The effect of prior influenza vaccination history on vaccine effectiveness was assessed in a community cohort over 8 seasons. Current- and previous-season vaccination generated similar levels of protection; vaccine-induced protection was greatest for individuals with no recent vaccination history.
Keywords: influenza, vaccine effectiveness
Abstract
Background. Recent studies suggest that influenza vaccination in the previous season may influence the effectiveness of current-season vaccination, but this has not been assessed in a single population over multiple years.
Methods. Patients presenting with acute respiratory illness were prospectively enrolled during the 2004–2005 through 2012–2013 influenza seasons. Respiratory swabs were tested for influenza and vaccination dates obtained from a validated registry. Vaccination status was determined for the current, previous, and prior 5 seasons. Vaccine effectiveness (VE) was calculated for participants aged ≥9 years using logistic regression models with an interaction term for vaccination history.
Results. There were 7315 enrollments during 8 seasons; 1056 (14%) and 650 (9%) were positive for influenza A(H3N2) and B, respectively. Vaccination during current only, previous only, or both seasons yielded similar protection against H3N2 (adjusted VE range, 31%–36%) and B (52%–66%). In the analysis using 5 years of historical vaccination data, current season VE against H3N2 was significantly higher among vaccinated individuals with no prior vaccination history (65%; 95% confidence interval [CI], 36%–80%) compared with vaccinated individuals with a frequent vaccination history (24%; 95% CI, 3%–41%; P = .01). VE against B was 75% (95% CI, 50%–87%) and 48% (95% CI, 29%–62%), respectively (P = .05). Similar findings were observed when analysis was restricted to adults 18–49 years.
Conclusions. Current- and previous-season vaccination generated similar levels of protection, and vaccine-induced protection was greatest for individuals not vaccinated during the prior 5 years. Additional studies are needed to understand the long-term effects of annual vaccination.
The Advisory Committee on Immunization Practices (ACIP) has recommended annual influenza vaccinations for all persons aged ≥6 months since 2010 [1]. Although annual vaccination has been recommended for adults aged ≥65 years and certain high-risk groups for decades, the impact of prior vaccination history on current-season vaccine effectiveness (VE) is unclear. Despite previous debates on the relevance of repeated vaccination [2, 3], few influenza VE studies have considered the effect of vaccinations received in prior seasons. In the 1980s, a multiseason randomized, placebo-controlled trial among healthy adults found no consistent differences in efficacy of primary vs repeated vaccination against serologically confirmed influenza [4, 5]. Recent observational studies have suggested that VE may be influenced by prior season vaccination [6–12]. However, these studies were limited to 1 or 2 seasons, historical vaccination data were limited, confounders related to repeated vaccination behavior may have gone unmeasured, and findings were inconsistent.
Given the universal recommendation for annual influenza revaccination in the United States, a better understanding of the relationship between prior vaccination history and current season VE is needed. Annual VE studies have been conducted in a Wisconsin community cohort since 2004–2005, and 5 years of vaccination history is available for most study participants. This provided an opportunity to explore the effects of prior vaccination during multiple seasons and to evaluate the relationship between propensity to be vaccinated and current-season vaccine effectiveness.
METHODS
Study Population and Enrollment
From 2004–2005 through 2012–2013, observational studies of influenza VE were conducted in a defined community cohort using consistent enrollment and laboratory methods [7, 13–17]. Community-dwelling residents of a 14-zip-code area around Marshfield, Wisconsin, with ≥12 months of continuous residency were eligible for enrollment each season. From 2004–2005 through 2006–2007, the cohort was restricted to individuals for whom vaccination was recommended based on age or the presence of a high-risk medical condition as recommended by ACIP [18–20]. In 2007–2008 and all subsequent seasons, the cohort was expanded to include all individuals aged ≥6 months living in the community.
During each influenza season, eligible individuals were actively recruited during a clinical encounter for acute respiratory illness (ARI). Participants completed an interview to assess illness onset and symptoms. Sample collection methods varied by season, but either a nasal, nasopharyngeal, or a combined shallow nasal swab and oropharyngeal swab was obtained from all participants for influenza testing.
Swab samples were tested for influenza (type and subtype) using real-time reverse transcription polymerase chain reaction and viral cultures at the Marshfield Clinic Research Foundation as previously described [13]. Culture alone was performed on samples collected in 2004–2005.
We restricted this analysis to enrolled persons aged ≥9 years at the beginning of each season (1 August). We excluded children aged 6 months through 8 years because the criteria to be considered fully vaccinated varied during the study period and were based on prior vaccination history. Most individuals were enrolled no more than once per season. For individuals with multiple enrollments in 1 season for distinct illness episodes, the analysis included either the first enrollment (if all were negative) or the first enrollment associated with a positive influenza test; 94.3% of all enrollment visits were included based on these criteria.
Study procedures were approved by the Marshfield Clinic Institutional Review Board. Informed consent was obtained from all adults and parents/guardians of children.
Vaccination History
Influenza vaccination history was obtained from a real-time, Internet-based immunization registry used by all vaccination providers serving the population (available at: http://www.recin.org). Validation studies during 2006–2007 and 2007–2008 [21], and 2011–2012 (unpublished) seasons demonstrated that the registry captured 95% of all influenza vaccinations received by study participants.
Vaccination status was determined for the current and previous seasons. Persons were considered vaccinated during the current season if they had received seasonal vaccine ≥14 days prior to illness onset. Previous season vaccination status was determined based on vaccination received 1 season prior (August–July). Five-year vaccination history was determined for individuals with ≥5 years of continuous residency in the community prior to study enrollment. Five-year vaccination history (excluding the current season) was classified into 3 categories: frequent vaccinees (vaccinated in 4–5 of the past 5 seasons), infrequent vaccinees (vaccinated in 1–3 of the past 5 seasons), and nonvaccinees (no vaccinations in the past 5 seasons). Persons who received live attenuated influenza vaccine were excluded.
Estimation of Vaccine Effectiveness
Vaccine effectiveness was assessed using a test-negative case-control study design [22–25]. Cases were persons with medically attended ARI that was laboratory confirmed as influenza. Controls were persons with ARI who tested negative for influenza. Two models were considered. The first model included exposure variables for current-season vaccination and previous-season vaccination, and an interaction term for current- and previous-season vaccination [6]. Using this model, VE was estimated for all combinations of vaccine exposure in the current and previous season: (1) vaccinated in both the current and previous season, (2) vaccinated in the current season only, (3) vaccinated in the previous season only, and (4) not vaccinated in either current or previous season (reference group).
The second model included exposure variables for current-season vaccination and 5-year vaccination history, and an interaction term for current-season vaccination and 5-year vaccination history. Using this model, VE was estimated for all combinations of vaccine exposure in the current season and 5-year vaccination history: (1) current-season vaccination and frequent vaccinee, (2) current-season vaccination and infrequent vaccinee, (3) current-season vaccination and nonvaccinee, (4) no current-season vaccination and frequent vaccinee, (5) no current-season vaccination and infrequent vaccinee, and (6) no current-season vaccination and nonvaccinee (reference group).
Vaccine effectiveness was estimated as 100% × (1 – odds ratio) using logistic regression models for each level of interaction. Like other VE studies using the test-negative approach [13, 14, 16], we adjusted for age (using a cubic B-spline with 3 equally spaced knots), high-risk medical conditions, interval from illness onset to specimen collection (<3 days, 3–4 days, and 5–7 days), season, and sex. In addition, any influenza diagnosis documented in the medical record in the previous season and prior 5 seasons was included in the adjusted model for examining the previous-season and 5-year vaccination history, respectively. High-risk conditions were classified into the following groups: cardiovascular, diabetes, pulmonary, and other. The latter included cancer, kidney disease, liver disease, blood disorders, immunosuppressive disorders, metabolic disorders, and neurological/musculoskeletal disorders (list of International Classification of Diseases codes available on request).
We estimated VE separately for influenza A(H3N2) and B infections. Only seasons with H3N2 (2004–2005 through 2007–2008, and 2010–2011 through 2012–2013) or B infections (2004–2005 through 2008–2009 and 2010–2011 through 2012–2013) in the community were included. We separately estimated VE against H3N2 for each of the 2 seasons (2007–2008 and 2012–2013) that accounted for the majority of H3N2 cases over the study period. The H3N2 component of the vaccine in 2007–2008 was the same as the previous season (2006–2007). In contrast, the 2012–2013 vaccine included a new H3N2 strain compared with the previous year. There were too few cases of seasonal H1N1 infection during the study period to estimate VE for H1N1. The 2009–2010 season was excluded because monovalent vaccine was not available before the local pandemic wave, and seasonal viruses were absent locally. We conducted sensitivity analyses restricting to certain age groups (9–49 years, 18–49 years, and ≥50 years), as repeated vaccination is common in older adults and unmeasured confounding is a particular concern [26]. We also performed an analysis restricted to participants swabbed within 4 days of illness onset because of potential misclassification bias with delayed sample collection.
Analyses were performed using SAS software (version 9.3; SAS Institute, Cary, North Carolina).
RESULTS
The analysis included 7315 persons aged ≥9 years with medically attended ARI during 8 influenza seasons: 1056 (14%) had influenza A(H3N2), 650 (9%) had influenza B, and 3 were coinfected with H3N2 and B. The median annual number of cases was 79 (range, 0–469) for H3N2 and 13.5 (range, 5–238) for influenza B. The median age was 27 and 40 years, respectively, for influenza B and H3N2 cases. The age distribution of controls was similar to that of H3N2 cases. Cases were more likely to be male, were less likely to have a high-risk condition, had a shorter interval from illness onset to sample collection, and were less likely to have had a prior influenza diagnosis compared with controls (Table 1).
Table 1.
Characteristics of Enrolled Participants by Case (Influenza A[H3N2] and B Virus Infection) Status, Age ≥9 years
| Characteristic | A(H3N2)a |
Bb |
||||
|---|---|---|---|---|---|---|
| H3N2 Cases (n = 1056) | Influenza-Negative Controls (n = 3238) | P Valuec | B Cases (n = 650) | Influenza-Negative Controls (n = 4293) | P Valuec | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Influenza season | <.001 | <.001 | ||||
| 2004–2005 | 108 (10) | 314 (10) | 8 (1) | 314 (7) | ||
| 2005–2006 | 17 (2) | 181 (6) | 14 (2) | 181 (4) | ||
| 2006–2007 | 16 (2) | 280 (9) | 5 (1) | 280 (7) | ||
| 2007–2008 | 469 (44) | 619 (19) | 169 (26) | 619 (14) | ||
| 2008–2009 | 0 | 0 | 238 (37) | 1055 (25) | ||
| 2010–2011 | 58 (5) | 847 (26) | 13 (2) | 847 (20) | ||
| 2011–2012 | 100 (9) | 501 (15) | 12 (2) | 501 (12) | ||
| 2012–2013 | 288 (27) | 496 (15) | 191 (29) | 496 (12) | ||
| Age | .2 | <.001 | ||||
| 9–19 y | 210 (20) | 664 (21) | 286 (44) | 979 (23) | ||
| 20–49 y | 445 (42) | 1246 (38) | 232 (36) | 1717 (40) | ||
| 50–59 y | 175 (17) | 524 (16) | 61 (9) | 657 (15) | ||
| 60–69 y | 96 (9) | 374 (12) | 46 (7) | 429 (10) | ||
| 70–79 y | 84 (8) | 269 (8) | 21 (3) | 325 (8) | ||
| ≥80 y | 46 (4) | 161 (5) | 4 (1) | 186 (4) | ||
| Male sex | 463 (44) | 1217 (38) | <.001 | 315 (48) | 1605 (37) | <.001 |
| High-risk conditiond | ||||||
| Any | 410 (39) | 1522 (47) | <.001 | 148 (23) | 1831 (43) | <.001 |
| Cardiovascular | 153 (14) | 576 (18) | .01 | 40 (6) | 684 (16) | <.001 |
| Diabetes | 94 (9) | 385 (12) | .007 | 35 (5) | 455 (11) | <.001 |
| Pulmonary | 164 (16) | 715 (22) | <.001 | 67 (10) | 858 (20) | <.001 |
| Other high-risk | 176 (17) | 670 (21) | .004 | 61 (9) | 804 (19) | <.001 |
| Interval from onset to enrollment | <.001 | <.001 | ||||
| <3 d | 493 (47) | 1086 (34) | 233 (36) | 1491 (35) | ||
| 3–4 d | 390 (37) | 1126 (35) | 284 (44) | 1488 (35) | ||
| 5–7 d | 173 (16) | 1026 (32) | 133 (20) | 1314 (31) | ||
| Influenza diagnosise | ||||||
| Previous season | 8 (1) | 77 (2) | .001 | 6 (1) | 134 (3) | .002 |
| Prior 5 seasons | 50 (5) | 288 (9) | <.001 | 37 (6) | 395 (9) | .003 |
a Excludes 2008–2009 and 2009–2010 seasons because H3N2 viruses were not identified in the study population.
b Excludes 2009–2010 season because type B viruses were not identified in the study population.
c P values were calculated with χ2 test.
d Presence of ≥1 medical record–documented high-risk code in prior year, as defined by the Advisory Committee on Immunization Practices guidance for conditions that increase risk for complications from influenza.
e Presence of International Classification of Diseases, Ninth Revision code for influenza infection in medical record.
Influenza vaccination history varied with age, sex, and high-risk condition (Table 2). Unvaccinated persons were more likely to be younger and male. Older adults and persons with a high-risk condition were more likely to be vaccinated during both the current and previous seasons, and they were more likely to be frequent vaccinees during the prior 5 years (Supplementary Table 1).
Table 2.
Characteristics of Enrolled Participants by Vaccination History, Age ≥9 Years
| Characteristic | Current- and Previous-Season Vaccination Status |
Current-Season Vaccination Status and 5-Year Vaccination Historya |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated in Both Current and Previous Season | Vaccinated in Current Season Only | Vaccinated in Previous Season Only | Not Vaccinated in Either Current or Previous Season | Current-Season Vaccination and Frequent Vaccinee | Current-Season Vaccination and Infrequent Vaccinee | Current-Season Vaccination and Nonvaccinee | No Current-Season Vaccination and Frequent Vaccinee | No Current-Season Vaccination and Infrequent Vaccinee | No Current-Season Vaccination and Nonvaccinee | |
| Age | ||||||||||
| 9–19 y | 308 (17) | 178 (10) | 149 (8) | 1211 (66) | 151 (10) | 169 (11) | 76 (5) | 42 (3) | 312 (21) | 758 (50) |
| 20–49 y | 759 (26) | 326 (11) | 292 (10) | 1557 (53) | 347 (17) | 327 (16) | 95 (5) | 69 (3) | 344 (17) | 813 (41) |
| 50–59 y | 486 (45) | 100 (9) | 98 (9) | 392 (36) | 317 (33) | 180 (19) | 24 (2) | 51 (5) | 121 (13) | 271 (28) |
| 60–69 y | 392 (58) | 63 (9) | 61 (9) | 160 (24) | 285 (46) | 116 (19) | 14 (2) | 29 (5) | 67 (11) | 110 (18) |
| 70–79 y | 381 (76) | 31 (6) | 30 (6) | 57 (11) | 318 (69) | 56 (12) | 4 (1) | 21 (5) | 23 (5) | 36 (8) |
| ≥80 y | 217 (76) | 19 (7) | 17 (6) | 31 (11) | 190 (75) | 20 (8) | 2 (1) | 14 (6) | 9 (4) | 19 (7) |
| Sex | ||||||||||
| Female | 1666 (38) | 470 (11) | 434 (10) | 1855 (42) | 1055 (31) | 552 (16) | 128 (4) | 141 (4) | 523 (15) | 1039 (30) |
| Male | 877 (30) | 247 (9) | 213 (7) | 1553 (54) | 553 (23) | 316 (13) | 87 (4) | 85 (4) | 353 (15) | 968 (41) |
| High-risk conditionb | ||||||||||
| Any | 1519 (53) | 296 (10) | 268 (9) | 760 (27) | 1058 (44) | 427 (18) | 70 (3) | 128 (5) | 280 (12) | 427 (18) |
| Cardiovascular | 635 (61) | 93 (9) | 86 (8) | 233 (22) | 471 (52) | 146 (16) | 25 (3) | 50 (6) | 75 (8) | 139 (15) |
| Diabetes | 463 (67) | 64 (9) | 65 (9) | 102 (15) | 324 (54) | 118 (20) | 13 (2) | 38 (6) | 50 (8) | 57 (10) |
| Pulmonary | 676 (52) | 143 (11) | 118 (9) | 355 (27) | 444 (42) | 205 (19) | 32 (3) | 54 (5) | 134 (13) | 190 (18) |
| Other | 743 (59) | 126 (10) | 106 (8) | 277 (22) | 532 (50) | 187 (18) | 26 (2) | 54 (5) | 99 (9) | 166 (16) |
| None | 1024 (23) | 421 (9) | 379 (8) | 2648 (59) | 550 (16) | 441 (13) | 145 (4) | 98 (3) | 596 (17) | 1580 (46) |
| Influenza diagnosis in previous seasonc | ||||||||||
| Yes | 46 (26) | 25 (14) | 19 (11) | 85 (49) | 27 (23) | 16 (14) | 7 (6) | 5 (4) | 20 (17) | 40 (35) |
| No | 2497 (35) | 692 (10) | 628 (9) | 3323 (47) | 1581 (28) | 852 (15) | 208 (4) | 221 (4) | 856 (15) | 1967 (35) |
| Influenza diagnosis in prior 5 seasonsc | ||||||||||
| Yes | 207 (35) | 75 (13) | 57 (10) | 252 (43) | 125 (28) | 79 (18) | 23 (5) | 18 (4) | 71 (16) | 127 (29) |
| No | 2336 (35) | 642 (10) | 590 (9) | 3156 (47) | 1483 (28) | 789 (15) | 192 (4) | 208 (4) | 805 (15) | 1880 (35) |
| Received monovalent pandemic vaccined | ||||||||||
| Yes | 696 (63) | 120 (11) | 132 (12) | 155 (14) | 482 (53) | 182 (20) | 25 (3) | 59 (6) | 95 (10) | 72 (8) |
| No | 655 (24) | 254 (9) | 253 (9) | 1560 (57) | 388 (18) | 278 (13) | 86 (4) | 83 (4) | 402 (19) | 932 (43) |
Data are presented as No. (row %).
a Among persons with ≥5 years of continuous residency.
b Presence of ≥1 medical record–documented high-risk code in prior year, as defined by the Advisory Committee on Immunization Practices guidance for conditions that increase risk for complications from influenza.
c Presence of International Classification of Diseases, Ninth Revision code for influenza infection in medical record.
d Among participants enrolled during or after the 2009–2010 season.
Vaccine Effectiveness Against Influenza A(H3N2)
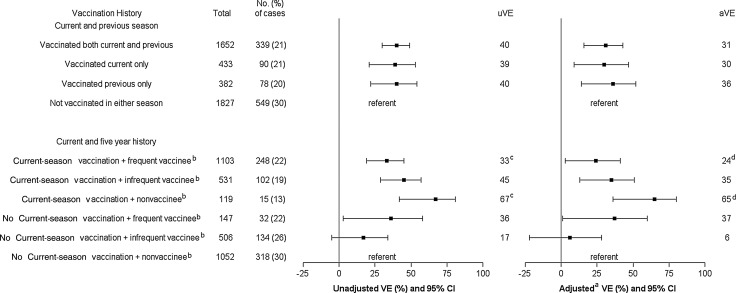
VE estimates against medically attended H3N2 infection was similar for vaccination in both current and previous season, current season only, and previous season only, but confidence intervals (CIs) were wide. The adjusted VE was 31% (95% CI, 16%–43%), 30% (95% CI, 8%–47%), and 36% (95% CI, 14%–52%), respectively, for each of these groups (Figure 1).
Figure 1.
Unadjusted and adjusted vaccine effectiveness (VE) of various vaccination histories against influenza A(H3N2) virus infection–associated medically attended acute respiratory illness, age ≥9 years. aAdjusted for age, sex, high-risk conditions, season, interval (days) from onset to sample collection, and influenza diagnosis code in prior seasons. bAmong persons with ≥5 years of continuous residency. cP = .01 for comparison between the 2 groups. dP = .01 for comparison between the 2 groups. Abbreviations: aVE, adjusted vaccine effectiveness; CI, confidence interval; uVE, unadjusted vaccine effectiveness.
The analysis including 5-year vaccination frequency was limited to 5800 individuals with at least 5 years of residency. Current-season VE against H3N2 was significantly higher (65%; 95% CI, 36%–80%) among nonvaccinees (ie, those vaccinated in the current season but not during the prior 5 years) compared with frequent vaccinees (24%; 95% CI, 3%–41%; P = .01). Current-season VE was 35% (95% CI, 13%–51%) among infrequent vaccinees.
The precision of VE estimates for the 2007–2008 and 2012–2013 seasons was lower, but we observed similar patterns compared with the 8-season analysis (Supplementary Figures 1 and 2). VE for vaccination in both seasons (current and previous) and in the current season only was higher during 2007–2008 compared with 2012–2013, but differences between seasons were not statistically significant. Data for 5-year vaccination history were limited in some groups, but current-season VE was lowest among frequent vaccinees, consistent with overall findings, although confidence intervals were wide and included zero for some groups.
Vaccine Effectiveness Against Influenza B
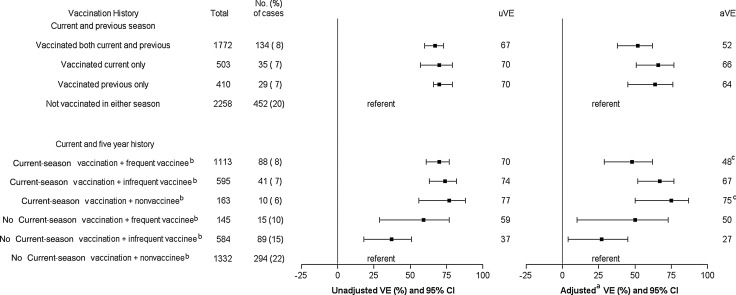
For protection against medically attended influenza B infections, VE was similar for vaccination in both current and previous season, current season only, and previous season only. The adjusted VE was 52% (95% CI, 38%–62%), 66% (95% CI, 51%–77%), and 64% (95% CI, 45%–76%), respectively, for each of these groups (Figure 2). In the analysis of 5-year vaccination history, adjusted VE against B was significantly higher (P = .05) among nonvaccinees with current-season vaccination (75%; 95% CI, 50%–87%) compared with frequent vaccinees with current-season vaccination (48%; 95% CI, 29%–62%).
Figure 2.
Unadjusted and adjusted vaccine effectiveness (VE) of various vaccination histories against influenza B virus infection–associated medically attended acute respiratory illness, age ≥9 years. aAdjusted for age, sex, high-risk conditions, season, interval (days) from onset to sample collection, and influenza diagnosis code in prior seasons. bAmong persons with ≥5 years of continuous residency. cP = .05 for comparison between the 2 groups. Abbreviations: aVE, adjusted vaccine effectiveness; CI, confidence interval; uVE, unadjusted vaccine effectiveness.
There was evidence of residual protection among frequent and infrequent vaccinees who did not receive current-season vaccine. The adjusted VE was 50% (95% CI, 10%–73%) and 27% (95% CI, 4%–45%) for these groups, respectively.
Sensitivity Analyses
VE estimates for H3N2 and B were similar when the analysis was restricted to individuals aged 9–49 years and 18–49 years, but sample size was smaller and VE differences between groups were not significant (Tables 3 and 4). VE estimates were also similar when restricting to participants who were swabbed within 4 days of illness onset. The absolute VE difference was ≤8%, with a few exceptions. However, CIs were wide (data not shown).
Table 3.
Unadjusted and Adjusted Vaccine Effectiveness Estimates Against Influenza A(H3N2) Virus Infection–Associated Medically Attended Acute Respiratory Illness Showing Effect of Individual Covariates
| Vaccination Status | Current and Previous Season |
Current-Season Vaccination Status and 5-Year Vaccination Historya |
||||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated Both | Vaccinated Current Season Only | Vaccinated Previous Season Only | Current-Season Vaccination + Frequent Vaccinee | Current-Season Vaccination + Infrequent Vaccinee | Current-Season Vaccination + Nonvaccinee | No Current-Season Vaccination + Frequent Vaccinee | No Current-Season Vaccination + Infrequent Vaccinee | |
| Age ≥9 y | ||||||||
| Unadjusted | 40 (30–49) | 39 (21–53) | 40 (22–54) | 33 (19–45) | 45 (29–57) | 67 (42–81) | 36 (3–58) | 17 (−5 to 34) |
| Adjusted for age | 42 (31–51) | 40 (23–53) | 41 (23–55) | 34 (18–47) | 46 (30–58) | 67 (42–81) | 37 (4–58) | 17 (−6 to 34) |
| Adjusted for sex | 39 (28–47) | 38 (20–52) | 39 (20–53) | 31 (17–43) | 44 (28–57) | 67 (42–81) | 35 (1–57) | 16 (−7 to 34) |
| Adjusted for season | 32 (19–42) | 32 (11–48) | 36 (16–52) | 25 (7–39) | 36 (16–51) | 65 (38–81) | 36 (1–59) | 10 (−16 to 30) |
| Adjusted for high-risk condition | 35 (24–45) | 36 (18–51) | 38 (19–53) | 27 (11–41) | 42 (25–55) | 66 (40–80) | 32 (−4 to 55) | 15 (−8 to 33) |
| Adjusted for interval from onset to sample collection | 36 (25–46) | 38 (20–52) | 39 (20–54) | 29 (13–41) | 44 (27–56) | 68 (44–82) | 33 (−2 to 56) | 15 (−8 to 33) |
| Adjusted for prior influenza historyb | 40 (30–49) | 38 (20–52) | 40 (22–54) | 33 (19–45) | 45 (29–57) | 66 (41–81) | 35 (2–57) | 17 (−6 to 34) |
| Fully adjustedc | 31 (16–43) | 30 (8–47) | 36 (14–52) | 24 (3–41) | 35 (13–51) | 65 (36–80) | 36 (1–60) | 6 (−22 to 28) |
| Age 9–49 y | ||||||||
| Unadjusted | 45 (31–56) | 49 (29–63) | 46 (25–62) | 37 (14–54) | 54 (34–67) | 74 (47–87) | 49 (4–73) | 10 (−18 to 32) |
| Fully adjustedc | 31 (11–47) | 43 (19–61) | 41 (15–59) | 17 (−19 to 42) | 48 (23–65) | 70 (36–86) | 41 (−19 to 71) | −3 (−40 to 24) |
| Age 18–49 y | ||||||||
| Unadjusted | 49 (33–61) | 37 (11–56) | 55 (33–70) | 42 (16–60) | 55 (32–70) | 66 (26–84) | 69 (25–87) | 16 (−20 to 41) |
| Fully adjustedc | 38 (15–54) | 37 (5–57) | 53 (26–70) | 26 (−15 to 52) | 53 (25–70) | 69 (28–87) | 68 (17–88) | 3 (−45 to 36) |
| Age ≥50 y | ||||||||
| Unadjusted | 30 (10–46) | 16 (−28 to 44) | 23 (−23 to 52) | 29 (4–48) | 34 (3–55) | 45 (−39 to 78) | 22 (−38 to 55) | 34 (−7 to 59) |
| Fully adjustedc | 28 (1–47) | 10 (−44 to 43) | 29 (−20 to 57) | 28 (−3 to 50) | 20 (−25 to 48) | 58 (−14 to 84) | 37 (−19 to 66) | 32 (−16 to 60) |
a Among persons with ≥5 years of continuous residency.
b Presence of International Classification of Diseases, Ninth Revision code for influenza infection in medical record for the previous season in model of vaccine exposure for the current and previous season and prior 5 seasons in model of vaccine exposure for current and 5-year vaccination history.
c Adjusted for age, sex, high-risk conditions, season, interval (days) from onset to sample collection, and influenza diagnosis code in prior seasons.
Table 4.
Unadjusted and Adjusted Vaccine Effectiveness Estimates Against Influenza B Virus Infection–Associated Medically Attended Acute Respiratory Illness Showing Effect of Individual Covariates
| Vaccination Status | Current and Previous Season |
Current-Season Vaccination Status and 5-Year Vaccination Historya |
||||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated Both | Vaccinated Current Season Only | Vaccinated Previous Season Only | Current-Season Vaccination + Frequent Vaccinee | Current-Season Vaccination + Infrequent Vaccinee | Current-Season Vaccination + Nonvaccinee | No Current-Season Vaccination + Frequent Vaccinee | No Current-Season Vaccination + Infrequent Vaccinee | |
| Age ≥9 y | ||||||||
| Unadjusted | 67 (60–73) | 70 (57–80) | 70 (55–79) | 70 (61–77) | 74 (63–82) | 77 (56–88) | 59 (29–77) | 37 (18–51) |
| Adjusted for age | 59 (49–67) | 69 (56–79) | 67 (51–78) | 58 (44–68) | 72 (60–80) | 79 (58–89) | 54 (20–74) | 38 (19–52) |
| Adjusted for sex | 66 (58–73) | 69 (56–78) | 68 (53–79) | 69 (59–76) | 73 (62–81) | 77 (55–88) | 58 (27–76) | 35 (16–50) |
| Adjusted for season | 62 (53–69) | 68 (54–78) | 66 (50–78) | 63 (52–72) | 70 (58–79) | 76 (53–88) | 57 (24–76) | 30 (8–47) |
| Adjusted for any high-risk condition | 59 (50–67) | 67 (53–77) | 67 (50–77) | 60 (48–69) | 69 (57–78) | 75 (53–87) | 50 (13–71) | 33 (13–48) |
| Adjusted for interval from onset to sample collection | 67 (59–73) | 70 (57–79) | 70 (55–79) | 69 (59–76) | 74 (63–81) | 77 (55–88) | 59 (28–76) | 36 (17–51) |
| Adjusted for prior influenza historyb | 68 (60–74) | 70 (57–79) | 70 (55–79) | 69 (61–76) | 74 (63–81) | 77 (55–88) | 59 (29–76) | 36 (17–51) |
| Fully adjustedc | 52 (38–62) | 66 (51–77) | 64 (45–76) | 48 (29–62) | 67 (53–77) | 75 (50–87) | 50 (10–73) | 27 (4–45) |
| Age 9–49 y | ||||||||
| Unadjusted | 54 (41–65) | 64 (47–76) | 67 (49–79) | 50 (30–65) | 62 (45–74) | 75 (51–88) | 40 (−14 to 68) | 34 (12–51) |
| Fully adjustedc | 42 (23–57) | 61 (41–74) | 60 (35–75) | 27 (−9 to 51) | 56 (33–71) | 70 (38–86) | 27 (−45 to 64) | 21 (−9 to 43) |
| Age 18–49 y | ||||||||
| Unadjusted | 55 (36–68) | 71 (47–84) | 61 (32–78) | 39 (5–62) | 70 (46–83) | 74 (28–91) | 40 (−43 to 75) | 33 (−4 to 57) |
| Fully adjustedc | 54 (32–69) | 70 (44–84) | 60 (27–78) | … | … | … | … | … |
| Age ≥50 y | ||||||||
| Unadjusted | 71 (58–80) | 85 (59–95) | 72 (35–88) | 74 (60–83) | 89 (74–95) | 86 (−4 to 98) | 79 (31–94) | 44 (−1 to 69) |
a Among persons with ≥5 years of continuous residency.
b Presence of International Classification of Diseases, Ninth Revision code for influenza infection in medical record for the previous season in model of vaccine exposure for the current and previous season and prior 5 seasons in model of vaccine exposure for current and 5-year vaccination history.
c Adjusted for age, sex, high-risk conditions, season, interval (days) from onset to sample collection, and influenza diagnosis code in prior seasons.
DISCUSSION
With our longitudinal influenza vaccination records, we were able to examine the independent and combined effectiveness of current-season vaccination and prior vaccination history over multiple seasons. Current-season vaccination was effective against medically attended H3N2 and B virus–associated illness regardless of prior vaccination history.
The observed protection among persons vaccinated during the previous season and not the current season suggests that influenza vaccines received >1 year earlier may provide some residual protection. A case-control study of influenza VE in pregnant women during the 2010–2011 and 2011–2012 seasons found patterns similar to our study with evidence of residual protection from previous-season vaccination [10]. VE results from the 2010–2011 season in Canada were also consistent with residual protection against A(H1N1)pdm09 among individuals who were previously vaccinated with an adjuvanted monovalent vaccine [9]. In our cohort, residual protection against H3N2 viruses occurred despite the fact that the predominant H3N2 viruses were antigenically distinct from the previous season H3N2 vaccine component in 5 of 7 seasons that were included in the H3N2 analysis. Similarly, the predominant circulating B lineage differed from the previous season vaccine lineage in 5 of 7 seasons, and both lineages circulated widely in 1 season (2012–2013). This residual protection may be conferred by immune responses elicited by previous vaccines that cross-react with current-season virus antigens. Consistent with this idea, several studies have shown evidence that seasonal influenza vaccination can induce antibodies and/or T cells capable of cross-reacting with antigenically distinct viruses [27–31].
Previous epidemiologic studies on clinical effectiveness of current-season influenza vaccination among persons with and without prior history of influenza vaccination have yielded inconsistent results [2, 3]. A placebo-controlled randomized clinical trial from 1984 to 1988 among healthy adults found no consistent differences in efficacy of primary vs repeated vaccination [4, 5]. However, the trial identified few virus-confirmed influenza cases and had limited power to detect differences between groups. More importantly, serologic endpoints were used to identify influenza cases in addition to virus culture. Use of serologic endpoints can cause bias in studies of influenza VE because vaccinated individuals are less likely to seroconvert after influenza infection compared with unvaccinated subjects [32, 33]. Although randomized clinical trials are less susceptible to confounding compared with observational studies, the negative results from this trial should be interpreted with caution due to these limitations.
Vaccine interference may be occurring if protection is lower in individuals who were vaccinated in both the current and previous season compared with those vaccinated in the current season only. Our analysis did not suggest any evidence of vaccine interference when we considered previous-season vaccination. However, the analysis using 5 years of historical vaccination data suggested a significant difference in current-season VE among frequent vaccinees compared with nonvaccinees. Interference due to repeated prior vaccination is one possible explanation for this difference, but unmeasured confounding could also account for the observed differences. The potential immunologic mechanisms for vaccine interference are not well understood. Contributing factors may include “original antigenic sin,” immune exhaustion, and/or antigenic drift of circulating influenza viruses with respect to vaccine antigens. The potential role of original antigenic sin is particularly intriguing as it postulates that exposure to influenza antigens can preferentially expand preexisting memory responses to historical virus antigens at the expense of de novo responses to the current vaccine or infecting strain [34–36]. In most people, vaccination appears to boost preexisting memory responses against antigenically related, previously circulating strains [28, 37]. Reviewing the available evidence, authors of a commentary suggested that the potential for original antigenic sin is greatest when “sequential viruses are of intermediate antigenic relatedeness; when they are antigenically complex; and when the sequential exposure intervals are long … .” [34]. The evidence of original antigenic sin is strongest for sequential natural influenza infections. It is unclear whether repeated influenza vaccination generates effects of similar magnitude.
This study had several limitations. Unmeasured confounding may have influenced VE estimates, as people who choose to get vaccinated every year may have different characteristics and susceptibility to influenza compared with those who do not seek vaccination every year. Although we adjusted for prior influenza using diagnosis codes, prior influenza infection history could be a source of unmeasured confounding as not all illnesses result in medical attention or documentation. However, we found similar trends for the effect of prior vaccination even when analysis was limited to younger persons who were less likely to be frequent vaccinees or to have prior infections. Also, the test-negative design has been shown to yield VE results that were virtually identical to those obtained using the gold standard clinical trial analysis in a recent reanalysis of influenza vaccine clinical trial data [25]. In addition, several other studies have indicated that it is a valid method for estimating VE under a wide range of assumptions [22–24]. Nonetheless, this study examined VE against medically attended influenza and results should not necessarily be interpreted as VE against influenza infection in general.
Despite data from 8 seasons, the sample size in some groups was limited. Furthermore, we were unable to examine VE patterns for each season to determine if patterns were consistent across all seasons. Although our findings represent aggregate effects across multiple seasons, it is apparent from the 2 largest H3N2 seasons that current-season VE is modified by prior vaccination history, irrespective of season or whether there was or was not a change in the vaccine component. We were not able to evaluate timing of past vaccinations for patients with an infrequent vaccination history. However, most patients in this group were vaccinated at least once in the most recent 2 seasons, so results for this group may reflect more recent vaccination history. Finally, this analysis was restricted to inactivated influenza vaccine, and it is unknown whether similar results would be observed with live attenuated influenza vaccine.
In conclusion, we found that vaccination provided protection against medically attended influenza infection, regardless of prior vaccination history. This is consistent with randomized clinical trials of influenza vaccine efficacy [38]. However, this study raises relevant questions about the potential interference of repeated annual influenza vaccination and possible residual protection from previous season vaccination that have not been considered in most trials. Further observational studies that include simultaneous assessment of immune response, both to vaccine antigens and previously circulating viruses, and clinical protection would be helpful. In addition, we believe the results of this study and others support the need for another multiseason randomized clinical trial. Although a randomized trial cannot be conducted in the United States where influenza vaccination is universally recommended, it may be feasible in countries that do not currently recommend influenza vaccination for healthy adults. This would provide valuable new information about the impact of repeated annual influenza vaccination, and it will help guide future vaccine policy recommendations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Burney Kieke, Sarah Kopitzke, Deanna Cole, Sandy Strey, Carol Beyer, and the Marshfield Clinic Research Foundation research coordinators for their contributions and support on this study.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention through a cooperative agreement (U01 IP000471).
Potential conflicts of interest. H. Q. M., M. E. S., J. K. M., D. L. M., and E. A. B. have received research grant support from MedImmune. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med. 1999;159:182–8. doi: 10.1001/archinte.159.2.182. [DOI] [PubMed] [Google Scholar]
- 3.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A. 1999;96:14001–6. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1988;127:353–64. doi: 10.1093/oxfordjournals.aje.a114809. [DOI] [PubMed] [Google Scholar]
- 5.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15:1114–22. doi: 10.1016/s0264-410x(97)00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56:1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis. 2013;57:474–6. doi: 10.1093/cid/cit255. [DOI] [PubMed] [Google Scholar]
- 9.Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010–2011 season. Clin Infect Dis. 2012;55:332–42. doi: 10.1093/cid/cis431. [DOI] [PubMed] [Google Scholar]
- 10.Thompson MG, Li DK, Shifflett P, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis. 2014;58:449–57. doi: 10.1093/cid/cit750. [DOI] [PubMed] [Google Scholar]
- 11.Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9:e92153. doi: 10.1371/journal.pone.0092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014;210:126–37. doi: 10.1093/infdis/jiu048. [DOI] [PubMed] [Google Scholar]
- 13.Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 14.Belongia EA, Kieke BA, Donahue JG, et al. Influenza vaccine effectiveness in Wisconsin during the 2007–08 season: comparison of interim and final results. Vaccine. 2011;29:6558–63. doi: 10.1016/j.vaccine.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Griffin MR, Monto AS, Belongia EA, et al. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One. 2011;6:e23085. doi: 10.1371/journal.pone.0023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treanor JJ, Talbot HK, Ohmit SE, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55:951–9. doi: 10.1093/cid/cis574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep. 2013;62:119–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Updated interim influenza vaccination recommendations—2004–05 influenza season. MMWR Morb Mortal Wkly Rep. 2004;53:1183–4. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Update: influenza vaccine supply and recommendations for prioritization during the 2005–06 influenza season. MMWR Morb Mortal Wkly Rep. 2005;54:850. [PubMed] [Google Scholar]
- 20.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-10):1–42. [PubMed] [Google Scholar]
- 21.Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine. 2009;27:6546–9. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–8. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Orenstein EW, De Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–31. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 24.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–9. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 25.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 26.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35:337–44. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 27.Pereira MS, Chakraverty P, Schild GC, Coleman MT, Dowdle WR. Prevalence of antibody to current influenza viruses and effect of vaccination on antibody response. Br Med J. 1972;4:701–3. doi: 10.1136/bmj.4.5842.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luytjes W, Enouf V, Schipper M, et al. HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non-homologous strains. Vaccine. 2012;30:5262–9. doi: 10.1016/j.vaccine.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 30.Ansaldi F, Zancolli M, Durando P, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28:4123–9. doi: 10.1016/j.vaccine.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Subbramanian RA, Basha S, Shata MT, Brady RC, Bernstein DI. Pandemic and seasonal H1N1 influenza hemagglutinin-specific T cell responses elicited by seasonal influenza vaccination. Vaccine. 2010;28:8258–67. doi: 10.1016/j.vaccine.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 32.Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203:1309–15. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald JC, Andrews BE. Diagnostic methods in an influenza vaccine trial. Br Med J. 1955;2:1232–5. doi: 10.1136/bmj.2.4950.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morens DM, Burke DS, Halstead SB. The wages of original antigenic sin. Emerg Infect Dis. 2010;16:1023–4. doi: 10.3201/eid1606.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167:584–92. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.