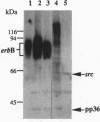
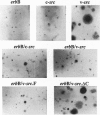
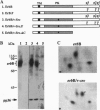
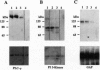
Abstract
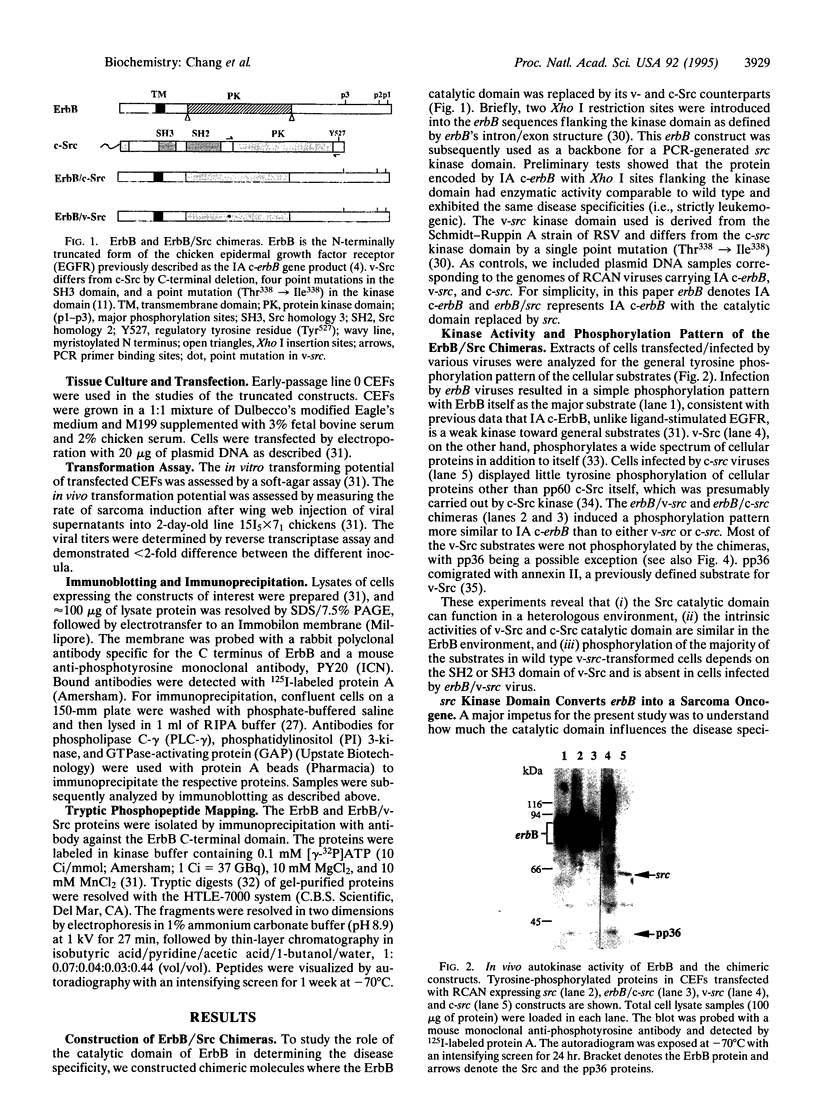
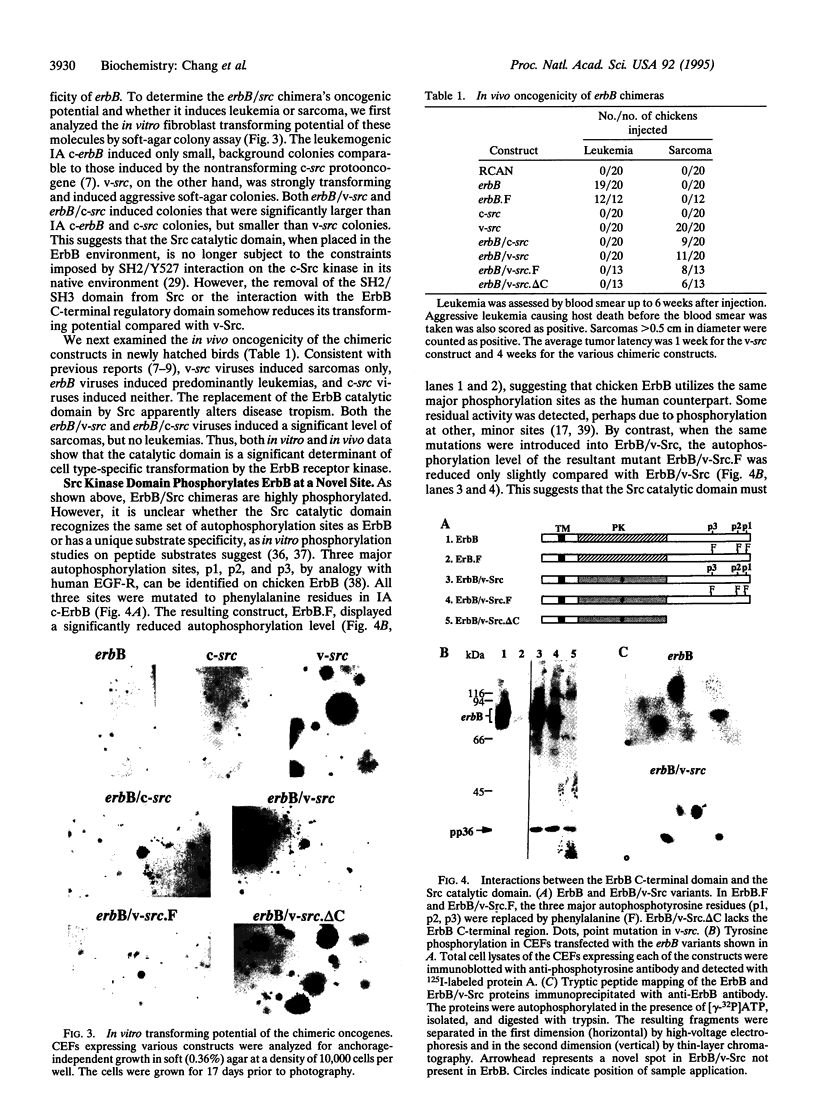
src and erbB are two tyrosine kinase-encoding oncogenes carried by retroviruses, which have distinct disease specificities. The former induces predominantly sarcomas, and the latter, leukemias. Src and ErbB have similar catalytic domains but have very different regulatory domains. A wealth of information exists concerning how different regulatory domains [Src homology 2 (SH2) and SH3 domains and autophosphorylation sites] control substrate and disease specificities. Whether the catalytic domain helps determine these specificities remains to be explored. Here we show that the Src catalytic domain is enzymatically active when substituted into the ErbB backbone and interacts with the ErbB regulatory domain. This ErbB/Src chimera displays autophosphorylation and substrate phosphorylation patterns different from those of both Src and ErbB. Neither SH2 and SH3 nor autophosphorylation sites are required for the Src catalytic domain to exert its fibroblast transforming ability. Most significantly, the catalytic domain can convert erbB from a leukemogenic oncogene into a sarcomagenic oncogene, suggesting that the leukemogenic determinants in part reside within the ErbB catalytic domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., el-Gendy K., Zhang S., Racker E. Control of src kinase activity by activators, inhibitors, and substrate chaperones. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7061–7065. doi: 10.1073/pnas.87.18.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. B., Hanafusa H. Closing in on SH2 specificity. Science. 1993 Dec 3;262(5139):1522–1524. doi: 10.1126/science.7504323. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Anderson S., Dexter T. M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984 Mar;36(3):763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Bouton A. H., Kanner S. B., Vines R. R., Wang H. C., Gibbs J. B., Parsons J. T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol. 1991 Feb;11(2):945–953. doi: 10.1128/mcb.11.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Callaghan T., Antczak M., Flickinger T., Raines M., Myers M., Kung H. J. A complete description of the EGF-receptor exon structure: implication in oncogenic activation and domain evolution. Oncogene. 1993 Nov;8(11):2939–2948. [PubMed] [Google Scholar]
- Carpenter G. Receptor tyrosine kinase substrates: src homology domains and signal transduction. FASEB J. 1992 Nov;6(14):3283–3289. doi: 10.1096/fasebj.6.14.1385243. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J. Transmembrane signaling by epidermal growth factor receptors lacking autophosphorylation sites. J Biol Chem. 1993 May 5;268(13):9176–9179. [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Requirement of phosphatidylinositol-3 kinase modification for its association with p60src. Mol Cell Biol. 1991 Apr;11(4):1972–1979. doi: 10.1128/mcb.11.4.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., O'Brien M. C., Hanafusa H. Deletions in the SH2 domain of p60v-src prevent association with the detergent-insoluble cellular matrix. Mol Cell Biol. 1991 Mar;11(3):1207–1213. doi: 10.1128/mcb.11.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991 May 15;266(14):8923–8931. [PubMed] [Google Scholar]
- Gotoh N., Tojo A., Muroya K., Hashimoto Y., Hattori S., Nakamura S., Takenawa T., Yazaki Y., Shibuya M. Epidermal growth factor-receptor mutant lacking the autophosphorylation sites induces phosphorylation of Shc protein and Shc-Grb2/ASH association and retains mitogenic activity. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):167–171. doi: 10.1073/pnas.91.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Helin K., Velu T., Martin P., Vass W. C., Allevato G., Lowy D. R., Beguinot L. The biological activity of the human epidermal growth factor receptor is positively regulated by its C-terminal tyrosines. Oncogene. 1991 May;6(5):825–832. [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Mutations in src homology regions 2 and 3 of activated chicken c-src that result in preferential transformation of mouse or chicken cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8592–8596. doi: 10.1073/pnas.87.21.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Most of the substrates of oncogenic viral tyrosine protein kinases can be phosphorylated by cellular tyrosine protein kinases in normal cells. Oncogene Res. 1988 Sep;3(2):105–115. [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Lee E. B., Beug H., Hayman M. J. Mutational analysis of the role of the carboxy-terminal region of the v-erbB protein in erythroid cell transformation. Oncogene. 1993 May;8(5):1317–1327. [PubMed] [Google Scholar]
- Liebl E. C., England L. J., DeClue J. E., Martin G. S. Host range mutants of v-src: alterations in kinase activity and substrate interactions. J Virol. 1992 Jul;66(7):4315–4324. doi: 10.1128/jvi.66.7.4315-4324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Cheng H. C., Wang J. H. Purification and characterization of a pp60c-src-related tyrosine kinase that effectively phosphorylates a synthetic peptide derived from p34cdc2. J Biol Chem. 1991 Feb 5;266(4):2557–2566. [PubMed] [Google Scholar]
- Margolis B. L., Lax I., Kris R., Dombalagian M., Honegger A. M., Howk R., Givol D., Ullrich A., Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem. 1989 Jun 25;264(18):10667–10671. [PubMed] [Google Scholar]
- McMahon M., Schatzman R. C., Bishop J. M. The amino-terminal 14 amino acids of v-src can functionally replace the extracellular and transmembrane domains of v-erbB. Mol Cell Biol. 1991 Sep;11(9):4760–4770. doi: 10.1128/mcb.11.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., LaBudda K., McGlade J., Hayman M. J. Analysis of the role of the Shc and Grb2 proteins in signal transduction by the v-ErbB protein. Mol Cell Biol. 1994 May;14(5):3253–3262. doi: 10.1128/mcb.14.5.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Nemeth S. P., Fox L. G., DeMarco M., Brugge J. S. Deletions within the amino-terminal half of the c-src gene product that alter the functional activity of the protein. Mol Cell Biol. 1989 Mar;9(3):1109–1119. doi: 10.1128/mcb.9.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pelley R. J., Moscovici C., Hughes S., Kung H. J. Proviral-activated c-erbB is leukemogenic but not sarcomagenic: characterization of a replication-competent retrovirus containing the activated c-erbB. J Virol. 1988 May;62(5):1840–1844. doi: 10.1128/jvi.62.5.1840-1844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A., Anderson S. M. Hematopoietic cell transformation by a murine recombinant retrovirus containing the src gene of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2374–2378. doi: 10.1073/pnas.81.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Radke K., Carter V. C., Moss P., Dehazya P., Schliwa M., Martin G. S. Membrane association of a 36,000-dalton substrate for tyrosine phosphorylation in chicken embryo fibroblasts transformed by avian sarcoma viruses. J Cell Biol. 1983 Nov;97(5 Pt 1):1601–1611. doi: 10.1083/jcb.97.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Lewis W. G., Crittenden L. B., Kung H. J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Moscovici M. G., Kung H. J. Molecular characterization of three erbB transducing viruses generated during avian leukosis virus-induced erythroleukemia: extensive internal deletion near the kinase domain activates the fibrosarcoma- and hemangioma-inducing potentials of erbB. J Virol. 1988 Jul;62(7):2444–2452. doi: 10.1128/jvi.62.7.2444-2452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Tracy S. E., Nair N., Taglienti-Sian C., Gamett D. C. Characterization of an angiosarcoma-inducing mutation in the erbB oncogene. Oncogene. 1992 Oct;7(10):2025–2030. [PubMed] [Google Scholar]
- Seidel-Dugan C., Meyer B. E., Thomas S. M., Brugge J. S. Effects of SH2 and SH3 deletions on the functional activities of wild-type and transforming variants of c-Src. Mol Cell Biol. 1992 Apr;12(4):1835–1845. doi: 10.1128/mcb.12.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. K., Chang C. M., Ravi L., Ling L., Castellano C. M., Walter E., Pelley R. J., Kung H. J. Modulation of erbB kinase activity and oncogenic potential by single point mutations in the glycine loop of the catalytic domain. Mol Cell Biol. 1994 Oct;14(10):6868–6878. doi: 10.1128/mcb.14.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. K., Pelley R. J., Kung H. J. Dissecting the activating mutations in v-erbB of avian erythroblastosis virus strain R. J Virol. 1991 Nov;65(11):6173–6180. doi: 10.1128/jvi.65.11.6173-6180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. K., Pelley R. J., Kung H. J. Tissue-specific transformation by epidermal growth factor receptor: a single point mutation within the ATP-binding pocket of the erbB product increases its intrinsic kinase activity and activates its sarcomagenic potential. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9103–9107. doi: 10.1073/pnas.87.23.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglienti-Sian C. A., Banner B., Davis R. J., Robinson H. L. Induction of renal adenocarcinoma by a nonmutated erbB oncogene. J Virol. 1993 Feb;67(2):1132–1136. doi: 10.1128/jvi.67.2.1132-1136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S. E., Woda B. A., Robinson H. L. Induction of angiosarcoma by a c-erbB transducing virus. J Virol. 1985 May;54(2):304–310. doi: 10.1128/jvi.54.2.304-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Chen W. S., Rosenfeld M. G., Gill G. N. Analysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J Biol Chem. 1990 Jan 25;265(3):1750–1754. [PubMed] [Google Scholar]
- Wang H. C., Parsons J. T. Deletions and insertions within an amino-terminal domain of pp60v-src inactivate transformation and modulate membrane stability. J Virol. 1989 Jan;63(1):291–302. doi: 10.1128/jvi.63.1.291-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A., Welsh J. B., Lazar C. S., Wiley H. S., Gill G. N., Rosenfeld M. G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990 Feb 23;247(4945):962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hihara H., Nishida T., Kawai S., Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983 Aug;34(1):225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]