Abstract
Thiazolidines are five-member heterocyclic having sulfur, nitrogen, and oxygen atoms in their ring structure and exhibiting potent as well as wide range of pharmacological activities. In this minireview, recent updates on synthesis and pharmacological evaluations of molecules based on 2,4-thiazolidine and rhodanine are discussed.
1. Introduction
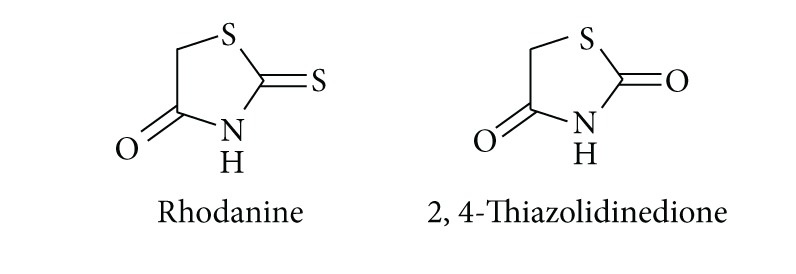
Five-membered heterocyclic molecules containing thiazole nucleus with carbonyl group on fourth carbon such as rhodanine and 2,4-thiazolidinedione derivatives have broad spectrum of pharmacological activities. In past two decades, rhodanines and 2,4-thiazolidinediones have emerged as potent antidiabetic agents. Some of them are clinically used such as ciglitazone, englitazone, pioglitazone, glitazones, epalrestat, and troglitazone for the treatment of type 2 diabetes mellitus and related complications. This is the reason why investigation/molecular modification and pharmacological evaluation of these molecules have attracted special attention of synthetic chemists and pharmacologists, respectively.
In recent years, a number of synthetic/pharmacological protocols based on these molecules have been emerged extensively and in witness available in the literature. These multifaceted molecules exhibit varied type of biological activities. Some recent developments in synthesis and pharmacology of these molecules are discussed in this section.
2. Recent Developments in Rhodanine Pharmacology
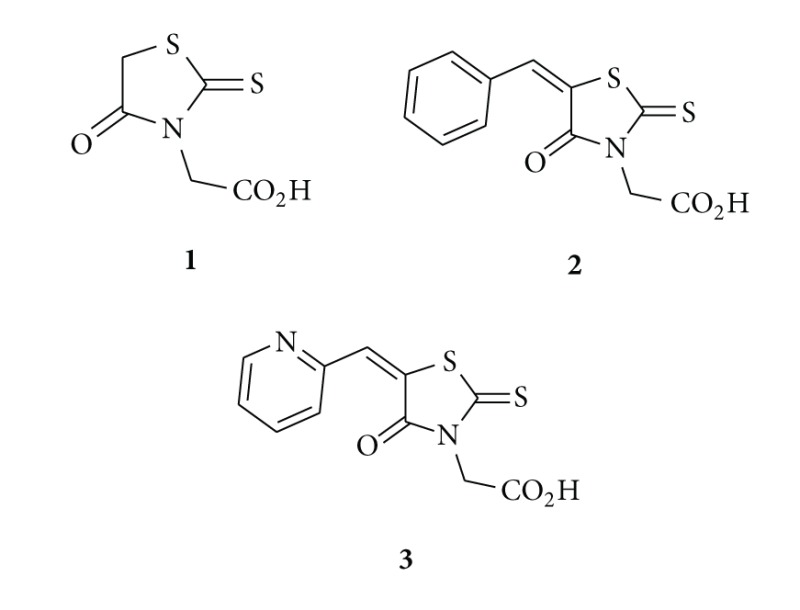
In 1997, Boyd carried out a study based on rhodanine-containing molecules of pharmaceutical interest and found pharmacological importance of these molecules is limited because of poor solubility of rhodanine derivatives in water (exception of rhodanine-3-acetic acids). However, these compounds exhibit a broad range of significant biological activities [1]. Rhodanine-3-acetic acid (RAA) 1 was prepared by Korner [2] in 1908, and Knoevenagel condensation products of the acid with various aldehydes, namely, [(5Z)-(5-benzylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)]acetic acids 2 were reported in the same year [3]. From 1960 onwards, studies revealed that such type of molecule exhibit potential antimycobacterial [4, 5], antifungal [6–15], pesticidal [16–18], antihypertensive [19], and antineoplastic [20, 21] activities. Their NMR characterization performed in 1982 [22]. In 2006, similar derivatives have been prepared under microwave irradiation [23]. Further, the Knoevenagel products of rhodanine-3-acetic acid with pyridinecarbaldehydes were prepared in 1961 and they possess potential antibacterial and antifungal activities [8]. {(5Z)-[4-Oxo-5-(pyridin-2-ylmethylidene)-2-thioxo-1,3-thiazolidin-3-yl]}acetic acid 3 were patented as a potential drug for the treatment of metabolic bone diseases [24, 25]. Later, it was found out that they stimulate parathyroid hormone receptor-mediated cAMP formation and could be useful for the local and systemic treatment of rheumatoid arthritis, osteoarthritis, and degenerative arthrosis [24, 25].
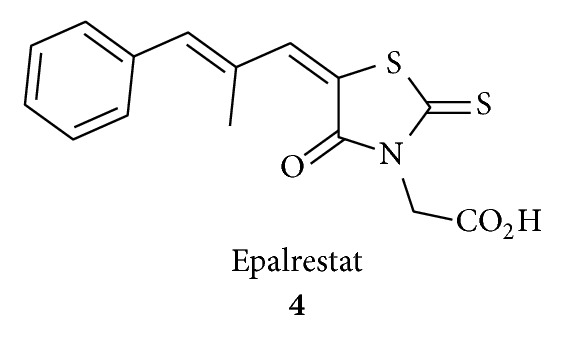
Trypanocidal activity of substituted rhodanine-3-acetic acids has been reported recently [26]. The only rhodanine acetic acid derivative that has been used clinically is the aldose reductase inhibitor epalrestat 4. It was marketed in Japan and used to slow eye damage associated with diabetes and to prevent diabetic peripheral neuropathy [1, 22, 27–29]. Aldose reductase is not the only enzyme inhibited by rhodanine carboxylic acids. It was found that many other enzymes are also inhibited by the derivatives of this structural class and may be responsible for their various biological effects [30]. Other rhodanine-based molecules have also been popular as small molecule inhibitors of numerous targets such as hepatitis C viral (HCV) NS3 protease [31], antidiabetic mechanism [32], aldose reductase [33], β-lactamase [34, 35], histidine decarboxylase [36], and JNK Stimulatory Phosphatase-1 (JSP-1) [37]. This section is a brief account on synthesis and biological effects and recent developments of newly prepared potential drugs based on nitrogen-sulphur containing heterocycles having rhodanine nucleus.
2.1. Rhodanine as Antidiabetic Agent
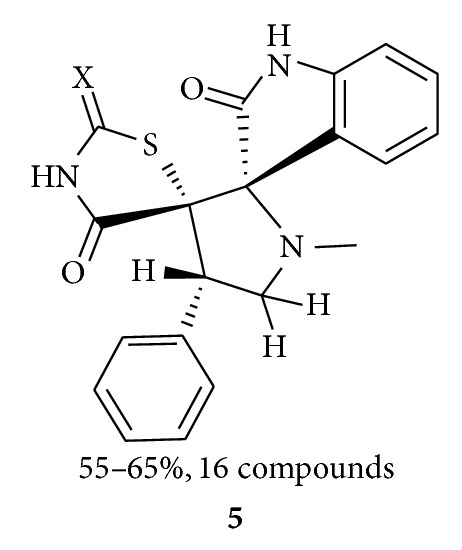
Murugana et al. synthesized [38] a series of dispiropyrrolidines 5 (16-compounds) by 1,3-dipolar cycloaddition reaction of azomethine ylides (in situ generated by the reaction of sarcosine with isatin) with 5-arylidene-1,3-thiazolidine-2,4-dione and 5-arylidene-4-thioxo-1,3-thiazolidine-2-one derivatives as dipolarophiles. They performed molecular docking studies on 1FM9 protein and screened synthesized compounds for their antidiabetic activity on male Wistar rats (after alloxan treatment). The synthesized compounds exhibited attractive antidiabetic properties and are more effective than rosiglitazone in ameliorating stress conditions.
2.2. Rhodanine as Antiapoptotic Agent
Xing and his co-worker synthesized, a series of BH3I-1 based dimeric modulators of 6. The overexpression of antiapoptotic Bcl-2 proteins which protects cells from apoptosis is one mechanism for tumours to acquire drug resistance. In this study they found dimeric modulators 7-8 have enhanced binding activity against antiapoptotic Bcl-2 proteins and proved dimerization of monomeric modulators is one practical approach to enhance the bioactivity of Bcl-2 antagonists [39].
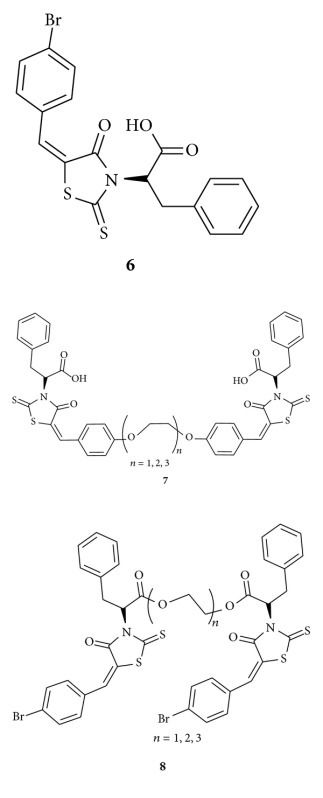
Moorthy and his group [40] designed and synthesized 5-isopropylidiene derivatives of 5-benzilidene-3-ethyl rhodanine (BTR-1) 9, 3-dimethyl-2-thio-hydantoin (ITH-1) 10, and 3-ethyl-2-thio-2,4-oxazolidinedione (ITO-1) 11 and tested their chemotherapeutic properties. They found all the compounds induced cytotoxicity in a time- and concentration-dependent manner on leukemic cell line, CEM. Among these compounds, BTR-1 9 was found to be manifold more potent in inducing cytotoxicity than ITH-1 10 and ITO-1 11 with an IC50 value of <10 μM and affected cell division by inducing a block at S phase, which finally led to the activation of apoptosis.
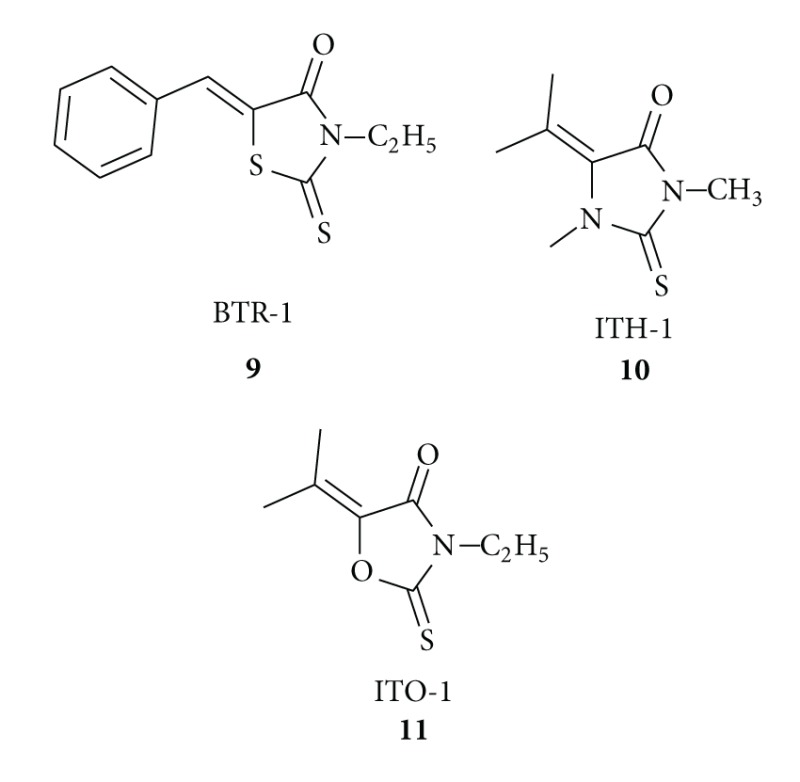
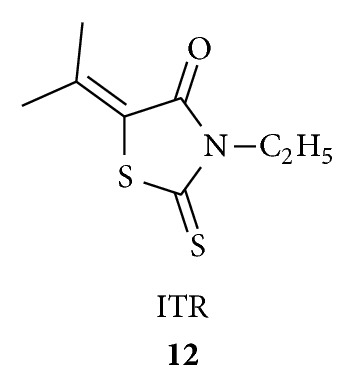
The same research group reported [41] the synthesis of 5-isopropylidene-3-ethyl rhodanine 12 by conventional and microwave-assisted method, and they found that rhodanine ITR 12 treatment led to cytotoxicity in leukemic cell line, CEM, by inducing apoptosis.
2.3. Rhodanine as Antimicrobial Agent
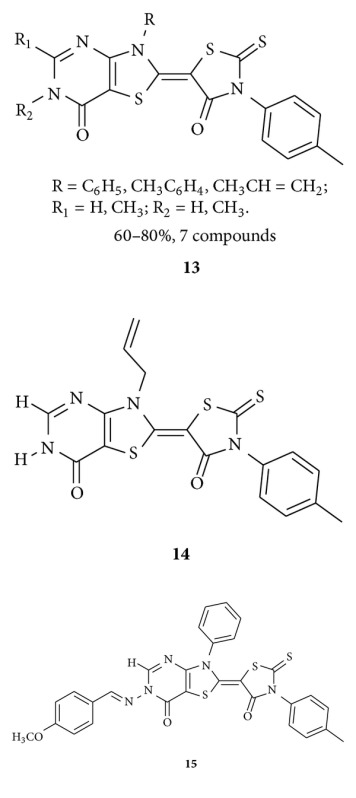
Habib et al. reacted [42] thiazolo[4,5-dlpyrimidines with rhodanines and investigated the obtained products (7 compounds with 60–80% yields) 13 for antimicrobial screening and they found antifungal activity against Aspergillus niger and Penicillium sp. with IZ 20–38 mm and MIC <50–<25 μg/mL. They claimed compound 14 is the most active against Aspergillus niger while compound 15 is the most active against Penicillium sp.; and 5-fold less active than the standard antibiotic clotrimazole. They concluded that the presence of an alkyl group at position 3 of the thiazolopyrimidine ring 14 is superior to that of other aromatic substituents; also the introduction of an arylideneamino group at position 6 of 15 enhanced the antifungal activity.
Opperman and his group disclosed [43] that aryl rhodanines 16–19 did not exhibit antibacterial activity against any of the bacterial strains tested and are not cytotoxic against HeLa cells. Their study revealed that the aryl rhodanines 16–19 specifically inhibit the early stages of biofilm development by preventing attachment of the bacteria (specifically inhibit biofilm formation of S. aureus, S. epidermidis, Enterococcus faecalis, E. faecium, and E. gallinarum but not the Gram-negative species Pseudomonas aeruginosa or Escherichia coli.) to surfaces.
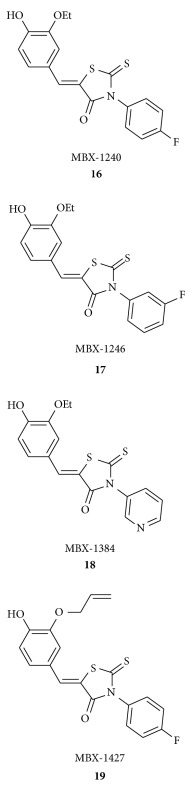
Sim and his associates reported [44] benzylidene rhodanines 20–22 as novel inhibitors of uridine diphospho-N-acetylmuramate/L-alanine ligase. They observed that compounds 20–22 exhibit selective whole-cell activity against the Gram-positive methicillin resistant Staphylococcus aureus (MRSA) but not against the Gram-negative Escherichia coli. They also evaluated their cytotoxic effect on mammalian Chinese hamster ovary (CHO) cells.
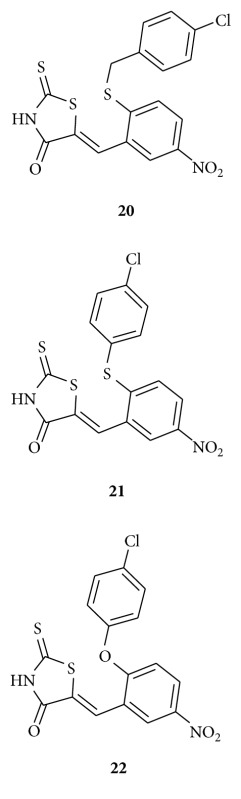
Hardej et al. synthesized [45] a series of glycine and phenylalanine-derived rhodanine analogs and evaluated their anti-MRSA activity. The antibacterial activity of compounds 23 and 24 against a panel of MRSA strains was significantly greater than that of the reference antibiotics penicillin G and ciprofloxacin. They claimed compound 24 exhibited only a 2–4-fold higher MIC value than that of vancomycin. They concluded from their study that the phenylalanine-derived compounds 23 and 24 are promising templates for the development of new drugs to treat MRSA infections.
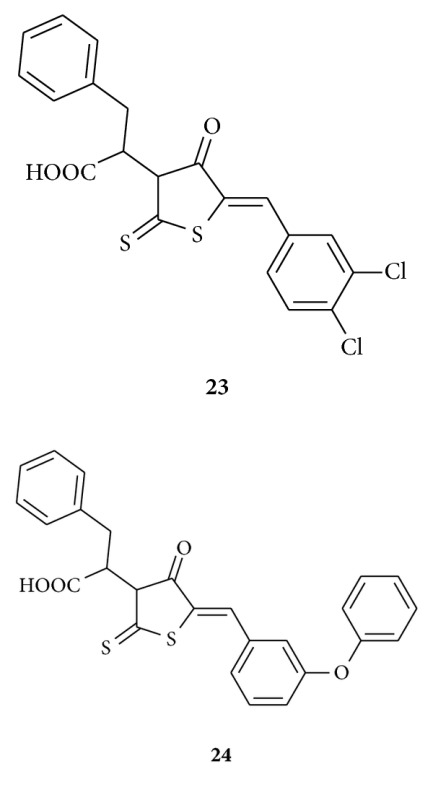
Chen and his group synthesized [46] several hybrid compounds (19 compounds with 31–58% yields) 25 having chalcone and rhodanine-3-acetic acid units and tested these compounds for their antibacterial activity. They found some compounds presented great antimicrobial activities against Gram-positive bacteria (including the multidrug-resistant clinical isolates) as active as the standard drug (norfloxacin) and less active than oxacillin.
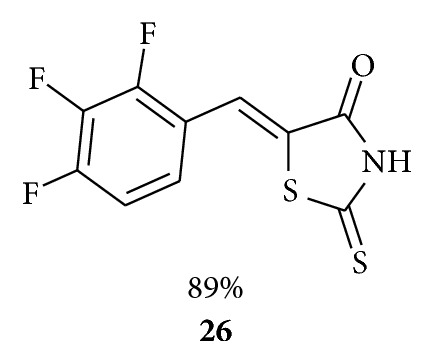
Tomasic et al. reported [47] the synthesis and antibacterial activity for a series of rhodanine-, rhodanine-N-acetic acid-, thiazolidine-2,4-dione-,barbituric-, and thiobarbituric acid-based compounds bearing an ylidene substituent at position 5. The most potent compound of the series, (Z)-5-(2,3,4-trifluorobenzylidene)rhodanine 26, inhibited the growth of S. aureus at 0.5 mg/mL and MRSA at 32 mg/mL.
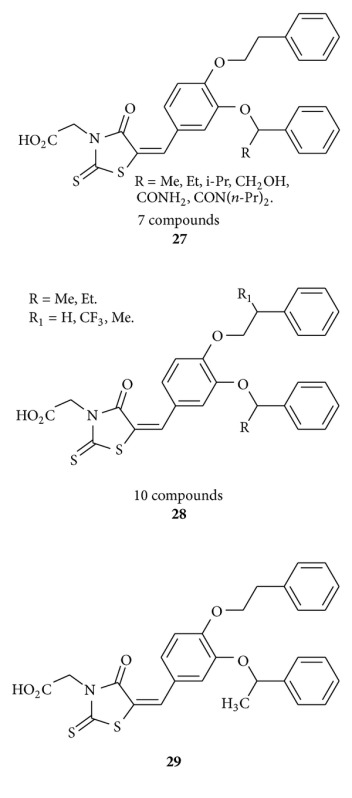
Orchard and his group [48] synthesized rhodanine-3-acetic acid-based compounds 27-28 and described as inhibitors of fungal protein: mannosyl transferase 1 (PMT1). They observed 5-[[3-(1-phenylethoxy)-4-(2-phenylethoxy)phenyl]methylene]-4-oxo-2-thioxo-3-thiazolidineacetic acid 29 inhibit Candida albicans PMT1 with IC50s in the range 0.2–0.5 μM. Members of the series are effective in inducing changes in morphology of C. albicans in vitro that have previously been associated with loss of the transferase activity. According to them, these compounds 27-28 could serve as useful tools for studying the effects of protein O-mannosylation and its relevance in the search for novel antifungal agents.
Sortino et al. reported [49] benzylidene-rhodanines 30 which act as antifungal agents. They evaluated that compounds 31 and 32 showed fungicidal activity and are the most active against Candida genus and C. neoformans including clinical isolates. Other compounds of this series showed a very good activity against dermatophytes.

2.4. Rhodanine as Antihepatitis C Virus (HCV) Agent
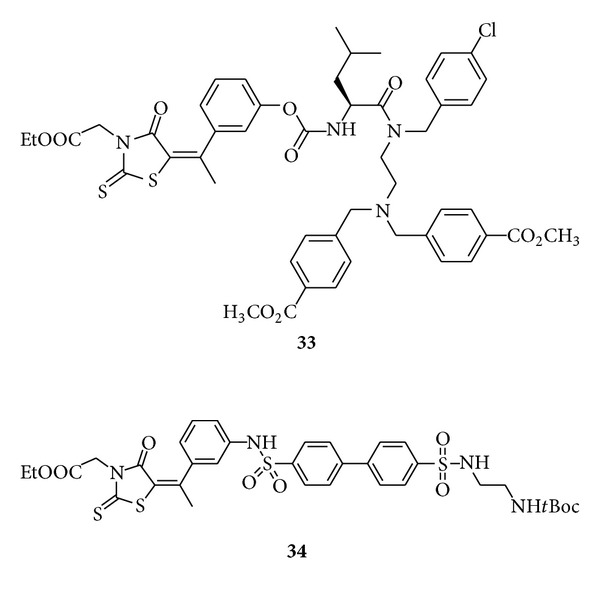
Sing et al. disclosed [50] arylalkylidene rhodanines 33-34 inhibit HCV NS3 protease at moderate concentrations. They claimed these rhodanine derivatives are better inhibitors of serine proteases such as chymotrypsin and plasmin. They concluded that selectivity of arylmethylidene rhodanines 33-34 with bulkier and more hydrophobic functional groups increases by 13- and 25-fold towards HCV NS3 protease, respectively.
2.5. Rhodanine as HIV-1 Integrase Inhibitors
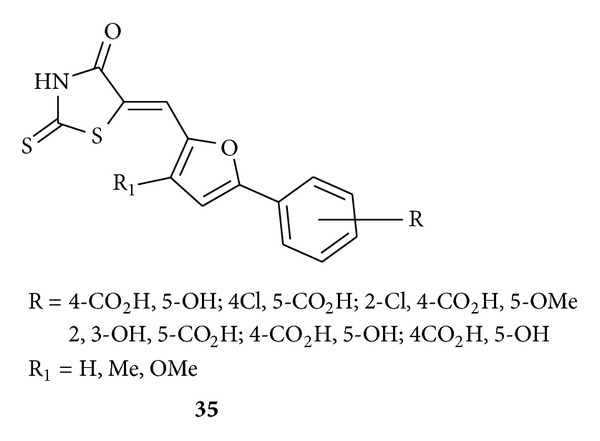
Rajamaki and his associate synthesized [51] and biologically evaluated rhodanine-based compounds 35 and identified these exhibiting anti-HIV-1 integrase activity and moderate inhibition of HIV-1 cell replication.
2.6. Rhodanine as Anti-Inflammatory Agent
Cutshall et al. reported [52] synthesis and evaluation of rhodanine-based compounds 36 as inhibitors of JSP-1. On SAR studies they demonstrated that stronger electron-withdrawing functional groups appended to the aryl-benzylidene position provided analogs with the greatest potencies as illustrated by compound 37. Compound 37 has also reversible and competitive bind with substrate and showed a high degree of enzyme selectivity against other phosphatases.
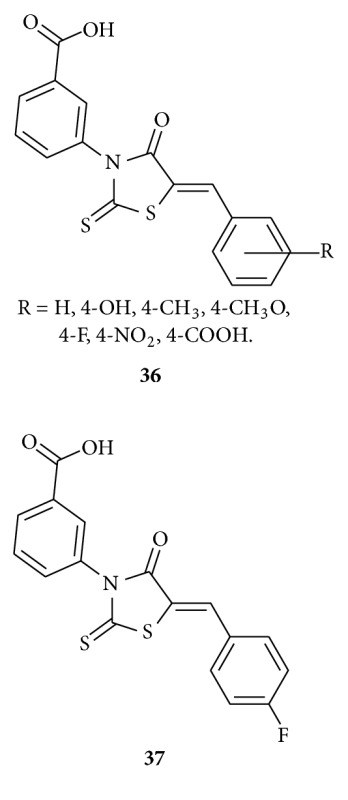
Irvine et al. identified [53] a series of rhodanine derivatives as novel inhibitors of phosphodiesterase type 4 (PDE4). Structures 39 and 40 displayed the most significant activity of the compounds synthesized, being some 20- and 24-fold more potent than lead compound 38.
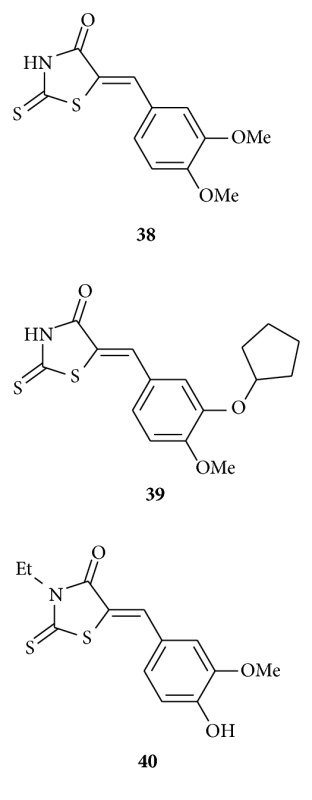
2.7. Rhodanines for Sleeping Sickness
Smith et al. developed [26] the first small molecular inhibitors of dolicholphosphate mannose synthase (DPMS), a mannosyltransferase critically involved in glycoconjugate biosynthesis in T. brucei. They claimed thiazolidinones 41, 42, and 43 in particular are promising candidates for further development because of their respective activities against trypanosomal DPMS and GPI anchor biosynthesis. They reported that these DPMS inhibitors prevent the biosynthesis of glycosylphosphatidylinositol (GPI) anchors and possess trypanocidal activity against live trypanosomes. Drug-like molecules 41–43 with activity against Trypanosoma brucei are urgently required as potential therapeutics for the treatment of African sleeping sickness.
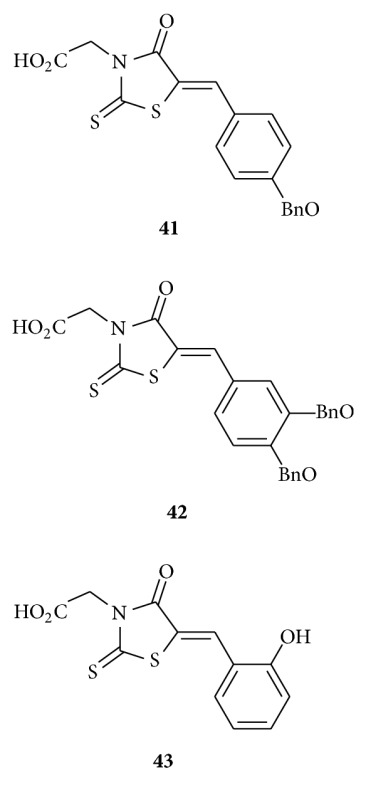
2.8. Rhodanines as Tyrosinase Inhibitors
Liu et al. synthesized [54] a series of dihydropyrimidin-(2H)-one analogues and rhodanine derivatives and evaluated their inhibitory effects on the diphenolase activity of mushroom tyrosinase. In results they found that some of the synthesized compounds exhibited significant inhibitory activities. Particularly, compound 44 bearing a hydroxyethoxyl group at position-4 of phenyl ring exhibited the most potent tyrosinase inhibitory activity with IC50 value of 0.56 mM. The inhibition mechanism analysis of compound 44 demonstrated that the inhibitory effect of the compound on the tyrosinase was irreversible. These results suggested that such compounds might be served as lead compounds for further designing new potential tyrosinase inhibitors.
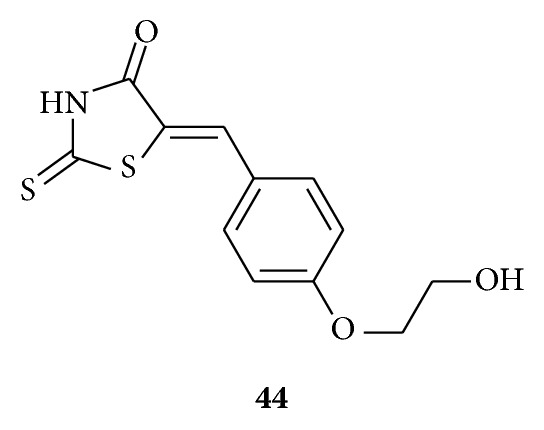
2.9. Rhodanines as PRL-3 Inhibitors
Ahn et al. synthesized [55] and evaluated a series of rhodanine derivatives 45 for their ability to inhibit oncolytic phosphatase (PRL-3). Benzylidene rhodanine derivative 45 showed good biological activity, while compound 46 is found to be the most active in this series exhibiting IC50 value of 0.9 Lm in vitro and showed a reduced invasion in cell-based assay.
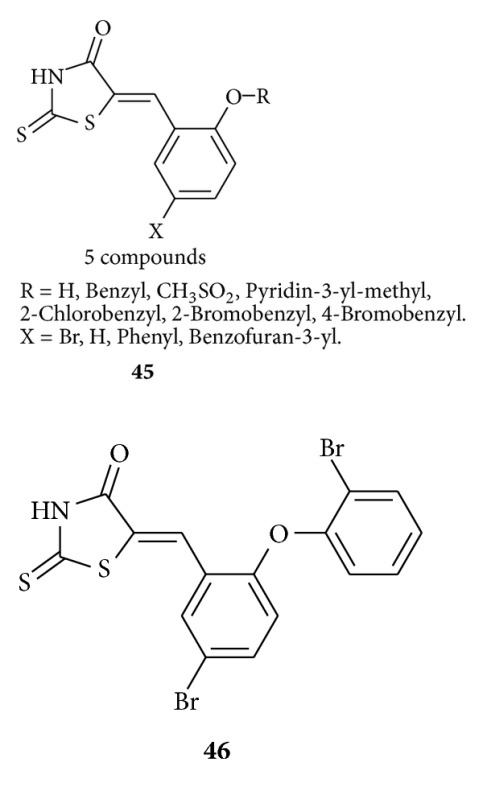
3. Pharmacological Developments in 2,4-Thiazolidinediones
The most commonly used antidiabetic agents have been sulfonylureas, metformin, and certain alphaglucosidase inhibitors and meglitinides. These agents increase insulin secretion from pancreatic β-cells but sometimes induce severe hypoglycemia and weight gain [56], and hyperinsulinemia is known to be a risk factor for ischemic heart disease [57]. In addition, high rates of both primary and secondary failure have been observed with these drugs [58–61]. Therefore, drugs that ameliorate the insulin resistance without stimulating insulin release from β-cells have been developed for the treatment of type 2 diabetes. Type 2 diabetes is a multifactorial disease defined by a high plasma glucose level and is characterized by both insulin resistance and impaired insulin secretion by pancreatic β-cells [62]. The prototypical 2,4-thiazolidinedione, ciglitazone 47 was discovered [63] by Takeda Chemical Industries, Ltd., Japan, and has antihyperglycemic activity in insulin-resistant animal models, KKAy mice [64], and Wistar fatty rats [65] but no effect in insulin-deficient animal models of diabetes [66, 67]. During structure-activity relationship studies on 2,4-thiazolidinediones and related compounds, they discovered highly potent compounds, such as pioglitazone 48 [68] and AD-5061 49 [69]. Since the discovery of ciglitazone 47, a number of pharmaceutical companies have been evaluating new 2,4-thiazolidinedione analogs as agents for improving insulin resistance. Troglitazone 50 [70] was launched first in the market but had been withdrawn because of liver toxicity and related deaths associated with the drug. Nowadays, two 2,4-thiazolidinedione class agents, pioglitazone 48 and rosiglitazone 51 [71], have been clinically used. Furthermore, many companies are still endeavoring to find a new glucose-lowering agent [72–84].
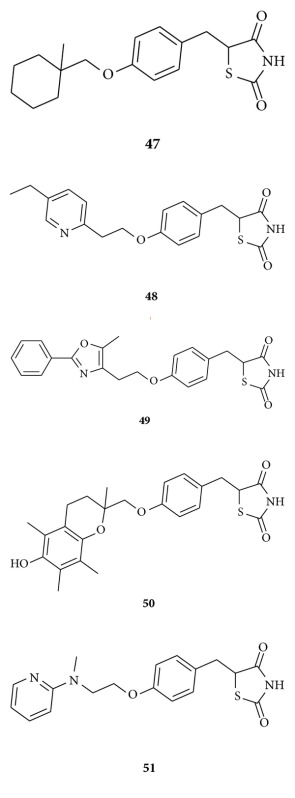
Although the precise mechanism of action of these drugs remains unknown, a recent study suggests that antidiabetic thiazolidinediones interact with a family of nuclear receptors known as peroxisome proliferator-activated receptor (PPAR)-γ [85]. PPARγ is one of a subfamily of PPARs encoded by independent genes. Three human PPARs, designated PPARα, PPARγ, and PPARδ, have been identified to date [86–88]. It was also observed that the potency for the activation of PPARγ in vitro mirrored the in vivo glucose-lowering activity in diabetic ob/ob mice [89]. This would indicate that the major mechanisms of action of 2,4-thiazolidinediones involve PPARγ. In case of those 2,4-thiazolidinediones already on the market, several side effects, such as anaemia, edema, and body weight gain, have been reported [90]. Therefore, search for new compounds with fewer side effects and a more advanced profile than existing drug molecules is the main focus of attention for chemists as well as for pharmacologists. Recent developments in the synthesis and evaluations of thiazolidinedione-based compounds for a variety of biological activities along with antidiabetes is discussed in the ongoing pages.
3.1. Thiazolidinedione as Antidiabetic Agent
Rakowitz et al. synthesised [91] and tested several 5-benzyl-2,4-thiazolidinediones 52-53 as in vitro aldose reductase inhibitors (ARIs). Their evaluation shows N-unsubstituted 5-benzyl-2,4-thiazolidinediones 52 and (5-benzyl-2,4-dioxothiazolidin-3-yl)acetic acids 53 displayed moderate-to-high inhibitory activity levels. The insertion of an acetic chain on N-3 significantly enhanced AR inhibitory potency, leading to acids 53 which proved to be the most effective among the tested compounds. In addition, in N-unsubstituted derivatives 52 the presence of an additional aromatic ring on the 5-benzyl moiety was generally beneficial.
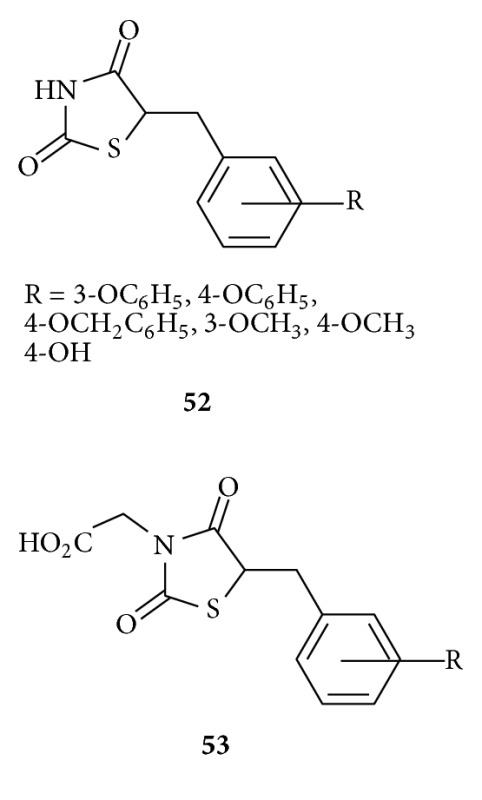
Madhavan et al. synthesized [92] and evaluated 2,4-thiazolidinedione derivatives of 1,3-benzoxazinone for their PPAR-α and -γ dual activation. They obtained that a compound DRF-2519 (54), through SAR of TZD derivatives of benzoxazinone, has shown potent dual PPAR activation. In ob/ob mice, it showed better efficacy than the comparator molecules. In fat fed rat model, it showed significant improvement in lipid parameters, which was better than fibrates.
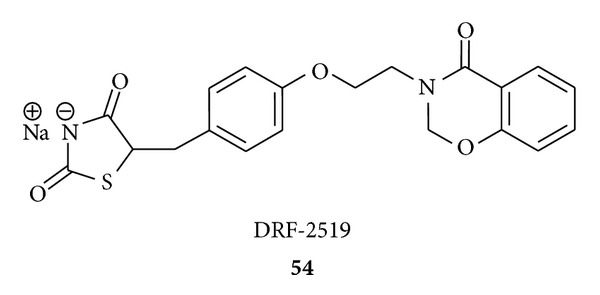
Bozdağ-Dündar et al. prepared [93] a new series of chromonyl-2,4-thiazolidinediones 55 aiming to reduce diabetic complications especially which have effect on the cataract formation. The synthesized compounds were tested for their ability to inhibit rat kidney AR by an in vitro spectrophotometric assay. Compound 56 showed the highest inhibitory activity. They concluded that the increasing inhibitory effect of compounds 55 might depend on the acetic acid side chain of 2,4-thiazolidinedione, and these compounds especially 56 could display therapeutic potential in the prevention and the treatment of diabetic complications as promising ARIs.
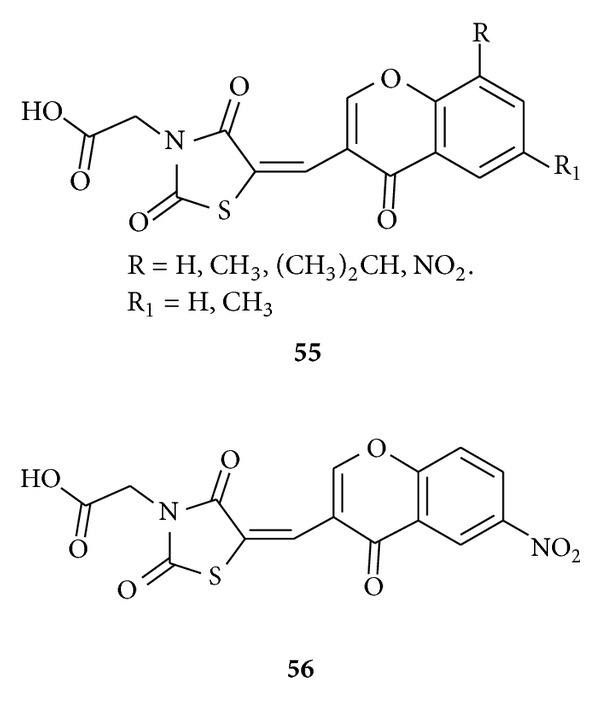
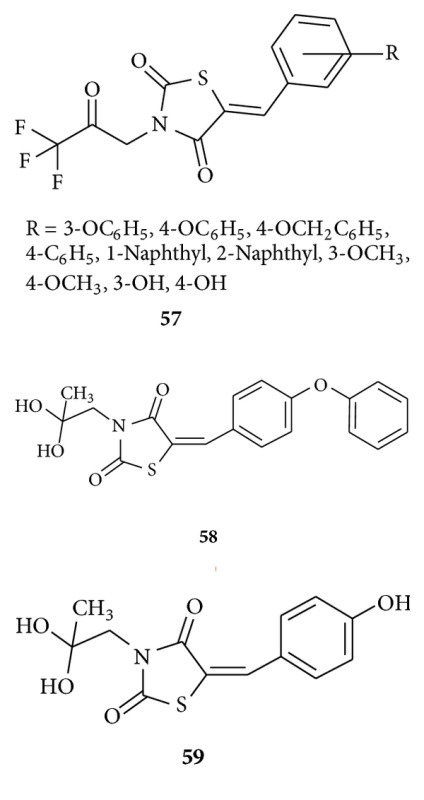
Maccari et al. reported [94] new ARIs via in vitro evaluation of a series of 5-arylidene-3-(3,3,3-trifluoro-2-oxopropyl)-2,4-thiazolidinediones 57 as ARIs allowed the identification of two new noncarboxylic acid containing 5-arylidene-2,4-thiazolidinedione derivatives (58 and 59) that found active at low-micromolar doses.
3.2. Thiazolidinedione as Anticancer Agent
Patil et al. synthesized [95] and evaluated ten derivatives of 5-benzylidene-2,4-thiazolidinediones 60 for their antiproliferative activity in a panel of 7 cancer cell lines using four concentrations at 10-fold dilutions. Sulforhodamine B (SRB) protein assay used to estimate cell stability or growth. These compounds showed varying degrees of cytotoxicity in the tested cell lines, most marked effect observed by compound 60 in MCF7 (breast cancer), K562 (leukemia), and GURAV (nasopharyngeal cancer) cell lines with log10 GI50 values of −6.7, −6.72, and −6.73, respectively.
3.3. Thiazolidinedione as Anti-Inflammatory Agent
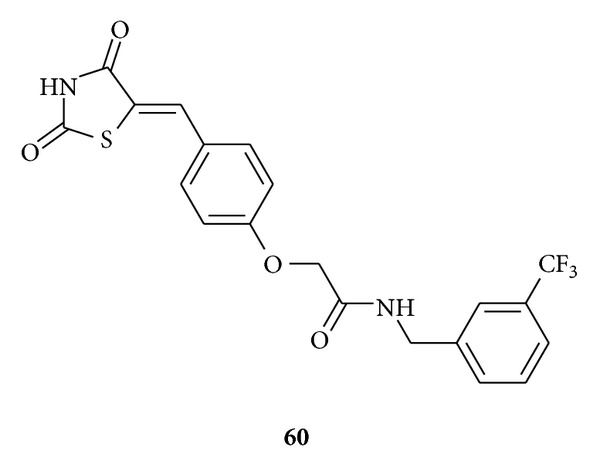
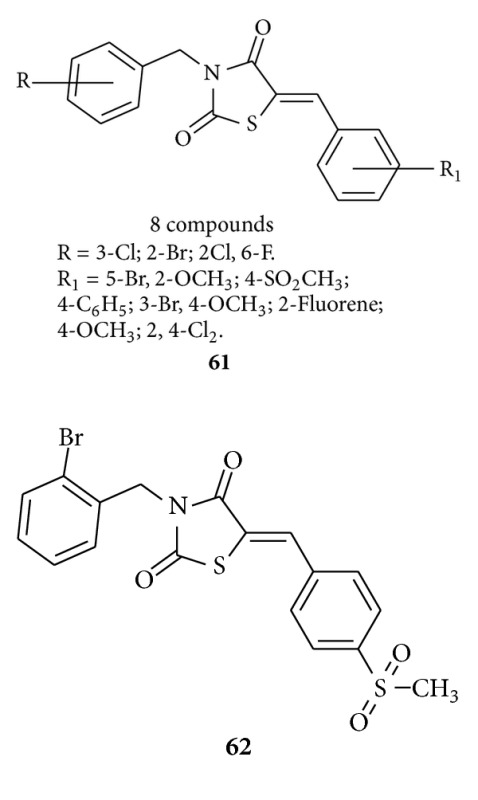
Barros et al. synthesized [96] 5-arylidene-3-benzyl-thiazolidine-2,4-diones 61 with halide groups on their benzyl rings (8 compounds) and assayed in vivo to investigate their anti-inflammatory activities, and 3-(2-bromo-benzyl)-5-(4-methanesulfonyl-benzylidene)-thiazolidine-2,4-dione, compound 62, showed higher anti-inflammatory activity than the rosiglitazone reference drug as it bound PPARγ with 200-fold lower affinity than the reference ligand.
Alagawadi and Alegaon described [97] the synthesis and antimicrobial activity of 5-substituted-2,4-thiazolidinedione derivatives. They evaluated all compounds for their preliminary in vitro antibacterial and antifungal activity. The investigation of antimicrobial screening revealed that some of the tested compounds showed moderate to good bacterial and fungal inhibition. Particularly, compounds 63–66 have shown good activity against S. aureus and E. faecalis with minimum inhibitory concentration (MIC) values between 4 and 32 μg/mL. They found all compounds active against tested fungal strains at 1–64 μg/mL concentration. Compounds 63-66 also showed good antifungal activity against C. albicans at 1–4 μg/mL and C. neoformans, A. flavus, A. niger at 2–8 μg/ml concentration.

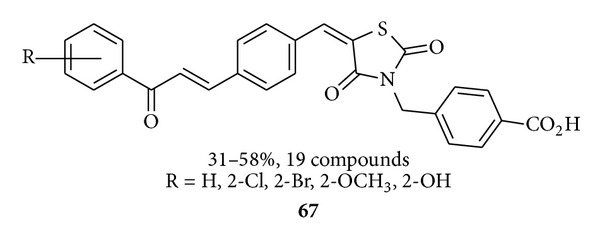
Liu et al. synthesized [98] a series of chalcone derivatives bearing the 2,4-thiazolidinedione and benzoic acid moieties 67 and evaluated for their antibacterial activity. In tested compounds, the most effective results obtained with MIC value in the range of 0.5–4 mg/mL against six Gram-positive bacteria (including multidrug-resistant clinical isolates).
3.4. Thiazolidinedione as Antioxidant
Jeong et al. synthesized [99] multisubstituted benzylidenethiazolidine-2,4-diones 68 by Knoevenagel condensation of di- or trisubstituted 4-hydroxybenzaldehydes with thiazolidine-2,4-dione and evaluated the antioxidant activities of Cu2+-induced oxidation of human low-density lipoproteins (LDLs). Among compounds, 69 was found to be superior to probucol in LDL-antioxidant activities and found to be 9-fold more active than probucol.

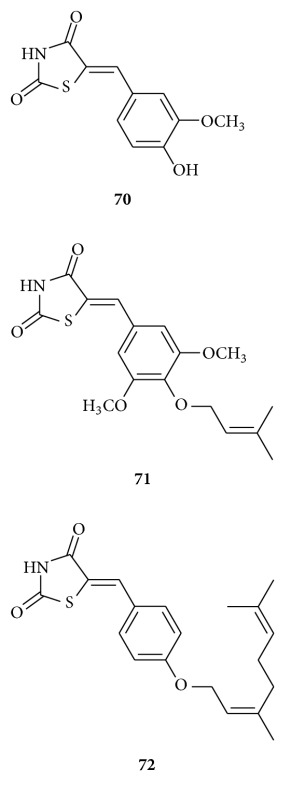
Hossain and Bhattacharya synthesized [100] a series of 5-arylidene-2,4-thiazolidinediones and its geranyloxy or prenyloxy derivative and studied their radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. They expressed comparable scavenging activities as IC50 value. Compounds 70–72 showed appreciable radical scavenging activities. The vanillin-based thiazolidinedione compound 70 displayed the highest activity comparable to that of α-tocopherol. But in vivo, compound 72 showed better results in inducing phase II detoxifying/antioxidative enzyme. The compounds 70–72 found to be effective in enhancing the host antioxidant defence system such as superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), and reduced Glutathione (GSH) and at the same time lowering the serum ALT and AST level at the preliminary screening dose of 3 mg/kg in normal Swiss albino mice given orally for 20 days as compared to the control animals. The hepatic lipid-peroxidation level (LPO) remained unchanged.
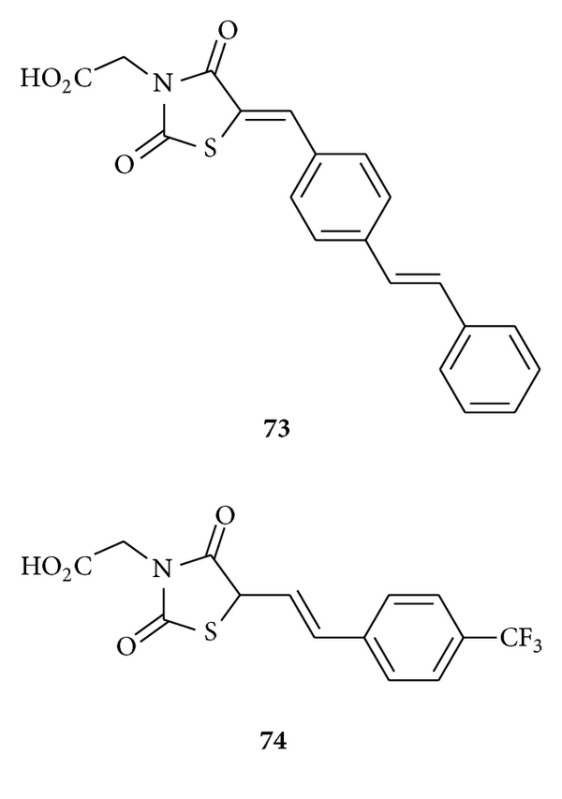
Ottanà et al. explored [101] 5-arylidene-4-thiazolidinones as antioxidant agents and aldose reductase inhibitors. They found that compounds 73 and 74 proved to be interesting inhibitors of the enzyme as well as excellent antioxidant agents that are potentially able to counteract the oxidative stress associated with both diabetic complications as well as other pathologies.
3.5. Thiazolidinedione as Antiobesity
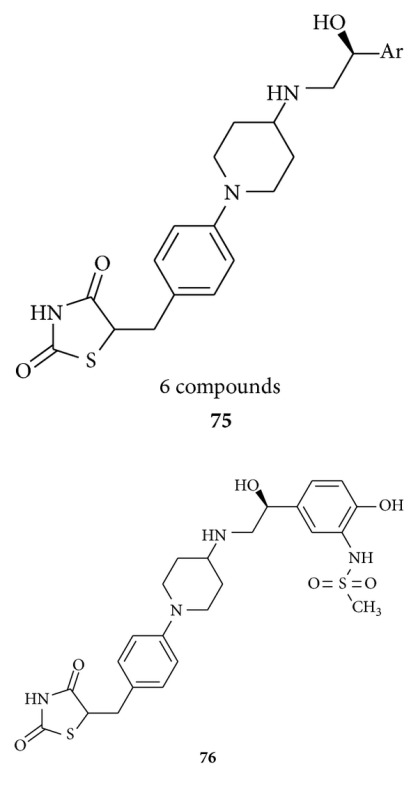
Hu et al. disclosed [102] synthesis of methylsulfonamide-substituted 2,4-thiazolidinedione (6 compounds) 75 and found 76 to be a potent (EC50 = 0.01 mM, IA = 1.19) and selective (more than 110-fold over β1 and β2 agonist activity) β3 agonist. This compound has also been proven to be active and selective in an in vivo mode.
Bhattarai et al. synthesized [103] benzylidene-2,4-thiazolidinedione derivatives (9 compounds) with substitutions on the phenyl ring at the ortho or para positions of the thiazolidinedione group 77 as protein tyrosine phosphatase (PTP1B) inhibitors with IC50 values in a low-micromolar range. Compound 78, the lowest, bores an IC50 of 5.0 μM. In vivo efficacy of 78 as an antiobesity and hypoglycemic agent evaluated in a mouse model system. This compound also significantly suppressed weight gain and significantly improved blood parameters such as TG, total cholesterol, and NEFA. Compound 78 also was found to activate peroxisome proliferator-activated receptors (PPARs) indicating multiple mechanisms of action.
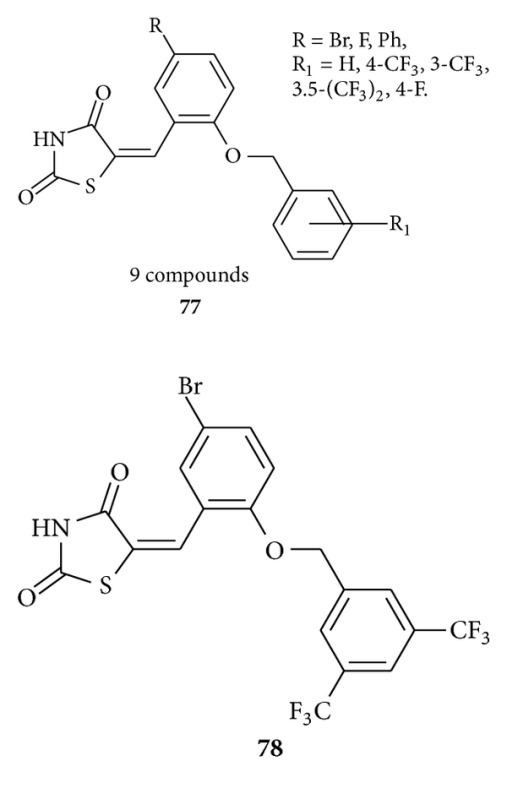
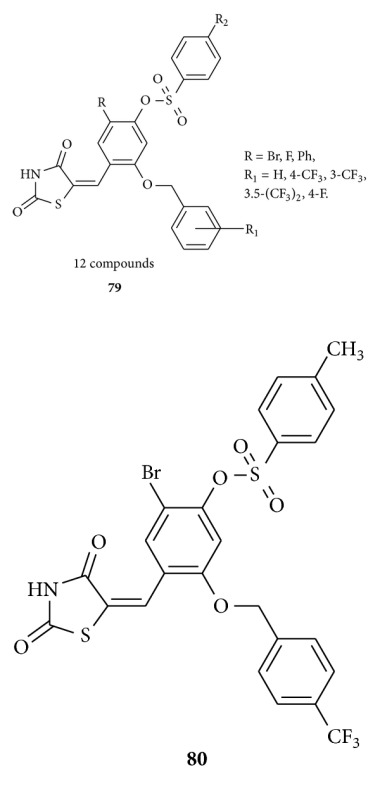
Same research group [104] synthesized benzylidene-2,4-thiazolidinedione derivatives (12 compounds) 79 as PTP1B inhibitors with IC50 values in a low-micromolar range. Compound 80, the lowest, bores an IC50 of 1.3 μM. In a peroxisome proliferator-activated receptor-γ (PPAR-γ) promoter reporter gene assay, 80 was found to activate the transcription of the reporter gene with potencies comparable to those of troglitazone, rosiglitazone, and pioglitazone. In vivo efficacy of 80 as an antiobesity and hypoglycemic agent was evaluated in a mouse model system. Compound 80 significantly suppressed weight gain and significantly improved blood parameters such as TG, total cholesterol, and NEFA without overt toxic effects.
3.6. Thiazolidinedione as Antiprostaglandins
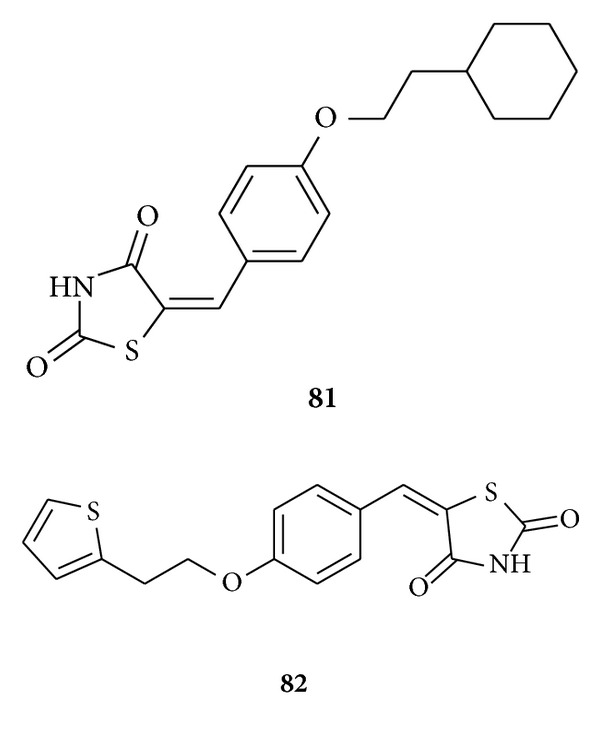
Ying et al. synthesized [105] a range of benzylidene thiazolidinedione derivatives (27 compounds with 75–88% yields) with different substituents on the phenyl ring and evaluated their inhibitory 15-hydroxyprostaglandin dehydrogenase (15-PGDH) activity. Based on the structures of the thiazolidinediones analogues and inhibitory activity, replacement of the cyclohexylethyl group of 81 with the hetero five-member ring increased the inhibitory potency. However, replacement of the cyclohexylethyl group with a hetero six-member ring decreased the inhibitory potency significantly. They found that compound 82 (5-(4-(2-(thiophen-2-yl)ethoxy)benzylidene)thiazolidine-2,4-dione) is the most potent inhibitor and effective in the nanomolar range.
3.7. Thiazolidinedione as Thyroid Hormone Receptor Antagonists
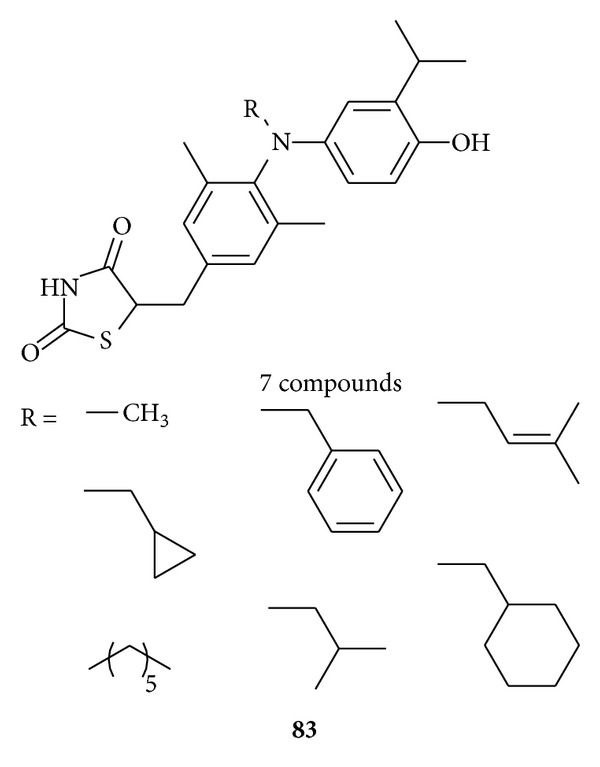
Komatsu et al. designed and synthesized [106] diphenylamine derivatives 83 with a thiazolidinedione moiety as the terminal polar group as thyroid hormone receptor (TR) antagonists. Thiazolidinedione derivatives 83 with N-alkyl group showed antagonistic activities towards both the hTRα1 and hTRβ1 subtypes.
4. Conclusion
In recent past, a variety of molecules based on rhodanine and thiazolidinedione have been synthesized and evaluated with improved pharmacological activities. Due to wide range of pharmacological activities and clinically used 2,4-thiazolidines, these molecules have attracted much attention and encouraged the chemists and biologists to be extensive investigations or molecular manipulations, and as a result further improved protocol with better observation is still under progress.
References
- 1.Boyd DBJ. On the rhodanines and their presence in biologically active ligands. Journal of Molecular Structure. 1997;401(3):227–234. [Google Scholar]
- 2.Korner H. Über einige derivate der dithiocarbamino-essaigs ure. Berichte der Deutschen Chemischen Gesellschaft. 1908;41(2):1901–1905. [Google Scholar]
- 3.Andreasch R. Über substituierte Rhodaninsäuren und deren Aldehydkondensationsprodukte . Monatshefte für Chemie und verwandte Teile anderer Wissenschaften. 1908;29(5):399–419. [Google Scholar]
- 4.Taniyama H, Yasui B, Takehara N, Uchida H. Chemotherapeutics for mycobacterium tuberculosis. XIX. Synthesis and antibacterial activity of some 3-substituted rhodanines. Yakugaku Zasshi. 1959;79:1465–1468. [Google Scholar]
- 5.Singh J, Nathan CF, Bryk R, Samy R, Pupek K, Gurney M. Cyclic carboxylic acid rhodanine derivatives for the treatment and prevention of tuberculosis. WO Pat. 2008;(2008005651)
- 6.Allan FJ, Allan GG, Crank G, Jack J. The condensation of rhodanine and derivatives with benzaldehyde sulphonic acids. Recueil des Travaux Chimiques des Pays-Bas. 1960;79:247–254. [Google Scholar]
- 7.Allan FJ, Allan GG. The condensation of rhodanine and derivatives with 4-antipyrinaldehyde. Canadian Journal of Chemistry. 1961;39(6):1397–1399. [Google Scholar]
- 8.Allan FJ, Allan GG, Thompson J. The condensation of rhodanine and derivatives with pyridine and quinoline aldehydes. Recueil des Travaux Chimiques des Pays-Bas. 1961;80:403–408. [Google Scholar]
- 9.Allan FJ, Allan GG. The condensation of rhodanine and derivatives with some indole aldehydes. Monatshefte für Chemie. 1963;94:1434–4475. [Google Scholar]
- 10.Allan FJ, Allan GG. The condensation of rhodanine and derivatives with aromatic aldehydes containing iodine. Recueil des Travaux Chimiques des PaysBas. 1962;82:177–181. [Google Scholar]
- 11.Allan FJ, Allan GG. Izoxazolylmethylenerhodanines. Recueil des Travaux Chimiques des Pays-Bas. 1964;83:1299–1300. [Google Scholar]
- 12.Orchard MG, Neuss JC, Galley CMS. Benzylidene thiazolidinediones and their use as antimycotic agents. WO Pat. 2002;(02017915)
- 13.Orchard MG, Neuss JD. Preparation of thiazolidines as antifungal agents. WO Pat. 2002;(0222612)
- 14.Orchard MG. Preparation of 5-benzylidene-4-thiazolidinone-2-thione antifungal agents. WO Pat. 2003;(03070238)
- 15.Orchard MG, Neuss JC, Galley CMS, et al. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1) Bioorganic and Medicinal Chemistry Letters. 2004;14(15):3975–3978. doi: 10.1016/j.bmcl.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Dovlatyan VV, Avetisyan FV. 2-Thioxo-4-imino(oxo)-1,3-thiazolidin-3-ylacetic acid and its alkyl esters. Armyanskii Khimicheskii Zhurnal. 1973;26:494–498. [Google Scholar]
- 17.Inamori Y, Muro C, Tanaka R, Adachi A, Miyamoto K, Tsujibo H. Phytogrowth-inhibitory activity of sulphur-containing compounds. I. Inhibitory activities of thiazolidine derivatives on plant growth. Chemical and Pharmaceutical Bulletin. 1992;40(10):2854–2856. [Google Scholar]
- 18.Muro C, Yasuda M, Sakagami Y, et al. Inhibitory activities of rhodanine derivatives on plant growth. Bioscience, Biotechnology and Biochemistry. 1996;60(8):1368–1371. [Google Scholar]
- 19.Frankov IA, Kirillov MV, Sokolova TN, Skupskaya R, Kharitonovich AN, Chizhevskaya II. Khimiko-Farmatsevticheskii Zhurnal. 1985;19:943–946. [Google Scholar]
- 20.Friebe WG, Krell HW, Woelle S, Wolff HP. Thiazolidine carboxylic acid derivatives and their use in the treatment of cancer. WO Pat. 2001;(0157006)
- 21.Singh R, Ramesh UV, Goff D, et al. Rhodanine derivatives and pharmaceutical compositions containing them. WO Pat. 2004;(2004043955)
- 22.Tanaouchi T, Kawamura M, Ajima A, et al. Rhodanine derivatives, process for their preparation, and aldose reductase inhibitor containing the rhodanine derivatives as active ingredient. European Patent. 1982;(0047109)
- 23.Zhou JF, Song YZ, Zhu FX, Zhu YL. Facile synthesis of 5-benzylidene rhodamine derivatives under microwave irradiation. Synthetic Communications. 2006;36(22):3297–3303. [Google Scholar]
- 24.Esswein A, Schaefer W, Tsaklakidis C, Honold K, Kaluza K. Rhodanine carboxylic acid derivatives for the treatment and prevention of metabolic bone disorders. US Patent Application. 2003;(2003032813)
- 25.Esswein A, Schaefer W, Tsaklakidis C, Honold K, Kaluza K. Rhodanine carboxylic acid derivatives for the treatment and prevention of metabolic bone disorders. US Patent Application. 2004;(6673816)
- 26.Smith TK, Young BL, Denton H, Hughes DL, Wagner GK. First small molecular inhibitors of T. brucei dolicholphosphate mannose synthase (DPMS), a validated drug target in African sleeping sickness. Bioorganic and Medicinal Chemistry Letters. 2009;19(6):1749–1752. doi: 10.1016/j.bmcl.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes care. 2008;31(supplement 2):S255–S261. doi: 10.2337/dc08-s263. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28(5):646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- 29.Tanaouchi T, Kawamura M, Ajima A, et al. Rhodanine derivatives, process for their preparation, and aldose reductase inhibitor containing the rhodanine derivatives as active ingredient. US Patent. 1984;(4464382)
- 30.Tomasic T, Masic LP. Rhodanine as a privileged scaffold in drug discovery. Current Medicinal Chemistry. 2009;16(13):1596–1629. doi: 10.2174/092986709788186200. [DOI] [PubMed] [Google Scholar]
- 31.Sing WT, Lee CL, Yeo SL, Lim SP, Sim MM. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorganic and Medicinal Chemistry Letters. 2001;11(2):91–94. doi: 10.1016/s0960-894x(00)00610-7. [DOI] [PubMed] [Google Scholar]
- 32.Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohada T. Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chemical & Pharmaceutical Bulletin. 1991;39(6):1440–1445. doi: 10.1248/cpb.39.1440. [DOI] [PubMed] [Google Scholar]
- 33.Fujishima H, Tuboto K. Improvement of corneal fluorescein staining in post cataract surgery of diabetic patients by an oral aldose reductase inhibitor, ONO-2235. British Journal of Ophthalmology. 2002;86(8):860–865. doi: 10.1136/bjo.86.8.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant EB, Guiadeen D, Baum EZ, et al. The synthesis and SAR of rhodanines as novel class C β-lactamase inhibitors. Bioorganic & Medicinal Chemistry Letters. 2000;10(19):2179–2181. doi: 10.1016/s0960-894x(00)00444-3. [DOI] [PubMed] [Google Scholar]
- 35.Zervosen A, Lu WP, Chen Z, White RE, Demuth TP, Jr., Frere JM. Interactions between penicillin-binding proteins (PBPs) and two novel classes of PBP inhibitors, arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones. Antimicrobial Agents and Chemotherapy. 2004;48(3):961–969. doi: 10.1128/AAC.48.3.961-969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Free CA, Majchrowich E, Hess SM. Mechanism of inhibition of histidine decarboxylase by rhodanines . Biochemical Pharmacology. 1971;20(7):1421–1428. doi: 10.1016/0006-2952(71)90269-3. [DOI] [PubMed] [Google Scholar]
- 37.Cutshall NS, O'Day C, Prezhdo M. Rhodanine derivatives as inhibitors of JSP-1. Bioorganic & Medicinal Chemistry Letters. 2005;15(14):3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Murugana R, Anbazhagan S, Narayanan SS. Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3 + 2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. European Journal of Medicinal Chemistry. 2009;44(8):3272–3279. doi: 10.1016/j.ejmech.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Kong F, Kokoski CL, Andrews DW, Xing C. Development of dimeric modulators for anti-apoptotic Bcl-2 proteins. Bioorganic & Medicinal Chemistry Letters. 2008;18(1):236–240. doi: 10.1016/j.bmcl.2007.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moorthy BT, Ravi S, Srivastava M, et al. Novel rhodanine derivatives induce growth inhibition followed by apoptosis. Bioorganic & Medicinal Chemistry Letters. 2010;20(21):6297–6301. doi: 10.1016/j.bmcl.2010.08.084. [DOI] [PubMed] [Google Scholar]
- 41.Ravi S, Chiruvella KK, Rajesh K, Prabhu V, Raghavan SC. 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. European Journal of Medicinal Chemistry. 2010;45(7):2748–2752. doi: 10.1016/j.ejmech.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Habib NS, Ridal SM, Badaweyl EAM, Fahmyl HTY, Ghozlan HA. Synthesis and antimicrobial activity of rhodanine derivatives . European Journal of Medicinal Chemistry. 1997;32(9):759–762. [Google Scholar]
- 43.Opperman TJ, Kwasny SM, Williams JD, et al. Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrobial Agents and Chemotherapy. 2009;53(10):4357–4367. doi: 10.1128/AAC.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sim MM, Ng SB, Buss AD, Crasta SC, Goh KL, Lee SK. Benzylidene rhodanines as novel inhibitors of UDP-N-acetylmuramate/l-alanine ligase. Bioorganic & Medicinal Chemistry Letters. 2002;12(4):697–699. doi: 10.1016/s0960-894x(01)00832-0. [DOI] [PubMed] [Google Scholar]
- 45.Hardej D, Ashby CR, Jr., Khadtare NS, Kulkarni SS, Singh S, Talele TT. The synthesis of phenylalanine-derived C5-substituted rhodanines and their activity against selected methicillin-resistant Staphylococcus aureus (MRSA) strains. European Journal of Medicinal Chemistry. 2010;45(12):5827–5832. doi: 10.1016/j.ejmech.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Chen ZH, Zheng CJ, Sun LP, Piao HR. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. European Journal of Medicinal Chemistry. 2010;45(12):5739–5743. doi: 10.1016/j.ejmech.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Tomasic T, Zidar N, Premru MM, Kikelj D, Masic LP. Synthesis and antibacterial activity of 5-ylidenethiazolidin-4-ones and 5-benzylidene-4,6-pyrimidinediones. European Journal of Medicinal Chemistry. 2010;45(4):1667–1672. doi: 10.1016/j.ejmech.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Orchard MG, Neuss JC, Galley CMS, et al. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT1) Bioorganic and Medicinal Chemistry Letters. 2004;14(15):3975–3978. doi: 10.1016/j.bmcl.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Sortino M, Delgado P, Juárez S, et al. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorganic and Medicinal Chemistry. 2007;15(1):484–494. doi: 10.1016/j.bmc.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 50.Sing WT, Lee CL, Yeo SL, Lim SP, Sim MM. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorganic & Medicinal Chemistry Letters. 2001;11(2):91–94. doi: 10.1016/s0960-894x(00)00610-7. [DOI] [PubMed] [Google Scholar]
- 51.Rajamaki S, Innitzer A, Falciani C, et al. Exploration of novel thiobarbituric acid-, rhodanine- and thiohydantoin-based HIV-1 integrase inhibitors. Bioorganic and Medicinal Chemistry Letters. 2009;19(13):3615–3618. doi: 10.1016/j.bmcl.2009.04.132. [DOI] [PubMed] [Google Scholar]
- 52.Cutshall NS, O'Day C, Prezhdo M. Rhodanine derivatives as inhibitors of JSP-1. Bioorganic & Medicinal Chemistry Letters. 2005;15(14):3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 53.Irvine MW, Patrick GL, Kewney J, Hastings SF, MacKenzie SJ. Rhodanine derivatives as novel inhibitors of PDE4. Bioorganic & Medicinal Chemistry Letters. 2008;18(6):2032–2037. doi: 10.1016/j.bmcl.2008.01.117. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Wu F, Chen L, et al. Evaluation of dihydropyrimidin-(2H)-one analogues and rhodanine derivatives as tyrosinase inhibitors. Bioorganic & Medicinal Chemistry Letters. 2011;21(8):2376–2379. doi: 10.1016/j.bmcl.2011.02.076. [DOI] [PubMed] [Google Scholar]
- 55.Ahn JH, Kim SJ, Park WS, et al. Synthesis and biological evaluation of rhodanine derivatives as PRL-3 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2006;16(11):2996–2999. doi: 10.1016/j.bmcl.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 56.Holman RR, Turner RC. In: Textbook of Diabetes. Pickup JC, Williams G, editors. London, UK: Blackwell Scientific Publications; 1991. pp. 462–476. [Google Scholar]
- 57.Depres JP, Lamarcha B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease . The New England Journal of Medicine. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 58.The Diabetes Control and Complications Trials (DCCT) Lymphocytic Infundibuloneurohypophysitis as a Cause of Central Diabetes Insipidus. The New England Journal of Medicine. 1993;329(supplement 2):683–689. doi: 10.1056/NEJM199309023291002. [DOI] [PubMed] [Google Scholar]
- 59.American Diabetic Association. Implications of the diabetes control and complications trial. Diabetes. 1993;43:1555–1558. doi: 10.2337/diab.42.11.1555. [DOI] [PubMed] [Google Scholar]
- 60.Harrower ADB. Comparison of efficacy, secondary failure rate, and complications of sulfonylureas . Journal of Diabetes and its Complications. 1994;8(4):201–203. doi: 10.1016/1056-8727(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 61.UK Prospective Diabetes Study Group. U.K. prospective diabetes study 16: overview of 6 tears' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 62.DeFronzo RA. Diabetes. 1998;37:255–297. [Google Scholar]
- 63.Sohda T, Mizuno K, Imamiya E, Sugiyama Y, Fujita T, Kawamatsu Y. Studies on antidiabetic agents. II. Synthesis of 5-[4-(1-Methylcyclohexylmethoxy)-benzyl] thiazolidine-2, 4-dione (ADD-3878) and its derivatives. Chemical & Pharmaceutical Bulletin. 1982;30(10):3580–3600. doi: 10.1248/cpb.30.3580. [DOI] [PubMed] [Google Scholar]
- 64.Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinologia Japonica. 1970;17(1):23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty) Diabetes. 1981;30(12):1045–1050. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- 66.Fujita T, Sugiyama Y, Taketomi S, et al. Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-Methylcyclohexylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, Ciglitazone), a new antidiabetic agent. Diabetes. 1983;32(9):804–810. doi: 10.2337/diab.32.9.804. [DOI] [PubMed] [Google Scholar]
- 67.Chang AY, Wyse BM, Gilchrist BJ. Ciglitazone, a new hypoglycemic agent. I. Studies in ob/ob and db/db mice, diabetic Chinese hamsters, and normal and streptozotocin-diabetic rats. Diabetes. 1983;32(9):830–838. doi: 10.2337/diab.32.9.830. [DOI] [PubMed] [Google Scholar]
- 68.Sohda T, Momose Y, Meguro K, Kawamatsu Y, Sugiyama Y, Ikeda H. Studies on antidiabetic agents. Synthesis and hypoglycemic activity of 5-[4-(pyridylalkoxy)benzyl]-2,4-thiazolidinediones. Arzneimittel-Forschung. 1990;40(1):37–42. [PubMed] [Google Scholar]
- 69.Sohda T, Mizuno K, Momose Y, Ikeda H, Fujita T, Meguro KJ. Studies on antidiabetic agents. 11. Novel thiazolidinedione derivatives as potent hypoglycemic and hypolipidemic agents. Journal of Medicinal Chemistry. 1992;35(14):2617–2626. doi: 10.1021/jm00092a012. [DOI] [PubMed] [Google Scholar]
- 70.Yoshioka T, Fujita T, Kanai T, et al. Studies on hindered phenols and analogs. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. Journal of Medicinal Chemistry. 1989;32(2):421–428. doi: 10.1021/jm00122a022. [DOI] [PubMed] [Google Scholar]
- 71.Cantello BCC, Cawthorne MA, Cottam GP, et al. [[ω-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. Journal of Medicinal Chemistry. 1994;37(23):3977–3985. doi: 10.1021/jm00049a017. [DOI] [PubMed] [Google Scholar]
- 72.Rami HK, Smith SA. Synthetic ligands for PPAR γ-review of patent literature 1994–1999. Expert Opinion on Therapeutic Patents. 2000;10(5):623–634. [Google Scholar]
- 73.Lohray BB, Bhushan V, Rao BP, et al. Novel euglycemic and hypolipidemic agents. Journal of Medicinal Chemistry. 1998;41(10):1619–1630. doi: 10.1021/jm970444e. [DOI] [PubMed] [Google Scholar]
- 74.Lohray BB, Lohray VB, Bajji AC, et al. (-)3-[4-[2-(phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxypropanoic acid [(-)DRF 2725]: a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. Journal of Medicinal Chemistry. 2001;44(16):2675–2678. doi: 10.1021/jm010143b. [DOI] [PubMed] [Google Scholar]
- 75.Oguchi M, Wada K, Honma H, et al. Molecular design, synthesis, and hypoglycemic activity of a series of thiazolidine-2,4-diones. Journal of Medicinal Chemistry. 2000;43(16):3052–3066. doi: 10.1021/jm990522t. [DOI] [PubMed] [Google Scholar]
- 76.Nomura M, Kinoshita S, Satoh H, et al. (3-Substituted benzyl)thiazolidine-2,4-diones as structurally new antihyperglycemic agents. Bioorganic & Medicinal Chemistry Letters. 1999;9(4):533–538. doi: 10.1016/s0960-894x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 77.Henke BR, Blanchard SG, Brackeen MF, et al. N-(2-benzoylphenyl)-L-tyrosine PPARγ agonists. 1. Discovery of a novel series of potent antihyperglycemic and antihyperlipidemic agents. Journal of Medicinal Chemistry. 1998;41(25):5020–5036. doi: 10.1021/jm9804127. [DOI] [PubMed] [Google Scholar]
- 78.Collins JL, Blanchard SG, Boswell GE, et al. N-(2-benzoylphenyl)-L-tyrosine PPARγ agonists. 2. Structure-activity relationship and optimization of the phenyl alkyl ether moiety. Journal of Medicinal Chemistry. 1998;41(25):5037–5054. doi: 10.1021/jm980413z. [DOI] [PubMed] [Google Scholar]
- 79.Cobb JE, Blanchard SG, Boswell EG, et al. N-(2-benzoylphenyl)-L-tyrosine PPARγ agonists. 3. Structure-activity relationship and optimization of the N-aryl substituent. Journal of Medicinal Chemistry. 1998;41(25):5055–5069. doi: 10.1021/jm980414r. [DOI] [PubMed] [Google Scholar]
- 80.Shinkai H, Onogi S, Tanaka M, et al. Isoxazolidine-3,5-dione and Noncyclic 1,3-Dicarbonyl Compounds as Hypoglycemic Agents. Journal of Medicinal Chemistry. 1998;41(11):1927–1933. doi: 10.1021/jm970771m. [DOI] [PubMed] [Google Scholar]
- 81.Reginato MJ, Bailey ST, Krakow SL, et al. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor γ-activating properties. The Journal of Biological Chemistry. 1998;273:32679–32684. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- 82.Hulin B, Newton LS, Lewis DM, Genereux PE, Gibbs EM, Clark DA. Hypoglycemic activity of a series of α-alkylthio and α-alkoxy carboxylic acids related to ciglitazone. Journal of Medicinal Chemistry. 1996;39(20):3897–3907. doi: 10.1021/jm960230h. [DOI] [PubMed] [Google Scholar]
- 83.Clark DA, Goldstein SW, Volkmann RA, et al. Substituted dihydrobenzopyran and dibydrobenzofuran thiazolidine-2,4-diones as hypoglycemic agents. Journal of Medicinal Chemistry. 1991;34(1):319–325. doi: 10.1021/jm00105a050. [DOI] [PubMed] [Google Scholar]
- 84.Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohda T. Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chemical & Pharmaceutical Bulletin. 1991;39(6):1440–1445. doi: 10.1248/cpb.39.1440. [DOI] [PubMed] [Google Scholar]
- 85.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. Sulfated glycans stimulate endocytosis of the cellular isoform of the prion protein, PrP, in cultured cells. Journal of Biological Chemistry. 1995;270:12953–12956. doi: 10.1074/jbc.270.50.30221. [DOI] [PubMed] [Google Scholar]
- 86.Isseman I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Molecular Endocrinology. 1992;6(10):1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 88.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willson TM, Cobb JE, Cowan DJ, et al. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. Journal of Medicinal Chemistry. 1996;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 90.Iwamoto Y, Kosaka K, Kuzuya T, Akanuma Y, Shigeta Y, Kaneko T. Effects of troglitazone: a new hypoglycemic agent in patients with NIDDM poorly controlled by diet therapy. Diabetes Care. 1996;19(2):151–156. doi: 10.2337/diacare.19.2.151. [DOI] [PubMed] [Google Scholar]
- 91.Rakowitz D, Maccari R, Ottana R, Vigorita MG. In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorganic & Medicinal Chemistry. 2006;14(2):567–574. doi: 10.1016/j.bmc.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 92.Madhavan GR, Chakrabarti R, Reddy KA, et al. Dual PPAR-α and -γ activators derived from novel benzoxazinone containing thiazolidinediones having antidiabetic and hypolipidemic potential . Bioorganic & Medicinal Chemistry. 2006;14(2):584–591. doi: 10.1016/j.bmc.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 93.Bozdağ-Dündar O, Evranos B, Daş-Evcimen N, Sarikaya M, Ertan R. Synthesis and aldose reductase inhibitory activity of some new chromonyl-2,4-thiazolidinediones. European Journal of Medicinal Chemistry. 2008;43(11):2412–2417. doi: 10.1016/j.ejmech.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Maccari R, Ciurleo R, Giglio M, et al. Identification of new non-carboxylic acid containing inhibitors of aldose reductase . Bioorganic & Medicinal Chemistry. 2010;18(11):4049–4055. doi: 10.1016/j.bmc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Patil V, Tilekar K, Mehendale-Munj S, Mohan R, Ramaa CS. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4- thiazolidinedione derivatives. European Journal of Medicinal Chemistry. 2010;45(10):4539–4544. doi: 10.1016/j.ejmech.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Barros CD, Amato AA, Oliveira TBD, et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorganic and Medicinal Chemistry. 2010;18(11):3805–3811. doi: 10.1016/j.bmc.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 97.Alagawadi KR, Alegaon SG. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-Thiazolidinediones bearing imidazo[2,1-b][1,3,4]thiadiazole moiety . Arabian Journal of Chemistry. 2011;4(4):465–472. [Google Scholar]
- 98.Liu X-F, Zheng C-J, Sun L-P, Liu X-K, Piao H-R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. European Journal of Medicinal Chemistry. 2011;46(8):3469–3473. doi: 10.1016/j.ejmech.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Jeong T-S, Kim J-R, Kim KS, Cho K-H, Bae K-H, Lee WS. Inhibitory effects of multi-substituted benzylidenethiazolidine-2,4-diones on LDL oxidation. Bioorganic & Medicinal Chemistry. 2004;12(15):4017–4023. doi: 10.1016/j.bmc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Hossain SU, Bhattacharya S. Synthesis of O-prenylated and O-geranylated derivatives of 5-benzylidene2,4-thiazolidinediones and evaluation of their free radical scavenging activity as well as effect on some phase II antioxidant/detoxifying enzymes. Bioorganic & Medicinal Chemistry Letters. 2007;17(5):1149–1154. doi: 10.1016/j.bmcl.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 101.Ottanà R, MacCari R, Giglio M, et al. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. European Journal of Medicinal Chemistry. 2011;46(7):2797–2806. doi: 10.1016/j.ejmech.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 102.Hu B, Ellingboe J, Gunawan I, et al. 2,4-Thiazolidinediones as potent and selective human β3 agonists. Bioorganic and Medicinal Chemistry Letters. 2001;11(6):757–760. doi: 10.1016/s0960-894x(01)00063-4. [DOI] [PubMed] [Google Scholar]
- 103.Bhattarai BR, Kafle B, Hwang J-S, et al. Thiazolidinedione derivatives as PTP1B inhibitors with antihyperglycemic and antiobesity effects. Bioorganic & Medicinal Chemistry Letters. 2009;19(21):6161–6165. doi: 10.1016/j.bmcl.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 104.Bhattarai BR, Kafle B, Hwang J-S, et al. Novel thiazolidinedione derivatives with anti-obesity effects: dual action as PTP1B inhibitors and PPAR-γ activators . Bioorganic & Medicinal Chemistry Letters. 2010;20(22):6758–6763. doi: 10.1016/j.bmcl.2010.08.130. [DOI] [PubMed] [Google Scholar]
- 105.Ying W, Tai H-H, Cho H. Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors . Bioorganic & Medicinal Chemistry. 2010;18(4):1428–1433. doi: 10.1016/j.bmc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 106.Komatsu T, Hirano T, Songkram C, Kawachi E, Kagechika H. Novel thyroid hormone receptor antagonists with an N-alkylated diphenylamine skeleton. Bioorganic & Medicinal Chemistry. 2007;15(9):3115–3126. doi: 10.1016/j.bmc.2007.02.053. [DOI] [PubMed] [Google Scholar]


