/weeks) expands BAT volume (25,26). Because BAT recruitment could reduce adiposity (26), it suggests that BAT may impact whole-body energy homeostasis. The corollary is that reduced cold exposure could suppress BAT/beige fat function in humans, with potential obesogenic consequences (27). To date, cold exposure is the best-known activator (15–17) and recruiter (25,26) of BAT, and associative data have linked higher BAT abundance with leanness and lower glycemia in humans (13,15,28,29). Whether BAT withers under warm exposure and whether BAT recruitment triggers compensatory metabolic and/or behavioral adaptations have not been investigated but are integral to BAT physiology. Rodent studies have revealed a complex interplay between housing temperature, BAT recruitment, and energy balance, which ultimately determines metabolic phenotype (30). For better appreciation of the metabolic significance of human BAT and the implications of BAT status on health, BAT recruitment interventions should be examined in the context of whole-body energy metabolism.
In this study, we investigated the effects of long-term mild cold and warm exposure by minimal overnight manipulation of ambient temperature on individual BAT status and the corresponding energy/substrate homeostatic responses. We hypothesized that human BAT exhibits plasticity and its activity modulates systemic energy metabolism.
Research Design and Methods
Subjects
Five healthy men were recruited through local advertisement and provided written informed consent. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)–National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board approved the study: Impact of Chronic Cold Exposure in Humans (ICEMAN). Supplementary Fig. 1 summarizes recruitment, allocation, and intervention.
Overall Design
This is a prospective crossover study consisting of four consecutive blocks of 1-month duration (Supplementary Fig. 2): it incorporates 1) sequential monthly thermal acclimation over 4 months and 2) acute thermometabolic evaluations at the end of each study temperature regimen. Volunteers were admitted to the Clinical Research Unit (NIDDK) in Bethesda, MD, (April–November 2013) for the entire 4 months.
Monthly Thermal Acclimation
Volunteers resided in a temperature-adjusted private room, engaged in usual daily activities, and returned to their room each evening. Room temperature was adjusted in the following sequence: 24°C (month 1) → 19°C (month 2) → 24°C (month 3) → 27°C (month 4). Volunteers were exposed to the study temperature for at least 10 h each night, wearing standardized hospital clothing with a combined thermal insulation value of 0.4 (clo). Only bedsheets were provided. Volunteers were asked to not deviate daily activity level over the study period. Each subject therefore acted as his own control. At 0800 h at the end of each month, volunteers were admitted to a whole-room indirect calorimeter for thermometabolic evaluation.
Temperature Monitoring
Volunteers wore two temperature data loggers (cat. no. RHT20; Extech, Nashua, NH): one “external to clothing” to track environmental temperature and the other “within clothing” to track immediate temperature changes in the “microenvironment” within clothing. We averaged individual exposed temperature every 30 min for the entire 4-month period, allowing us to record environmental temperature variations and the “true temperatures” the individual was being exposed to.
Diet
All meals, including prepacked lunches/snacks, were provided with the following composition: 50% carbohydrate, 20% protein, and 30% fat. The first month was an equilibration period, during which volunteers followed a weight-maintenance diet. After month 1, subjects ate according to hunger. Caloric/macronutrient content was calculated based on weight-maintenance requirements, determined during equilibration month. Any unconsumed foods were returned/weighed for energy/macronutrient intake calculation. Subjects met study dietitians twice weekly to verify food diaries/compliance. Total intake/macronutrient was computed/analyzed using three-dimensional food models (ProNutra, version 3.4.0.0.; Viocare Technologies, Princeton, NJ).
Appetite/Hunger Assessment
Subjects completed questionnaires assessing appetite twice a week before/after breakfast by marking on a visual analog scale (VAS) (10 cm long) responses to the following questions: 1) How hungry are you? 2) How full are you? 3) How much food can you consume? These questions gauged hunger, satiety, and desire to eat before/after meals.
Before each monthly thermaometabolic evaluation, volunteers underwent an ad libitum meal test, consisting of a selection of food items displayed in a vending machine. Subjects ate until they felt “comfortably full.” Total energy/macronutrient intake were recorded, together with ratings of appetite (hunger, satiety, and desire to eat) using the same weekly questionnaire at T = −10, 0, 60, 120, 180, 240, and 300 min where initiation of the meal was defined as T = 0 min.
Acute Thermometabolic Evaluations
Thermometabolic evaluation was scheduled at the end of each month (Supplementary Fig. 2), modeled on our previous published methods (24,31), with total EE calculated as previously described (24,31). Volunteers underwent two 24-h sessions in a whole-room calorimeter, exposed to first 24°C (day 1) and then 19°C (day 3), with a resting 24-h period in between. The temperature order was not randomized because our previous study did not reveal a sequence effect (31). Testing at the two temperatures allowed us to evaluate how monthly acclimation modulated EE/metabolism at both thermoneutral and mild cold conditions. Lunch (Boost Plus; Nestlé Healthcare Nutrition, Inc., Vevey, Switzerland) and dinner (selected from Metabolic Menu) were provided at 1300 and 1900 h, consisting of one-third and two-thirds of daily caloric intake, respectively, based on calculation from equilibration month. CIT was calculated as difference in total EE between 24°C and 19°C and DIT as difference in pre- (0800–1300 h) and post- (1300–1900 h) lunch EE. As the test meal carried identical caloric and macronutrient content, we attributed any changes observed to arise from adaptive thermogenesis because the facultative component (i.e., digestion/absorption) should be relatively unaltered. Shivering response was quantified by surface electromyography (EMG), as previously described (32), and volunteers reported perception to cold each month during EE testing using VAS. Hormonal/metabolic parameters were measured in venous samples. Postprandial insulin sensitivity was calculated after a mixed meal (33) and adipose resistance index as the product of free fatty acid × insulin. At the conclusion of the thermometabolic study, body composition was measured, as previously published (24,31).
PET/CT Scanning
PET/CT was performed using Siemens Biograph mCT (Siemens Healthcare) (32). PET/CT was undertaken at 0800 h the morning after the 19°C testing day at the end of each acclimation month. Attenuation-corrected PET/CT images were analyzed using custom software built with IDL (Exelis Visual Information Solutions, Inc., Boulder, CO). A three-dimensional region of interest (ROI) was defined cranially by a horizontal line parallel to the base of the C4 vertebra and caudally by an oblique line traversing the manubriosternal joint and T8 transverse process (Supplementary Fig. 3). BAT was defined as tissue with Hounsfield units (HU) −300 to −10 on CT (i.e., fat density) with a lean body mass standardized uptake value (SUV) of ≥2 (i.e., high glucose uptake). The chosen ROI captures major BAT depots in the cervical, supraclavicular, axillary, superior mediastinal, and paravertebral areas. This ROI was chosen because spurious myocardial/renal excretory fluorodeoxyglucose (FDG) uptake could not be reliably excluded from BAT. This approach allowed examination of BAT evolution within a well-defined region of adipose tissue across 4 months.
PET/CT Parameters
The following parameters were analyzed: BAT volume, mean SUV, and activity. BAT volume, defined as the sum of the volume of all voxels that met HU-SUV criteria, represents activated BAT. Mean SUV (normalized by FDG dose and lean body mass) of ROI represents mean metabolic activity within BAT-harboring region. BAT activity represents the total radioactivity (in MBq) within ROI and captures both changes in volume and mean FDG uptake. Furthermore, because fat exhibits metabolic activity as a continuum and the chosen SUV threshold of ≥2 is arbitrary and may potentially exclude more diffuse enhancement of adipose metabolic activity, we also quantified mean SUV in the entire ROI within tissue of fat density (HU: −300 to −10). Mean SUV of whole fat depot estimates overall metabolic activity and may capture both BAT and diffuse beige fat activity. This is particularly relevant in subjects with lower BAT abundance (Supplementary Figs. 5 and 7). While BAT, defined with an SUV threshold of ≥2, was not visually apparent in these two subjects, whole fat activity followed the same pattern of acclimated BAT changes. When a lower SUV was used (≥1) (Supplementary Fig. 8), adipose activity changes were visually concordant with overall fat activity. Mean SUV uptake in liver and skeletal muscle (rectus femoris) was quantified to compare temperature-acclimation impact on BAT and other metabolic organs. All images were analyzed twice by an investigator (P.L.) blinded to subject identity and acclimation temperature. Intrascan coefficient of variation of BAT volume, mean SUV, BAT activity, and mean whole fat activity were 0.7%, 3.0%, 1.3%, and 2.4%, respectively.
Tissue Biopsies
Paired subcutaneous adipose/muscle biopsies were obtained at the end of each month from abdomen and rectus femoris, respectively (31). RNA extraction and cDNA synthesis were performed using standard methods, and genes governing thermogenesis and glucose metabolism were examined using TaqMan Gene Expression assays (Applied Biosystems) (Supplementary Table 2).
Laboratory Measurements
Plasma adiponectin, leptin, and fibroblast growth factor 21 (FGF21) were measured by ELISA (R&D Systems, Minneapolis, MN, and BioVendor, Oxford, U.K.), according to manufacturer’s protocol, with intra-assay/interassay coefficients of variation between 2.5 and 4.8%. Remaining tests were measured by the Department of Laboratory Medicine, National Institutes of Health (NIH).
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Inc., Chicago, IL). Data are expressed as means ± SD. Trend changes of physiologic and hormonal parameters during temperature acclimation across 4 months, expressed as fold change over baseline, were analyzed by one-way ANOVA with Bonferroni correction. Areas under the curve (AUCs) were calculated using the trapezoidal rules incorporating sampling points across a 24-h period from 0800 to 0700 h the next morning (Supplementary Fig. 2). Postprandial glucose and insulin AUCs were calculated in the period after lunch starting at 1300 h (T = 0, 60, 120, 240, and 360 min). Pearson correlation coefficients were used to examine associations between variables. An α-error of 0.05 was considered statistically significant.
Results
Baseline Acute Thermometabolic Evaluation
Five men (21 ± 2 years old, BMI 22 ± 1 kg/m2, body fat 21 ± 2%) participated in the study. Volunteers were first evaluated at baseline for BAT status and thermometabolic responses to temperature changes. Compared with 24°C, mild cold exposure at 19°C increased total EE by 6 ± 4% (P < 0.05), representing CIT response. Baseline cold-activated BAT volume was 55 ± 61 mL, with a mean SUV of 3.2 ± 0.8. EE at 19°C correlated positively with BAT volume (R2 = 0.82, P = 0.03). These results replicated findings in our previous overnight cold exposure studies (24,31) and validated the methodology in the investigation of temperature acclimation–associated metabolic and physiologic consequences. Hereafter, we describe changes in physiologic and metabolic parameters at each monthly thermometabolic evaluation, with results stratified to either the 19°C or the 24°C testing condition to decipher impact of acclimation on metabolism under thermoneutrality and mild cold exposure.
Metabolic Consequences of Monthly Acclimation
Tables 1–4 summarize changes in BAT and physiologic, dietary, body compositional, and hormonal parameters across the 4-month acclimation. Hormone/metabolite AUC results are shown in Table 4 and fasting levels in Supplementary Table 3. Results from each domain are described in the following subsections.
Table 1.
PET/CT parameters across 4 months of acclimation
| Month 1: 24°C | Month 2: 19°C | Month 3: 24°C | Month 4: 27°C | Ptrend | |
|---|---|---|---|---|---|
| BAT volume (mL) | 55 ± 61 | 78 ± 84a | 63 ± 81 | 58 ± 81 | 0.036 |
| BAT mean SUV | 3.2 ± 0.8 | 3.8 ± 1.3 | 3.4 ± 1.0 | 3.4 ± 0.8 | 0.35 |
| BAT activity (MBq) | 0.65 ± 0.76 | 1.0 ± 1.3a | 0.8 ± 1.1 | 0.7 ± 1.0 | 0.038 |
| BAT radiodensity (HU) | −58.8 ± 7.2 | −44.2 ± 6.8 | −55.4 ± 6.5 | −69.2 ± 6.8 | <0.01 |
| Whole fat mean SUV | 0.61 ± 0.13 | 0.68 ± 0.18a | 0.63 ± 0.17 | 0.59 ± 0.16 | 0.035 |
| Muscle mean SUV | 0.46 ± 0.08 | 0.41 ± 0.04 | 0.43 ± 0.05 | 0.48 ± 0.08 | 0.52 |
| Liver mean SUV | 1.68 ± 0.08 | 1.50 ± 0.14 | 1.61 ± 0.15 | 1.67 ± 0.16 | 0.15 |
Data are means ± SD. At the end of each testing month, subjects underwent acute thermometabolic evaluation at either 24°C or 19°C.
aP < 0.05 (month 1 vs. 2).
Table 4.
Hormonal and metabolic parameters across 4 months of acclimation
| Month 1: 24°C |
Month 2: 19°C |
Month 3: 24°C |
Month 4: 27°C |
Ptrend |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | |
| Sympathoadrenal | ||||||||||
| Urinary epinephrine (μg/day) | 7.5 ± 5.0 | 8.0 ± 4.8 | 7.5 ± 3.9 | 8.3 ± 5.6 | 7.5 ± 4.4 | 7.7 ± 4.6 | 8.3 ± 6.1 | 7.7 ± 5.1 | 0.97 | 0.94 |
| Urinary norepinephrine (μg/day) | 46 ± 29 | 53 ± 14 | 56 ± 30 | 64 ± 20 | 35 ± 9 | 61 ± 18a | 37 ± 5 | 58 ± 24 | 0.42 | 0.56 |
| Glucocorticoid axis | ||||||||||
| ACTH AUC (pg ⋅ min/mL) | 207 ± 61 | 199 ± 73 | 199 ± 51 | 197 ± 65 | 203 ± 74 | 176 ± 61 | 209 ± 71 | 199 ± 83 | 0.20 | 0.40 |
| Cortisol AUC (μg ⋅ min/mL) | 0.96 ± 0.14 | 0.91 ± 0.13 | 0.87 ± 0.10 | 0.95 ± 0.12 | 0.81 ± 0.13 | 0.84 ± 0.17 | 0.90 ± 0.15 | 0.88 ± 0.22 | 0.14 | 0.39 |
| Urinary cortisol (μg/day) | 49 ± 9 | 36 ± 13 | 49 ± 27 | 39 ± 9 | 39 ± 11 | 38 ± 11 | 39 ± 22 | 49 ± 21 | 0.42 | 0.25 |
| Thyroid axis | ||||||||||
| TSH AUC (μIU ⋅ min/mL) | 7.8 ± 3.5 | 8.0 ± 2.8 | 7.5 ± 3.6 | 7.3 ± 2.1 | 8.6 ± 4.3 | 7.3 ± 2.2 | 8.8 ± 3.5c | 7.6 ± 1.9 | 0.38 | 0.39 |
| Free T4 AUC (pg ⋅ min/mL) | 95 ± 13 | 95 ± 9 | 93 ± 12 | 92 ± 12 | 93 ± 9 | 93 ± 11 | 90 ± 9 | 94 ± 9 | 0.39 | 0.54 |
| Free T3 AUC (pg ⋅ min/mL) | 26 ± 1 | 27 ± 1 | 29 ± 2b | 28 ± 1 | 28 ± 3 | 27 ± 2 | 26 ± 2d | 28 ± 2 | 0.16 | 0.59 |
| Free T3/free T4 AUC | 2,381 ± 490 | 2,410 ± 345 | 2,642 ± 574b | 2,556 ± 393 | 2,513 ± 449 | 2,511 ± 466 | 2,515 ± 481 | 2,491 ± 403 | 0.06 | 0.41 |
| Glucose and lipid metabolism | ||||||||||
| Total glucose AUC (mg ⋅ min/mL) | 7.38 ± 0.64 | 7.32 ± 0.37 | 7.22 ± 0.51 | 7.25 ± 0.63 | 7.22 ± 0.73 | 7.32 ± 0.63 | 7.14 ± 0.51 | 7.11 ± 0.74 | 0.75 | 0.71 |
| Postprandial glucose AUC (mg ⋅ min/mL) | 2.73 ± 0.27 | 2.68 ± 0.34 | 2.59 ± 0.13 | 2.68 ± 0.29 | 2.64 ± 0.40 | 2.57 ± 0.29 | 2.64 ± 0.15 | 2.53 ± 0.27 | 0.79 | 0.63 |
| Total insulin AUC (IU ⋅ min/L) | 170 ± 102 | 210 ± 83 | 198 ± 97 | 143 ± 49 | 212 ± 118 | 186 ± 123 | 171 ± 87 | 182 ± 128 | 0.08 | 0.44 |
| Postprandial insulin AUC (IU ⋅ min/L) | 106 ± 64 | 133 ± 57 | 111 ± 52 | 77 ± 22 | 132 ± 85 | 114 ± 80 | 103 ± 55 | 109 ± 79 | 0.19 | 0.31 |
| Total free fatty acid AUC (mEq ⋅ min/L) | 3.53 ± 0.70 | 3.76 ± 1.05 | 3.36 ± 0.31 | 3.37 ± 1.16 | 2.73 ± 0.86 | 3.20 ± 0.40 | 3.65 ± 1.09 | 3.76 ± 0.41 | 0.36 | 0.68 |
| Fasting total cholesterol (mg/dL) | 120 ± 24 | 132 ± 24 | 117 ± 16 | 136 ± 11 | 0.13 | |||||
| Fasting LDL (mg/dL) | 71 ± 21 | 75 ± 21 | 62 ± 13 | 76 ± 10 | 0.20 | |||||
| Fasting TG (mg/dL) | 57 ± 16 | 68 ± 27 | 68 ± 15 | 65 ± 22 | 0.31 | |||||
| Fasting HDL (mg/dL) | 38 ± 7 | 44 ± 7 | 41 ± 7 | 46 ± 4d | 0.03 | |||||
| Adipokine | ||||||||||
| Leptin AUC (ng ⋅ min/mL) | 16 ± 5 | 15 ± 6 | 14 ± 3b | 12 ± 2a,b | 29 ± 15 | 26 ± 11 | 25 ± 12 | 25 ± 11c | 0.01 | 0.002 |
| Adiponectin AUC (pg ⋅ min/mL) | 99 ± 38 | 103 ± 37 | 117 ± 51b | 127 ± 49a,b | 78 ± 31 | 77 ± 32 | 74 ± 32c | 82 ± 38c | 0.0007 | 0.0003 |
| FGF21 AUC (pg ⋅ min/mL) | 333 ± 57 | 411 ± 104 | 343 ± 46 | 460 ± 91a,b | 350 ± 25 | 400 ± 80 | 370 ± 37 | 435 ± 75 | 0.28 | 0.10 |
Data are means ± SD. At the end of each testing month, subjects underwent acute thermometabolic evaluation at either 24°C or 19°C. ACTH, adrenocortotropic hormone.
aP < 0.05 compared with 24°C during acute thermometabolic evaluation each month;
bP < 0.05 (month 1 vs. 2),
cP < 0.05 (months 2 vs. 4), and
dP < 0.05 (months 1 vs. 4) compared with matching measurement at the same temperature performed at respective months as indicated.
BAT Changes
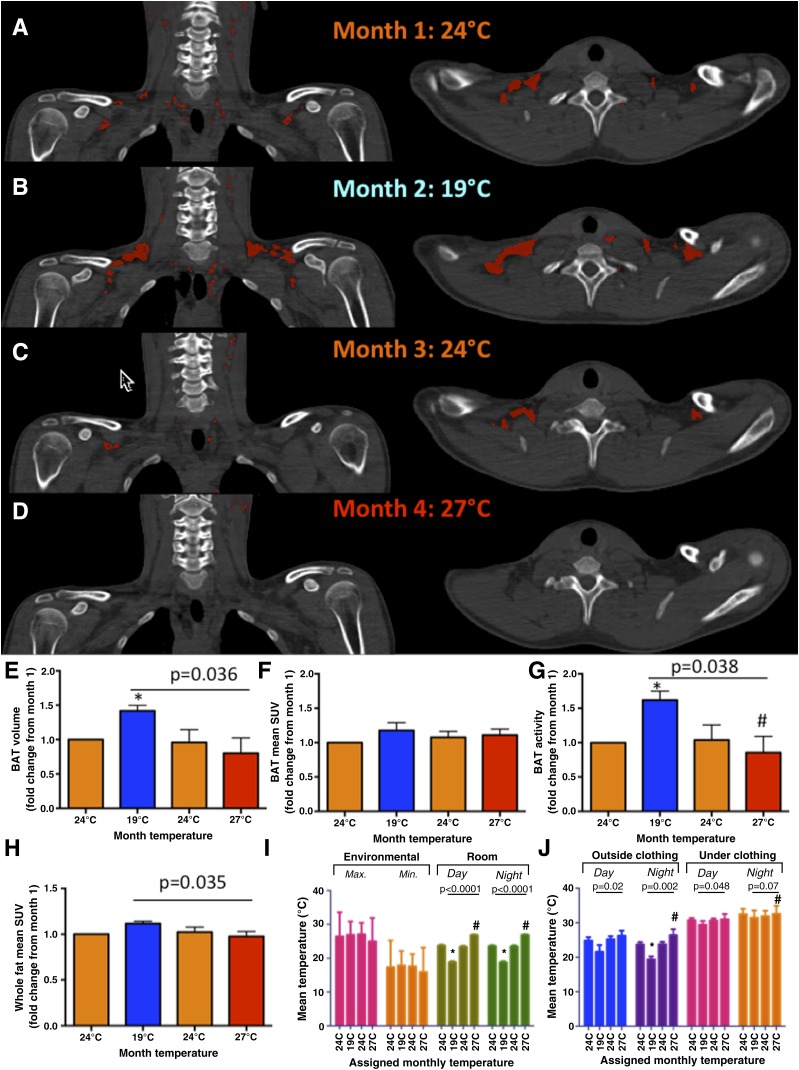
Figure 1A–D demonstrates BAT evolution in one representative subject throughout the 4-month sequential acclimation. Supplementary Figs. 4–7 show individual results. Figure 1E–H displays mean changes in BAT volume and overall fat metabolic activity, which increased upon cold acclimation (19°C) by 42 ± 18% (P < 0.05) and 10 ± 11% (P < 0.05), respectively; decreased after the thermoneutral month (24°C) to nearly baseline level; and completely muted at the end of the 1-month warm exposure period (27°C). BAT radiodensity, measured in HU, responded to acclimation with the same pattern (P < 0.01) (Table 1). BAT HUs increased by 25 ± 8% after cold acclimation, reversed after the thermoneutral month, and by the end of warm acclimation in month 4, HU was 18 ± 11% lower than baseline values in month 1 (Table 1). In contrast, mean SUV of skeletal muscle and liver remained unchanged during acclimation (Table 1). Room (P < 0.05) and individually exposed temperatures (P < 0.01), but not outdoor temperatures, correlated with BAT changes during the study period (Fig. 1I and J and Supplementary Table 1).
Figure 1.
Temperature-dependent BAT acclimation. A–D: Representative PET/CT fused images of the cervical-supraclavicular region (left panels: coronal view; right panels: transverse view) of one subject during monthly temperature acclimation. BAT (HU: −300 to −10 and SUV ≥2) is shown in red. Baseline BAT volume and mean SUV and activity were 26 mL and 2.65 and 0.238 MBq, respectively (A). All BAT parameters increased after 1 month of mild cold acclimation (19°C) (B), decreased to nearly baseline level after the thermoneutral month (24°C) (C), and BAT was nearly completely muted at the end of the 1-month mild warm exposure in the final month (27°C) (D). Mean fold changes (N = 5) of BAT volume (E) and mean SUV (F) and BAT activity (G), relative to month 1 (24°C), were significant across 4-month acclimation. Whole fat activity, as defined by 18F-fluodeoxyglucose uptake within tissue of fat density (HU: −300 to −10), followed the same pattern (H) and interacted significantly with temperature acclimation. Room (I) and individual exposed temperatures (J), but not environmental seasonal fluctuations (I), tracked BAT and whole fat metabolic changes in the predicted temperature-dependent manner. Correlative analysis between BAT parameters and temperature exposure is shown in Supplementary Table 1. Individual PET/CT images and temperature profiles are shown in Supplementary Figs. 4–7. *P < 0.05 compared with month 1 (24°C); #P < 0.05 compared with month 2 (19°C).
Cold- and Diet-Induced Thermogenesis
We next explored metabolic consequences of BAT acclimation. CIT response did not change significantly during temperature acclimation (Table 2). In contrast, DIT measured at 19°C rose by 32 ± 35% (P = 0.03) after cold acclimation. Progressive rewarming suppressed 19°C DIT response at months 3 and 4 to nearly baseline level. DIT measured at 24°C was unaltered (Table 2).
Table 2.
Physiologic parameters across 4 months of acclimation
| Month 1: 24°C |
Month 2: 19°C |
Month 3: 24°C |
Month 4: 27°C |
Ptrend |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | 24°C | 19°C | ||
| Total EE (kcal) | 2,472 ± 180 | 2,624 ± 198a | 2,366 ± 358 | 2,543 ± 410a | 2,400 ± 252 | 2,555 ± 346a | 2,341 ± 255 | 2,505 ± 322a | 0.45 | 0.46 | |
| Respiratory quotient | 0.84 ± 0.03 | 0.84 ± 0.01 | 0.84 ± 0.02 | 0.83 ± 0.02 | 0.85 ± 0.02 | 0.84 ± 0.02 | 0.84 ± 0.03 | 0.85 ± 0.02 | 0.72 | 0.47 | |
| Total activity (units) | 8.4 ± 1.6 | 8.4 ± 2.8 | 7.6 ± 2.9 | 7.1 ± 2.4 | 6.8 ± 3.7 | 7.1 ± 4.1 | 7.9 ± 5.0 | 7.2 ± 4.7 | 0.54 | 0.38 | |
| Surface electromyography (×10−6 RMS) | 2.8 ± 0.4 | 2.5 ± 1.3 | 2.7 ± 0.3 | 2.6 ± 0.2 | 2.8 ± 0.5 | 2.7 ± 0.2 | 2.8 ± 0.3 | 2.6 ± 0.4 | 0.98 | 0.83 | |
| CIT (%) | 6.2 ± 4.1 | 7.4 ± 3.1 | 6.2 ± 3.9 | 6.8 ± 3.2 | 0.16 | ||||||
| DIT (%) | 10.3 ± 13.1 | 33.4 ± 18.2 | 19.0 ± 15.4 | 42.2 ± 17.4a,b | 19.1 ± 16.0 | 37.1 ± 19.6a | 22.5 ± 11.0 | 34.4 ± 19.2a | 0.30 | 0.36 | |
Data are means ± SD. At the end of each testing month, subjects underwent acute thermometabolic evaluation at either 24°C or 19°C. RMS, root mean square.
aP < 0.05 compared with 24°C during acute thermometabolic evaluation each month;
bP < 0.05 (month 1 vs. 2) compared with matching measurement at same temperature performed at respective months as indicated.
Shivering Response and Cold Sensitivity
Surface EMG recordings of muscle fasciculation/shivering measured at 19°C and 24°C were not different (Table 2), indicating absence of significant shivering and validating our model in capturing nonshivering thermogenesis. Monthly acclimation did not alter EMG recordings, and subjects did not report changes in cold perception at 19°C during monthly calorimeter testing (Supplementary Fig. 9).
Diet and Body Composition
Neither total caloric nor macronutrient content of intake changed during acclimation (Table 3). Biweekly hunger and satiety scores did not change significantly (Table 3); however, volunteers reported an increase in desire to eat and reduction in satiety during ad libitum meal test after cold acclimation, which reversed during the warm months (Supplementary Fig. 10). Body composition was unaltered across the study period (Table 3).
Table 3.
Nutritional and body compositional parameters across 4 months of acclimation
| Month 1: 24°C | Month 2: 19°C | Month 3: 24°C | Month 4: 27°C | Ptrend | |
|---|---|---|---|---|---|
| Dietary intake | |||||
| Caloric (kcal/day) | 2,530 ± 321 | 2,620 ± 412 | 2,623 ± 342 | 2,514 ± 359 | 0.32 |
| Protein (g/day) | 126 ± 16 | 131 ± 19 | 131 ± 15 | 127 ± 18 | 0.35 |
| Fat (g/day) | 88 ± 9 | 91 ± 13 | 93 ± 11 | 86 ± 10 | 0.16 |
| Carbohydrate (g/day) | 319 ± 42 | 331 ± 51 | 329 ± 44 | 320 ± 48 | 0.44 |
| Appetite/hunger VAS | |||||
| Hunger AUC | 13.1 ± 6.3 | 20.7 ± 7.0 | 17.5 ± 6.1 | 15.5 ± 3.5 | 0.13 |
| Satiety AUC | 35.8 ± 4.6 | 25.7 ± 8.5 | 25.5 ± 5.4 | 27.9 ± 7.9 | 0.09 |
| Desire to eat AUC | 14.6 ± 4.3 | 20.1 ± 3.4a,b | 17.9 ± 4.2 | 16.5 ± 5.3 | 0.003 |
| Body composition | |||||
| Body weight (kg) | 74.4 ± 7.3 | 74.8 ± 7.5 | 74.9 ± 7.4 | 74.7 ± 7.7 | 0.72 |
| Lean mass (kg) | 55.8 ± 6.0 | 56.3 ± 6.1 | 56.6 ± 6.3 | 56.1 ± 6.4 | 0.56 |
| Fat mass (kg) | 14.6 ± 0.5 | 14.5 ± 0.8 | 14.6 ± 1.4 | 14.7 ± 1.7 | 0.95 |
| % body fat | 20.92 ± 2.00 | 20.62 ± 1.64 | 20.64 ± 2.22 | 20.88 ± 2.51 | 0.99 |
Data are means ± SD. At the end of each testing month, subjects underwent acute thermometabolic evaluation at either 24°C or 19°C.
aP < 0.05 (month 1 vs. 2),
bP < 0.05 (months 2 vs. 4) compared with matching measurement at same temperature performed at respective months as indicated.
Pituitary-Thyroid-Adrenal Axis
To elucidate potential endocrine mediators of BAT acclimation, we profiled pituitary-thyroid-adrenal axes (Table 4). Cold acclimation increased free triiodothyronine (T3) AUC measured at 24°C but not at 19°C. Free T3 to free thyroxine (T4) ratio (an indicator of peripheral T4 to T3 conversion [34]) was greater by 11 ± 5% (P = 0.01), measured at 24°C. No significant changes were observed in thyrotropin (thyroid-stimulating hormone [TSH]) or the pituitary-adrenal axis.
Insulin Sensitivity
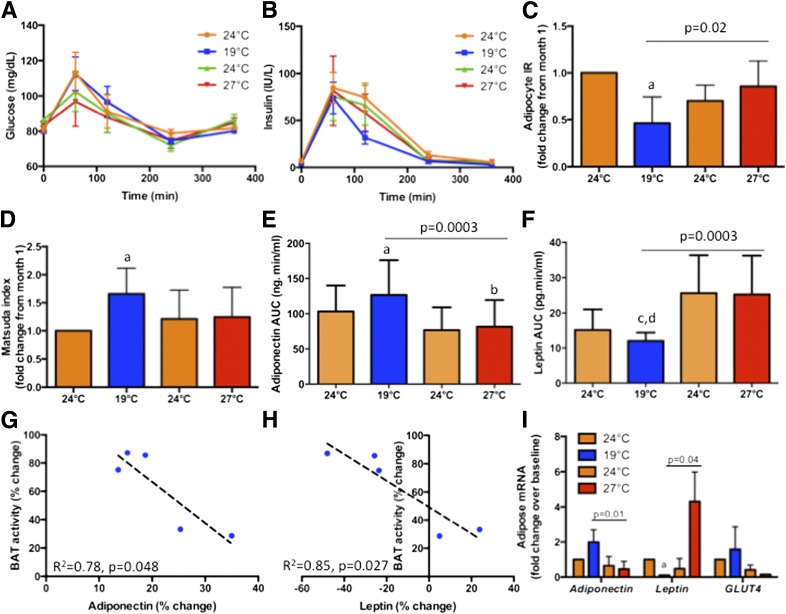
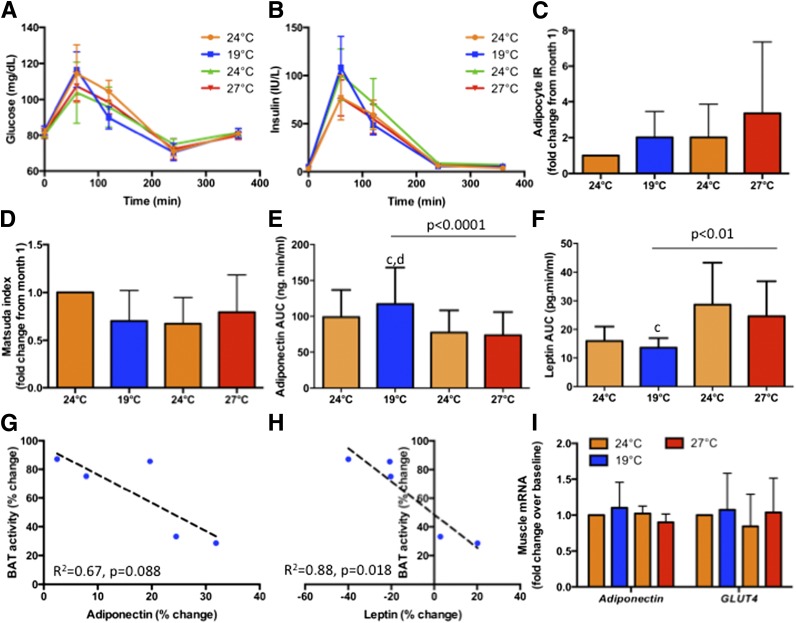
Total glucose and insulin AUCs did not change during acclimation (Table 4). In contrast, postprandial insulin excursion measured at 19°C reached a nadir after cold acclimation, without significant changes to glucose excursion (Fig. 2A and B). Indices of insulin sensitivity and resistance showed significant reciprocal changes during cold and warm acclimation, consistent with an improvement of postprandial whole-body insulin sensitivity after cold acclimation (Fig. 2C and D). These changes were absent during measurements at 24°C (Fig. 3A–D).
Figure 2.
Metabolic consequences of BAT acclimation at 19°C. A and B: Comparison of postprandial glucose and insulin excursions after a mixed meal at 1300 h before and after cold acclimation, respectively, measured at 19°C. Glucose excursions were unchanged but insulin levels decreased, with a significant reduction in AUC, after mild cold acclimation (month 2). Accordingly, adipocyte insulin resistance (IR) was the lowest (C) and Matsuda index (an indicator of insulin sensitivity) was the highest (D) after cold acclimation (month 2). These changes in glucose metabolism were accompanied by an increase in circulating adiponectin (E) and a decrease in circulating leptin (F). Cold acclimation–induced changes (months 1 and 2) in circulating adiponectin (G) and leptin levels (H) correlated negatively with changes in BAT activity. Adiponectin and leptin mRNA displayed concordant changes in subcutaneous adipose tissue biopsies with circulating levels, and changes in GLUT4 tracked those of adiponectin (I). aP < 0.05 compared with month 1 (24°C), bP < 0.05 compared with month 2 (19°C), cP < 0.05 compared with month 3 (24°C), and dP < 0.05 compared with month 4 (27°C).
Figure 3.
Metabolic consequences of BAT acclimatization at 24°C. A and B: Comparison of postprandial glucose and insulin excursions after a mixed meal at 1300 h before and after cold acclimatization, respectively, measured at 24°C. Unlike measurements at 19°C (Fig. 2A and B), no significant changes were observed in glucose or insulin excursions. Accordingly, adipocyte insulin resistance (IR) (C) and Matsuda index (an indicator of insulin sensitivity) (D) were unchanged. Circulating adiponectin increased (E), while leptin decreased (F), identical to measurements observed at 19°C (Fig. 2E and F). Cold acclimatization–induced changes (months 1 and 2) in circulating adiponectin (G) and leptin levels (H) correlated negatively with changes in BAT activity. In contrast to that observed in adipose tissue (Fig. 2I), adiponectin and GLUT4 mRNA did not change significantly in muscle (I). cP < 0.05 compared with month 3 (24°C); dP < 0.05 compared with month 4 (27°C).
Adipokine Changes
Given our recent demonstration of BAT as an endocrine organ in humans (19,32,35), we probed adipokine changes during acclimation. Adiponectin AUC was augmented by 22 ± 9% (P < 0.001) after cold acclimation (Fig. 2E). Not only was enhancement of adiponectin levels observed at 19°C during acute thermometabolic evaluation, but similar increase also occurred at 24°C (P < 0.001) (Fig. 3E). In contrast, cold acclimation reduced leptin AUC by 14 ± 28% (P < 0.001), evident at both 19°C (Fig. 2F) and 24°C (Fig. 3F). These dichotomized changes returned almost to baseline during the thermoneutral third month, trending to the opposite directions at the end of the fourth month at 27°C (P < 0.05). Changes in circulating adiponectin and leptin correlated negatively with changes in BAT activity after cold acclimation (Fig. 2G and H and Fig. 3G and H). FGF21 AUC rose after cold acclimation, although overall trend did not reach significance (Table 4).
Fat and Muscle Gene Expression
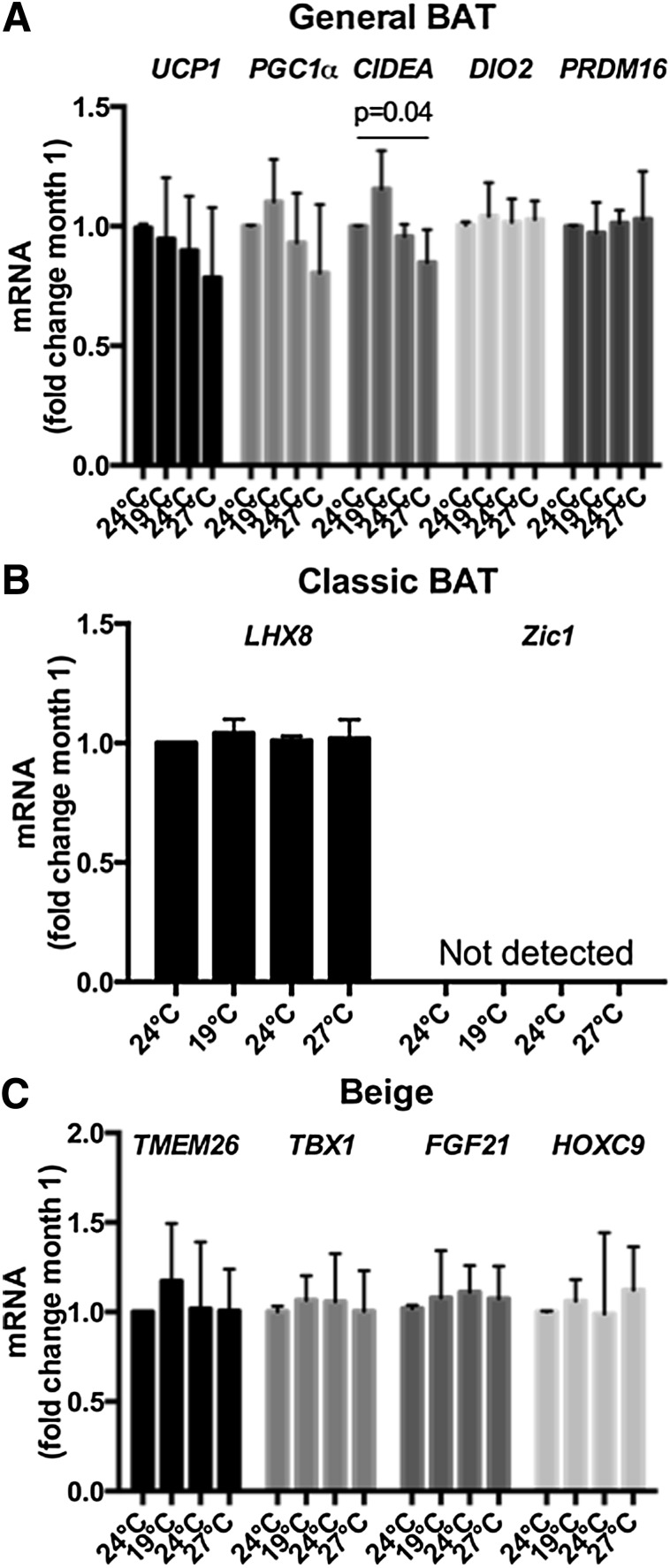
For exploration of sources of adipokine and origins of metabolic changes, fat and muscle biopsies were obtained from four volunteers at the end of each month. Adiponectin and GLUT4 expression in adipose tissue (Fig. 2I), but not muscle (Fig. 3I), rose after cold acclimation, while expression of leptin fell, and their respective trends reversed after thermoneutral and warm acclimation months (P < 0.05). Expression of CIDEA, a BAT gene governing lipid mobilization (36), increased after cold acclimation but decreased during rewarming (Fig. 4). No other BAT/beige fat gene changes were observed.
Figure 4.
BAT and beige fat gene changes in adipose tissue biopsies across 4-month acclimatization. A: Changes in general BAT gene expression (general BAT genes are defined as genes ascribed to general BAT function and do not indicate their developmental origin). Expression of CIDEA, but not others, changed significantly (P = 0.04) during acclimatization across 4-month period. B: Changes in classic BAT gene expression. Classic BAT genes are defined as those expressed in interscapular BAT in animals or human infants (50). C: Changes in beige fat gene expression. Beige fat genes are defined as those expressed in inducible brown adipocytes, also known as brite or beige adipocytes, found within WAT depots. No significant changes were observed in classic BAT or beige fat genes across temperature acclimation.
Discussion
The major finding of our study is the demonstration of BAT acclimation and its metabolic consequences by minimal manipulation of overnight temperature exposure while allowing usual daily activities. Human BAT is inducible and suppressible by controlled mild cold and warm exposure, respectively, independent of seasonal fluctuations. BAT acclimation is accompanied by boosting of DIT and postprandial insulin sensitivity. Mechanistically, this is associated with reciprocal changes of circulating adiponectin and leptin, mirrored by corresponding transcriptosomal changes in adipose tissue ex vivo. These results provide the first evidence linking ambient temperature, BAT acclimation, and whole-body energy/substrate metabolism in humans.
Consistent with previous reports (25,26), we confirmed BAT recruitability by cold exposure but did not observe significant CIT response augmentation; the latter could be a type 2 error. Despite tentalizing associative data linking BAT abundance with favorable energy metabolism in humans, it remains unclear, to date, whether BAT recruitment is accompanied by metabolic benefits. We specifically sought to determine the significance of BAT recruitment and revealed an association of BAT acclimation with enhancement of postprandial energy metabolism and insulin sensitization. Within the allowance and feasibility of human research, we explored underlying mechanisms through blood and tissue analyses.
First, within the pituitary-thyroid-adrenal axis, we observed an increase in T3-to-T4 ratio, which indicates enhanced T3 synthesis. Given the enrichment of BAT with type 2 deiodinase (37) and our previous report showing severe insulin resistance amelioration by thyroid hormone-mediated BAT activation (38), we hypothesize heightened T3 synthesis within BAT to be one plausible mechanism underlying acclimated-BAT associated metabolic changes. Such a pattern of increased thyroid hormone turnover in the absence of TSH changes is reminiscent of cold adaptation observed among Arctic residents (39).
Second, our adipokine profiling uncovered an intriguing relation between BAT, adiponectin, and leptin. Cold acclimation augmented circulating adiponectin but decreased leptin. It is tempting to speculate that cold-induced adiponectin, a potent insulin sensitizer, contributes to glucose metabolism improvement and leptin reduction, with the latter as a result of improved tissue sensitivity. Concordant gene changes in adipose adiponectin and leptin, absent in muscle, argue adipose to be the primary effector. Surprisingly, circulating adiponectin related negatively with BAT activity, suggesting that PET-detectable BAT was not the source of cold-induced adiponectin. As BAT exhibits insulin-independent glucose uptake capacity (40), lesser BAT expansion could have triggered alternative glucose utilizing pathways in WAT during cold acclimation, evident by observed WAT GLUT4 upregulation. Interestingly, such changes in circulating adiponectin and leptin were not limited to the cold-exposed condition (Fig. 2) but persisted at thermoneutrality (Fig. 3), indicating that the temperature-acclimated hormonal milieu was not totally dependent on BAT activation. The corollary is that acclimated BAT could be serving beneficial metabolic functions not related to temperature regulation per se.
Third, newly identified cytokines, such as FGF21, may mediate temperature-acclimated tissue cross-talk. Recent identification of a FGF21-adiponectin feed-forward axis (41) led us to wonder whether FGF21 augmentation after cold acclimation could have brought forth the adiponectin rise. When BAT was muted at the end of warm acclimation and adiponectin dwindled, FGF21 did not fall, suggesting that non-BAT FGF-secreting tissues might have compensated in states of relative BAT deficiency.
Fourth, although we did not observe an increase in beige fat gene expression, possibly due to the small sample size, we speculate fat browning to be a possibility. This is corroborated by finding an increased expression of the BAT gene CIDEA in adipose tissue after cold acclimation. Although ethics considerations prohibited serial neck fat biopsies in our volunteers, changes in radiodensity within BAT by PET/CT have offered insight on tissue changes. Adipose tissue is typically characterized by HU between −10 and −300, in contrast to muscle tissue, whose HU is within the positive range. Compared with WAT, BAT has relatively less lipid, as it is filled with abundant mitochondria and blood vessels. This is exemplified by water-fat separated magnetic resonance imaging revealing a lower fat fraction in activated BAT both in humans (42) and in rodents (43). We speculate that the rise and fall in BAT radiodensity with cold and warm acclimation, respectively, could be reflections of WAT → BAT transformation (or fat browning). This is also supported by previous studies demonstrating cell-autonomous (44) and endocrine-mediated (19) cold-induced WAT browning in humans. Further studies are required to ascertain whether WAT browning contributes to cold-acclimated BAT-induced metabolic changes.
Collectively, our results infer a complex concerted BAT-WAT response to cold acclimation, which could involve interplay between CIDEA-mediated lipid mobilization (45,46), GLUT4-enhanced glucose utilization, and FGF21/adiponectin-induced insulin sensitization. Most importantly, all these changes occurred in the absence of measureable EE, caloric intake, or body compositional alterations, suggesting such responses to be primary cold-induced metabolic sequelae rather than compensatory physiologic adaptations. Nonetheless, because the desire to eat heightened after cold acclimation, we cannot exclude the possibility that appetite stimulation could diminish metabolic benefits of BAT recruitment if it increases caloric intake in longer-term studies.
The inducibility, suppressibility, and plasticity of human BAT entail implications beyond thermoregulatory physiology. The translation of recently discovered BAT activators in the laboratory to pharmacologic BAT stimulants available for clinical use is not a trivial process (23). Our study substantiates, in contrast, a simple BAT-modulating strategy: a mild reduction in environmental temperature is capable of recruiting BAT and yielding associated metabolic benefits; conversely, even a small elevation in ambient temperature could impair BAT and dampen previously attained metabolic benefits. Such reversible metabolic switching, occurring within a temperature range achievable in climate-controlled buildings, therefore carries therapeutic implications of BAT acclimation both on an individual and a public health level. Bedroom temperature has gradually increased from 19°C to 21.5°C over the last three decades in the U.S. (47). The blunting of BAT function due to widespread use of indoor climate control could be a neglected contribution to the obesity epidemic. Moderate downward adjustment of indoor temperature could represent a simple and plausible strategy in dampening the escalation of obesity on a population level. Our volunteers reported satisfactory sleep during acclimation, although more formal assessment of sleep quality is required in future studies.
Our findings should be viewed as a proof of concept illustrating human BAT plasticity. We acknowledge the small sample size to be a limitation of our study. Unfortunately, the conduct of long-term acclimation study necessitated substantial resources and regrettably prohibited a large sample size. Despite a small study population, the investigations were undertaken in a tightly monitored and controlled yet real-life simulating and applicable setting encompassing the most comprehensive spectrum of energy balance/metabolism to date to tackle a question fundamental to human BAT research: What is the significance of BAT recruitment? The unveiled positive relation between acclimated BAT and glucose homeostasis is clinically relevant. Glucose intolerance is an independent risk factor of cardiovascular mortality, and postprandial hyperglycemia is its earliest manifestation (48). We emphasize that a causal linkage could not be definitely ascertained between BAT recruitment and postprandial insulin sensitivity improvement; however, our study provides compelling circumstantial evidence supporting a potential therapeutic role of BAT in impaired glucose metabolism and calls for the investigation of similar temperature acclimation in individuals with impaired glycemia. Our observation of BAT recruitment accompanied by insulin sensitization in the absence of significant weight loss echoes animal findings showing glucose homeostasis improvement after fat browning to be greater than expected from adiposity reduction alone (49,50). Whether it was indeed a result of fat phenotypic and/or adipokine changes merits further studies.
In summary, temperature acclimation modulates BAT abundance and activity, subsequently impacting energy and substrate metabolism in humans. BAT exhibits thermal plasticity intimately related to glucose homeostasis. Harnessing BAT by simple adjustment of ambient temperature could be a new strategy in the combat against obesity, diabetes, and related disorders.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Peter Herscovitch and Dr. Corina Millo, both from the PET Department, Clinical Center, NIH, for advice on PET/CT scanning; Rachel Perron, Christopher Idelson, Sarah Smyth, Jacob Hattenbach, and Juan Wang, all from Diabetes, Endocrinology, and Obesity Branch, NIDDK, NIH, for technical assistance; Dilalat Bello and Oretha Potts, from the Clinical Center, NIH, for dietary counseling/monitoring; and all nurses in the Clinical Metabolic Unit, NIH, for their nursing care.
Funding. This study was supported by the Intramural Research Program Z01-DK047057-07 of NIDDK and the NIH Clinical Center. P.L. was supported by an Australian National Health and Medical Research Council Early Career Fellowship, the Diabetes Australia Fellowship and Bushell Travelling Fellowship, and the School of Medicine, University of Queensland.
The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of data; or preparation, review, or approval of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.L. participated in the development of the study concept and design, research, acquisition of data, and analysis and discussion of results; wrote the manuscript; participated in critical revision; and approved the final version of the manuscript. S.S., J.L., A.B.C., R.J.B., K.Y.C., and F.S.C. participated in the development of the study concept and design, research, acquisition of data and analysis and discussion of results; participated in critical revision; and approved the final version of the manuscript. W.D. and C.D.W. researched and analyzed data, contributed to discussion of results, participated in critical revision, and approved the final version of the manuscript. P.L. and F.S.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at ICE/ENDO 2014, Chicago, IL, 22 June 2014.
Footnotes
Clinical trial reg. no. NCT01730105, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0513/-/DC1.
References
- 1.Keith SW, Redden DT, Katzmarzyk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594 [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB, Bachman ES. Beta-adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 2003;278:29385–29388 [DOI] [PubMed] [Google Scholar]
- 3.Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J 2013;37:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 5.Petrovic N, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARgamma agonist. Am J Physiol Endocrinol Metab 2008;295:E287–E296 [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013;27:234–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972;112:35–39 [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillaud F, Combes-George M, Ricquier D. Mitochondria of adult human brown adipose tissue contain a 32 000-Mr uncoupling protein. Biosci Rep 1983;3:775–780 [DOI] [PubMed] [Google Scholar]
- 12.Tanuma Y, Tamamoto M, Ito T, Yokochi C. The occurrence of brown adipose tissue in perirenal fat in Japanese. Arch Histol Jpn 1975;38:43–70 [DOI] [PubMed] [Google Scholar]
- 13.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee P, Zhao JT, Swarbrick MM, et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011;96:2450–2455 [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 17.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 2011;152:3597–3602 [DOI] [PubMed] [Google Scholar]
- 19.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 2014;38:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012;122:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev 2013;34:413–438 [DOI] [PubMed] [Google Scholar]
- 24.Chen KY, Brychta RJ, Linderman JD, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab 2013;98:E1218–E1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito M. Human brown adipose tissue: regulation and anti-obesity potential [Review]. Endocr J 2014;61:409–416 [DOI] [PubMed] [Google Scholar]
- 28.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010;299:E601–E606 [DOI] [PubMed] [Google Scholar]
- 29.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2014;38:812–817 [DOI] [PubMed] [Google Scholar]
- 30.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–209 [DOI] [PubMed] [Google Scholar]
- 31.Celi FS, Brychta RJ, Linderman JD, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol 2010;163:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014;19:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 2014;63:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 2005;115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab 2013;98:E98–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barneda D, Frontini A, Cinti S, Christian M. Dynamic changes in lipid droplet-associated proteins in the “browning” of white adipose tissues. Biochim Biophys Acta 2013;1831:924–933 [DOI] [PubMed] [Google Scholar]
- 37.Celi FS. Brown adipose tissue--when it pays to be inefficient. N Engl J Med 2009;360:1553–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skarulis MC, Celi FS, Mueller E, et al. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab 2010;95:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen S, Kleinschmidt K, Hvingel B, Laurberg P. Thyroid hyperactivity with high thyroglobulin in serum despite sufficient iodine intake in chronic cold adaptation in an Arctic Inuit hunter population. Eur J Endocrinol 2012;166:433–440 [DOI] [PubMed] [Google Scholar]
- 40.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 41.Holland WL, Adams AC, Brozinick JT, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013;17:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu HH, Wu TW, Yin L, et al. MRI detection of brown adipose tissue with low fat content in newborns with hypothermia. Magn Reson Imaging 2014;32:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu HH, Smith DL, Jr, Nayak KS, Goran MI, Nagy TR. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging 2010;31:1195–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L, Wu J, Cohen P, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A 2013;110:12480–12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordström EA, Rydén M, Backlund EC, et al. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes 2005;54:1726–1734 [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Zhou L, Chen C, et al. Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci 2014;57:107–116 [DOI] [PubMed] [Google Scholar]
- 47.Johnson F, Mavrogianni A, Ucci M, Vidal-Puig A, Wardle J. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes Rev 2011;12:543–551 [DOI] [PubMed] [Google Scholar]
- 48.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617–621 [PubMed] [Google Scholar]
- 49.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.